Abstract
Human umbilical cord blood‐derived mesenchymal stem cells (hUCB‐MSCs) exhibit potency for the regeneration of infarcted hearts. Vascular endothelial growth factor (VEGF) is capable of inducing angiogenesis and can boost stem cell‐based therapeutic effects. However, high levels of VEGF can cause abnormal blood vessel growth and hemangiomas. Thus, a controllable system to induce therapeutic levels of VEGF is required for cell therapy. We generated an inducible VEGF‐secreting stem cell (VEGF/hUCB‐MSC) that controls the expression of VEGF and tested the therapeutic efficacy in rat myocardial infarction (MI) model to apply functional stem cells to MI. To introduce the inducible VEGF gene cassette into a safe harbor site of the hUCB‐MSC chromosome, the transcription activator‐like effector nucleases system was used. After confirming the integration of the cassette into the locus, VEGF secretion in physiological concentration from VEGF/hUCB‐MSCs after doxycycline (Dox) induction was proved in conditioned media. VEGF secretion was detected in mice implanted with VEGF/hUCB‐MSCs grown via a cell sheet system. Vessel formation was induced in mice transplanted with Matrigel containing VEGF/hUCB‐MSCs treated with Dox. Moreover, seeding of the VEGF/hUCB‐MSCs onto the cardiac patch significantly improved the left ventricle ejection fraction and fractional shortening in a rat MI model upon VEGF induction. Induced VEGF/hUCB‐MSC patches significantly decreased the MI size and fibrosis and increased muscle thickness, suggesting improved survival of cardiomyocytes and protection from MI damage. These results suggest that our inducible VEGF‐secreting stem cell system is an effective therapeutic approach for the treatment of MI. Stem Cells Translational Medicine 2017;6:1040–1051
Keywords: Myocardial infarction, Angiogenesis, Stem cells, Umbilical cord blood, Genome editing
Significance Statement.
Human umbilical cord‐derived mesenchymal stem cells harboring an angiogenesis gene, vascular endothelial growth factor (VEGF), in a safe harbor were generated. This gene cassette was specifically designed in a drug inducible manner. The engineered stem cells were able to produce the growth factor at a physiologically relevant concentration. Transplantation of the stem cells harboring the gene in a rat myocardial infarction model showed greatly improved protection from heart damage and amelioration of the tissue damage in the infarcted hearts. This study indicates that inducible VEGF‐secreting stem cells represent a potent therapeutic approach for the treatment of myocardial infarction.
Introduction
Myocardial infarction (MI) is a serious health and economic problem and a primary cause of mortality in developed countries. A primary therapy for this disease is cardiac regeneration, which represents a difficult and immense challenge 1, 2. During the process of MI, the coronary artery is occluded after plaque rupture. After a few weeks, the ischemic region is replaced with a thin fibrotic area that finally causes cardiac dysfunction 3. The cardiomyocytes in the ischemic site have a limited capacity for self‐regeneration, and their repopulation is almost impossible 4. Thus, other sources of cells and factors are required to regenerate the infarcted region of the heart.
Recently, stem cell‐based therapy including human umbilical cord blood‐derived mesenchymal stem cells (hUCB‐MSCs) has attracted interest for heart regeneration and restored the injured myocardium in certain degrees. Various strategies including biomaterials 5, inducing vasculogenesis 6, and tissue engineering 7 have been applied and the therapy showed positive effects, restoration of damaged cardiomyocytes, enhancement of cardiac function and a reduced infarct size to certain degrees, in preclinical trials 5, 8, 9. However, stem cell therapy has encountered various problems, such as low survival and proliferation rates of implanted cells and the reason of these limitations is an insufficient supply of oxygen and nutrient and the hostile microenvironment of infarcted region. Therefore, to acquire efficient and rapid therapeutic efficacy of stem cells, providing an intrinsic vascularization stimulus by growth factors is demanded.
Vascular endothelial growth factor (VEGF) is an important angiogenic factor that promotes the survival of endothelial cells (EC) and prevents EC apoptosis 10. VEGF can be used as a therapeutic reagent in ischemic injuries. Rat mesenchymal stem cells expressing VEGF transiently by adenovirus induced angiogenesis and protected the remaining cardiomyocytes in MI animal models 11. VEGF protein with hepatocyte growth factor (HGF) has also been demonstrated to have a cardioprotective effect by increasing the tolerance of cardiomyocytes to ischemia and reducing cardiomyocyte apoptosis 12. However, transient gene expression and protein injection are limited for long‐term therapeutic effect accomplishment in MI. Thus, for long‐term gene expression, retrovirus‐mediated gene delivery has been attempted. However, gene delivery through a retroviral vector for cell transduction also carries a risk of stem cell neoplastic transformation and oncogene activation by the long term repeat (LTR) promoter 13. Therefore, this long‐term expression system has a limit for therapeutic application and may cause tumors and insertional mutagenesis via the incorporation of the growth factor gene into a critical region of the host chromosome. Moreover, high and uncontrollable level of VEGF itself also has the potential to cause abnormal blood vessel formation and hemangiomas 14.
One way to overcome these issues is to use a controllable system for the transgene and to ensure integration into a safe harbor site in the chromosome. The tetracycline(Tet)‐controlled system is commonly used for gene regulation in mammalian cells 15. In this system, rtTA binds to the Tet‐On promoter in the presence of doxycycline (Dox) and activates Tet‐On/CMV‐driven gene transcription 16. For safe gene insertion into a safe harbor site of human chromosome, genome editing technologies have been used; zinc‐finger nucleases, transcription activator‐like effector nucleases (TALENs), and recent clustered regulatory interspaced short palindromic repeat (CRISPR)/Cas9 17. TALENs are targetable nucleases composed of a FokI nuclease domain and customizable DNA binding domain 18. The DNA‐binding domain has conserved repeats that originate from transcription activator‐like effectors found in Xanthomonas plant pathogens. TALENs have rapidly emerged as a genome editing technology with high efficiency and low toxicity that allow safe, targeted nonviral gene delivery to a specific chromosome locus 19. It is known that the human chromosome 19 adeno‐associated virus integration site 1 (AAVS1) integration site is a “safe harbor site” for the targeted insertion of foreign genes by producing DNA double‐strand breaks (DSBs) and subsequent repair via homologous recombination (HR) 20.
In this study, we developed inducible VEGF‐secreting hUCB‐MSCs by TALEN‐mediated VEGF gene integration into a safe harbor site of the chromosome. These engineered stem cells were capable of secreting VEGF at physiological concentration upon induction by Dox treatment. VEGF/hUCB‐MSCs were implanted in a MI rat model to assess whether they could enhance angiogenesis and provide a cardioprotective effect.
Materials and Methods
Isolation and Culture of Human UCB‐MSCs
Human UCB‐MSCs were isolated as previously described 21, 22. Human UCB‐MSC isolation was performed according to the procedure approved by the Borame Hospital Institutional Review Board and Seoul National University (IRB No. 0603/001‐002‐07C1). Briefly, UCB samples from term and preterm deliveries were harvested at the time of birth with the mother's informed consent (Seoul City Borame Hospital Cord Blood Bank). The UCB samples were mixed with the HetaSep solution (StemCell Technologies, Vancouver, Canada, https://www.stemcell.com) at a ratio of 5:1 and were then incubated at room temperature to deplete erythrocyte counts. The supernatant was carefully collected and mononuclear cells were obtained using Ficoll‐Paque PLUS (GE Healthcare Life Sciences, Marlborough, MA, http://www.gehealthcare.com) density‐gradient centrifugation at 2,500 rpm for 20 minutes. The cells were washed twice in phosphate buffered saline (PBS) and seeded in growth media consisted of RPMI 1640 medium (11875; Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com) containing 10% fetal bovine serum. The medium was changed at 48‐hour intervals and the cells were subcultured after they reached 90% confluence, unless described.
Designing New TALEN Vectors and Inducible VEGF Donor Vectors
Supporting Information Figure 1A shows the TALEN domains that consisted of specific binding sequences targeting the AAVS1 locus (safe harbor site) and the FokI nonspecific endonuclease domains. The left and right TALEN plasmids were newly constructed as follows. The target sequences of the chromosome 19 AAVS1‐targeting TALENs were: 5′‐TGGAGCCATCTCTCTCCTT‐3′ (Left)—gccagaacctctaa (spacer)—5′‐GGTTTGCTTACGATGGA‐3′ (Right). The plasmids encoding the TALENs targeting this sequence were prepared as previously described 23. To prepare the efficient targeting donor DNA, we designed several new 800 base pair (bp) homology arms. The initial targeting vector with the left and right homology arm (HA‐L and HA‐R) cassette containing the inducible VEGF gene did not produce a sufficient number of cells integrated with the gene cassette. Therefore, we redesigned several TALEN‐L/R targeting vectors along with several HA‐Ls and HA‐Rs in donor vectors and tested their efficiency. Finally, we could generate an efficient combination of TALEN‐L/R targeting vector and HA‐L and HA‐R donor vector cassette for the AAVS1 locus by giving a 50 bp space apart between the homology arms and the TALEN target sites. The homology arms were polymerase chain reaction (PCR)‐amplified from human genomic DNA and cloned into the pGEM T‐Easy vector. The left and right homology arms were isolated using pairs of restriction enzymes (KpnI/AgeI/NotI for the left and NotI/EcoRI/SphI for the right homology arms) and cloned into the KpnI/SphI site of the pUC19 vector.
The inducible donor vectors were constructed using three DNA fragments. The Tet‐on mini‐CMV promoter (Addgene, Cambridge, MA, https://www.addgene.org), VEGF cDNA (synthesized by Bioneer, Daejeon, Korea, http://www.bioneer.co.kr), and hEF1a‐rtTA‐pA (Addgene, Cambridge, MA, https://www.addgene.org) were amplified by PCR using specific primers containing the flanking sequences. Each amplified DNA fragment was cloned into the pZDonor‐AAVS1‐puromycin DNA vector (Sigma‐Aldrich, St. Louis, MO, https://www.sigmaaldrich.com) digested with the AgeI and EcoRI restriction enzymes (New England BioLabs, Ipswich, MA, https://www.neb.com) using the In‐Fusion HD Cloning Kit (TaKaRa Bio, Inc., Kusatsu, Japan, http://www.takara-bio.com) (Fig. 1). Then, the whole insert TetO‐CMV‐VEGF‐hEF1a‐rtTA was transferred intothe pUC19‐AAVS1 donor vector that contained newly designed HA‐L and HA‐R sequences to target to the chromosome 19 AAVS1 site.
Figure 1.
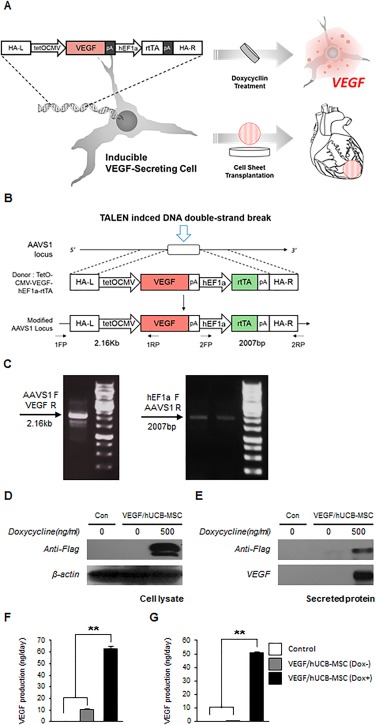
Generation of VEGF/hUCB‐MSCs and confirmation of conditional VEGF secretion. (A): Schematic illustration of producing inducible VEGF‐secreting hUCB‐MSC cells and cell sheet transplantation in the rat myocardial infarction model. The human UCB‐MSCs secreting VEGF were seeded onto a cell sheet for transplantation to the myocardial infarction site of rat hearts. (B): The integration of the inducible VEGF secretion DNA cassette into the AAVS1 site following the TALEN‐mediated DNA double‐strand break. HR (L) and HR (R), the left and right arms for homologous recombination. FP and RP, forward primer and reverse primer binding sites for the PCR primers. (C): Junction PCR analysis for the confirmation of the integrated inducible VEGF secretion cassette with two different PCR primer sets. For validation of the gene integration into the UCB‐MSC genome, two sets of primers were used to detect the specific sites; a 2.16 kb fragment with the AAVS1 (forward) and VEGF (reverse) region primers and a 2007 base pair (bp) fragment with thehEF1a (forward) and AAVS1 (reverse) region primers. (D): Western blotting analysis detected inducible VEGF expression in VEGF/hUCB‐MSC cell lysates and (E) inducible VEGF secretion in the VEGF/hUCB‐MSC culture media. The Dox treatment was maintained for 48 hours. (F): Quantification of the secreted VEGF in the VEGF/hUCB‐MSC (Dox+) culture media 2 days after transient transfection and (G) 2 weeks after stable transduction. The amount of VEGF production is indicated as ng per 2 × 106 cells per day on the Y‐axis. The error bars indicate the SEM of three replicate measurements per group. (*p < .05, **p < .01). Abbreviations: AAVS1, adeno‐associated virus integration site 1; Dox, doxycycline; hUCB‐MSCs, human umbilical cord blood‐derived mesenchymal stem cells; HA‐L, left homology arm; HA‐R, right homology arm; HR, homologous recombination; TALEN, transcription activator‐like effector nuclease; VEGF, vascular endothelial growth factor.
TALEN‐Mediated Homologous Recombination
TALEN‐mediated AAVS1 site integration of an inducible VEGF‐secreting cassette into a safe harbor site hUCB‐MSCs was accomplished in two steps (Fig. 1B). First, the TALEN left (L) and right (R) arms bind to their target sequences in the host chromosome and the FOKI endonuclease induces a DNA DSB. Then, the DSB is repaired by the HR machinery using a pUC19‐TetO‐CMV‐VEGF‐hEF1a‐rtTA donor vector. Cells (8 × 105) seeded on a 60‐mm dish were transfected with TALEN left (L), right (R) (1.5 μg) and the pUC19‐TetOn‐CMV‐VEGF‐hEF1a‐rtTA donor vector (3 μg). At 5 days post‐transfection, the medium was changed; then, the cells were subcultured two times at 4.5 day intervals. After 2 weeks, genomic DNA was isolated from the hUCB‐MSCs by adding 1 ml of the SNET extraction buffer per 106 cells (20 mM Tris‐HCl [pH 8], 5 mM EDTA [pH 8], 400 mM NaCl, and 1% SDS) containing 100mg/ml proteinase K (Sigma‐Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). The DNA samples were incubated at 55°C for 2 hours. Proteinase K was inactivated by incubating the DNA samples at 98°C for 10 minutes; then, RNase (1 mg/ml) was added and the cells were incubated at 37°C for 30 minutes. The DNA was precipitated and its concentration was determined by spectrophotometry. TALEN‐mediated HR was detected using PCR genotyping. PCR amplification of the genomic DNA was performed using the following parameters: an initial denaturation step at 94°C for 5 minutes, followed by 35 cycles at 94°C for 15 seconds, 65°C for 45 seconds, and 72°C for 150 seconds with a final extension step at 72°C for 10 minutes. The amplified products were analyzed on a 1% agarose gel. The amplification of 2160 bp and 2007 bp PCR fragments indicated the site‐specific integration of the inducible VEGF‐secreting donor cassette into the AAVS1 locus (Fig. 1C).
Preparation of Secreted Proteins and Western Blotting
Secreted proteins were collected from the VEGF/hUCB‐MSCs and hUCB‐MSCs. After culturing the cells in growth medium for 3 days, the cells were washed five times with serum‐free media and incubated with serum‐free media for 24 hours. Proteins in the conditioned media were precipitated with 10% (vol/vol) trichloroacetic acid (Sigma‐Aldrich) at 4°C. The protein pellets were air dried prior to use. Western blot analysis was performed as previously described 24. Briefly, hUCB‐MSCs were washed twice with cold PBS and lysed with RIPA buffer containing protease inhibitors (Roche, Basel, Switzerland, http://www.roche.com). For primary antibody labeling, rabbit anti‐flag antibody (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com) and rabbit anti‐human VEGF polyclonal antibody (1:2,000, AbClon, Seoul, Korea, http://abclon.com) were used. An horse radish peroxidase (HRP)‐conjugated anti‐rabbit IgG antibody (1:2,000, Enzo Life Sciences, Farmingdale, NY, http://www.enzolifesciences.com) was used as a secondary antibody.
VEGF165‐a Enzyme‐Linked Immunosorbent Assay (ELISA) Measurements
VEGF165‐a in the cell culture supernatants was quantified using a Quantikine mouse VEGF immunoassay ELISA kit (R&D Systems, Minneapolis, MN, https://www.rndsystems.com). The cells were washed twice with PBS, and the media was changed to keratinocyte serum‐free medium (KSFM) for the hUCB‐MSC and VEGF/hUCB‐MSC (Dox−) groups. For the VEGF/hUCB‐MSCs (Dox+) group, 500 ng/ml of Dox was added. After incubation for 1 day, 1 ml of medium in a 60‐mm dish was harvested from each group. The conditioned media was filtered and analyzed by ELISA in duplicate. The results were normalized for the number of cells in the dish and the time of exposure to the cells.
Subcutaneous Transplantation of VEGF/hUCB‐MSCs on Cell Sheets
VEGF/hUCB‐MSC cell sheets were subcutaneously transplanted on the mouse dorsal skin as previously described 25. Briefly, VEGF/hUCB‐MSCs were cultured on the UpCell surface at a density of 1 × 106 cells per 35‐mm dish. Six female mice were anesthetized and the dorsal skin was lifted and cut vertically. The cell sheet was transferred onto a polyvinylidene difluoride (PVDF) membrane and the PVDF membrane was slowly removed. Doxycycline was added to the drinking water at a concentration of 1 µg/ml. Eight days after transplantation, the animals were sacrificed for analysis.
Cell‐Matrigel Transplantation into Severe Combined Immunodeficiency (SCID) Mice
The hUCB‐MSC and VEGF/hUCB‐MSCs cell suspension was washed once with PBS to remove all traces of trypsin. After centrifugation at 300g for 5 minutes, the supernatant was decanted and the cell pellets were resuspended to yield a final concentration of 1 × 106 cells per 100 µl in Matrigel for transplantation into mice. The animal protocol was approved by the Seoul National University Institutional Animal Care and Use Committee (IACUC) prior to the experiment. Vascular formation was evaluated in 7‐ to 8‐week‐old female SCID mice (OrientBio, Seongnam, SouthKorea, www.orient.co.kr). Matrigel was loaded with a final concentration of 1 × 106 cells per 100 µl. The injection sites were cleaned with ethanol twice prior to injection of Cell‐Matrigel into each site using 1 ml syringes and 27 G needles. Eight days after the injections, the animals were sacrificed for analysis.
MI Rat Model and Cell Transplantation
The MI rat models were surgically induced as previously described 5. Briefly, Male Sprague‐Dawley rats weighting 260–300 g (OrientBio, Seongnam, SouthKorea, www.orient.co.kr) were used for the MI model. All animal procedures were performed in accordance with the guidelines of the SNU IACUC. After MI induction, the rats were divided into four treatment groups: rats that received VEGF/hUCB‐MSCs (n = 12, later divided into two groups), hUCB‐MSCs (n = 6), or a sham operation (Control, n = 6).
Immediately after the MI induction surgery, the UpCell sheets layered with VEGF/hUCB‐MSC or hUCB‐MSC cells on PIPAAm‐grafted surfaces were detached from the plates by incubating at 20°C for 20 minutes. Then, the cell sheets were attached to polypropylene supporting membranes (Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com) and transplanted onto the injured epicardial anterolateral area. Seven minutes after transplantation, the supporting membrane was removed and the cell sheets showed stable attachment. The same procedures were repeated to make a bilayer cell sheet composed of 2 × 106 hUCB‐MSCs and VEGF/hUCB‐MSCs cells. To prevent the rat's immune rejection of the human cells, all rats received cyclosporine as previously described 5.
Functional Assessment of the Infarcted Myocardium
The cardiac functional assessment was performed as previously reported by our group 5. Briefly, cardiac functions were assessed by transthoracic echocardiography prior to MI surgery (normal base line) and 1 week and 3 weeks after MI for each cell transplantation group.
Histological and Immunohistochemical Examinations
Histological and Immunohistochemical examinations were performed as previously reported by our group 5. Briefly, Hearts were harvested and fixed in 4% paraformaldehyde for 24 hours and then cut into four transverse slices through the infarcted area. The slices were embedded in paraffin, and 5 μm histologic sections were stained with Masson's Trichrome. The infarct size, fibrosis, and scar thickness were quantified using the ImageJ software (NIH, Bethesda, MD, USA).
Statistical Analysis
All statistical analyses were performed with SPSS version 20.0 (IBM, Armonk, NY, http://www.ibm.com). A Kruskal‐Wallis test was used to assess differences among the groups. The Mann‐Whitney U test was performed as the post hoc test. A p value less than .05 was considered to be statistically significant.
Results
Genetic Engineering of an Inducible VEGF‐Expressing DNA Cassette Into the hUCB‐MSC Chromosome by TALEN‐Mediated Genome Editing
Figure 1A illustrates production of inducible VEGF‐secreting hUCB‐MSC cells and cell sheet transplantation in the rat MI model. To confirm whether the inducible VEGF expression system from the VEGF/hUCB‐MSCs is properly induced by treatment with Dox, western blotting was performed on VEGF/hUCB‐MSC samples. VEGF expression and secretion were detected in the cell lysates (Fig. 1D) and secreted media (Fig. 1E) after Dox treatment for 48 hours. In the transient episomal plasmid transfection experiment, we observed the induction of the VEGF secretion into the media after Dox treatment by ELISA (Fig. 1F). Similar VEGF levels were measured in the VEGF/hUCB‐MSC cells with the inducible system integrated into the chromosome by TALEN‐mediated genome editing (Fig. 1G). Too high a continuous level of VEGF is known to result in angioma formation, which is one of the main obstacles for VEGF therapy. Thus, the VEGF concentration is the main consideration for the treatment of ischemic disease with VEGF. In our study, the VEGF concentration secreted into the media following induction was 50.74 ng/106 cells per day, which was within the physiological concentration range (32.3–70 ng/106 cells per day). It is known that the VEGF expression by viral infection continuously secreted VEGF but resulted in large variations (100–191.2 ng/106 cells per day) and the concentration could easily cause the hemangiomas 14. From our results, we confirmed that VEGF/hUCB‐MSCs harboring an inducible VEGF gene in a safe harbor site secreted VEGF into the cell culture media in the physiological therapeutic concentration range.
Increased Angiogenic Markers and Decreased Cell Cycle Inhibitor Expression by VEGF Induction
To examine the biological effects of VEGF secreted from VEGF/hUCB‐MSCs, we compared the expression levels of angiogenic markers and cell cycle‐related genes in each group by real‐time RT‐PCR. At 48 hours after the induction of VEGF secretion by Dox, the VEGF/hUCB‐MSCs showed a significant increase in the expression of the angiogenic markers FLK1, NRP1, and ANGPT1, and a reduction in the expression of the cell cycle arrest‐ and senescence‐related genes p21 and p16 (Fig. 2). These results indicate that the VEGF/hUCB‐MSCs exhibit EC‐like marker characteristics through VEGF autocrine effects.
Figure 2.
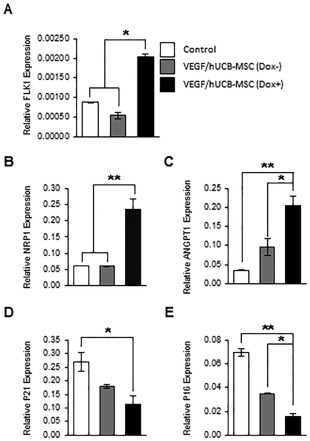
Effect of VEGF secretion from VEGF/hUCB‐MSCs on the gene expression of neoangiogenesis markers and cell cycle regulators. (A–C): Induced VEGF enhanced the expression of angiogenesis‐related markers. The mRNA levels of Flk‐1, NRP1, and ANGPT1 in VEGF/hUCB‐MSCs (Dox+) were evaluated by comparing to control hUCB‐MSCs (Con) and VEGF/hUCB‐MSCs (Dox−) using real‐time RT‐PCR. (D, E): Cell cycle regulation in VEGF/hUCB‐MSCs in response to secreted VEGF. Secreted VEGF from VEGF/hUCB‐MSCs (Dox+) decreased cell cycle inhibitors (p21 and p16), suggesting that VEGF had a positive effect on hUCB‐MSC survival. The experiments were performed in triplicates, and the data were presented as the means ± SE. (*p < .05, **p < .01, n = 3). Abbreviations: Dox, doxycycline; hUCB‐MSCs, human umbilical cord blood‐derived mesenchymal stem cells; clease; SE, standard error; VEGF, vascular endothelial growth factor.
Functional Assessment of Transplanted VEGF/hUCB‐MSCs in a Mouse Model
Prior to the application of VEGF/hUCB‐MSCs to the MI animal model, we first tested: (a) whether VEGF was secreted properly by the VEGF/hUCB‐MSCs upon Dox treatment in vivo and (b) whether VEGF secreted from VEGF/hUCB‐MSCs could enhance neoangiogenesis.
To confirm VEGF secretion by the implanted VEGF/hUCB‐MSCs, we divided the mice into three groups: the hUCB‐MSC cell only group, the VEGF/hUCB‐MSC without Dox group and the VEGF/hUCB‐MSC with Dox group. Using an UpCell transplantation system, hUCB‐MSC or VEGF/hUCB‐MSC cell sheets were transplanted onto the skin on the backs of the mice. For the VEGF/hUCB‐MSC (Dox+) group, the mice were freely administered Dox in their drinking water (1 μg/ml) for 4 days starting one day after UpCell transplantation of VEGF/hUCB‐MSCs (Fig. 3A). VEGF expression was detected in the transplanted subcutaneous tissues in the Dox (+) group mice by Western blot analysis using anti‐Flag and anti‐VEGF antibodies (Fig. 3B). The amount of secreted VEGF from the extracted subcutaneous tissue was quantified by ELISA. Significantly higher levels of VEGF were detected in the VEGF/hUCB‐MSC (Dox+) group mice (15.4 ng/mg) compared to the Dox− and cell only groups (Fig. 3C).
Figure 3.
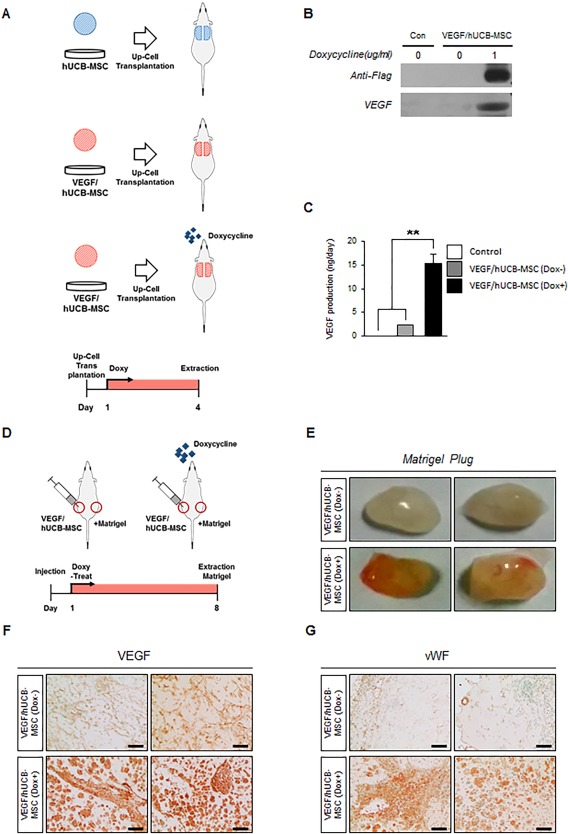
The induction of VEGF expression by VEGF/hUCB‐MSCs transplanted on mouse skin and neoangiogenesis by secreted VEGF in Matrigel plugs. (A): In vivo test design for the transplantation of the UpCell seeded with hUCB‐MSCs secreting VEGF. (B, C): Western blot and ELISA analysis for VEGF secreted at the UpCell transplantation site. Error bars indicate the SEM of three replicate measurements per clone or population. (**p < .01, n = 3). (D): Design of the blood vessel induction test in Matrigels with VEGF/hUCB‐MSCs. (E): Blood vessels formed in the Matrigel containing VEGF/hUCB‐MSCs without doxycycline (Dox−) and with doxycycline (Dox+). (F, G): Immunohistochemical staining for VEGF and newly formed vessels by vWF in Matrigel with VEGF/hUCB‐MSCs (Dox− or Dox+). (Scale bar: 100 μm). Abbreviations: Dox, doxycycline; hUCB‐MSCs, human umbilical cord blood‐derived mesenchymal stem cells; VEGF, vascular endothelial growth factor; vWF, von Willibrand factor.
To test the angiogenetic function of the VEGF/hUCB‐MSCs, we subcutaneously injected the mice with Matrigel mixed with VEGF/hUCB‐MSCs (Fig. 3D). The injected Matrigel plugs were removed 8 days after implantation. The plugs of the Dox‐fed mice showed a more reddish color compared with the group not provided Dox, indicating greatly enhanced angiogenesis (Fig. 3E). Immunostaining of the Matrigel with an anti‐VEGF antibody (Fig. 3F) and an anti‐von Willibrand factor (vWF) antibody (Fig. 3G) showed high levels of VEGF secretion and higher levels of blood vessel formation in the doxycycline‐treated group. These results demonstrated that the inducible VEGF‐secreting hUCB‐MSC cells secreted VEGF within normal physiological ranges and induced neo‐vascularization in vivo following doxycycline treatment.
Enhanced Cardiac Function by VEGF Secretion from VEGF/hUCB‐MSCs in the Rat MI Model
Next, we tested whether the VEGF/hUCB‐MSCs could enhance cardiac function upon VEGF induction in the rat MI model. Twenty‐four rats received MI surgery and the rats were divided into four group; Control, hUCB‐MSCs or VEGF/hUCB‐MSCs (Dox−), VEGF/hUCB‐MSCs (Dox+). After confirming the infarct by visual inspection, the cell sheets were transplanted into the infarcted region 30 minutes after MI induction. Echocardiography was performed to evaluate the therapeutic efficacy of VEGF secretion in combination with VEGF/hUCB‐MSC cells in the rat MI‐induced heart. To monitor the consistency of the infarction procedure and size, we compared left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) values of Pre‐MI (baseline) and Post‐MI using echocardiography and confirmed the consistency of infracted heart surgery (Supporting Information Fig. 2). Then, healthy heart function indicators (i.e., ejection fraction [EF], fractional shortening [FS], left ventricle inner diameter at diastole [LVIDd], and left ventricle inner diameter at systole [LVIDs]) were measured 1 week and 3 weeks after the coronary artery ligation and implantation (Fig. 4A, 4B). One week after transplantation of VEGF/hUCB‐MSCs (Dox+), the values of LVIDd, LVIDs, EF, and FS showed improved heart function compared with the hUCB‐MSC alone and VEGF/hUCB‐MSC (Dox−) groups. Further improved cardiac functions were observed 3 weeks after the treatment. Left ventricle (LV) dilation and dysfunction was reduced in the VEGF/hUCB‐MSC (Dox+) group compared to the control and other groups (Fig. 4C). Three weeks after implantation, the EF in the VEGF/hUCB‐MSC (Dox+) group (65.39 ± 3.5%) was much higher than the EF in the control (35.31 ± 4.10%), hUCB‐MSC (49.95 ± 4.19%), and VEGF/hUCB‐MSC (Dox−) (49.24 ± 2.92%) groups (Fig. 4D). Similarly, fraction shortening in the VEGF/hUCB‐MSC (Dox+) group (31.87 ± 1.94%) was significantly higher compared to the control (14.61 ± 1.88%), hUCB‐MSC (22 ± 1.87%), and VEGF/hUCB‐MSC (Dox−) (21.80 ± 2.28%) groups (Fig. 4E). Although the LVIDd values in the hUCB‐MSC alone (8.71 ± 0.32 mm) and VEGF/hUCB‐MSC (Dox−) (8.43 ± 0.87 mm) groups were slightly reduced compared to the control group (9.83 ± 0.61 mm), the VEGF/hUCB‐MSC (Dox+) group (6.42 ± 0.26 mm) showed a significant reduction (p < .01).
Figure 4.
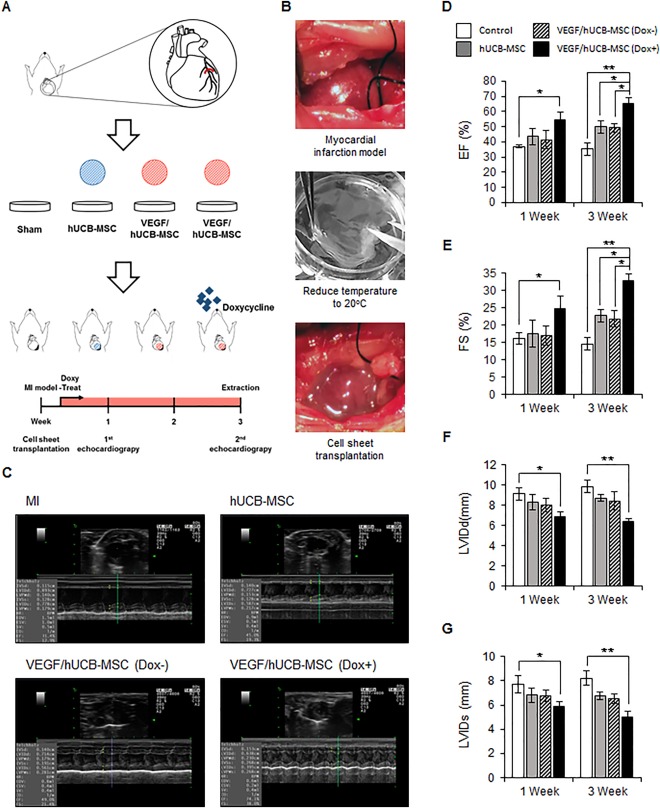
Engraftment of VEGF/hUCB‐MSCs and recovery of cardiac function. (A): Schematic illustration of treatment of MI by VEGF/hUCB‐MSCs. Echocardiography measurements performed 1 week and 3 weeks after MI induction and cell transplantation. (B): The image of an MI and the transplantation process of the UpCell system. (C): Representative echocardiogram images in the MI site after 3 weeks. The infarcted rat heart served as the control for transplantation with hUCB‐MSCs, VEGF/hCUB‐MSC (Dox−), and VEGF/hUCB‐MSC (Dox+). In the quantitative analysis, treatment of VEGF/hUCB‐MSCs with doxycycline significantly enhanced the cardiac function in 1 week and 3 weeks. The VEGF/hUCB‐MSC (Dox+) group showed (D) an increase in the EF and (E) FS and (F) a decrease in the LVIDd and (G) LVIDs. (*p < .05, **p < .01, n = 6 each group). All values represent the mean ± SE. Abbreviations: Dox, doxycycline; EF, ejection fraction; FS, fractional shortening; hUCB‐MSCs, human umbilical cord blood‐derived mesenchymal stem cells; LVIDd, left ventricle inner diameter at diastole; LVIDs, left ventricle inner diameter at systole; MI, myocardial infarction; SE, standard error; VEGF, vascular endothelial growth factor.
Reduced MI Size and Fibrosis but Thicker Left Ventricle Due to VEGF Secretion by VEGF/hUCB‐MSCs in the Rat MI Model
We conducted histological and histomorphometric analyses to better understand the improvement of cardiac function by Masson's trichrome staining of the rat hearts at 3 weeks after implantation. Fibrosis due to MI appears blue and the preserved myocardium appears red in Masson's trichrome staining. Dramatic LV wall thinning was detected in the MI model in the hUCB‐MSC alone group. The VEGF/hUCB‐MSC(Dox−) group showed a minor protective effect. However, an improved anti‐fibrosis effect was observed in the LV wall in the rats treated with VEGF/hUCB‐MSCs (Dox+) (Fig. 5A, Supporting Information Fig. 3). The MI size was greatly reduced in the VEGF/hUCB‐MSC (Dox+) group compared to the MI control, hUCB‐MSC alone, and VEGF/hUCB‐MSC (Dox−) groups (Fig. 5B). The ventricular fibrosis rate was significantly reduced in the VEGF/hUCB‐MSC (Dox+)‐treated rats compared with the other groups (Fig. 5B). However, the LV wall thickness of the VEGF/hUCB‐MSC (Dox+) group was significantly thicker than the MI, hUCB‐MSC alone, and VEGF/hUCB‐MSC (Dox−) groups (Fig. 5B).
Figure 5.
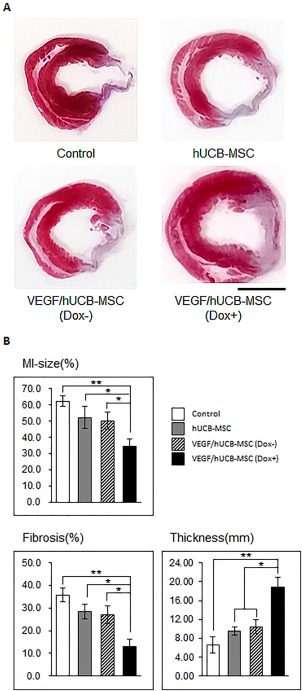
Evaluation of MI size, left ventricle (LV) fibrosis and wall thickness after the transplantation of VEGF/hUCB‐MSCs. (A): Representative images of heart sections stained with Masson's trichrome show fibrosis and wall thinning at the infarcted area. Fibrotic areas are colored in blue and the viable myocardium is colored in red. (B): Infarct size, percentage of LV fibrosis, and LV wall thickness were compared among the different groups. (*p < .05, **p < .01). All values represent the mean ± SE. (Scale bar = 5 mm). Abbreviations: Dox, doxycycline; hUCB‐MSCs, human umbilical cord blood‐derived mesenchymal stem cells; MI, myocardial infarction; SE, standard error; VEGF, vascular endothelial growth factor.
Immunohistochemical staining was performed to investigate Dox‐induced VEGF secretion in the infarcted area following treatment with VEGF/hUCB‐MSC + Dox. Heart tissues were stained with a VEGF antibody. The infarcted area in the VEGF/hUCB‐MSC (Dox+) group showed high expression of VEGF, which was not observed in the control, hUCB‐MSC, and VEGF/hUCB‐MSC (Dox−) groups (Fig. 6A). These results suggested that VEGF was secreted properly by the VEGF/hUCB‐MSC cells upon doxycycline treatment. Furthermore, anti‐vWF staining showed that the VEGF/hUCB‐MSC (Dox+) group had significantly increased vessel density in the infarcted area, whereas the hUCB‐MSC alone and VEGF/hUCB‐MSC (Dox−) groups showed small increases in vessel density compared to the control group (Fig. 6B). These results suggest that VEGF secreted from VEGF/hUCB‐MSCs greatly enhanced neoangiogenesis compared to stem cell therapy alone by directly and indirectly stimulating angiogenic factors or EC cell‐like factors.
Figure 6.
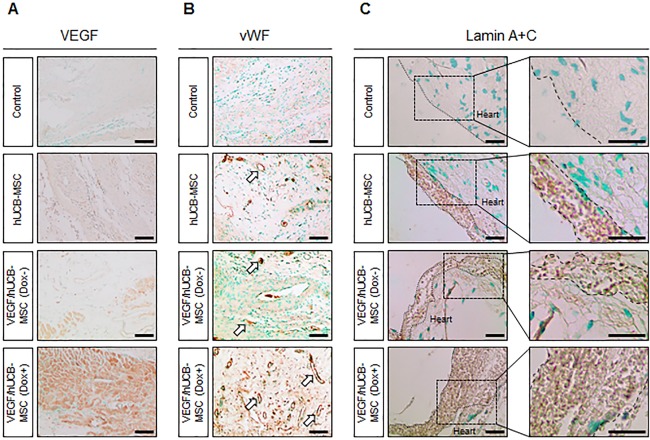
Immunohistochemical staining of VEGF, blood vessel formation by VEGF/hUCB‐MSCs and implanted human cell engraftment. Myocardial infarction (MI)‐induced rats were divided into four groups. Control (MI alone), treated with hUCB‐MSCs, VEGF/hUCB‐MSCs (Dox−), and VEGF/hUCB‐MSCs (Dox+). (A): VEGF staining was performed at the infarcted area. High levels of VEGF were detected only in the VEGF/HUCB‐MSC (Dox+) group. (B): Generation of blood vessels in the MI regions. More blood vessels were observed in VEGF/hUCB‐MSC (Dox+) group than other groups. (C): An anti‐human Lamin A+C antibody was used to visualize the transplanted human stem cells. More human Lamin A+C positive cells were observed in rat hearts transplanted with VEGF/hUCB‐MSC (Dox+). (Scale bar = 100 μm). Abbreviations: AAVS1, adeno‐associated virus integration site 1; Dox, doxycycline; hUCB‐MSCs, human umbilical cord blood‐derived mesenchymal stem cells; VEGF, vascular endothelial growth factor; vWF, von Willibrand factor.
Prolonged Cell Survival of Transplanted Human VEGF/hUCB‐MSCs in the Infarcted Region
To determine the effects of VEGF secreted from VEGF/hUCB‐MSCs (Dox+) on the survival of transplanted human stem cells, we detected transplanted VEGF/hUCB‐MSC in rat hearts via immunohistochemical staining. An anti‐lamin A+C antibody was used to detect human cells in the infarcted region. Lamin A+C‐positive cells were not detected in the MI control group (Fig. 6). Significant numbers of transplanted human cells were detected above the myocardium of the hUCB‐MSC alone and VEGF/hUCB‐MSC(Dox−)‐transplanted groups. However, in the VEGF/hUCB‐MSC (Dox+) group, a large number of Lamin A+C‐positive cells were detected above the myocardium (Fig. 6). These results indicate that secreted VEGF also has a positive effect on stem cell survival and thus leads to the induction of enhanced paracrine effects and improved protection from cardiac damage.
Discussion
Stem cell‐based therapy has been widely investigated for the treatment of MI. In recent studies, stem cell therapy using MSCs offered a positive result for the treatment of MI due to paracrine effects and revascularization 26. However, the application of cell therapy faces many obstacles due to the low survival of the implanted cells and insufficient oxygen and nutrients. Moreover, cardiomyocytes have a limited regeneration capacity 27. To overcome these obstacles with ischemic disease, combined therapy using vascular stimulating growth factor and MSCs is required. In our previous study, VEGF protein delivery with stem cells showed a therapeutic effect on ischemic disease 28. However, the protein therapy highlighted the limitation of the application periods due to the short half‐life of the VEGF proteins 29. To overcome this limitation, several studies reported genetic modifications using retroviruses for the integration of transgenes into the host chromosome to induce their long‐term expression 30, 31. However, retroviruses can produce oncogene activation or tumorigenesis due to the random and unpredictable integration of the transgene, which is a major side effect. Furthermore, uncontrolled VEGF expression also causes side effects such as hemangioma and nonstandard blood vessel formation. To address these issues, we generated an inducible VEGF‐secreting stem cell using TALEN‐mediated transgene integration into a specific safe harbor site by AAVS1 locus‐directed HR. With this TALEN‐mediated safe harbor gene delivery strategy, the hUCB‐MSCs were able to secrete VEGF upon doxycycline treatment, protect the host from cardiac damage and improve cardiac function in a rat MI model.
There have been a number of studies utilizing the UCB‐MSCs to treat the infracted heart. Various strategies such as biomaterials, growth factors, and tissue engineering have been applied and many progress were shown, but therapeutic limits existed in these studies 5, 6, 7. However, UCB‐MSCs were reported promising cell sources for cardiac repair and good candidate for vascular growth 6. To increase the therapeutic potential of UCB‐MSCs, it requires stimulation by growth factors such as VEGF. In our results, the VEGF signal was easily adjustable. Furthermore, various materials have been applied to enhance the implanted cell survival, and these materials enhance the effectiveness of hUCB‐cell therapy. However, these materials remained the retention and low cell survival problems. In this study, we used a cell patch that delivers cells only because the cell sheets are removed after cell implantation. The needle injection of stem cells into infarcted myocardium was some routine procedures in the preclinical studies. However, the needle injection has some unsafe concerns for implantation of stem cells on the cardiac tissue. It shows the low transplant rates and difficulty for treating the large areas 32, and the needle may induce the mechanical injury and myocardial damages 33, 34. Conversely, the positive effect of the cell sheet has been validated previously 35. It easily grafted and the transplantation method is safe and a high rate of successful transplantation was accomplished in our study.
TALENs have been successfully applied to edit targeted genomes in various cell types 19, 36. However, the limitations associated with engineered nucleases remain. The expression of TALEN pairs could cause additional DNA cleavage in human cells, and this unexpected DNA cleavage at off‐target sites could reduce cell survival and cell cycle arrest 18. To predict off‐target cleavage activities, high‐throughput sequencing assessment is required. In previous reports, the off‐target cleavage activities of AAVS1‐specific TALENs were investigated and revealed to be approximately 0.13% 20, 37. Although we did not profile TALEN off‐target activity using whole genome sequencing in a VEGF/hUCB‐MSC cell population in this study, similar activities were expected. When we tested the three sites with the highest homology to AAVS1 safe harbor site by PCR, the results showed no off‐target integration.
The apparent improvement observed for this combination therapy can be explained as follows. First, the impaired cardiac tissue may have been regenerated better by the transplanted stem cells. According to our previous results and this result, transplanted MSCs could regenerate the impaired cardiac tissue and promote the proliferation of cardiac progenitor cells near the transplanted area 5. This is supported by our results that higher vWF staining is observed in the VEGF/hUCB‐MSCs (Dox+) group and also human MSC cells are remained in the implanted site as demonstrated by human Lamin A+C staining. However, it was hard to show that the human MSCs actually formed vascular EC or not. However, the cardiac regenerative effect was not clearly observed in our MSC alone group, and other researchers also reported that the frequency of the occurrence of these regenerative processes by MSC was also very low 38. However, our combinatorial approaches of induced VEGF and VEGF/hUCB‐MSCs greatly contributed to the prevention of LV remodeling and the protection of the remaining cardiomyocytes as well as cardiac regeneration. Thus, as previously reported by others and our studies, the major therapeutic effect might come from VEGF paracrine effect of the engineered VEGF/hUCB‐MSCs (Dox+) cells in addition to the MSC paracrine effect itself. The second explanation for these positive results is the paracrine effects of the combination therapy. Previous studies also suggested that the main therapeutic effect of transplanted MSCs occurred due to paracrine effects 4, 39. The transplanted cells secrete many cytokines, and these cytokines activate cell survival, alleviate the fibrosis, and contribute to cardiomyocyte preservation. Furthermore, tuning the cytokine secretion toward angiogenic factors will further help the therapeutic effect of stem cells in MI. Indeed, our data showed that the induced VEGF could enhance the release of cytokines related to the angiogenic paracrine such as cytokine Angiopoietin 1 (Fig. 2), and finally prevent cardiac remodeling by improving implanted cell survival in the harshly infarcted region. These VEGF stimulating paracrine effects were also demonstrated in myoblast and EC in mouse model 40, 41. Third, VEGF/hUCB‐MSCs and secreted VEGF promoted neovascularization in the implanted area and this neovascularization might maintain VEGF/hUCB‐MSC survival after cell sheet integration and the cells' therapeutic functions in the MI microenvironment. Our data showed that more neovascularization occurred in the infarcted area when the engineered cells were boosted with secreted VEGF (Fig. 6). Other studies also reported that the therapeutic concentration of VEGF was able to induce stable capillary formation and protect the remaining cardiomyocytes in MI model 30, 41. Therefore, the newly formed vessels also increased the cell survival of the implanted hUCB/MSCs, thereby contributing to the formation of a compact cell sheet in the myocardial surface of the infarcted area as demonstrated by anti‐human Lamin A+C staining (Fig. 6). Furthermore, the increased vessel formation may contribute to protection against negative LV remodeling, the deterioration of heart tissues and myocardial functional enhancement through an improved nutrient and oxygen supply.
Conclusion
We developed VEGF/hUCB mesenchymal stem cells that could regulate VEGF secretion. We used TALENs, which offered safe and high genome editing efficiency, to insert the inducible VEGF gene cassettes into the AAVS1 safe harbor site. In our study, the secreted VEGF induced neoangiogenesis and increased cell survival. Furthermore, the combined therapy of mesenchymal stem cells and controllable VEGF secretion from the cells significantly enhanced angiogenesis, reversed the MI remodeling process and improved cardiac function. Therefore, inducible VEGF‐secreting safe‐harbored VEGF/hUCB‐MSC transplantation provides an effective therapeutic modality for MI.
Author Contributions
H.‐M.C.: conception and design, perform experiment, collection and assembly of data, manuscript writing; P.‐H.K., B.‐J.K., and M.‐c.C.: data analysis and interpretation; H.‐K.C.: provision of study material; Y.‐m.S., K.B., and S.‐Y.Y.: perform experiment, collection of data; J.‐H.K.: data collection; S.‐Y.L.: provision of study material, data interpretation; H.K.: provision of study material, data interpretation; G.J.: provision of study material, manuscript writing; J.‐Y.C.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Supporting information
Supporting Information Figures.
Acknowledgments
We thank Hyung‐Jung Oh in Department of Veterinary Radiology, College of Veterinary Medicine, Seoul National University for her help with echocardiograph imaging. This research was supported by grants from the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (2012M3A9C6049716, 2014M3A9D5A01073598, 2016M3A9B6026771).
References
- 1. Coulombe KL, Bajpai VK, Andreadis ST et al. Heart regeneration with engineered myocardial tissue. Annu Rev Biomed Eng 2014;16:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jessup M, Brozena S. Heart failure. N Engl J Med 2003;348:2007–2018. [DOI] [PubMed] [Google Scholar]
- 3. Vallee JP, Hauwel M, Lepetit‐Coiffe M et al. Embryonic stem cell‐based cardiopatches improve cardiac function in infarcted rats. Stem Cells Translational Medicine 2012;1:248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Segers VF, Lee RT. Stem‐cell therapy for cardiac disease. Nature 2008;451:937–942. [DOI] [PubMed] [Google Scholar]
- 5. Kang BJ, Kim H, Lee SK et al. Umbilical‐cord‐blood‐derived mesenchymal stem cells seeded onto fibronectin‐immobilized polycaprolactone nanofiber improve cardiac function. Acta Biomater 2014;10:3007–3017. [DOI] [PubMed] [Google Scholar]
- 6. Roura S, Bago JR, Soler‐Botija C et al. Human umbilical cord blood‐derived mesenchymal stem cells promote vascular growth in vivo. PLoS One 2012;7:e49447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roura S, Soler‐Botija C, Bago JR et al. Postinfarction functional recovery driven by a three‐dimensional engineered fibrin patch composed of human umbilical cord blood‐derived mesenchymal stem cells. Stem Cells Translational Medicine 2015;4:956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karam JP, Muscari C, Montero‐Menei CN. Combining adult stem cells and polymeric devices for tissue engineering in infarcted myocardium. Biomaterials 2012;33:5683–5695. [DOI] [PubMed] [Google Scholar]
- 9. Shi RZ, Li QP. Improving outcome of transplanted mesenchymal stem cells for ischemic heart disease. Biochem Biophys Res Commun 2008;376:247–250. [DOI] [PubMed] [Google Scholar]
- 10. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–676. [DOI] [PubMed] [Google Scholar]
- 11. Matsumoto R, Omura T, Yoshiyama M et al. Vascular endothelial growth factor‐expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc Biol 2005;25:1168–1173. [DOI] [PubMed] [Google Scholar]
- 12. Deuse T, Peter C, Fedak PW et al. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell‐based myocardial salvage after acute myocardial infarction. Circulation 2009;120:S247–S254. [DOI] [PubMed] [Google Scholar]
- 13. Baum C, Dullmann J, Li Z et al. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood 2003;101:2099–2114. [DOI] [PubMed] [Google Scholar]
- 14. Ozawa CR, Banfi A, Glazer NL et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest 2004;113:516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline‐responsive promoters. Proc Natl Acad Sci USA 1992;89:5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watanabe T, Saito D, Tanabe K et al. Tet‐on inducible system combined with in ovo electroporation dissects multiple roles of genes in somitogenesis of chicken embryos. Dev Biol 2007;305:625–636. [DOI] [PubMed] [Google Scholar]
- 17. Gaj T, Gersbach CA, Barbas CF, 3rd ZFN, TALEN, and CRISPR/Cas‐based methods for genome engineering. Trends Biotechnol 2013;31:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bogdanove AJ, Voytas DF. TAL effectors: Customizable proteins for DNA targeting. Science 2011;333:1843–1846. [DOI] [PubMed] [Google Scholar]
- 19. Joung JK, Sander JD. TALENS: A widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol 2013;14:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mussolino C, Alzubi J, Fine EJ et al. TALENs facilitate targeted genome editing in human cells with high specificity and low cytotoxicity. Nucleic Acids Res 2014;42:6762–6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seo Y, Yang SR, Jee MK et al. Human umbilical cord blood‐derived mesenchymal stem cells protect against neuronal cell death and ameliorate motor deficits in niemann pick type C1 mice. Cell Transplant 2011;20:1033–1047. [DOI] [PubMed] [Google Scholar]
- 22. Yu KR, Lee JY, Kim HS et al. A p38 MAPK‐mediated alteration of cox‐2/PGE2 regulates immunomodulatory properties in human mesenchymal stem cell aging. PLoS One 2014;9:e102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim Y, Kweon J, Kim A et al. A library of TAL effector nucleases spanning the human genome. Nat Biotechnol 2013;31:251–258. [DOI] [PubMed] [Google Scholar]
- 24. Lee JH, Kim BG, Ahn JM et al. Role of PI3K on the regulation of BMP2‐induced beta‐catenin activation in human bone marrow stem cells. Bone 2010;46:1522–1532. [DOI] [PubMed] [Google Scholar]
- 25. Obokata H, Yamato M, Tsuneda S et al. Reproducible subcutaneous transplantation of cell sheets into recipient mice. Nat Protoc 2011;6:1053–1059. [DOI] [PubMed] [Google Scholar]
- 26. Williams AR, Hare JM. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res 2011;109:923–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mangi AA, Noiseux N, Kong D et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 2003;9:1195–1201. [DOI] [PubMed] [Google Scholar]
- 28. Kim PH, Yim HG, Choi YJ et al. Injectable multifunctional microgel encapsulating outgrowth endothelial cells and growth factors for enhanced neovascularization. J Control Release 2014;187:1–13. [DOI] [PubMed] [Google Scholar]
- 29. Henry TD, Annex BH, McKendall GR et al. The viva trial: Vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 2003;107:1359–1365. [DOI] [PubMed] [Google Scholar]
- 30. Misteli H, Wolff T, Fuglistaler P et al. High‐throughput flow cytometry purification of transduced progenitors expressing defined levels of vascular endothelial growth factor induces controlled angiogenesis in vivo. Stem Cells 2010;28:611–619. [DOI] [PubMed] [Google Scholar]
- 31. Chen HK, Hung HF, Shyu KG et al. Combined cord blood stem cells and gene therapy enhances angiogenesis and improves cardiac performance in mouse after acute myocardial infarction. Eur J Clin Invest 2005;35:677–686. [DOI] [PubMed] [Google Scholar]
- 32. Shimizu T, Yamato M, Kikuchi A et al. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials 2003;24:2309–2316. [DOI] [PubMed] [Google Scholar]
- 33. Pagani FD, DerSimonian H, Zawadzka A et al. Autologous skeletal myoblasts transplanted to ischemia‐damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol 2003;41:879–888. [DOI] [PubMed] [Google Scholar]
- 34. Suzuki K, Murtuza B, Fukushima S et al. Targeted cell delivery into infarcted rat hearts by retrograde intracoronary infusion: Distribution, dynamics, and influence on cardiac function. Circulation 2004;110:II225–II230. [DOI] [PubMed] [Google Scholar]
- 35. Hata H, Matsumiya G, Miyagawa S et al. Grafted skeletal myoblast sheets attenuate myocardial remodeling in pacing‐induced canine heart failure model. J Thorac Cardiovasc Surg 2006;132:918–924. [DOI] [PubMed] [Google Scholar]
- 36. Carroll D. Genome engineering with zinc‐finger nucleases. Genetics 2011;188:773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Niu J, Zhang B, Chen H. Applications of TALENs and CRISPR/Cas9 in human cells and their potentials for gene therapy. Mol Biotechnol 2014;56:681–688. [DOI] [PubMed] [Google Scholar]
- 38. Cashman TJ, Gouon‐Evans V, Costa KD. Mesenchymal stem cells for cardiac therapy: Practical challenges and potential mechanisms. Stem Cell Rev 2013;9:254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fedak PW. Paracrine effects of cell transplantation: Modifying ventricular remodeling in the failing heart. Semin Thorac Cardiovasc Surg 2008;20:87–93. [DOI] [PubMed] [Google Scholar]
- 40. Cucina A, Borrelli V, Randone B et al. Vascular endothelial growth factor increases the migration and proliferation of smooth muscle cells through the mediation of growth factors released by endothelial cells. J Surg Res 2003;109:16–23. [DOI] [PubMed] [Google Scholar]
- 41. Marsano A, Maidhof R, Luo J et al. The effect of controlled expression of vegf by transduced myoblasts in a cardiac patch on vascularization in a mouse model of myocardial infarction. Biomaterials 2013;34:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figures.


