Abstract
One‐way endobronchial valves (EBV) insertion to reduce pulmonary air trapping has been used as therapy for chronic obstructive pulmonary disease (COPD) patients. However, local inflammation may result and can contribute to worsening of clinical status in these patients. We hypothesized that combined EBV insertion and intrabronchial administration of mesenchymal stromal cells (MSCs) would decrease the inflammatory process, thus mitigating EBV complications in severe COPD patients. This initial study sought to investigate the safety of this approach. For this purpose, a phase I, prospective, patient‐blinded, randomized, placebo‐controlled design was used. Heterogeneous advanced emphysema (Global Initiative for Chronic Lung Disease [GOLD] III or IV) patients randomly received either allogeneic bone marrow‐derived MSCs (108 cells, EBV+MSC) or 0.9% saline solution (EBV) (n = 5 per group), bronchoscopically, just before insertion of one‐way EBVs. Patients were evaluated 1, 7, 30, and 90 days after therapy. All patients completed the study protocol and 90‐day follow‐up. MSC delivery did not result in acute administration‐related toxicity, serious adverse events, or death. No significant between‐group differences were observed in overall number of adverse events, frequency of COPD exacerbations, or worsening of disease. Additionally, there were no significant differences in blood tests, lung function, or radiological outcomes. However, quality‐of‐life indicators were higher in EBV + MSC compared with EBV. EBV + MSC patients presented decreased levels of circulating C‐reactive protein at 30 and 90 days, as well as BODE (Body mass index, airway Obstruction, Dyspnea, and Exercise index) and MMRC (Modified Medical Research Council) scores. Thus, combined use of EBV and MSCs appears to be safe in patients with severe COPD, providing a basis for subsequent investigations using MSCs as concomitant therapy. Stem Cells Translational Medicine 2017;6:962–969
Keywords: Spirometry, Mesenchymal stromal cells, Endobronchial valves, Lung volume reduction, Chronic obstructive pulmonary disease
Significance Statement.
This article describes a phase I, prospective, patient‐blinded, randomized, placebo‐controlled study of 10 patients with severe emphysema. One‐way endobronchial valve insertion was combined with mesenchymal stromal cells (MSCs) administration in half of these patients. Cell therapy proved to be safe, thus not associated with higher overall number of adverse events, frequency of chronic obstructive pulmonary disease (COPD) exacerbations, serological toxicity or worsening of disease. Secondarily, MSCs administration was able to reduce systemic inflammation and COPD predictors and to improve life quality of these patients 30 and 90 days after treatment.
Introduction
Chronic obstructive pulmonary disease (COPD), which encompasses small airway disease (obstructive bronchiolitis) and parenchymal destruction (emphysema), will be the third leading cause of death worldwide by the year 2020, representing a substantial economic and social burden. COPD is inexorably progressive despite available pharmacologic treatments, which are mostly geared toward symptomatic relief. For more severe and end‐stage disease, lung transplantation is only a limited option, given the shortage of donor lungs and possibility of short‐ and long‐term graft rejection 1, 2. Therefore, new therapeutic strategies for emphysema are being intensely investigated. One novel approach, the use of mesenchymal stromal cells (MSCs) 3, appears particularly promising. MSCs harvested from bone marrow (BM), adipose tissue, or other sources have demonstrated potent anti‐inflammatory actions following systemic administration in a wide range of preclinical models of inflammatory and autoimmune diseases, as well as in a growing number of clinical trials 4. Intratracheal MSC administration has been shown to reduce inflammation and injury in rodent and large‐animal models, including explanted human lungs and models of lung injury 5. Preclinical studies have demonstrated reduction of inflammation and fibrosis in different animal models of emphysema 6. In the clinical setting, a prospective, randomized, double‐blind trial in patients with moderate‐to‐severe emphysema 7 demonstrated that four monthly intravenous administrations of allogeneic BM‐derived MSCs obtained from healthy volunteers induced no acute infusion‐related toxicities and were safe over a 2‐year follow‐up period 7. Moreover, MSC administrations did not lead to COPD exacerbation and were associated with a reduction in circulating levels of C‐reactive protein (CRP) over 2‐year follow‐up 7. However, the trial was underpowered to detect any potential efficacy, and did not demonstrate any beneficial effects on pulmonary function or quality of life (QoL) 7.
Concomitantly, a novel therapeutic approach using one‐way endobronchial valves (EBVs) to deflate emphysematous sections and reduce air trapping, to allow better expansion and mechanics in the functional lung, has exhibited beneficial results on pulmonary function, exercise capacity, and QoL measures 2, 8, 9, 10. Despite these promising findings, such bronchoscopic lung volume reduction (BLVR) has important limitations: valve placement may induce granuloma formation and localized inflammation, with subsequent mucus hypersecretion, which can increase the risk of infection and contribute to worsening of clinical status in these patients 2.
Based on the anti‐inflammatory, microbicidal, and antifibrotic properties of MSCs, we hypothesized that a combination of BLVR and intrabronchial local administration of MSCs at the EBV site could ameliorate both the underlying inflammatory process and possible side effects of EBV placement, and thus improve lung function and QoL, in patients with severe COPD. For this purpose, the present study was designed to evaluate the safety of combination MSC and EBV therapy in severe emphysema. The secondary goal was to evaluate whether this combination of therapies might reduce systemic inflammation and improve lung function and QoL in severe COPD patients.
Materials and Methods
Study Design and Oversight
A phase I, prospective, nonrandomized, patient‐blinded, placebo (vehicle)‐controlled design was used. Participants with advanced emphysema (Global Initiative for Chronic Lung Disease [GOLD] stage III or IV) were recruited from Hospital de Clinicas de Porto Alegre, Brazil. The study was approved by the Brazilian National Research Council and by the Ethics Committees of Pontifícia Universidade Católica do Paraná (CAAE: 10952412.2.0000) and Hospital de Clínicas de Porto Alegre/Universidade Federal do Rio Grande do Sul (CAAE: 10952412.2.0000.5327), and written informed consent was obtained from each participant. An independent data and safety monitoring board approved all amendments to the protocol and oversaw conduction of the trial. The study (ClinicalTrials.gov identifier: NCT01872624) was conducted in accordance with the amended Declaration of Helsinki 11.
Patient Selection
Patients aged 40 to 80 years, of both sexes, with severe heterogeneous emphysema (GOLD stage III or IV) 1 were eligible for the study. The selection criteria included dyspnea during usual tasks in the presence of available optimal care for COPD, absence of other diseases that could cause dyspnea, a radiologic diagnosis of emphysema (at least 20% of lung parenchyma with density <−950 HU and 25% of target lobe parenchyma with density <−950 HU), no smoking for at least 6 months prior to the EBV procedure, post‐bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio < 70%, post‐bronchodilator FEV1 below 45% of predicted value 2, heterogeneity score > 15 pp 12 and fissure integrity > 75%, and oral corticosteroid dose < 20 mg per day. The inclusion and exclusion criteria are listed in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| 1. Diagnosis of severe heterogeneous emphysema (heterogeneity > 15 pp), with heterogeneity defined as the difference between lobes in the percent area covered by parenchymal density greater than −950 HU |
| 2. Estimation of collateral ventilation based on fissure integrity ≥75% |
| 3. Total lung capacity >100% of predicted |
| 4. Residual volume >150% of predicted |
| 5. Forced expiratory volume at 1 minute <45% of predicted |
| 6. Diffusing capacity of the lungs for carbon monoxide <45% of predicted |
| 7. Optimal medical treatment |
| 8. Limitations in daily physical activities |
| 9. No smoking for at least 6 months |
| 10. Modified Medical Research Council Dyspnea Scale stage ≥2 |
| Exclusion criteria |
| 1. Homogeneous emphysema |
| 2. Presence of collateral ventilation |
| 3. Systemic corticosteroid therapy at a dose >20 mg per day prednisone or equivalent |
| 4. Pulmonary or extrapulmonary infection |
| 5. Coronary heart disease and/or severe ventricular dysfunction |
| 6. Significant renal or liver disease |
| 7. Active smoking |
| 8. Cancer with survival prognosis <2 years |
| 9. Psychosocial problems |
| 10. Pregnancy |
From May 7, 2013, through October 27, 2014, Digital Imaging and Communications in Medicine‐compliant computed tomography (CT) images from 216 patients were evaluated by a single radiologist employing a specific protocol for CT scan analysis in Apollo software (VIDA Diagnostics, Coralville, IA, USA) 13, 14. As 182 of the patients screened were from other countries or states, we chose to evaluate only those 34 patients from the state in which the study facility is located. Of these, only 22 patients were eligible for treatment using one‐way EBVs (heterogeneity score > 15 pp and fissure integrity > 75%). Seven patients were excluded for oral corticosteroid therapy at a dose >20 mg per day and/or active pulmonary infection and five patients refused consent. Thus, 10 patients were included in this study, which began in December 2013.
Collection of BM
Human BM was collected (60 ml) from the iliac crest of a single healthy donor who had provided informed consent, following guidelines on the use of human subjects approved by the Pontifícia Universidade Católica do Paraná Ethics Committee (approval number 518.599).
Isolation and Culture of Adherent Cells
The aspirate was diluted 1:3 with Iscove's Modified Dulbecco's Medium (IMDM) (Gibco, Invitrogen, Grand Island, NY, USA, https://www.thermofisher.com/order/catalog/product/12440079) and carefully loaded onto Histopaque (1·077 g/ml; Sigma Chemical, St. Louis, CA, USA, http://www.sigmaaldrich.com/catalog/product/sigma/10771?lang=pt®ion=BR) to obtain BM‐derived mononuclear cells (MNCs). MNCs were isolated after a density‐gradient centrifugation (400g, 30 minutes, room temperature) 15 and washed twice with IMDM. BM‐derived MNCs were cultured at a density of 1 × 105 cells per cm2 in T75 culture flasks (TPP, Trasadingen, Switzerland, http://www.tpp.ch/) at 37°C in a humidified chamber containing 5% CO2, using IMDM supplemented with 15% fetal bovine serum (Gibco Invitrogen, Grand Island, NY, USA, https://www.thermofisher.com/br/en/home/life-science/cell-culture/mammalian-cell-culture/fbs.html?gclid=CP-d9umQwtACFYIJkQod-QoM1Q&s_kwcid=AL!3652!3!93193105348!b!!g!!%2Bgibco%20%2Bfbs&ef_id=V8Qu1gAABcPxOQnr:20161124193127:s), penicillin (100 units per ml), and streptomycin (100 µg/ml) (Gibco Invitrogen, Grand Island, NY, USA, https://www.thermofisher.com/order/catalog/product/10378016?ICID=search-product). The culture medium was changed to remove the remaining nonadherent cells 2 days after the initial plating. Thereafter, the culture medium was replaced twice a week. After cultures had reached 80%–90% confluence, BM‐derived MSCs were subcultured; MSCs were detached with 0.25% trypsin/EDTA (Invitrogen, Grand Island, NY, USA, https://www.thermofisher.com/order/catalog/product/25200056?ICID=search-product) and replated as passage‐1 cells; the process was then continued as previously described. MSCs were continually expanded and transplanted at third or fourth passages. Samples were taken for microbiological testing, immunophenotyping, and cytogenetic studies according to Brazilian National Health Surveillance Agency Resolution 09/2011 16. Cell‐surface antigen profile 3, 17, stemness, and cytogenetics 18 were evaluated (Supporting Information data). Cells in culture were harvested for use, diluted in saline solution (0.9% sodium chloride, Cristália, São Paulo, SP, Brazil, http://2cristalia.com.br/2015/detalhe_produto.php?id=67), and placed in infusion bags. Finally, 108 cells were administered to each patient just before valve insertion.
MSC Administration
Following informed consent, local anesthesia of the oropharynx, larynx and vocal cords, and major airways was achieved with topical application of 1% lidocaine (Nonorap, Biolab, São Paulo, SP, Brazil, http://www.biolabfarma.com.br/produtos.php). Conscious sedation was induced with a single 100 μg bolus of fentanyl (Janssen‐Cilag, SP, São Paulo, Brazil, http://www.janssen.com/brasil/sites/www_janssen_com_brazil/files/product/pdf/fentanil_pub02_vps.pdf) and continuous intravenous infusion of propofol (4 mg/kg/h, Diprivan 1%, AstraZeneca, Cotia, SP, Brazil, http://www.astrazeneca.com.br/arquivos/bulas-encriptadas/Diprivan.pdf). Patients were kept under spontaneous ventilation with supplemental oxygen delivered by nasal cannula to ensure normal ranges of SpO2. A video bronchoscope (VB1830, Pentax, Montvale, NJ, USA, https://pentaxmedical.com/pentax/en/99/1/Bronchoscopes) with a 2.8‐mm instrument channel was used.
Of the 10 study patients, 5 were randomly chosen to receive fresh BM‐MSCs (108 cells in 30 ml saline, at a rate of 2.0 × 107 cells per min, infused over 5 minutes), and 5 to receive saline (vehicle control). MSCs or saline were infused through a polyethylene catheter (Olympus PR‐2B1) placed through the working bronchoscopy channel. The catheter tip was placed 3–5 cm in the segmental or subsegmental bronchus of all branches of the target lobe, in the region where the EBVs were to be placed. A total volume of 30 ml of either MSCs or of saline vehicle control was instilled into each subsegmental airway division visualized, immediately prior to insertion of each EBV into these same subsegments.
Valve Placement
Following MSC or saline infusion, endobronchial lobar occlusion was performed using Zephyr valves placed in segmental or subsegmental bronchi of all branches of the target lobe. Pretreatment analysis of airway morphology and virtual bronchoscopy using the Apollo software was used to facilitate treatment planning 19, 20 by determining, a priori, the size and number of valves to be utilized in each patient 10.
Postoperative Care and Follow‐Up
A chest radiograph was performed immediately after the procedure. Patients were kept under cardiopulmonary monitoring throughout the procedure, which lasted no longer than 15 minutes.
Study Outcomes
Patients remained in hospital for 2 days due to the possible risk of COPD exacerbation or pneumothorax. Heart and respiratory rates, body temperature, blood pressure, and oxygen saturation were measured continuously during this period. On discharge, patients were instructed to call the investigators or return to the hospital if any acute event occurred. Safety was characterized by the occurrence of adverse events (Table 2) during MSC infusion, EBV implantation, or at 1‐ and 3‐month follow‐up evaluations. Blood tests (arterial blood gas analysis, complete blood count, urea, creatinine, glucose, and electrolytes) were performed immediately before the procedure and 1, 7, 30, and 90 days thereafter.
Table 2.
Adverse events
| System organ class/Preferred term | EBV (n = 5) | EBV + MSC (n = 5) | p value |
|---|---|---|---|
| Asthenia | 1 (20%) | 0 | .29 |
| Arrhythmia | 0 | 0 | .29 |
| Respiratory, thoracic, and mediastinal disorders | |||
| Cough | 1 (20%) | 0 | .29 |
| Bronchospasm | 0 | 0 | 1.00 |
| Hemoptysis | 0 | 0 | 1.00 |
| Pulmonary tuberculosis | 1 (20%) | 0 | .29 |
| Pneumothorax | 1 (20%) | 0 | .29 |
| Valve replacement | 1 (20%) | 0 | .29 |
| Valve removal | 1 (20%) | 0 | .29 |
| COPD exacerbation/Pneumonia | 2 (40%) | 2 (40%) | 1.00 |
| Empyema | 1 (20%) | 0 | .29 |
| Respiratory failure | 1 (20%) | 0 | .29 |
Data are presented as n (%).
Abbreviations: COPD, chronic obstructive pulmonary disease; EBV, endobronchial valve; MSC, mesenchymal stromal cells.
Chest CT scans were performed before and 30 and 90 days after treatment. To assess systemic inflammation, circulating levels of transforming growth factor (TGF)‐β, keratinocyte growth factor (KGF), and vascular endothelial growth factor (VEGF), were measured as modulator factors induced by MSCs 6. Systemic tumor necrosis factor (TNF)‐α, interleukin (IL)−8, IL‐10, and CRP levels were assessed in serial blood samples obtained throughout the study period 21.
Secondarily, efficacy measures assessed improvement from baseline in pulmonary function parameters: (a) FEV1, FVC, FEV1/FVC 22, and total lung capacity (TLC), assessed by body plethysmography, (Jaeger, Hoechberg, Germany) 22; (b) single‐breath carbon monoxide diffusing capacity (DLCO) 22; (c) the Body mass index, airway Obstruction, Dyspnea, and Exercise (BODE) index, which incorporates four factors known to be independent predictors of disease severity in COPD: body mass index (BMI); exercise capacity assessed by the 6‐minute walking distance (6MWD) test; the degree of airflow obstruction, as assessed by the FEV1; and functional dyspnea, as assessed by the Modified Medical Research Council (MMRC) questionnaire 23; and (d) health‐related QoL, as measured with the standardized St. George's Respiratory Questionnaire (SGRQ) 24.
Statistical Analysis
The number of patients was selected for initial assessment of safety in a phase I investigation. However, this study was ultimately underpowered for efficacy; only 10 patients were randomized, in a 1:1 ratio of MSCs to placebo. For all endpoints, statistical analyses were performed using two‐sided hypothesis tests, including t tests, Wilcoxon rank‐sum tests, and Fisher exact tests, as appropriate. All were performed at the .05 level of significance 25.
Results
Patients
All 10 randomized patients completed the scheduled follow‐up period (Supporting Information Fig. 1). The main baseline characteristics of the two populations are summarized in Supporting Information Tables 1 and 2. In general, the patients were well matched by age, sex, race, BMI, cumulative pack‐year smoking history, and comorbid conditions. Additionally, the enrolled population had relatively advanced disease, with 100% of patients being categorized as having severe COPD at study entry, with an average of 23% of predicted FEV1 and an average smoking history of 62.9 pack years. All patients had at least one significant clinical comorbidity (Supporting Information Table 2).
Procedure
Endobronchial valve implantation data are summarized in Supporting Information Table 3. Briefly, valve number, total emphysema area (area with density <−950 HU), treated lobar volume, percentage of treated lobe volume over ipsilateral lung volume, and gradient of emphysema area of target lobe compared with adjacent lobes from the ipsilateral lung did not differ between groups.
Outcomes
BLVR was well‐tolerated in all patients but one, who developed pneumothorax, pneumonia, empyema, and respiratory failure, resulting in removal of all valves. Intrabronchial MSC administration was well‐tolerated and no serious or clinically significant symptoms or signs were observed during instillation. Three patients (60%) in the placebo group and two (40%) in the MSC group experienced an adverse event during the study period. Only one patient (25%) in the EBV group experienced a serious adverse event, as noted above. This patient underwent valve removal, recovered from complications (pneumothorax and empyema), and was referred for lung transplantation. No patient in the EBV‐MSC group experienced serious adverse events. Table 2 provides a detailed list of adverse events stratified by system organ class.
Serial toxicologic outcomes, such as arterial oxygen partial pressure (PaO2), arterial oxygen dioxide partial pressure (PaCO2), and serum glucose, sodium, potassium, urea, creatinine levels did not differ significantly between the two groups and did not change significantly over time (Table 3). Levels of circulating TGF‐β, KGF, VEGF, TNF‐α, IL‐8, and IL‐10 were at or below assay detection limits in most patients, thus precluding analysis for significant differences (data not shown). Circulating CRP levels did not differ significantly between groups at baseline or at days 1 and 7; however, CRP levels were significantly reduced in EBV + MSC compared with EBV + SAL at days 30 and 90 (Fig. 1).
Table 3.
Laboratory outcomes
| Laboratory outcomes in the intent‐to‐treat study populations | Groups | ||||
|---|---|---|---|---|---|
| EBV (n = 5) | EBV + MSC (n = 5) | ||||
| Time point | Baseline | D90 | Baseline | D90 | p value |
| PaO2 (mmHg) | 75 (22.7) | 66.6 (27.5) | 60.8 (5.5) | 67.2 (11.2) | .82 |
| PaCO2 (mmHg) | 60 (30.8) | 52.2 (24.6) | 43.3 (0.2) | 40.5 (7.1) | .56 |
| Glucose (mg/dl) | 85 (17.1) | 104.3 (25.7) | 105 (13.9) | 104.3 (25.7) | .93 |
| Sodium (mmol/l) | 141 (1.4) | 140.8 (2.6) | 140.8 (2.6) | 140.8 (1.6) | .99 |
| Potassium (mmol/l) | 4.6 (0.8) | 4.4 (0.9) | 4.4 (0.3) | 4.4 (0.5) | .94 |
| Urea (mg/dl) | 46.2 (23.8) | 38.8 (17.9) | 42.3 (4.0) | 42 (13.4) | .93 |
| Creatinine (mg/dl) | 0.4 (0.1) | 0.7 (0.1) | 0.9 (0.3) | 0.9 (0.2) | .52 |
Data are presented as mean (SD).
Abbreviations: EBV, endobronchial valve; MSC, mesenchymal stromal cells.
Figure 1.
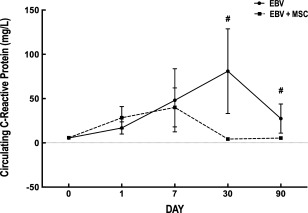
Changes in C‐reactive protein levels in the study populations. Values represent mean ± SD. #, Significantly different from EBV group (p < .05). Abbreviations: EBV, endobronchial valve; MSC, mesenchymal stromal cells.
No statistically significant between‐group differences in FEV1, FEV1% predicted, FVC, FVC% predicted, TLC, TLC% predicted, or DLCO were observed (Table 4). Lung scintigraphy findings were similar in both groups over 90 days (Table 5).
Table 4.
Pulmonary function testing
| EBV (n = 5) | EBV + MSC (n = 5) | |||||
|---|---|---|---|---|---|---|
| Baseline | D30 | D90 | Baseline | D30 | D90 | |
| TLC | 7.1 (5.8–8.0) | 6.7 (5.9–8.1) | 6.4 (5.2–7.7) | 8.0 (6.8–9.1) | 8.1 (7.4–9.2) | 8.8 (7.4–9.9) |
| Predicted TLC% | 129.5 (110.2–172.6) | 124.7 (119.1–138.4) | 123.9 (111.8–165.8) | 126.5 (110.6–148.0) | 127.7 (122.5–154.8) | 148.7 (119.4–165.1) |
| RV | 5.0 (4.7–6.6) | 4.5 (4.2–5.8) | 4.9 (3.6–5.9) | 5.2(4.6–6.2) | 5·1 (4.3–6.4) | 6.0 (4.3–8.4) |
| Predicted RV% | 273.1 (225.9–411.0) | 254.1 (238.5–255.0) | 244.2 (25.7–361.6) | 257.3 (194.8–291.8) | 221.0 (203.3–263.0) | 223.0 (194.4–413.9) |
| FVC | 1.4 (0.8–2.1) | 2.2 (1.7–2.3) | 1.4 (1.2–1.6) | 1.9 (0.9–4.0) | 2.5 (1.6–3.1) | 2.3 (1.4–4.2) |
| Predicted FVC% | 44.1 (26.2–72.2) | 56.2 (51–74.2) | 48.4 (35.8–61.5) | 45.1 (27.2–91.0) | 69.2 (47.7–75.6) | 63.7 (38.1–97.2) |
| FEV1 | 0.6 (0.3–0.9) | 0.8 (0.5–1.3) | 0.5 (0.5–1.6) | 0.7 (0.4–1.6) | 1.0 (0.6–1.5) | 1.0 (0.5–1.9) |
| Predicted FEV1 (%) | 24.1 (13.3–33.5) | 33.0 (20.7–38.0) | 24.2 (20.1–35.8) | 22.0 (13.4–49.1) | 36.4 (22.2–46.0) | 35.0 (17.1–57.2) |
| DLCO | 7.9 (4.8–11.1) | 4.3 (1.2–11.3) | 5.1 (0.9–9.1) | 5.6 (2.0–11.2) | 7.7 (3.1–9.7) | 7.6 (4.2–11.4) |
| Predicted DLCO (%) | 27.1 (19.4–34.7) | 24.9 (15.5–31.8) | 24.8 (12.3–35.2) | 24.3 (7.7–42.7) | 30.8 (12.9–35.7) | 31.9 (17.7–37.3) |
Data are presented as median (range).
Abbreviations: DLCO, diffusing capacity of the lungs for carbon monoxide; EBV, endobronchial valve; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; MSC, mesenchymal stromal cells; RV, residual volume; TLC, total lung capacity.
Table 5.
Scintigraphy findings
| Groups | ||||||
|---|---|---|---|---|---|---|
| EBV (n = 5) | EBV + MSC (n = 5) | |||||
| Lobe | Baseline | D90 | % Change | Baseline | D90 | % Change |
| Right lung | 45.8 (8.5 to 57.0) | 50.7 (10.0 to 55.1) | 7.2 (−6.3 to 30.1) | 34.1 (15.7 to 62.7) | 44.2 (30.8 to 66.5) | 8.5 (3.3 to 18.5) |
| Upper | 7.5 (2.7 to 12.5) | 6.4 (3.4 to 14.7) | 17.7 (−14.0 to 25.9) | 6.8 (1.7 to 15.8) | 6.9 (2.5 to 20.1) | 22.2 (0.9 to 58.8) |
| Middle | 16.15 (2.7 to 25.0) | 23.0 (4.9 to 24.0) | 45.9 (−5.2 to 81.5) | 17.7 (3.1 to 32.24) | 24.0 (14.7 to 34.9) | 4.7 (−4.6 to 674.2) |
| Lower | 16.2 (2.2 to 31.3) | 17.0 (1.8 to 24.7) | −16.3 (−35.5 to 31.8) | 14.9 (4.0 to 17.27) | 17.5 (2.9 to 23.2) | 20.0 (−27.5 to 62.0) |
| Left lung | 54.2 (43.0 to 91.5) | 49.3 (44.9 to 90.0) | 1.4 (−20.5 to 6.8) | 65.9 (37.3 to 84.3) | 55.8 (33.5 to 69.2) | −0.2 (−33.8 to −1.4) |
| Upper | 14.7 (3.7 to 27.4) | 10.9 (4.8 to 27.3) | −11.0 (−23.9 to 29.7) | 16.7 (8.8 to 19.8) | 13.5 (4.8 to 19.2) | −18.0 (−48.6 to 25.0) |
| Middle | 24.7 (19.5 to 46.2) | 24.0 (19.1 to 42.2) | −5.4 (−17.2 to 9.9) | 24.0 (19.1 to 42.2) | 38.7 (12.3 to 40.5) | −11.4 (−30.9 to 39.8) |
| Lower | 17.2 (6.2 to 25.6) | 18·0 (10.8 to 28.2) | 12.4 (−24.7 to 74.2) | 13.4 (8.4 to 25.3) | 13.7 (2.6 to 17.7) | −13.4 (−69.1 to −2.0) |
Data are presented as median (range).
Abbreviations: EBV, endobronchial valve; MSC, mesenchymal stromal cells.
The study was underpowered for assessment of potential efficacy outcomes. As such, no statistically significant differences between the two treated groups were observed in 6MWD or BMI. EBV + MSC patients had a significant lower BODE index at day 90 compared with baseline, as well as lower MMRC scores at day 90 compared with baseline and to day 30. Significant decreases in St. George's Respiratory Questionnaire scores (SGRQS, SGRQA, SGRQI, SGRQT) were observed in the EBV + MSC group at 90 (Table 6).
Table 6.
Quality‐of‐life indicators
| Groups | ||||||
|---|---|---|---|---|---|---|
| EBV (n = 5) | EBV + MSC (n = 5) | |||||
| Parameter | Baseline | D30 | D90 | Baseline | D30 | D90 |
| BODE (D.U.) | 8 (6 to 9) | 4 (2 to 9) | 5 (3 to 9) | 8 (6 to 9) | 5 (4/7) | 3 (1/6)a |
| MMRC (D.U.) | 4.0 (3 to 4) | 2.0 (1 to 4) | 2.0 (1 to 4) | 4.0 (3 to 4) | 2.0 (2/3)a | 1.0 (1/2)a, b |
| 6 MWT (mt) | 356 (225 to 435) | 394 (387 to 432) | 403 (356 to 429) | 182 (162 to 285) | 327 (164/332) | 351 (270/375) |
| ΔTLVR (ml) | — | −663 (−1,510 to −176) | −540 (−1,510 to −147) | — | −686 (−1,840/−171) | −998 (−3,284/−57) |
| EBV group (n = 5) | EBV + MSC group (n = 5) | |||
|---|---|---|---|---|
| Baseline | D90 | Baseline | D90 | |
| SGRQS (D.U.) | 57 (31 to 85) | 34 (22 to 93) | 73 (38 to 97) | 35 (14 to 35)a |
| SGRQA (D.U.) | 92.49 (79.7 to 93.4) | 66.29 (23.3 to 99.9) | 99.9 (61.3 to 99.9) | 60.5 (12.2 to 79.1)a |
| SGRQI (D.U.) | 63.3 (54.5 to 74.4) | 35.9 (11.2 to 74.0) | 58.4 (32.9 to 88.8) | 14.0 (9.2 to 41.5)a |
| SGRQT (D.U.) | 68.8 (61.5 to 81.9) | 44.9 (18.8 to 85.1) | 67.7 (48.3 to 93.6) | 29.9 (14.6 to 48.6)a |
Data are presented as median (range).
Significantly different from baseline (p < .05).
Significantly different from D30 (p < .05).
Abbreviations: 6MWT, 6‐minute walk test; BODE, Body mass index, airway Obstruction, Dyspnea, and Exercise index; DU, dimensionless unit; EBV, endobronchial valve; MMRC, Modified Medical Research Council dyspnea scale; MSC, mesenchymal stromal cells; MWT, Minutes Walk Test; SGRQ, Saint George Respiratory Questionnaires; TLVR, total lung volume reduction.
Discussion
In this sample, combined placement of one‐way EBV and MSC administration was not associated with serious adverse events in patients with advanced heterogeneous emphysema. Neither group experienced significant improvement in ventilation/perfusion rates, laboratory outcomes, expiratory flow rates, or 6‐minute walk distance. Nevertheless, in the EBV + MSC group, patients experienced a significant reduction in CRP levels at days 30 and 90 after transplant, in conjunction with a decrease in BODE, MMRC, and SGRQs scores, reflecting improvement in overall health, daily life, and perceived well‐being.
Circulating CRP levels can be a strong and independent predictor of hospitalization, exacerbation, and mortality in individuals with COPD 26. As MSC administration was associated with reduced systemic CRP levels, we speculate that MSC administration might reduce these outcomes in this patient population. However, in a previous study, no such reduction was observed following systemic MSC administration over a 2‐year follow‐up period. Moreover, it is unclear how localized intrabronchial MSC administration could modify circulating CRP levels.
The BODE index has proven to be a good predictor of mortality, hospitalization, and number and severity of exacerbations 23. The SGRQs are a standardized set of self‐report measures for assessment of impaired health and perceived well‐being in patients with lung diseases 24. In this initial study, patients who received MSCs had better BODE and SGRQ scores, suggesting improved self‐perception of QoL. Larger‐scale trials are warranted to elucidate these initial findings.
Promising new approaches to treat COPD continue to be developed. One example, lung volume reduction surgery (LVRS), was initially regarded as a therapeutic alternative for patients with severe COPD, but was complicated by adverse effects and perioperative complications, long‐term respiratory complications, high mortality rates, and increased health care expenditures 8. Non‐operative BLVR with EBVs is a simpler, relatively safe, and more accessible approach that has demonstrated growing success 9, 10. The main complications following BLVR are pneumothorax and the formation of small granulomas around the valves, possibly due to mucosal trauma generated by the insertion technique 10. An anti‐inflammatory and antifibrogenic adjuvant, administrated concomitantly with BLVR insertion, could prevent granuloma formation and subsequent functional compromise.
MSCs have low immunogenicity 3 and may suppress immune‐effector cells, such as T and B lymphocytes 4. Such features indicate that allogeneic transplantation of MSCs might be feasible. Preclinical data have shown the great potential of MSCs for modulation of inflammatory parameters (immune cell polarization and activity, production of inflammatory cytokines, and growth factors) in several models of respiratory diseases 27, including COPD 6, 27.
Systemic administration of autologous and allogeneic MSCs has been uniformly demonstrated as safe in a wide range of diseases, without infusion‐related toxicity or significant adverse effects over at least 5 years after administration, as shown in recent meta‐analyses 28. Preclinical studies have also demonstrated the safety of intrabronchial MSC administration, and a small number of trials support the clinical safety of this approach 27. Preclinical data are strongest for acute, severe lung diseases and critical illnesses, such as the acute respiratory distress syndrome and septic shock, and these conditions are expected to be those most amenable to MSC‐based cell therapies 29. Whether chronic diseases such as COPD are also amenable to cell‐based therapies remains to be determined. The present study does not directly propose MSCs as a therapy for COPD, but rather as an adjunct to decrease potential complications of BLVR with EBV placement. Following our initial safety investigation, future larger‐scale trials should be conducted to determine the potential efficacy of this approach.
A growing number of clinical investigations of MSC‐based cell therapies are being conducted in an ever‐widening spectrum of critical illnesses and lung diseases 7, 27, 29, including COPD. The PROCHYMAL study tested the safety of BM‐MSCs infused every 4 months in patients with moderate‐to‐severe emphysema, and found that MSC therapy was safe and promoted significant reductions in CRP levels, with no significant differences in pulmonary function tests. However, contrary to our findings, there was no improvement in QoL indicators 7. In the PROCHYMAL study, MSCs were administered through the intravenous route. In the present study, we decided to administer MSCs through the intrabronchial route (3–5 cm distal to segmental bronchi) in an attempt to deliver these cells close to the primarily injured tissue, so as to enhance local anti‐inflammatory, antifibrotic, and microbicidal effects. Additionally, the cells were administered in combination with EBVs, which ensure their proximal retention in the damaged parenchyma.
In a conceptually related study, Stolk et al. assessed the safety and feasibility of intravenous administration of autologous BM‐MSCs after one‐sided LVRS and prior to a second LVRS procedure for patients with severe pulmonary emphysema 30. Briefly, LVRS was first performed on one lung without preoperative infusion of BM‐MSCs, followed by a second surgical procedure on the contralateral lung, which was preceded by two IV of BM‐MSCs (3 and 4 weeks before the second surgery). Although the results showed that autologous MSC administration in patients with severe emphysema was feasible and safe, the study had several limitations, including its open‐label, nonrandomized, nonblinded, prospective design. The study also lacked standardization of the number of cells in each dose across patients, and timing of MSC administration after the first treatment varied. The main limitation, however, was the lack of a placebo group. As most results were attributed to cell therapy, but patients were not compared with a control group, outcomes may in fact have been due to LVRS and not necessarily due to effects of cell therapy. Thus, its results should be interpreted with caution 30.
Our group was the first to test the effect of MSC administration in the distal airway combined with EBV‐based BLVR in a placebo‐controlled, randomized design. Limitations of our study include the small number of recruited patients, the short duration of follow‐up (up to 90 days), and measurement of inflammatory mediators only in plasma, but not in bronchoalveolar lavage fluid. Furthermore, as our design was underpowered for efficacy, our primary objective was limited to assessment of the safety of MSC administration concomitant to EBV insertion.
Conclusion
In summary, the combined use of intrabronchial MSC administration and BLVR through endobronchial valve placement appears to be safe and may decrease systemic inflammation in patients with compromised lung function due to severe COPD. These results provide a basis for subsequent investigations of MSCs as adjunctive therapy in patients with COPD.
Author Contributions
H.G.O., F.F.C., M.A.A., and P.R.M.R.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; A.V.M.N.: conception and design, collection and or assembly of data, final approval of manuscript; G.A.O., F.M.S., T.B., and C.L.K.R.: collection and/or assembly of data, final approval of manuscript; D.J.W.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript; P.R.S.B., M.M.M., and J.R.L.S.: conception and design, financial support, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
Hugo Goulart de Oliveira is PI of the Brazilian Liberate Study Site (ClinicalTrials NCT01796392). Valves were donated by PulmonX and Ciclomed. Other authors have indicated no potential conflicts of interests. Disclaimer: The views expressed in the submitted article are the authors' own and are not an official position of their institutions or funders.
Supporting information
Supplemental Figure 1
supplemental figure 1 legend
Supplemental data
Supplemental Table 1
Supplemental Table 2
Supplemental Table 3
Supplemental Table 1
Supplemental Table 5
Acknowledgments
We would like to express our gratitude to PulmonX and Ciclomed for donating the valves used in this trial, and to Moira Elizabeth Schöttler and Filippe Vasconcellos for their assistance in editing the manuscript. This work was supported by Brazilian Council for Scientific and Technological Development (CNPq), Rio de Janeiro State Research Foundation (FAPERJ), Coordination for the Improvement of Higher Level Personnel (CAPES), and the Department of Science and Technology, Brazilian Ministry of Health (DECIT/MS).
References
- 1. Global Strategy for the Diagnosis, Management and Prevention of COPD . Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. Available at http://www.goldcopd.org/. Acessed May 12, 2016.
- 2. Sciurba FC, Ernst A, Herth FJ et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233–1244. [DOI] [PubMed] [Google Scholar]
- 3. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 4. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013;13:392–402. [DOI] [PubMed] [Google Scholar]
- 5. Lee JW, Fang X, Gupta N et al. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin‐induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 2009;106:16357–16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antunes MA, Abreu SC, Cruz FF et al. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir Res 2014;15:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiss DJ, Casaburi R, Flannery R et al. A placebo‐controlled, randomized trial of mesenchymal stem cells in COPD. Chest 2013;143:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Criner GJ, Cordova F, Sternberg AL et al. The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011;184:881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davey C, Zoumot Z, Jordan S et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR‐HIFi trial): Study design and rationale. Thorax 2015;70:288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Oliveira HG, Macedo‐Neto AV, John AB et al. Transbronchoscopic pulmonary emphysema treatment: 1‐month to 24‐month endoscopic follow‐up. Chest 2006;130:190–199. [DOI] [PubMed] [Google Scholar]
- 11. World Medical Association (WMA). Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 12. van Rikxoort EM, Goldin JG, Galperin‐Aizenberg M et al. A method for the automatic quantification of the completeness of pulmonary fissures: Evaluation in a database of subjects with severe emphysema. Eur Radiol 2012;22:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oliveira HG, Rambo RR, Macedo‐Neto AV et al. Fissure integrity as a non‐invasive method to predict volume reduction in endobronchial valve treatment of emphysema. Paper presented at: ATS Internatinal Conference; 2013; Philadelphia, PA.
- 14. Yin Y, Newell J, Raffy P. Automatic quantification of lung fissure integrity in subjects with severe emphysema. Paper presented at: ATS International Conference; 2013; Philadelphia, PA.
- 15. Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 1968;97:77–89. [PubMed] [Google Scholar]
- 16. Barbano DBA. Resolução RDC n° 9, 14 de março de 2011. Agência Nacional de Vigilância Sanitária (ANVISA); Diário Oficial da União no 187, Brasília-DF, Brazil. 2011.
- 17. Rebelatto CK, Aguiar AM, Moretao MP et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008;233:901–913. [DOI] [PubMed] [Google Scholar]
- 18. Borgonovo T, Vaz IM, Senegaglia AC et al. Genetic evaluation of mesenchymal stem cells by G‐banded karyotyping in a Cell Technology Center. Rev Bras Hematol Hemoter 2014;36:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herth FJ, Eberhardt R, Gompelmann D et al. Radiological and clinical outcomes of using Chartis to plan endobronchial valve treatment. Eur Respir J 2013;41:302–308. [DOI] [PubMed] [Google Scholar]
- 20. Oliveira HG, Macedo‐Neto AV, Valle E. Using Virtual Bronchoscopy for Emphysema Evaluation and Treatment Planning. 17th World Congress for Bronchology and Interventional Pulmonology, World Association for Bronchology and Interventional Pulmonology. Cleveland, OH, USA; 2012. [Google Scholar]
- 21. Celli BR, Locantore N, Yates J et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;185:1065–1072. [DOI] [PubMed] [Google Scholar]
- 22. Rabe KF, Hurd S, Anzueto A et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555. [DOI] [PubMed] [Google Scholar]
- 23. Celli BR, Cote CG, Marin JM et al. The body‐mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005–1012. [DOI] [PubMed] [Google Scholar]
- 24. Jones PW, Quirk FH, Baveystock CM et al. A self‐complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321–1327. [DOI] [PubMed] [Google Scholar]
- 25. Zar JH. Biostatistical Analysis. Upper Saddle River, NJ: Prentice‐Hall, Inc, 1974. [Google Scholar]
- 26. Dahl M, Vestbo J, Lange P et al. C‐reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:250–255. [DOI] [PubMed] [Google Scholar]
- 27. Weiss DJ. Concise review: Current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells 2014;32:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McIntyre LA, Moher D, Fergusson DA et al. Efficacy of mesenchymal stromal cell therapy for acute lung injury in preclinical animal models: A systematic review. PLoS One 2016;11:e0147170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilson JG, Liu KD, Zhuo H et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir Med 2015;3:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stolk J, Broekman W, Mauad T et al. A phase I study for intravenous autologous mesenchymal stromal cell administration to patients with severe emphysema. QJM 2016;109:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1
supplemental figure 1 legend
Supplemental data
Supplemental Table 1
Supplemental Table 2
Supplemental Table 3
Supplemental Table 1
Supplemental Table 5


