Abstract
Osteoarthritis (OA) is a degenerative cartilage disease that is characterized by a local inflammatory reaction. Consequently, many studies have been performed to identify suitable prevention and treatment interventions. In recent years, both arthroscopic microfracture (AM) and stem cell therapy have been used clinically to treat OA. This study aimed to evaluate the clinical effects of AM in the presence and absence of a stromal vascular fraction (SVF) injection in the management of patients with OA. Thirty patients with grade 2 or 3 (Lawrence scale) OA of the knee participated in this study. Placebo group patients (n = 15) received AM alone; treatment group patients (n = 15) received AM and an adipose tissue‐derived SVF injection. The SVF was suspended in platelet‐rich plasma (PRP) before injection into the joint. Patient groups were monitored and scored with the Western Ontario and McMaster Universities Arthritis Index (WOMAC), Lysholm, Visual Analog Pain Scale (VAS), and modified Outerbridge classifications before treatment and at 6, 12, and 18 months post‐treatment. Bone marrow edema was also assessed at these time points. Patients were evaluated for knee activity (joint motion amplitude) and adverse effects relating to surgery and stem cell injection. Treatment efficacy was significantly different between placebo and treatment groups. All treatment group patients had significantly reduced pain and WOMAC scores, and increased Lysholm and VAS scores compared with the placebo group. These findings suggest that the SVF/PRP injection efficiently improved OA for 18 months after treatment. This study will be continuously monitored for additional 24 months. Stem Cells Translational Medicine 2017;6:187–195
Keywords: Osteoarthritis, Stromal vascular fraction, Platelet‐rich plasma, Arthroscopic microfracture
Significance Statement.
Arthroscopic microfracture (AM) and stem cell therapy have been used clinically to treat osteoarthritis (OA). This study evaluated the clinical effects of AM in the presence (treatment group) and absence (placebo group) of a stromal vascular fraction (SVF) injection in the knee for OA. The SVF was suspended in platelet‐rich plasma (PRP) before injection. Treatment efficacy differed significantly between placebo and treatment groups. All treatment group patients had significantly improved pain and arthritis index scores compared with the placebo group. These findings suggest that the SVF/PRP injection efficiently improved OA after 18 months. This study will be continuously monitored for 24 months.
Introduction
Osteoarthritis (OA) is a chronic progressive disease characterized by cartilage degeneration, osteophyte formation, bone reorganization, and loss of joint function 1. OA is the most frequent cause of disability among adults in the United States, and it occurred in >10% of the U.S. adult population in 2009. In 2009, 905,000 knee and hip replacements were carried out in OA patients, costing approximately $42.3 billion in total.
At present, OA is mainly treated with pharmaceuticals 2, 3, hyaluronic acid 4, and neridronate 5, 6. However, these treatments only reduce symptoms and pain or control the inflammation process 7 8 9; none of these drugs actually prevents the progression of OA 10, 11.
Arthroscopic microfracture (AM) has recently gained popularity as a therapy for OA 12 13 14, with some studies reporting significant symptom and functional improvement following the procedure 15. Consequently, AM is indicated as a routine treatment for OA. However, meta‐ and systematic analyses indicate that although AM initially improves OA symptoms 16, 17, this effect is only short term 16. In some cases, particularly among older people, AM can be harmful 16, 18, 19.
As an alternative approach, OA has been treated using platelet‐rich plasma (PRP). PRP contains the pool of cytokines and growth factors stored in platelets 20. Some studies have shown that PRP improves OA symptoms 21, 22. However, this effect has not been not observed for a prolonged period 22 23 24 25 26 27. To improve the effects of PRP, previous studies have investigated the combined injection of PRP with stem cells. Mesenchymal stem cells (MSCs) in conjunction with PRP have been found to mildly improve cartilage healing, and had improved Knee Injury and Osteoarthritis Outcome Score subscores and visual analog pain scores (VAS) compared with PRP‐only therapy 28. Using this approach, it is hypothesized that MSCs differentiate into chondrocytes, which participate directly in cartilage repair and also contribute to immune modulation to inhibit knee joint inflammation.
To date, various stem cell sources have been used to treat OA, such as bone marrow‐derived MSCs (BM‐MSCs) for autograft 29 30 31 32 or allograft 33, adipose‐derived stem cells (ADSCs) 34 35 36, and peripheral blood‐derived stem cells 37 38 39. Other MSC sources include enriched mononuclear cells (MNCs) from bone marrow or umbilical cord blood, stromal vascular fractions (SVFs) from adipose tissue (AT) and purified MSCs obtained from culture‐expanded MNCs.
In their published study, Enea et al. 40 combined autologous bone marrow‐derived cells with microfracture to repair cartilage defects. Their results showed that single‐stage treatment of focal cartilage defects of the knee with microfracture followed by coverage with a polyglycolic acid (PGA)‐hyaluronic acid (HA) matrix augmented with autologous BMCs (PGA‐HA‐CMBMC) was safe and improved knee function. To date, no clinical studies have compared the efficacy of arthroscopic surgery with and without SVF injection in the treatment of OA. This study, therefore, aimed to evaluate the clinical effects of AM alone and in combination with SVF injection on the function and satisfaction of patients with OA.
Materials and Methods
All experimental protocols were approved by the National Ethical Committee Ministry of Health, Vietnam. This study was registered at clinicaltrials.gov with identifier NCT02142842.
Inclusion and Exclusion Criteria
All patients enrolled in this study were required to sign a consent form. Patient inclusion criteria were as follows: patients must be older than 18 years, have OA with grade 2 to 3 cartilage degeneration at the time of presentation, failed drug treatment and autologous cartilage transplantation, a Lysholm score less than 65, committed with an artheroplasty condition, and be HIV negative.
A total of 30 patients were enrolled in the study: 15 patients were treated using traditional AM and 15 patients were treated with AM plus an injected mixture of SVF and PRP. The follow‐up time was 18 months for all patients.
Liposuction
Patients were restricted from taking corticosteroids, aspirin, nonsteroidal anti‐inflammatory drugs and oriental herbal medications for a minimum of 1 week before liposuction. For the liposuction, patients were given spinal anesthesia with 2–3 ml (5 g/L) of bupivacaine hydrochloride. The lower abdomen was also anesthetized. Liposuction was performed using a tumescent solution (500 ml of normal saline and 0.5 ml of 1:1,000 epinephrine). We used a TriPort Harvester cannula (Tulip Medical Products, San Diego, CA, http://www.tulipmedical.com) and a 60‐ml BD Luer‐Lock syringe (BD Biosciences, East Rutherford, NJ, http://www.bd.com) to harvest 100–500 ml of adipose tissue from each patient.
SVF Isolation
The SVF was isolated from the abdominal adipose tissue of each patient. Approximately 100 ml of lipoaspirate collected from each patient was divided into two 50‐ml sterile syringes. The syringes were stored in a sterile box at 2–8°C and immediately transferred to the laboratory. The SVF was isolated using an ADSC Extraction Kit (GeneWorld, Ho Chi Minh City, Vietnam, http://geneworld.vn) according to the manufacturer's instructions. Briefly, 100 ml of lipoaspirate was placed in a sterile, disposable 250‐ml conical centrifuge tube (Corning Life Sciences, Tewksbury, MA, https://www.corning.com) and washed twice with sterile phosphate‐buffered saline (PBS) by centrifugation at 400g for 5 minutes at room temperature. The adipose tissue was then digested using SuperExtract Solution (GeneWorld) containing collagenase at 37°C, for 30 minutes with agitation at 5‐minute intervals. The suspension was centrifuged again at 800g for 10 minutes, and the SVF was harvested as a pellet. The pellet was washed twice with PBS to remove any residual enzyme, and resuspended in PBS so that the cell quantity and viability could be measured using an automatic cell counter (NucleoCounter; Chemometec, Lillerød, Denmark, https://chemometec.com).
Activated PRP Preparation
Activated PRP was derived from the peripheral blood of the same patients as the adipose tissue, using a New‐PRP Pro Kit (GeneWorld) according to the manufacturer's guidelines. Briefly, 20 ml of peripheral blood was collected in vacuum tubes and centrifuged at 800g for 10 minutes. The plasma fraction was collected and centrifuged at 1,000g for 5 minutes to produce a platelet pellet. Most of the plasma was then removed, leaving 3 ml of plasma for resuspension of the platelets. The inactivated PRP was then activated using activating tubes containing 100 µl of 20% CaCl2.
Preparation of Product for Injection
The final injection product was composed of a mixture of the harvested SVF and activated PRP. Activated PRP was used to dilute the SVF to achieve a suitable dose for injection at 107 SVF cells/ml.
AM and SVF/PRP Injection
All patients in both groups received AM, which was used to confirm the degree of OA in each patient. Local chondral lesions were removed using medical instruments and an arthroscopic shaver. Microfractures were performed in accordance with the methods described by Steadman et al. 41. The 30 patients were grouped into a treatment group and a placebo group (n = 15 per group). After arthroscopic marrow stimulation by AM, the water flow was stopped and excess water was aspirated from the joint cavity. In the treatment group, the SVF and activated PRP mixture (5 ml per knee) was injected. Patients in the placebo group were injected with saline.
Follow‐Up and Evaluation
Patients were monitored in the hospital for 1 week postinjection. During this time, all complications, including shock, infection, and inflammation, were noted. After this, patients were followed for 18 months. Western Ontario and McMaster Universities Arthritis Index (WOMAC), Lysholm, and VAS scores were assessed 1, 6, 12, and 18 months after surgery. Radiographic imaging and magnetic resonance imaging (MRI) were performed 6 and 12 months post‐treatment. In this study, we used the modified VAS scores. with 4 indicating no pain; 3, mild pain; 2, moderate pain; 1, severe pain; and 0, worst pain possible.
Patients began continuous passive motion 4–5 days post‐treatment. Partial weight bearing was permitted at 2 weeks, progressing to full weight bearing 4 weeks after surgery. Isometric quadriceps and hamstring training with straight‐leg raises were advised during the non‐weight‐bearing period. Light sport activities such as swimming, cycling, or jogging on even, soft ground were permitted at 6 months. Permission to participate in unrestricted sports activity was given after 12 months.
Statistical Analysis
Results were expressed as the mean ± SD. One‐way analysis of variance and two‐tailed t tests were used for all statistical analyses, which were performed with GraphPad Prism 4.0 (GraphPad Software, La Jolla, CA, https://www.graphpad.com). p values <.05 were considered statistically significant.
Results
Patient Characteristics
This study was performed from April 2013 to September 2015 at two hospitals (Van Hanh General Hospital and 115 Hospital, both in Ho Chi Minh City, Vietnam). The 30 patients who satisfied the study standard were divided into 2 groups: placebo (n = 15) and treatment (n = 15). Demographic analysis found that these groups had an equivocal age, body mass index, sex, and Kellgren‐Lawrence OA grade (Table 1). The Kellgren‐Lawrence grade was based on x‐rays, and was confirmed during AM ( supplemental online Fig. 1).
Table 1.
Study participant demographic characteristics
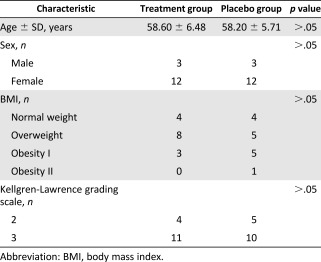
|
Adverse Effects
No adverse events were observed during the study in either group. We identified four cases with complications not related to the AM or SVF injection; these complications included high blood pressure, chest pain, dyspnea, and urinary retention.
Changes in WOMAC Scores
Figure 1 shows the WOMAC score results. Pretreatment WOMAC scores were equivocal, with a small nonsignificant difference observed between the placebo and treatment group (47.27 ± 17.13 vs. 42.87 ± 16.29, respectively; p > .05). At 6 and 12 months after treatment, the WOMAC scores in both groups significantly decreased compared with the pretreatment scores. In the placebo group, WOMAC scores decreased from 47.27 ± 17.13 to 23.27 ± 15.61 and 25.60 ± 19.69 at 6 and 12 months after surgery, respectively. In the treatment group, WOMAC scores decreased from 42.87 ± 16.19 to 19.27 ± 14.87 and 17.33 ± 14.91 at 6 and 12 months after surgery, respectively. At 6 and 12 months after surgery, the differences in the WOMAC scores between the treatment and placebo groups were nonsignificant (p > .05). However, a slight difference was observed between the 2 groups 12 months after surgery. WOMAC scores in the treatment group gradually decreased at 6 and 12 months compared with the pretreatment scores, although the WOMAC score 12 months after surgery was slightly increased compared with the score 6 months after the procedure.
Figure 1.
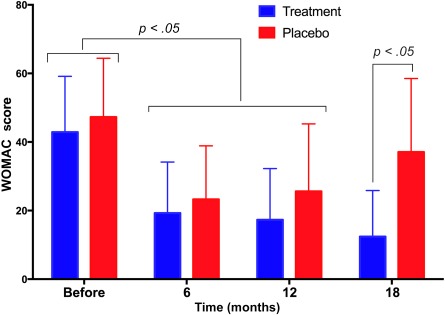
WOMAC scores in placebo and treatment groups at 6, 12, and 18 months post‐treatment. After 6 months, WOMAC scores significantly decreased in both the treated and placebo groups. At 12 and 18 months, WOMAC scores continued to decrease in the treatment group and increased in the placebo group. Abbreviation: WOMAC, Western Ontario and McMaster Universities Arthritis Index.
The difference in the WOMAC scores of the placebo and treatment groups became more pronounced after 18 months of monitoring. In the placebo group, the WOMAC score increased from 25.60 ± 19.69 at 12 months to 37.08 ± 21.45 at 18 months. More importantly, WOMAC scores at 18 months in the placebo group were not significantly different compared with pretreatment scores. The WOMAC scores of the treatment group decreased at 6, 12, and 18 months (19.27 ± 14.87, 17.33 ± 14.91, and 12.40 ± 13.44, respectively) after surgery compared with the pretreatment score (42.87 ± 16.29). The 18‐month WOMAC scores were also significantly different between the placebo and treatment groups (p < .05; Fig. 1).
Changes in Lysholm Scores
The results presented in Figure 2 show that Lysholm scores changed in both the treatment and placebo groups, but in opposite directions. The Lysholm scores increased significantly in both groups 6 months post‐treatment compared with the pretreatment score (p < .05). In the placebo group, however, the Lysholm scores were decreased dramatically 18 months after surgery to a level comparable to the pretreatment score (75.80 ± 16.05, 76.47 ± 12.44, and 65.17 ± 14.74 at 6, 12, and 18 months, respectively, compared with 64.13 ± 10.19 pretreatment). In the treatment group, the Lysholm scores gradually increased over 6, 12, and 18 months compared with pretreatment scores (80.53 ± 7.86, 82.13 ± 8.98, 84.73 ± 19.54, and 53.47 ± 14.56, respectively). At 18 months, the mean Lysholm score of the placebo and treatment groups was significantly different (p < .05).
Figure 2.
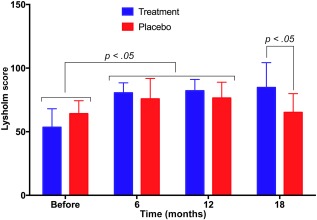
Lysholm scores in placebo and treatment groups at 6, 12, and 18 months post‐treatment. In both treated and placebo groups, the Lysholm score significantly increased at 6 months post‐treatment. At 12 and 18 months post‐treatment, the Lysholm scores of the treatment group continued to increase, whereas those of the placebo group gradually decreased.
Changes in VAS Scores
Similar to the Lysholm scores, VAS scores in both the treatment and placebo groups changed, but in opposite directions (Fig. 3). In the placebo group, VAS scores significantly increased after 6 months compared with those at pretreatment (2.67 ± 0.62 vs. 1.40 ± 0.51, respectively; p < .05). However, the scores then decreased from 12 to 18 months (2.53 ± 0.83 and 2.08 ± 1.08, respectively). In the treatment group, VAS scores continuously increased from 1.60 ± 0.83 at pretreatment to 3.01 ± 0.59, 3.20 ± 0.68, and 3.47 ± 0.74 at 6, 12, and 18 months, respectively (p < .05).
Figure 3.
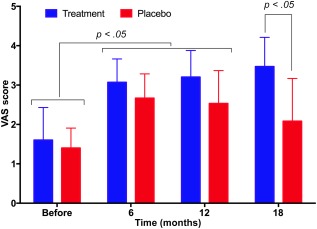
VAS scores at pretreatment and 6, 12, and 18 months post‐treatment in the placebo and treatment groups. VAS scores in the treatment group gradually increased post‐treatment. In the placebo group, scores increased after 6 months and gradually decreased at 12 and 18 months. Abbreviation: VAS, Visual Analog Pain Scale.
Cartilage Injury Evaluation by MRI
Based on the MRI results and the Outerbridge classification system (OS), changes in cartilage injury were recorded and are presented in Figure 4A. OS scores gradually increased in the placebo group from pretreatment to 6, 12, and 18 months post‐treatment (2.67 ± 1.35, 2.93 ± 1.34, and 3.20 ± 1.08, respectively). However, scores decreased in the treatment group from pretreatment to 12 months post‐treatment (3.33 ± 0.97 vs. 2.93 ± 0.88, respectively).
Figure 4.
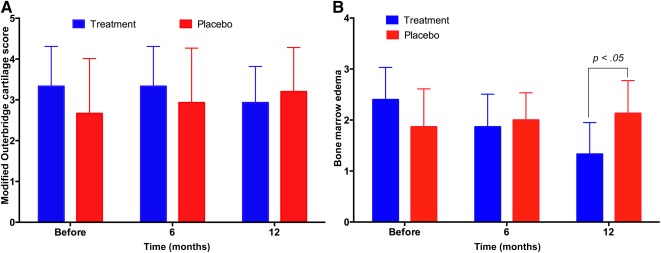
OS and BME scores at pretreatment and 6 and 12 months post‐treatment. Although the changes were nonsignificant, OS scores increased in the placebo group and decreased in the treatment group (A); and BME was significantly decreased in the treatment group 12 months after surgery, and only slightly increased in the placebo group (B). Abbreviations: BME, bone marrow edema; OS, Outerbridge classification system.
Although differences in OS scores were nonsignificant (p > .05), the trend was clearly different between the two groups: OS scores increased in the placebo group over time but decreased in the treatment group. MRI imaging demonstrated that the cartilage layer was thicker in the treatment group 12 months after AM ( supplemental online Fig. 3).
Bone Marrow Edema
Bone marrow edema (BME) was also recorded based on the MRI results. The results presented in Figure 4B and supplemental online Figure 2 show that BME was considerably deceased 12 months after surgery in the treatment group, although it was moderately increased in the placebo group. In the treatment group, BME gradually decreased from pretreatment to 6 and 12 months post‐treatment (2.40 ± 0.63, 1.86 ± 0.64, and 1.33 ± 0.62, respectively), with a significant difference at 12 months (p < .05).
In the placebo group, BME increased moderately at 6 to 12 months post‐treatment compared with pretreatment measurements (1.87 ± 0.74 at pretreatment vs. 2.00 ± 0.53; 2.13 ± 0.64 at 6 to 12 months post‐treatment, respectively).
Correlating OA Stage With Treatment Efficacy
Although the number of patients included in this study was low, we were able to evaluate the relative efficacy of AM plus SVP/PRP treatment between patients with stage 2 (n = 4) and stage 3 (n = 11) OA.
The results presented in Figure 5A and 5B shows that the SVF/PRP injection affected patients with stage 2 and 3 OA differently with respect to both WOMAC and Lysholm scores, with significant differences observed at 18 months post‐treatment. Although the WOMAC and Lysholm scores were significantly improved in both stage 2 and 3 OA groups at 18 months post‐treatment compared with pretreatment, only in stage 2 OA patients were both WOMAC and Lysholm scores significantly improved at 18 months compared with 12 months post‐treatment (p < .05).
Figure 5.
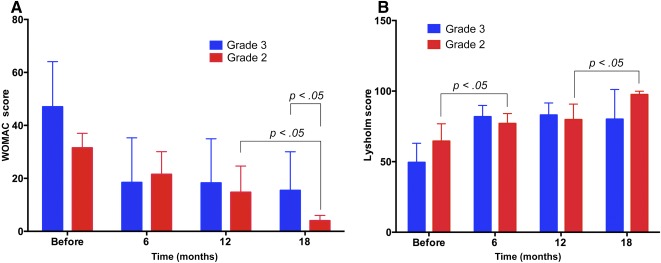
WOMAC and Lysholm scores in stage 2 and 3 osteoarthritis (OA; treatment group). Stromal vascular fraction and platelet‐rich plasma injection significantly improved WOMAC and Lysholm scores in patients with stage 2 OA compared with those with stage 3 disease. Abbreviations: OA, osteoarthritis; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
When we separately compared the stage 2 and stage 3 treatment groups with the placebo group, the differences became clearer (Fig. 6). Compared with the stage 2 OA members of the placebo group, the stage 2 treatment group had significantly improved WOMAC and Lysholm scores. Compared with the stage 3 OA placebo group, the stage 3 treatment group was improved but to a lesser extent. Patients in the stage 2 treatment group continuously improved in both their WOMAC and Lysholm scores at 12 and 18 months post‐treatment, whereas the improvement rate was slower in the stage 3 OA group.
Figure 6.
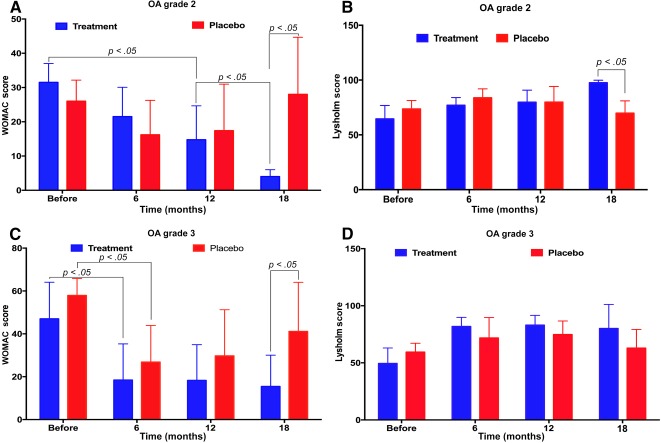
WOMAC and Lysholm scores in stage 2 and 3 OA treated and placebo groups. Stage 2 patients improved more rapidly compared with stage 3 patients. Abbreviations: OA, osteoarthritis; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
Changes in Knee Joint Function
The knee joint function of treated patients was significantly improved at 18 months post‐treatment, and their joint motion amplitude (JMA) increased from 116.2 ± 27.1 at pretreatment to 138.8 ± 12.0 at 18 months post‐treatment. JMA also increased in the placebo group from 120.6 ± 24.3 pretreatment to 133.3 ± 17.9 at 18 months post‐treatment but to a lesser extent than in the treatment group.
Discussion
AM is the conventional method to treat cartilage degeneration, including OA lesions. However, the benefits of AM are gradually lost in the 18 months following treatment. This study aimed to combine the AM approach with an injection of SVF and PRP to improve treatment efficacy. Autologous ADSCs and autologous PRP from the peripheral blood were used in this study. Although previous studies used allogeneic‐derived MSCs to effectively improve OA, we used an autologous source to minimize the side effects relating to host factors, specifically inflammation.
Both SVF and ADSCs (the purified form of SVF) have been used clinically in the treatment of conditions such as multiple sclerosis 42, femoral head necrosis 43, 44, chronic myocardial ischemia 45, critical limb ischemia, progressive supranuclear palsy 46, and acute respiratory distress syndrome 47. Our results indicate that AM with a combined SVF/PRP injection significantly improved and prolonged the treatment efficacy of AM for OA. At 6 months post‐treatment, the WOMAC, Lysholm, and VAS scores were significantly improved compared with pretreatment scores. These scores were further and significantly improved at 12 and 18 months post‐treatment in the SVF/PRP group. Some of the patients obtained scores similar to that of healthy individuals. The WOMAC is a widely used, proprietary set of standardized questionnaires used by health professionals to evaluate the condition of patients with OA of the knee and hip, including pain, stiffness, and joint function. Higher WOMAC scores correspond with a higher level of pain, stiffness, and functional limitation. In the treatment group, the mean WOMAC score was 12.40 ± 13.44 at 18 months after surgery. The WOMAC Index is sensitive to change and, therefore, is considered a suitable scale to assess OA.
In addition to the WOMAC Index, the Lysholm scale is one of the most commonly used scoring systems for measuring OA. It was first published in 1982 and comprises 8 questions designed to evaluate joint instability in younger patients. This scale measures disability and focuses on the patient's perception of their ability to perform activities of daily living, as well as various intensities of physical activity 48. According to this scale, a score of 84–90 is considered a good result. The average Lysholm score of patients in the AM plus SVF/PRP group 18 months after treatment was 84.73 ± 19.54.
Supporting the change seen in the WOMAC and Lysholm scores, the VAS scale scores also showed clear improvements in the treatment group. The VAS is a psychometric response scale that can be used in questionnaires. It is a measurement approach for subjective characteristics or attitudes that cannot be directly measured. The VAS scale for pain is divided into 4 points: 4 (no pain), 3 (mild pain), 2 (moderate pain), and 0 (severe pain). The WOMAC, Lysholm, and VAS scores demonstrated that at 18 months post‐treatment, all patients in the treatment group had significantly improved pain, movement, and capacity for physical activity. Some patients’ scores appeared similar to those of healthy individuals.
AM resulted in significantly reduced pain and improved knee function 6 months after the procedure, and these persisted for up to 12 months. However, by 18 months post‐AM, the symptoms of OA in the majority of patients reverted back to pretreatment levels. These results support those of several published studies. Thorlund et al. 16 reviewed 1,789 reports of AM used in degenerative knees. They found that AM had a small, inconsequential benefit in the management of OA, was effective for a limited time, and any benefits were absent 1 to 2 years after surgery. Furthermore, in patients with moderate to severe OA of the knee, Risberg 18 showed that the addition of arthroscopy to a regimen of physiotherapy and medication did not improve the physical function, pain, or health‐related quality of life of patients with OA.
Our results showed that SVF in combination with PRP significantly improved the outcomes of AM for OA of the knee. SVF and PRP not only maintained and prolonged the effects of AM, but also increased overall treatment efficacy. All WOMAC, Lysholm, and VAS scores were noticeably improved compared with AM alone at 6 and 12 months post‐treatment.
From the MRI results, we showed that OS scores and BME were significantly improved at 12 months post‐treatment. Whereas OS scores and BME improved after AM in the placebo group, both of these indicators were decreased in the treatment group. In particular, BME was significantly decreased at 12 months post‐treatment. OS classification is a grading system for joint cartilage breakdown: grade 0 represents normal joint cartilage; grade 1 represents cartilage with softening and swelling; grade 2 represents a partial‐thickness defect with fissures on the surface that do not reach the subchondral bone or exceed 1.5 cm in diameter; grade 3 represents fissuring to the level of subchondral bone in an area with a diameter more than 1.5 cm; and grade 4 represents exposed subchondral bone. Our results showed that the OS scores decreased from 3.33 ± 0.97 pretreatment to 2.93 ± 0.88 at 12 months post‐treatment in the treatment group. These results showed that the cartilage layer was thicker 12 months after the knee was injected with SVF and PRP, a finding congruent with our previously published study 36. Other studies have shown that SVF in combination with PRP stimulates cartilage regeneration, with a thicker cartilage layer observed using post‐treatment MRI evaluation 34, 44. We have shown in a mouse model that SVF and PRP can stimulate knee cartilage regeneration 49. The impact of a SVF/PRP injection in our study was also similar to effects noted in canine 50 51 52, rabbit 53, 54, horse 55, rat 56, and goat 57 models. Cartilage regeneration in these models was attributed to neocartilage triggered by SVF and PRP. In a rabbit model, Dragoo et al. 58 showed that autologous ADSCs were able to re‐establish the joint surface in rabbits. They found neocartilage was present in 100% of treated rabbits (12 of 12), whereas only 8% of control rabbits (1 of 12) had neocartilage.
The mechanisms of action of SVF and ADSCs have been investigated in previous studies. In 2003, Gimble and Guilak 57 showed that injected ADSCs were able to protect and heal injured cartilage. Other benefits of ADSCs have been reported for cartilage regeneration, including anti‐inflammatory properties 59, 60 and immune modulation. ADSCs can produce and secrete cytokines and growth factors that can trigger chondrogenesis, including transforming growth factor‐β (TGF‐β), bone morphogenic protein 2 (BMP‐2), BMP‐4, BMP‐7, insulin‐like growth factor 1, and fibroblast growth factor 2 (FGF‐2). ADSCs also produce cytokines that modulate the recipient immune system, including TGF‐β, hepatocyte growth factor, nitric oxide, indolamine‐2,3‐dioxygenase, TNF‐α 61 and interferon‐γ 62, 63. In vitro, cultured ADSCs suppress the host's immune response and the T‐cell proliferation as effectively as do BM‐MSCs 61, 64. Further studies have demonstrated that ADSCs actually stimulate a lesser proliferative response than do allogeneic PBMCs, but a similar response to BM‐MSCs 65 66 67. These findings suggest that ADSCs can replace BM‐MSCs in the field of regenerative medicine 61.
The anti‐inflammatory roles of ADSCs and PRP were also confirmed in our study by the obvious improvement of BME in the treatment group. BME is a condition characterized by the accumulation of excessive fluid in bone marrow‐related structures. BME is a predictor for the progression of knee OA in the compartment ipsilateral to the bone marrow lesion 68. BME was significantly reduced and the cartilage layer thickness was increased in the SVF/PRP‐treatment group, indicating that OA was significantly improved. The increased BME observed in the placebo group may have been related to the progression of OA and inflammation after AM.
Cartilage regeneration in OA knees following AM and the combined SVF/PRP injection was likely because of the combination of SVF and PRP. However, SVF is likely to be the main contributor to this healing response. PRP has been used to treat knee OA in previous studies 69 70 71, but almost all of these studies showed that PRP significantly reduced short‐term pain without concurrent cartilage regeneration 21, 69, 71, 72. In combination with ADSCs, PRP can improve chondrogenesis in vitro and in vivo 73. The components of PRP play important roles in stimulating grafted and endogenous cell growth and differentiation. PRP contains at least six known growth factors, including: platelet‐derived growth factor, which promotes blood vessel growth and cell division; TGF‐β, which promotes cell mitosis and bone metabolism; vascular endothelial growth factor, which promotes blood vessel formation; epidermal growth factor, which promotes cell growth and differentiation, angiogenesis, and collagen formation; FGF‐2, which promotes cell differentiation and angiogenesis; and IGF, which is a regulator of all of the body's cell types 74 75 76.
We also observed that the regeneration response of cartilage to injected SVF/PRP was different between patients with grade 2 and 3 OA. Both WOMAC and Lysholm scores showed that the recovery of patients with grade 2 OA was faster than that of those with grade 3 disease. In particular, the improvement of WOMAC and Lysholm scores in patients with OA grade 2 were significant at 18 months compared with 12 months post‐treatment. This demonstrated that OA grade 2 was treated with higher efficacy than OA grade 3 following SVF/PRP injection. Although this study was limited with respect to the sample size of patients with either grade 2 or 3 OA, these results are similar to other treatment options for OA, such as HA and PRP injections 24, 25.
Finally, JMA was compared between treated and placebo group patients. JMA was clearly increased in the treatment group compared with the placebo group, which agrees with both our subjective and radiographic analyses. More importantly, almost all patients in the treatment group exhibited a JMA similar to healthy individuals. The mean JMA was 138.8 ± 12 at 18 months post‐treatment. The mean JMA of healthy individuals has been reported to be 140.0 (range, 113.9–166.4) 77.
We believe that our study is the first to evaluate AM with and without SVF for OA treatment with an 18‐month follow‐up time. Although Freitag et al. 78 recently performed a similar study to ours, their follow‐up time was only 12 months.
Conclusion
This study showed that AM with SVF/PRP injection was effective for knee OA and had better long‐term outcomes than AM alone. Our preliminary analysis also showed that grade 2 knee OA was improved to a greater extent than grade 3 disease following AM with SVF injection. AM with SVF injection significantly improved WOMAC, Lysholm, and VAS scores over the entire 18‐month study period. MRI findings showed that the regenerated cartilage layer of patients treated with AM and SVF was thicker at 12 and 18 months after the procedure. Furthermore, the JMA of SVF/PRP‐treatment patients 18 months after surgery was significantly improved and comparable with that of healthy individuals. No adverse effects were recorded in any treated patients. From these findings, we conclude that AM with SVF/PRP injection may be a suitable treatment for grade 2 and 3 OA of the knee.
Author Contributions
P.D.N.: conception and design, administrative support, provision of study material or patients; T.D.‐X.T., H.T.‐N.N., and H.T.V.: provision of study material or patients, collection and/or assembly of data; P.T.‐B.L.: conception and design, administrative support, provision of study material or patients, collection and/or assembly of data; N.L.‐C.P. and N.B.V.: provision of study material or patients; N.K.P.: conception and design, data analysis and interpretation; P.V.P.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Supporting Information
Acknowledgments
This study was funded in part by GeneWord Ltd.
Authored by a member of IFATS.
References
- 1. Wieland HA, Michaelis M, Kirschbaum BJ et al. Osteoarthritis ‐ an untreatable disease?. Nat Rev Drug Discov 2005;4:331–344. [DOI] [PubMed] [Google Scholar]
- 2. Subedi N, Chew NS, Chandramohan M et al. Effectiveness of fluoroscopy‐guided intra‐articular steroid injection for hip osteoarthritis. Clin Radiol 2015;70:1276–1280. [DOI] [PubMed] [Google Scholar]
- 3. Debbi EM, Agar G, Fichman G et al. Efficacy of methylsulfonylmethane supplementation on osteoarthritis of the knee: A randomized controlled study. BMC Complement Altern Med 2011;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Monticone M, Frizziero A, Rovere G et al. Hyaluronic acid intra‐articular Injection and exercise therapy: Effects on pain and disability in subjects affected by lower limb joints osteoarthritis. The Italian Society of Physical and Rehabilitation Medicine (SIMFER) systematic review. Eur J Phys Rehabil Med 2016;52:389–399. [PubMed] [Google Scholar]
- 5. Varenna M, Zucchi F, Failoni S et al. Intravenous neridronate in the treatment of acute painful knee osteoarthritis: A randomized controlled study. Rheumatology (Oxford) 2015;54:1826–1932. [DOI] [PubMed] [Google Scholar]
- 6. Varenna M, Adami S, Rossini M et al. Treatment of complex regional pain syndrome type I with neridronate: A randomized, double‐blind, placebo‐controlled study. Rheumatology (Oxford) 2013;52:534–542. [DOI] [PubMed] [Google Scholar]
- 7. Li X, Shah A, Franklin P et al. Arthroscopic debridement of the osteoarthritic knee combined with hyaluronic acid (Orthovisc) treatment: A case series and review of the literature. J Orthop Surg 2008;3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kotevoglu N, Iyibozkurt PC, Hiz O et al. A prospective randomised controlled clinical trial comparing the efficacy of different molecular weight hyaluronan solutions in the treatment of knee osteoarthritis. Rheumatol Int 2006;26:325–330. [DOI] [PubMed] [Google Scholar]
- 9. Varenna M, Zucchi F, Failoni S et al. Intravenous neridronate in the treatment of acute painful knee osteoarthritis: A randomized controlled study. Rheumatology (Oxford) 2015;54:1826–1832. [DOI] [PubMed] [Google Scholar]
- 10. Altman R, Fredericson M, Bhattacharyya SK et al. Association between hyaluronic acid injections and time‐to‐total knee replacement surgery. J Knee Surg 2015. (in press). [DOI] [PubMed] [Google Scholar]
- 11. Waddell DD, Bricker DC. Total knee replacement delayed with Hylan G‐F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm 2007;13:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moseley JB, O'Malley K, Petersen NJ et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002;347:81–88. [DOI] [PubMed] [Google Scholar]
- 13. Dervin GF, Stiell IG, Rody K et al. Effect of arthroscopic débridement for osteoarthritis of the knee on health‐related quality of life. J Bone Joint Surg Am 2003;85‐A:10–19. [DOI] [PubMed] [Google Scholar]
- 14. Laupattarakasem W, Laopaiboon M, Laupattarakasem P et al. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev 2008;1:CD005118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diduch DR, Scuderi GR, Scott WN et al. The efficacy of arthroscopy following total knee replacement. Arthroscopy 1997;13:166–171. [DOI] [PubMed] [Google Scholar]
- 16. Thorlund JB, Juhl CB, Roos EM et al. Arthroscopic surgery for degenerative knee: Systematic review and meta‐analysis of benefits and harms. Br J Sports Med 2015;49:1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giri S, Santosha Singh CA et al. Role of arthroscopy in the treatment of osteoarthritis of knee. J Clin Diagn Res 2015;9:RC08–RC11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Risberg MA. Arthroscopic surgery provides no additional benefit over physiotherapy and medication for the treatment of knee osteoarthritis. Aust J Physiother 2009;55:137. [DOI] [PubMed] [Google Scholar]
- 19. Kirkley A, Birmingham TB, Litchfield RB et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2008;359:1097–1107. [DOI] [PubMed] [Google Scholar]
- 20. Ra Hara G, Basu T. Platelet‐rich plasma in regenerative medicine. Biomed Res Ther 2014;1:25–31. [Google Scholar]
- 21. Gobbi A, Karnatzikos G, Mahajan V et al. Platelet‐rich plasma treatment in symptomatic patients with knee osteoarthritis: Preliminary results in a group of active patients. Sports Health 2012;4:162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kon E, Mandelbaum B, Buda R et al. Platelet‐rich plasma intra‐articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: From early degeneration to osteoarthritis. Arthroscopy 2011;27:1490–1501. [DOI] [PubMed] [Google Scholar]
- 23. Meheux CJ, McCulloch PC, Lintner DM et al. Efficacy of intra‐articular platelet‐rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy 2016;32:495–505. [DOI] [PubMed] [Google Scholar]
- 24. Raeissadat SA, Rayegani SM, Hassanabadi H et al. Knee osteoarthritis injection choices: Platelet‐rich plasma (PRP) versus hyaluronic acid (a one‐year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord 2015;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Filardo G, Kon E, Di Martino A et al. Platelet‐rich plasma vs hyaluronic acid to treat knee degenerative pathology: Study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord 2012;13:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khoshbin A, Leroux T, Wasserstein D et al. The efficacy of platelet‐rich plasma in the treatment of symptomatic knee osteoarthritis: A systematic review with quantitative synthesis. Arthroscopy 2013;29:2037–2048. [DOI] [PubMed] [Google Scholar]
- 27. Vaquerizo V, Plasencia MA, Arribas I et al. Comparison of intra‐articular injections of plasma rich in growth factors (PRGF‐Endoret) versus Durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: A randomized controlled trial. Arthroscopy 2013;29:1635–1643. [DOI] [PubMed] [Google Scholar]
- 28. Koh YG, Kwon OR, Kim YS et al. Comparative outcomes of open‐wedge high tibial osteotomy with platelet‐rich plasma alone or in combination with mesenchymal stem cell treatment: A prospective study. Arthroscopy 2014;30:1453–1460. [DOI] [PubMed] [Google Scholar]
- 29. Buda R, Castagnini F, Cavallo M et al. “One‐step” bone marrow‐derived cells transplantation and joint debridement for osteochondral lesions of the talus in ankle osteoarthritis: Clinical and radiological outcomes at 36 months. Arch Orthop Trauma Surg 2016;136:107–116. [DOI] [PubMed] [Google Scholar]
- 30. Emadedin M, Ghorbani Liastani M, Fazeli R et al. Long‐term follow‐up of intra‐articular injection of autologous mesenchymal stem cells in patients with knee, ankle, or hip osteoarthritis. Arch Iran Med 2015;18:336–344. [PubMed] [Google Scholar]
- 31. Orozco L, Munar A, Soler R et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation 2013;95:1535–1541. [DOI] [PubMed] [Google Scholar]
- 32. Davatchi F, Abdollahi BS, Mohyeddin M et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis 2011;14:211–215. [DOI] [PubMed] [Google Scholar]
- 33. Vega A, Martín‐Ferrero MA, Del Canto F et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015;99:1681–1690. [DOI] [PubMed] [Google Scholar]
- 34. Koh YG, Jo SB, Kwon OR et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 2013;29:748–755. [DOI] [PubMed] [Google Scholar]
- 35. Jo CH, Lee YG, Shin WH et al. Intra‐articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof‐of‐concept clinical trial. STEM CELLS 2014;32:1254–1266. [DOI] [PubMed] [Google Scholar]
- 36. Van Pham P, Bui KH‐T, Duong TD et al. Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet‐rich plasma: A clinical study. Biomed Res Ther 2014;1:02–08. [Google Scholar]
- 37. Skowroński J, Skowroński R, Rutka M. Cartilage lesions of the knee treated with blood mesenchymal stem cells ‐ results. Ortop Traumatol Rehabil 2012;14:569–577. [DOI] [PubMed] [Google Scholar]
- 38. Saw KY, Anz A, Merican S et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: A report of 5 cases with histology. Arthroscopy 2011;27:493–506. [DOI] [PubMed] [Google Scholar]
- 39. Saw KY, Anz A, Siew‐Yoke Jee C et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: A randomized controlled trial. Arthroscopy 2013;29:684–694. [DOI] [PubMed] [Google Scholar]
- 40. Enea D, Cecconi S, Calcagno S et al. Single‐stage cartilage repair in the knee with microfracture covered with a resorbable polymer‐based matrix and autologous bone marrow concentrate. Knee 2013;20:562–569. [DOI] [PubMed] [Google Scholar]
- 41. Steadman JR, Briggs KK, Rodrigo JJ et al. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11‐year follow‐up. Arthroscopy 2003;19:477–484. [DOI] [PubMed] [Google Scholar]
- 42. Riordan NH, Ichim TE, Min WP et al. Non‐expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med 2009;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Namazi H.. Autologous adipose tissue‐derived stem cells induce persistent bone‐like tissue in osteonecrotic femoral heads: A molecular mechanism. Pain Physician 2012;15:E345; author reply E345. [PubMed] [Google Scholar]
- 44. Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose‐tissue‐derived stem cells: A case series. J Med Case Reports 2011;5:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Qayyum AA, Haack‐Sørensen M, Mathiasen AB et al. Adipose‐derived mesenchymal stromal cells for chronic myocardial ischemia (MyStromalCell Trial): study design. Regen Med 2012;7:421–428. [DOI] [PubMed] [Google Scholar]
- 46. Choi SW, Park KB, Woo SK et al. Treatment of progressive supranuclear palsy with autologous adipose tissue‐derived mesenchymal stem cells: A case report. J Med Case Reports 2014;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zheng G, Huang L, Tong H et al. Treatment of acute respiratory distress syndrome with allogeneic adipose‐derived mesenchymal stem cells: A randomized, placebo‐controlled pilot study. Respir Res 2014;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med 1982;10:150–154. [DOI] [PubMed] [Google Scholar]
- 49. Van Pham P, Hong‐Thien Bui K, Quoc Ngo D et al. Transplantation of nonexpanded adipose stromal vascular fraction and platelet‐rich plasma for articular cartilage injury treatment in mice model. J Med Eng 2013;2013:832396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Black LL, Gaynor J, Adams C et al. Effect of intraarticular injection of autologous adipose‐derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther 2008;9:192–200. [PubMed] [Google Scholar]
- 51. Black LL, Gaynor J, Gahring D et al. Effect of adipose‐derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: A randomized, double‐blinded, multicenter, controlled trial. Vet Ther 2007;8:272–284. [PubMed] [Google Scholar]
- 52. Guercio A, Di Marco P, Casella S et al. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol Int 2012;36:189–194. [DOI] [PubMed] [Google Scholar]
- 53. Toghraie FS, Chenari N, Gholipour MA et al. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit. Knee 2011;18:71–75. [DOI] [PubMed] [Google Scholar]
- 54. Oliveira JT, Gardel LS, Rada T et al. Injectable gellan gum hydrogels with autologous cells for the treatment of rabbit articular cartilage defects. J Orthop Res 2010;28:1193–1199. [DOI] [PubMed] [Google Scholar]
- 55. Frisbie DD, Kisiday JD, Kawcak CE et al. Evaluation of adipose‐derived stromal vascular fraction or bone marrow‐derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res 2009;27:1675–1680. [DOI] [PubMed] [Google Scholar]
- 56. Lee J‐M, Im G‐I. SOX trio‐co‐transduced adipose stem cells in fibrin gel to enhance cartilage repair and delay the progression of osteoarthritis in the rat. Biomaterials 2012;33:2016–2024. [DOI] [PubMed] [Google Scholar]
- 57. Gimble JM, Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol 2003;58:137–160. [DOI] [PubMed] [Google Scholar]
- 58. Dragoo JL, Carlson G, McCormick F et al. Healing full‐thickness cartilage defects using adipose‐derived stem cells. Tissue Eng 2007;13:1615–1621. [DOI] [PubMed] [Google Scholar]
- 59. Caimi PF, Reese J, Lee Z et al. Emerging therapeutic approaches for multipotent mesenchymal stromal cells. Curr Opin Hematol 2010;17:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Singer NG, Caplan AI. Mesenchymal stem cells: Mechanisms of inflammation. Annu Rev Pathol 2011;6:457–478. [DOI] [PubMed] [Google Scholar]
- 61. Yoo KH, Jang IK, Lee MW et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol 2009;259:150–156. [DOI] [PubMed] [Google Scholar]
- 62. Chan JL, Tang KC, Patel AP et al. Antigen‐presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon‐γ. Blood 2006;107:4817–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Krampera M, Cosmi L, Angeli R et al. Role for interferon‐γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. STEM CELLS 2006;24:386–398. [DOI] [PubMed] [Google Scholar]
- 64. McIntosh K, Zvonic S, Garrett S et al. The immunogenicity of human adipose‐derived cells: temporal changes in vitro. STEM CELLS 2006;24:1246–1253. [DOI] [PubMed] [Google Scholar]
- 65. Augello A, Tasso R, Negrini SM et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol 2005;35:1482–1490. [DOI] [PubMed] [Google Scholar]
- 66. Mitchell JB, McIntosh K, Zvonic S et al. Immunophenotype of human adipose‐derived cells: temporal changes in stromal‐associated and stem cell‐associated markers. STEM CELLS 2006;24:376–385. [DOI] [PubMed] [Google Scholar]
- 67. Klyushnenkova E, Mosca JD, Zernetkina V et al. T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. J Biomed Sci 2005;12:47–57. [DOI] [PubMed] [Google Scholar]
- 68. Felson DT, McLaughlin S, Goggins J et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med 2003;139:330–336. [DOI] [PubMed] [Google Scholar]
- 69. Sampson S, Reed M, Silvers H et al. Injection of platelet‐rich plasma in patients with primary and secondary knee osteoarthritis: A pilot study. Am J Phys Med Rehabil 2010;89:961–969. [DOI] [PubMed] [Google Scholar]
- 70. Patel S, Dhillon MS, Aggarwal S et al. Treatment with platelet‐rich plasma is more effective than placebo for knee osteoarthritis: A prospective, double‐blind, randomized trial. Am J Sports Med 2013;41:356–364. [DOI] [PubMed] [Google Scholar]
- 71. Filardo G, Kon E, Buda R et al. Platelet‐rich plasma intra‐articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2011;19:528–535. [DOI] [PubMed] [Google Scholar]
- 72. Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop 2014;5:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Van Pham P, Bui KH, Ngo DQ et al. Activated platelet‐rich plasma improves adipose‐derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Res Ther 2013;4:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Marx RE. Platelet‐rich plasma (PRP): What is PRP and what is not PRP?. Implant Dent 2001;10:225–228. [DOI] [PubMed] [Google Scholar]
- 75. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet‐rich plasma. Am J Sports Med 2011;39:2135–2140. [DOI] [PubMed] [Google Scholar]
- 76. Zhu Y, Yuan M, Meng HY et al. Basic science and clinical application of platelet‐rich plasma for cartilage defects and osteoarthritis: A review. Osteoarthritis Cartilage 2013;21:1627–1637. [DOI] [PubMed] [Google Scholar]
- 77. Walker CRC, Myles C, Nutton R et al. Movement of the knee in osteoarthritis. The use of electrogoniometry to assess function. J Bone Joint Surg Br 2001;83:195–198. [DOI] [PubMed] [Google Scholar]
- 78. Freitag J, Ford J, Bates D et al. Adipose derived mesenchymal stem cell therapy in the treatment of isolated knee chondral lesions: Design of a randomised controlled pilot study comparing arthroscopic microfracture versus arthroscopic microfracture combined with postoperative mesenchymal stem cell injections. BMJ Open 2015;5:e009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


