Summary
Mesenchymal stem (stromal) cells (MSCs) are being investigated for treating degenerative and inflammatory disorders because of their reparative and immunomodulatory properties. Intricate mechanisms relate cell death processes with immune responses, which have implications for degenerative and inflammatory conditions. We review the therapeutic value of MSCs in terms of preventing regulated cell death (RCD). When cells identify an insult, specific intracellular pathways are elicited for execution of RCD processes, such as apoptosis, necroptosis, and pyroptosis. To some extent, exacerbated RCD can provoke an intense inflammatory response and vice versa. Emerging studies are focusing on the molecular mechanisms deployed by MSCs to ameliorate the survival, bioenergetics, and functions of unfit immune or nonimmune cells. Given these aspects, and in light of MSC actions in modulating cell death processes, we suggest the use of novel functional in vitro assays to ensure the potency of MSCs for preventing RCD. Such analyses should be associated with existing functional assays measuring the anti‐inflammatory capabilities of MSCs in vitro. MSCs selected on the basis of two in vitro functional criteria (i.e., prevention of inflammation and RCD) could possess optimal therapeutic efficacy in vivo. In addition, we underline the implications of these perspectives in clinical studies of MSC therapy, with particular focus on acute respiratory distress syndrome. Stem Cells Translational Medicine 2017;6:713–719
Keywords: Mesenchymal stem cells, Cell death, Functional potency, Cellular therapy, Degenerative disorder, Inflammatory disorder, Clinical translation, Selection technologies
Significance Statement.
Most studies of mesenchymal stem (stromal) cells (MSCs) focus on their anti‐inflammatory, trophic and differentiation abilities, but their ability to prevent regulated cell death (RCD) remains undefined. However, this last function could explain both the regenerative and anti‐inflammatory therapeutic effect of MSCs observed in preclinical and clinical studies. The present report reviews the role of MSCs in preventing RCD, with implications for enhancing their therapeutic efficacy in the clinic. Development of in vitro assays to assess MSC functional potency in preventing RCD is suggested and criteria for selecting MSCs for therapeutic use are proposed. Furthermore, in vivo biomarkers of RCD that can be used for prompt evaluation of the therapeutic effects of MSCs are suggested.
Introduction
Mesenchymal stem (stromal) cells (MSCs), in humans, are principally derived from bone marrow and adipose tissues in adults and in neonatal tissues from umbilical cord blood and placenta [1, 2, 3]. Regardless of their origin, in vitro‐expanded MSCs possess a common phenotype and share mutual biological properties [4, 5, 6, 7, 8]. However, we lack specific biomarkers to distinguish MSCs phenotypically and exclusively in vivo or in MSCs expanded in vitro. This situation is further complicated by the fact that in vitro‐expanded MSC cultures are not derived from a single clone but rather several fibroblastic colony forming units [9, 10] with probable functional heterogeneities [8, 11]. To address this complexity, researchers use a combination of cell surface markers [7, 8] that are often associated with functional assessment of MSCs in differentiating into osteoblasts, chondroblasts, and adipocytes to confirm the MSC identity [8] (Fig. 1).
Figure 1.
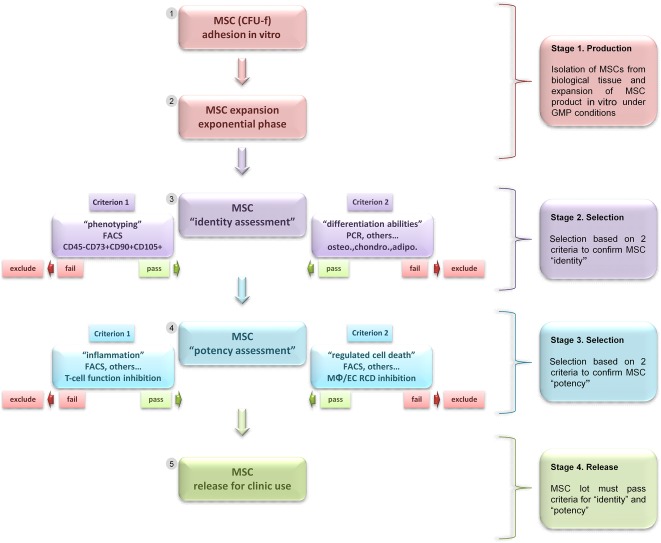
Schematic diagram summarizing the concept of MSC selection based on identity and double functional potency for preventing inflammation and RCD before use as therapy. This schematic shows four essential stages, from isolation to release of MSC product for use in clinic. Stage 1: optimal methods for MSC isolation, expansion, and production by GMP with severe control in cell sterility and genetic stability. Stage 2: selection of MSCs based on two criteria, phenotype and potential for differentiation, for assessing MSC “identity” in vitro. Stage 3: selection of MSCs based on two criteria, inhibition of inflammation and inhibition of RCD, for assessing MSC “potency” in vitro. Stage 4: for approval of MSCs for therapy and monitoring of in vivo actions of MSCs. Abbreviations: adipo., adipocytes; CFU‐f, colony‐forming unit fibroblast; chondro., chondroblasts; EC, epithelial cell; FACS, fluorescence‐activated cell sorting; GMP, Good Manufacturing Practices; MΦ, macrophage; MSC, mesenchymal stem (stromal) cell; osteo., osteoblasts; PCR, polymerase chain reaction; RCD, regulated cell death.
Today, MSCs are under intense clinical investigation for regenerative medicine because of their differentiation and trophic abilities [12, 13, 14] and for treatment of inflammatory diseases because of their immunosuppressive properties [15, 16]. MSCs delivered in vivo can home to inflammatory sites [17, 18] and produce anti‐inflammatory and growth factors; therapeutic effects have been demonstrated in preclinical and clinical studies of various disorders [19, 20]. Hence, the clinical use of MSCs for treating severe degenerative and inflammatory diseases lacking appropriate treatments is expected to increase exponentially [8].
Substantial efforts have been undertaken by the translational community to standardize methods for producing, selecting, and using MSCs in the clinic [5, 6]. Notably, general guidance has been proposed for developing in vitro assays for selecting MSCs with potent therapeutic ability based on functional criteria [20, 21]. These assays require identifying MSC functions to predict clinical efficacy [6]. Some clinical observations have confirmed the relevance of in vitro assays to measure anti‐inflammatory MSC potency, which was found consistent with in vivo effects [21]. Challenges remain in improving and using pertinent functional in vitro assays to identify MSCs with bona fide optimal efficiency in vivo [5, 6]. Thus, the ability of MSCs to prevent cell death processes could be tested in vitro to identify functional MSCs for clinical use.
Regulated Cell Death as a Therapeutic Target
Emerging evidences indicate a critical role for regulated cell death (RCD) in the pathogenesis of various diseases [22]. By definition, RCD is opposite to accidental cell death (ACD), whose effects are often identified as necrosis [23] (Tables 1, 2). ACD results from sudden trauma and occurs in an uncontrolled manner [23]. Nonetheless, ACD occurring in cells and through the release of intracellular content might trigger RCD in bystander cells [23]. RCD includes several processes [24, 25], among which the most distinct are apoptosis, necroptosis, and pyroptosis [23] (Tables 1, 2). Thus, RCD is caused after cells sense danger or inflammatory mediators, in sterile or nonsterile conditions, which has implications for the pathogenesis of degenerative and inflammatory disorders [22, 24, 25, 26, 27, 28].
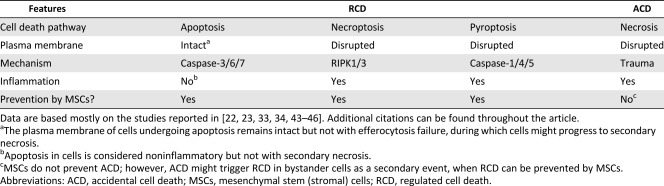
Table 1.
Features of RCD and ACD with the role of MSCs in preventing RCD in terminally differentiated third‐party cells
|
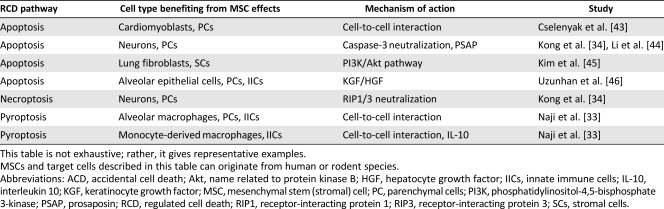
Table 2.
MSC prevention of RCD processes occurring in terminally differentiated parenchymal, stromal, and immune cells
|
The RCD processes differ by their molecular triggers, molecular pathways engaged and mode of execution [23]. Apoptosis has been considered a programmed cell death (PCD) both during physiological and pathological processes. The term “PCD” is now preferred to indicate cell death from physiological processes, such as during development and maintenance of tissue homeostasis [29]. However, when cell death occurs during pathological conditions, RCD rather than PCD appears more appropriate [23, 25, 29]. Apoptosis is executed via a mechanism involving caspase‐3/6/7 and results in cell death without plasma membrane rupture [22]. With disrupted plasma membrane, apoptosis might culminate in secondary necrosis [24, 30, 31]. Thus, apoptosis can be considered nonimmunogenic but not occurring in particular pathological conditions [26, 30, 31], whereas RCD such as necroptosis and pyroptosis are intrinsically immunogenic [24].
Necroptosis is mediated by a mechanism that depends on receptor‐interacting protein kinase 1/3 and mixed lineage kinase‐like protein, whereas pyroptosis is executed in cells by a mechanism involving caspase‐1/4/5 and gasdermin D [24, 26, 27]. Both necroptosis and pyroptosis conclude with a rapid rupture of the plasma membrane, release of intracellular content and often with harmful consequences [24, 27]. Hence, RCD can be detrimental because it can sustain inflammation, tissue damage, and loss of function of the affected organ [22, 28]. Furthermore, exacerbated RCD can cause inflammation, and intense inflammation can elicit RCD, with, in all cases, pathological consequences [22]. Therefore, targeting RCD in addition to inflammation is needed to improve the efficacy of existing anti‐inflammatory therapeutics [22, 28, 32].
Brief Insights Into the Prospective Mode of Action of MSCs in Preventing RCD
MSCs are known to improve cell survival and prevent apoptosis, necroptosis and pyroptosis (Tables 1, 2) occurring in various parenchymal or nonparenchymal cells and immune cells under unfavorable conditions [19, 33, 34, 35]. Mechanistically, MSCs are thought to promote cell survival via the secretion and paracrine actions of various cytokines and growth factors [20, 36]. They may also promote survival, bioenergetics, and functions of distressed cells, by mitochondria transfer through tunneling nanotubes (TNT), or microRNA/protein transfer through extracellular vesicles [37, 38, 39, 40]. The mechanism may involve gap‐junction communication via connexin 43 between MSCs and unfit cells [38, 41]. Consistently, mitochondrial transfer from MSCs to immune cells occurs in vivo and results in enhanced cell survival, phagocytic activity, and antimicrobial effects in preclinical models of acute lung injury and acute respiratory distress syndrome (ARDS) [38, 39]. The mechanisms MSCs use to achieve improved survival, bioenergetics, and functions of unfit cells are diverse and sophisticated and may reflect their vital importance, such as preventing RCD. Of note, TNT‐mediated transfer of mitochondria from healthy to apoptotic neuroblastic PC12 cells can reverse apoptosis, with implications for the survival mechanisms of damaged cells [42]. By comparison, this proposes that transfer of mitochondria from MSCs to distressed cells through a TNT‐dependent mechanism might prevent the execution of RCD.
Therefore, innovative therapeutic interventions should simultaneously target RCD and inflammation to optimize cure [22]. The abundant success of MSC therapy in certain degenerative and inflammatory disorders, observed in preclinical and clinical studies, might be because of the intrinsic properties of MSCs to simultaneously modulate RCD and inflammation. Further dissecting the mechanisms MSCs use to prevent RCD is fundamental, but the use of such functional attributes as selection criteria for MSCs intended for therapy is of immediate practical importance for the clinic.
MSC Function to Modulate RCD as Criteria for Therapeutic Use
The antiapoptotic properties of MSCs toward immune and nonimmune cells have been demonstrated in some contexts [35, 36, 43, 44, 45, 46]. Emerging studies suggest that MSCs can inhibit RCD such as necroptosis [34], and we recently showed that MSCs could prevent pyroptosis in macrophages [33]. We focused on the pathogenesis of severe occupational lung diseases such as interstitial lung disease and pulmonary alveolar proteinosis, which could involve pyroptosis of lung macrophages caused by inhalation of inorganic particles [33]. This pyroptosis is characterized by the production of inflammatory cytokines and cell death by cytolysis, events depending on the inflammasome NACHT, LRR, and PYD domain‐containing protein 3–apoptosis‐associated speck‐like protein containing a CARD–Caspase‐1 (NLRP3‐ASC‐Caspase‐1) [33]. Blockade of inflammatory pathways with pharmacological inhibitors such as dexamethasone and genetic knockdown of essential inflammasome protein components (i.e., NLRP3 or ASC) reduced the production of inflammatory cytokines but were ineffective in preventing cell death. However, coculture of MSCs with macrophages undergoing pyroptosis resulted in both inflammation and cell death inhibition [33].
Therefore, we suggest that to optimize the efficiency of MSC therapy, the ability of MSCs to prevent RCD should be evaluated by in vitro functional assays before the cells are used in clinical interventions (Fig. 1). The assays can be established rapidly and suitably in conventional biology laboratories (Tables 3–5). These functional assays should be implemented by coculturing MSCs with cells of innate immunity, including macrophages and epithelial cells, because RCD in innate immune cells are likely responsible for triggering an exacerbated inflammatory response, such as in sepsis [22]. Thus, macrophages and epithelial cells, challenged with specific cell death inducers, can be cocultured with MSCs at varying cell ratios to estimate the ability of MSCs to modulate RCD. These in vitro functional assays can be used to measure markers of cell death in cells or supernatant (Table 4) within hours [33]. As well, they can allow for quantifying pro‐ and anti‐inflammatory cytokines (i.e., tumor necrosis factor α and interleukin 10) released in the supernatant in assessing MSC function to modulate RCD and inflammation [33]. To further compare the MSC potency of various products to modulate RCD, MSCs should be tested in dilution series with limiting dilution analysis (LDA) [47] to measure the amplitude of potency of a given MSC culture in preventing RCD. Hence, LDA established for each MSC product might help estimate the MSC frequency with actual function to prevent RCD to predict the MSC therapeutic benefit in vivo. However, these functional assays must be accompanied by in vitro evaluation of the MSC anti‐inflammatory potency for cells of adaptive immunity, such as T cells [48]. The selection of MSCs based on in vitro functional criteria for modulating both RCD and inflammation of innate and adaptive immune cells might lead to an optimal therapeutic effect in vivo (Tables 3–5).
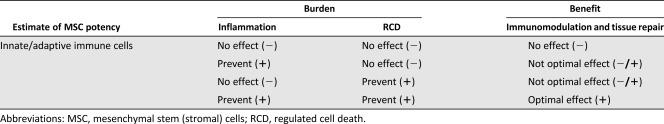
Table 3.
Evaluation of MSC potency based on two functional criteria: inflammation and RCD
|
Table 5.
Evaluating RCD and ACD in vivo with specific biomarkers
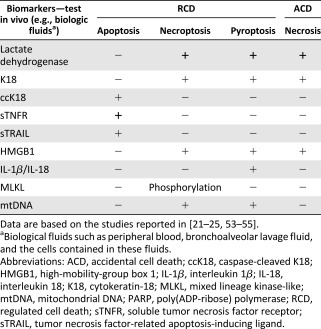
|
Table 4.
Evaluating RCD and ACD in vitro with specific RCD biomarkers
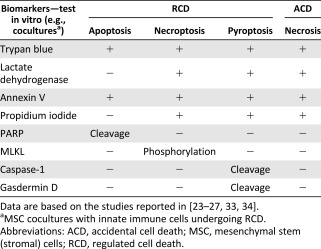
|
Because the therapeutic effects of MSCs often result from multiple pathways, with or without redundant actions [8], the in vitro potency of MSCs must be assessed in terms of two functional criteria to ensure the optimal in vivo effect. Assessing the MSC potency to prevent both RCD and inflammation, with the assumption that both functions can be determined by independent mechanisms, is critical to ensure the optimal therapeutic effect of MSCs, and particularly for diseases in which cell death is closely related to inflammatory processes, such as ARDS or other devastating disorders [22, 32]. Studies have suggested that the systemic administration of MSCs in preclinical ARDS models improves respiratory conditions [39, 49]. Recently, a phase 1 clinical trial demonstrated the safety and tolerability of intravascular infusion of allogeneic MSCs in nine patients with ARDS [16, 37]. A phase 2 clinical trial in progress [20, 50] is assessing the clinical efficacy of MSC infusion in patients with moderate to severe ARDS [50]. Therefore, selecting MSCs intended for use in treatment of ARDS has clinical relevance in terms of the in vitro potency to modulate both RCD and inflammation.
Clinical Relevance for Identifying MSCs With Optimal Therapeutic Actions
Indeed, RCD represents a therapeutic target for attenuating both tissue damage and inflammation in various disorders [22] such as ARDS [49]. ARDS represents severe lung injury, a serious and life‐threatening condition that often results from intense trauma, pneumonia infection or sepsis [49]. The pathogenesis of ARDS is characterized by diffuse alveolar damage complicated by intense inflammation [51]. Diffuse alveolar damage is associated with rapid and massive myeloid and epithelial cell death, which is detected by molecular markers such as activated caspases and cleavage of cytokeratin 18 (K18) [21, 49]. Hence, in advanced‐phase clinical trials, the MSC potency in preventing RCD in myeloid and epithelial cells could be evaluated as supplementary selection criteria for MSCs intended for patients with ARDS. This suggestion is motivated by patients with ARDS being particularly affected by intense cell death and inflammation within the lung parenchyma [51]. Furthermore, molecular markers of RCD should be tested in vivo (Table 5) to measure the beneficial effects of MSC adoptive transfer, as an integral part of monitoring MSC therapy, especially for patients with ARDS.
A study by Leblanc and colleagues [21] showed improvement with MSC infusion in severe cases of ARDS, with resolution of respiratory, hemodynamic, and organ failure [21]. These improvements were associated with decreased levels of markers of inflammation. Moreover, the authors evaluated in vitro the immunomodulation potency of the MSCs used. The in vitro potency assays included functional assays for determining the anti‐inflammatory properties of MSCs and proteomic analysis of both MSCs and extracellular vesicles released by MSCs. Encouraging results were observed in two patients with ARDS who received an intravascular infusion of MSCs on a compassionate basis [21]. In these two cases, adoptive transfer of MSCs demonstrated that the in vivo actions of MSCs agreed with most of the MSC actions measured in vitro [21].
Improvements in patients with ARDS who received adoptive transfer of MSCs were associated with a rapid decrease in levels of markers of cell death [21]. Significantly, Leblanc and colleagues analyzed bronchoalveolar lavage fluid (BALF) for monitoring molecular makers of apoptosis and necrosis of alveolar epithelial cells. The analysis of cell death in BALF was based on detection of epithelial apoptosis by measuring caspase‐cleaved K18 and other forms of cell death with features of necrosis, detected by measuring uncleaved K18 [21]. The results revealed a rapid decrease in both apoptosis and necrosis of lung epithelial cells, assessed within only few hours after the adoptive transfer of MSCs in patients [21]. This finding might indicate a sequential mechanism of the MSC action, the first effect being to home to the site of tissue damage, to prevent RCD, before or concomitant with the assessable action of MSCs in modulating inflammation.
Thus, RCD biomarkers could be measured to monitor and rapidly predict the outcomes of a given MSC treatment in patients with ARDS. This analysis is crucial to readily evaluate the response of the intervention in patients and could be used to adapt and appropriately improve the treatment. Leblanc and colleagues suggested that MSCs have therapeutic efficacy for ARDS [21]. Furthermore, the authors demonstrated the advantage of in vitro assessment of the MSC anti‐inflammatory potency while providing critical molecular insights into the processes of cell death as pertinent in vivo biomarkers [21]. Thenceforth, such assessments appear critical in order to rapidly monitor and evaluate the therapeutic effects of MSCs.
Conclusion
MSCs are remarkable from therapeutic perspectives, given the ease with which we can obtain a significant number of genetically stable MSCs and the number of diseases that can be treated because of the intrinsic properties of MSCs [36]. Today, MSCs are used in advanced‐phase clinical trials of therapy to inhibit the degenerative and inflammatory processes in various disorders [6, 14, 36]. Thus, we increasingly need to standardize, optimize, and ensure the success of MSC therapy in such advanced‐phase clinical trials [5, 6, 9, 13, 14, 20, 21, 48, 50, 52]. The challenges and perspectives lie in implementing appropriate functional assays in vitro that could assess the therapeutic potential of MSCs intended for clinical use. To this end, the efforts of the translational community have focused on providing release criteria for MSCs based on their anti‐inflammatory function, usually toward T‐cell activation and proliferation, in vitro [5, 6, 48]. In this review, we suggest that in addition to developing easy‐to‐use and rapid functional assays for MSCs, we should develop assays to evaluate their ability to modulate RCD and in particular innate immune cells such as macrophages and epithelial cells. However, functional assays for MSCs in modulating RCD of other cell types, such as parenchymal cells or organ‐specific cell subtypes, could be applied; pertinent target cells should be identified according to a known pathogenesis implying RCD for a given disease. In addition, we suggest monitoring RCD biomarkers in patients, including specific markers for apoptosis, necroptosis, and pyroptosis, because these RCD have a direct effect on the pathogenesis of a number of diseases [22, 23, 28]. Of note, RCD may not be relevant in the pathogenesis of all diseases treated with MSCs, in which case other pertinent markers should be evaluated. Nonetheless, targeting both inflammation pathways and RCD pathways as therapeutic objectives might help improve MSC treatments intended for degenerative and inflammatory diseases. The assessment of the potency of MSCs in modulating both inflammation and RCD in vitro and the monitoring of both inflammation and RCD biomarkers in vivo [23, 25, 53, 54, 55] would certainly benefit patients receiving MSC therapy, particularly those with ARDS currently in advanced‐phase clinical trials [20, 21, 39, 50, 51].
Author Contributions
A.N., conception and design, figure/table design, manuscript writing, final approval of manuscript; N.S., N.E., K‐i.Y., and N.B.: manuscript writing, final approval of manuscript; L.S. and F.D.: conception and design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
Support was provided by the Program to Disseminate Tenure Tracking System of the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by intramural funding from Kochi Medical School, Kochi University, Japan to A.N. Additional support was provided by Grant 12/12/12.11 to L.S., “Nouveaux Marqueurs de Sécurité des Cellules Souches,” Région Midi‐Pyrénées, Toulouse, France.
References
- 1. Kern S, Eichler H, Stoeve J et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294–1301. [DOI] [PubMed] [Google Scholar]
- 2. Hass R, Kasper C, Böhm S et al. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue‐derived MSC. Cell Commun Signal 2011;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Via AG, Frizziero A, Oliva F. Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J 2012;2:154–162. [PMC free article] [PubMed] [Google Scholar]
- 4. Le Blanc K, Ringdén O. Mesenchymal stem cells: Properties and role in clinical bone marrow transplantation. Curr Opin Immunol 2006;18:586–591. [DOI] [PubMed] [Google Scholar]
- 5. Galipeau J, Krampera M. The challenge of defining mesenchymal stromal cell potency assays and their potential use as release criteria. Cytotherapy 2015;17:125–127. [DOI] [PubMed] [Google Scholar]
- 6. Galipeau J, Krampera M, Barrett J et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy 2016;18:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 8. Ma S, Xie N, Li W et al. Immunobiology of mesenchymal stem cells. Cell Death Differ 2014;21:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Phinney DG. Functional heterogeneity of mesenchymal stem cells: Implications for cell therapy. J Cell Biochem 2012;113:2806–2812. [DOI] [PubMed] [Google Scholar]
- 10. Shoshani O, Ravid O, Massalha H et al. Cell isolation induces fate changes of bone marrow mesenchymal cells leading to loss or alternatively to acquisition of new differentiation potentials. Stem Cells 2014;32:2008–2020. [DOI] [PubMed] [Google Scholar]
- 11. Wagner W, Feldmann RE Jr, Seckinger A et al. The heterogeneity of human mesenchymal stem cell preparations—evidence from simultaneous analysis of proteomes and transcriptomes. Exp Hematol 2006;34:536–548. [DOI] [PubMed] [Google Scholar]
- 12. Zahorec P, Koller J, Danisovic L et al. Mesenchymal stem cells for chronic wounds therapy. Cell Tissue Bank 2015;16:19–26. [DOI] [PubMed] [Google Scholar]
- 13. Mineda K, Feng J, Ishimine H et al. Therapeutic potential of human adipose‐derived stem/stromal cell microspheroids prepared by three‐dimensional culture in non‐cross‐linked hyaluronic acid gel. Stem Cells 2015;4:1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samsonraj RM, Rai B, Sathiyanathan P et al. Establishing criteria for human mesenchymal stem cell potency. Stem Cells 2015;33:1878–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–736. [DOI] [PubMed] [Google Scholar]
- 16. Naji A, Rouas‐Freiss N, Durrbach A et al. Concise review: Combining human leukocyte antigen G and mesenchymal stem cells for immunosuppressant biotherapy. Stem Cells 2013;31:2296–2303. [DOI] [PubMed] [Google Scholar]
- 17. De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy?. World J Stem Cells 2016;8:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ren G, Chen X, Dong F et al. Concise review: Mesenchymal stem cells and translational medicine: Emerging issues. Stem Cells 2012;1:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JW, Fang X, Krasnodembskaya A et al. Concise review: Mesenchymal stem cells for acute lung injury: Role of paracrine soluble factors. Stem Cells 2011;29:913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: Mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med 2014;2:1016–1026. [DOI] [PubMed] [Google Scholar]
- 21. Simonson OE, Mougiakakos D, Heldring N et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells 2015;4:1199–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Linkermann A, Stockwell BR, Krautwald S et al. Regulated cell death and inflammation: an auto‐amplification loop causes organ failure. Nat Rev Immunol 2014;14:759–767. [DOI] [PubMed] [Google Scholar]
- 23. Galluzzi L, Bravo‐San Pedro JM, Vitale I et al. Essential versus accessory aspects of cell death: Recommendations of the NCCD 2015. Cell Death Differ 2015;22:58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tait SW, Ichim G, Green DR. Die another way‐‐non‐apoptotic mechanisms of cell death. J Cell Sci 2014;127:2135–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kroemer G, Galluzzi L, Vandenabeele P et al.; Nomenclature Committee on Cell Death 2009 . Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009;16:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murphy JM, Silke J. Ars Moriendi: The art of dying well—new insights into the molecular pathways of necroptotic cell death. EMBO Rep 2014;15:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wallach D, Kang TB, Dillon CP et al. Programmed necrosis in inflammation: Toward identification of the effector molecules. Science 2016;352:aaf2154. [DOI] [PubMed] [Google Scholar]
- 28. Ashkenazi A, Salvesen G. Regulated cell death: Signaling and mechanisms. Annu Rev Cell Dev Biol 2014;30:337–356. [DOI] [PubMed] [Google Scholar]
- 29. Galluzzi L, López‐Soto A, Kumar S et al. Caspases connect cell‐death signaling to organismal homeostasis. Immunity 2016;44:221–231. [DOI] [PubMed] [Google Scholar]
- 30. Poon IK, Lucas CD, Rossi AG et al. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat Rev Immunol 2014;14:166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Medina CB, Ravichandran KS. Do not let death do us part: ‘Find‐me’ signals in communication between dying cells and the phagocytes. Cell Death Differ 2016;23:979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fullerton JN, Gilroy DW. Resolution of inflammation: A new therapeutic frontier. Nat Rev Drug Discov 2016;15:551–567. [DOI] [PubMed] [Google Scholar]
- 33. Naji A, Muzembo BA, Yagyu K et al. Endocytosis of indium‐tin‐oxide nanoparticles by macrophages provokes pyroptosis requiring NLRP3‐ASC‐Caspase1 axis that can be prevented by mesenchymal stem cells. Sci Rep 2016;6:26162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kong D, Zhu J, Liu Q et al. Mesenchymal stem cells protect neurons against hypoxic‐ischemic injury via inhibiting parthanatos, necroptosis, and apoptosis, but not autophagy. Cell Mol Neurobiol 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raffaghello L, Bianchi G, Bertolotto M et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: A model for neutrophil preservation in the bone marrow niche. Stem Cells 2008;26:151–162. [DOI] [PubMed] [Google Scholar]
- 36. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 2013;45:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahmad T, Mukherjee S, Pattnaik B et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J 2014;33:994–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Islam MN, Das SR, Emin MT et al. Mitochondrial transfer from bone‐marrow‐derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 2012;18:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jackson MV, Morrison TJ, Doherty DF et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells 2016;34:2210–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vallabhaneni KC, Penfornis P, Dhule S et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget 2015;6:4953–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Han J, Kim B, Shin JY et al. Iron oxide nanoparticle‐mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano 2015;9:2805–2819. [DOI] [PubMed] [Google Scholar]
- 42. Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ 2015;22:1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cselenyák A, Pankotai E, Horváth EM et al. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell‐to‐cell connections. BMC Cell Biol 2010;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li N, Sarojini H, An J et al. Prosaposin in the secretome of marrow stroma‐derived neural progenitor cells protects neural cells from apoptotic death. J Neurochem 2010;112:1527–1538. [DOI] [PubMed] [Google Scholar]
- 45. Kim SY, Lee JH, Kim HJ et al. Mesenchymal stem cell‐conditioned media recovers lung fibroblasts from cigarette smoke‐induced damage. Am J Physiol Lung Cell Mol Physiol 2012;302:L891–L908. [DOI] [PubMed] [Google Scholar]
- 46. Uzunhan Y, Bernard O, Marchant D et al. Mesenchymal stem cells protect from hypoxia‐induced alveolar epithelial‐mesenchymal transition. Am J Physiol Lung Cell Mol Physiol 2016;310:L439–L451. [DOI] [PubMed] [Google Scholar]
- 47. Dozmorov I, Eisenbraun MD, Lefkovits I. Limiting dilution analysis: from frequencies to cellular interactions. Immunol Today 2000;21:15–18. [DOI] [PubMed] [Google Scholar]
- 48. Salem B, Miner S, Hensel NF et al. Quantitative activation suppression assay to evaluate human bone marrow‐derived mesenchymal stromal cell potency. Cytotherapy 2015;17:1675–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moon HG, Cao Y, Yang J et al. Lung epithelial cell‐derived extracellular vesicles activate macrophage‐mediated inflammatory responses via ROCK1 pathway. Cell Death Dis 2015;6:e2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilson JG, Liu KD, Zhuo H et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir Med 2015;3:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kao KC, Hu HC, Chang CH et al. Diffuse alveolar damage associated mortality in selected acute respiratory distress syndrome patients with open lung biopsy. Crit Care 2015;19:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Munneke JM, Spruit MJ, Cornelissen AS et al. The potential of mesenchymal stromal cells as treatment for severe steroid‐refractory acute graft‐versus‐host disease: A critical review of the literature. Transplantation 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 53. Jouan‐Lanhouet S, Riquet F, Duprez L et al. Necroptosis, in vivo detection in experimental disease models. Semin Cell Dev Biol 2014;35:2–13. [DOI] [PubMed] [Google Scholar]
- 54. Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: The release of damage‐associated molecular patterns and its physiological relevance. Immunity 2013;38:209–223. [DOI] [PubMed] [Google Scholar]
- 55. Vanden Berghe T, Demon D, Bogaert P et al. Simultaneous targeting of IL‐1 and IL‐18 is required for protection against inflammatory and septic shock. Am J Respir Crit Care Med 2014;189:282–291. [DOI] [PubMed] [Google Scholar]


