Abstract
Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis is a hallmark of major depressive disorder. A number of studies have shown that this dysregulation is correlated with impaired forebrain glucocorticoid receptor (GR) function. To determine whether a primary, acquired deficit in forebrain GR signaling is an etiologic factor in the pathogenesis of depression, we generated a line of mice with time-dependent, forebrain-specific disruption of GR (FBGRKO). These mice develop a number of both physiological and behavioral abnormalities that mimic major depressive disorder in humans, including hyperactivity of the HPA axis, impaired negative feedback regulation of the HPA axis and, increased depression-like behavior. Importantly, a number of these abnormalities are normalized by chronic treatment with the tricyclic antidepressant, imipramine. Our findings suggest that imipramine's proposed activities on forebrain GR function are not essential for its antidepressant effects, and that alteration in GR expression may play a causative role in disease onset of major depressive disorder.
Keywords: knockout mice
Major depression is a serious neuropsychiatric illness that the World Health Organization predicts will soon be the world's greatest public health burden (www.nimh.nih.gov/publicat/burden). Although both genetic and environmental factors are known to contribute to its pathogenesis, two fundamental questions remain unanswered. First, what are the primary genetic factors that contribute to a predisposition for depression? Second, what interacting biochemical pathways lead to the disease state? There are a number of lines of evidence that suggest that dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis may be a primary factor in the pathogenesis of depression (1). Depressed patients show hyperactivity of the HPA axis that may result from impairments in negative feedback regulation of glucocorticoid release (2). Moreover, normalization of these HPA axis abnormalities is associated with successful antidepressant treatment, and patients whose HPA abnormalities do not normalize are significantly more likely to relapse (3).
Research both in humans and in animal models have implicated forebrain glucocorticoid receptors (GRs) in HPA axis regulation and depression (4, 5). Studies examining postmortem tissue from suicide victims and individuals with major depressive disorder (MDD) have revealed decreased GR mRNA expression in the hippocampus and cortex (6). Importantly, GR mRNA expression and hormone-binding activity are both increased after antidepressant treatment (7). In addition, a number of animal models of depression have been shown to be associated with decreased forebrain GR expression. In rodents, exposure to early life maternal neglect, which, in humans, is known to increase the risk of depression, leads to a depression like-phenotype (8) that is associated with decreased hippocampal GR expression (9). Conversely, an early nurturing environment led to increased hippocampal GR expression and decreased susceptibility to depression in adulthood (10). These studies suggest that early life environment modulates hippocampal GR expression, which may contribute to the expression of stress related behaviors in adulthood. Although these studies do not provide a direct causal link between GR expression and depression-related behaviors, they do provide strong correlational evidence in support of the hypothesis that altered GR activity is an etiologic factor in depression.
To more directly address this question, several groups have generated mice with targeted GR deficiencies to investigate the role of GR in HPA axis regulation and depression. Mice with a global deletion of GR die shortly after birth because of impaired lung development, preventing analysis of the endocrine and behavioral consequences of GR deletion (11). Transgenic mice have been produced in which antisense GR mRNA is expressed in neuronal and nonneuronal cells and leads to a 50% decrease in GR protein (12). These mice show no differences in nonstressed, basal HPA axis activity. However, these GR mRNA antisense transgenic mice show impaired inhibition in response to the GR-specific agonist dexamethasone (DEX), in the DEX suppression test (DST) and decreased expression of corticotropin-releasing hormone (CRH) in the hypothalamus. When tested for behavioral measures of stress and depression, they were found to be less anxious in the elevated plus maze, and they showed less floating behavior (a measure of despair) in a forced swim task (13). These mice provide important insights into the role of GR in HPA axis regulation and depression; however, this mutation affects GR throughout the brain and body starting early in development, and it is unclear how developmental effects and GR signaling in areas outside of the forebrain influence these results.
To specifically examine the role of GR in the brain, Tronche et al. (14) generated mice with central nervous system-specific deletion of GR (GRnes/cre). These mice show a number of alterations in the HPA axis, including 10 times greater basal (morning) plasma concentration of corticosterone, increased CRH in the paraventricular nucleus (PVN), and decreased adrenocorticotropic hormone (ACTH) in the anterior pituitary. Unfortunately, in these mice, GR is deleted in the PVN, a site of major negative feedback inhibition of the HPA axis, leading to severe hyperadrenalism and wasting, confounding behavioral analysis. Furthermore, in the GRnes/cre mice, GR is deleted early in development, again, making it difficult to separate effects resulting from alterations that occur during development from those resulting from an acute requirement for GR.
To test the hypothesis that acquired primary disruption of GR action will lead to dysregulation of the HPA axis and depression-like behavior, we used the Cre/LoxP system to generate forebrain-specific GR knockout (FBGRKO) mice. In this system, Cre recombinase is expressed under the control of the calcium-stimulated calmodulin kinase IIα (CaMKIIα) promoter. This Cre transgene is not active until ≈3 weeks of age (15), allowing us to avoid deletion during early brain development. Deletion of forebrain GR is not complete in the FBGRKO mice until ≈4–6 months of age. This time course is highly relevant to the natural progression of depression in humans, which is most commonly diagnosed between the ages of 25 and 35 years (16). We demonstrate that GR action in the forebrain regulates the circuits controlling neuroendocrine and behavioral effectors that impact upon the phenotype characteristic of MDD.
Materials and Methods
Generation of FBGRKO Mice. Mice homozygous for a GRloxPneo allele (17) were crossed with mice expressing a transgene in which Cre recombinase is expressed under the control of the forebrain-specific CaMKIIα promoter (T50 Cre) (15) to generate mice with forebrain-specific disruption of GR (FBGRKO) and Cre-negative littermate controls. Mice were of a mixed C57BL/6 × 129 × CBA background. Littermate knockout and control mice were used for all experiments.
Immunohistochemistry. Coronal sections were processed for immunohistochemistry with antibodies recognizing GR (1:200, M-20, Santa Cruz Biotechnology) and NeuN (1:200, Chemicon). See Supporting Text, which is published as supporting information on the PNAS web site, for details.
Pituitary ACTH/GR Immunostaining. Pituitary sections from 6-month-old FBGRKO and control mice were stained with antibodies specific for GR (as described above) and ACTH (guinea pig anti-ACTH antibody, Peninsula Laboratories, San Carlos, CA). See Supporting Text for details.
Long-Term Depression (LTD). Transverse slices (500 μm thick) from 6-month-old FBGRKO and control mice were maintained in an incubation chamber for 1 h at 37°C in a standard solution, transferred to a submersion recording chamber at 30°C. Extracellular recordings were obtained from the dendritic layer of the CA1 region with the use of glass electrodes filled with 2 M NaCl. A bipolar electrode was placed in stratum radiatum to stimulate the Schaffer collateral/commissural pathway. Stimuli (50 μs in duration) were applied every minute. The stimulus intensity was set to evoke 40–50% of the maximal amplitude of field excitatory postsynaptic potentials. To induce LTD, 1-Hz low-frequency stimulation was delivered continuously for 15 min. See Supporting Text for details.
In Situ Hybridization. RNA probes complementary to mRNA for the mineralocorticoid receptor (MR), CRH, arginine vasopressin (AVP), or proopiomelanocortin were radiolabeled with [α-33P]UTP, hybridized to sections at an annealing temperature of 60°C, and washed after hybridization. See Supporting Text for details.
DEX Suppression Test. FBGRKO and control mice were injected i.p. with DEX sodium phosphate (0.1 mg/kg, American Regent Laboratories, Shirley, NY) or sterile normal saline at 12 p.m. Six hours later, plasma was collected by retroorbital phlebotomy.
Radioimmunoassays. Plasma concentrations of corticosterone and ACTH were determined by radioimmunoassay from blood collected by retroorbital phlebotomy at circadian nadir (8 a.m.) and peak (6 p.m.) from adult male mice as described (18).
Behavioral Analysis. All behavioral analyses were performed by an observer blinded to genotype. Despair related behaviors were measured with the forced swim test and the tail suspension test. Graphs were generated by calculating the percentage of bins that each mouse was active during the trial. Anhedonia was measured in 6-month-old FBGRKO and control mice by using a two-bottle sucrose preference test. The data are presented as the percentage of sucrose consumed out of the total fluid consumed. A general motor battery including the inclined screen, ledge, and accelerating rotorod tests was performed as described (19). See Supporting Text for a full description of behavioral testing paradigms.
Antidepressant Treatment. Four-month-old FBGRKO and control mice were treated daily with Imipramine HCl (16 mg/kg i.p., Sigma) or normal saline. After 3 weeks, blood was collected by retroorbital phlebotomy at circadian nadir (8 a.m.) and peak (6 p.m.) and assayed for corticosterone concentration. One week later, these mice were tested with the DST (as described above). One week after the last blood collection, mice were tested in the tail suspension test and forced swim test. On the day of testing, mice were not injected until after completion of testing.
Statistical Analysis. Results were expressed as mean ± SEM. Statistical comparisons were performed with the use of one- or two-way ANOVA with the post hoc Student–Newmann–Keuls test to identify significant differences. In all cases, P < 0.05 was considered statistically significant.
Results
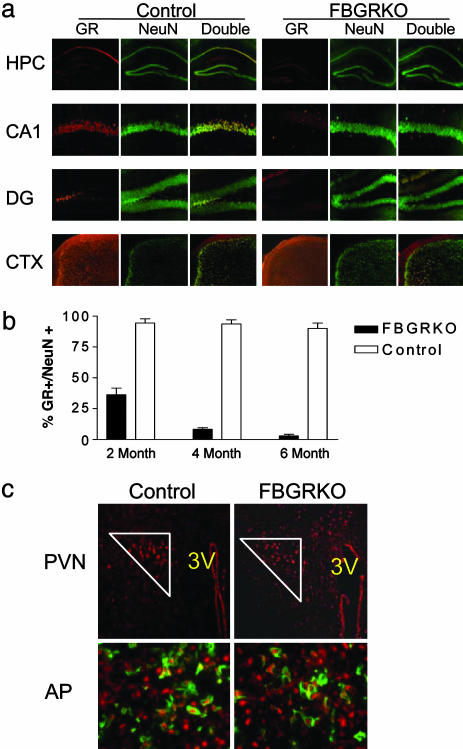
GR Is Specifically Disrupted Throughout the Forebrain of the FBGRKO Mice in a Time-Dependent Manner. We generated mice homozygous for a GR allele in which loxP sites had been inserted around exons 1C and 2 (17) and expressing a transgene in which Cre recombinase is driven by the forebrain-specific CaMKIIα promoter (15). To demonstrate that GR is disrupted specifically in forebrain neurons, coronal sections from 2-, 4-, and 6-month-old FBGRKO and control mice were double-labeled for GR and the neuronal-specific marker, NeuN (Fig. 1a; for images at 2 and 4 months, see Fig. 6, which is published as supporting information on the PNAS web site). This CaMKIIα-Cre transgene is not active until ≈3 weeks of age (15). Consistent with this observation, we found that, at 2 months of age, ≈60% of hippocampal neurons lack GR immunoreactivity in the FBGRKO mice. By 4–6 months of age, the GR immunoreactivity is lost in 90–100% of hippocampal neurons (Fig. 1b). As seen in Fig. 1a, GR is deleted first in the outer layers of cortex and in the hippocampus. By 6 months, GR is deleted throughout the hippocampus and in most of the cortex; however, some deeper cortical neurons remain GR positive even at this later time point.
Fig. 1.
GR immunoreactivity is lost throughout the forebrain of the FBGRKO mice in a time-dependent manner. (a) Immunohistochemical analysis of GR expression in forebrain neurons of 6-month-old FBGRKO and control mice. Each panel shows immunoreactivity for GR (red), NeuN (green), and a merged image in which double labeled cells appear yellow. Top row shows the hippocampus (HPC) at ×100 magnification. Middle rows show area CA1 and the dentate gyrus (DG) at ×400 magnification. Bottom row shows a ×100 magnification image of cortex (CTX). (b) Quantification of the number of CA1 NeuN-positive GR-expressing neurons in FBGRKO and control mice at 2, 4, and 6 months of age (n = 4–6). (c) GR expression remains intact in the PVN and anterior pituitary. (Upper) Representative sections of the PVN from FRGRKO and control mice stained for GR (red). GR-positive PVN neurons are enclosed within the white triangle. (Lower) Representative sections of anterior pituitary (AP) stained for GR (red, nuclear) and proopiomelanocortin (green, cytoplasmic).
Although the forebrain has been implicated in regulation of the HPA axis, the two major sites for negative feedback inhibition of the HPA axis are the PVN of the hypothalamus and corticotrophs of the anterior pituitary. Therefore, we analyzed GR expression in the PVN and anterior pituitary to ensure that deletion had not occurred at these sites. No differences were found between control and FBGRKO mice in the levels of GR expression in PVN neurons or proopiomelanocortin-expressing cells of the anterior pituitary (Fig. 1c). Additionally, we examined GR expression in the amygdala and found no differences in the number of GR-positive neurons in the FBGRKO and control mice (FBGRKO, 135.3 ± 41; control, 156 ± 20; P = 0.33, n = 3, three sections per animal).
The brain contains two types of receptors responsive to glucocorticoids: the type I, or MR, and the type II, or GR. MR has also been implicated in regulation of the HPA axis. However, in situ analysis of control and FBGRKO mice (at 6 months of age) demonstrated that disruption of GR did not lead to a baseline compensatory change in MR mRNA expression (data not shown).
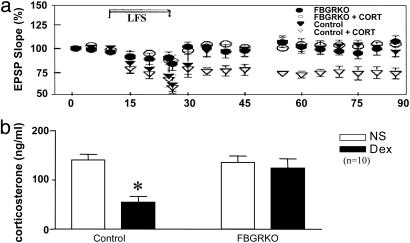
Hippocampal Slices from FBGRKO Mice Are Glucocorticoid-Resistant. LTD has previously been shown to occur as a consequence of stress or glucocorticoid action (20). To demonstrate the functional loss of GR action in FBGRKO mice, we examined corticosterone-induced LTD in hippocampal slices. Hippocampal slices from 6-month-old FBGRKO and control mice were incubated in the presence or absence of 1 μM corticosterone. Low-frequency stimulation in the presence of corticosterone induced LTD in the controls but could not induce LTD in the slices from FBGRKO mice, demonstrating glucocorticoid resistance (Fig. 2a). To further determine the degree of functional glucocorticoid resistance in the FBGRKO mice, we performed a DST, a measure of negative feedback inhibition of the HPA axis in response to exogenous steroids. Whereas the control mice show significant suppression of corticosterone release in response to DEX challenge, the FBGRKO mice show no suppression (Fig. 2b). This finding provides strong support for a role of forebrain GR in negative feedback regulation of the HPA axis.
Fig. 2.
Functional glucocorticoid resistance in FBGRKO mice. (a) Low-frequency stimulation (LFS) of the commissural pathway in the presence of corticosterone (cort) elicits significant long-term depression of excitatory postsynaptic field potentials in area CA1 of control (P = 0.0076) but not FBGRKO mice. (b) Injection of DEX produces a significant suppression of corticosterone release in control mice (*, P < 0.0001) but produces no suppression in FBGRKO mice in the DST.
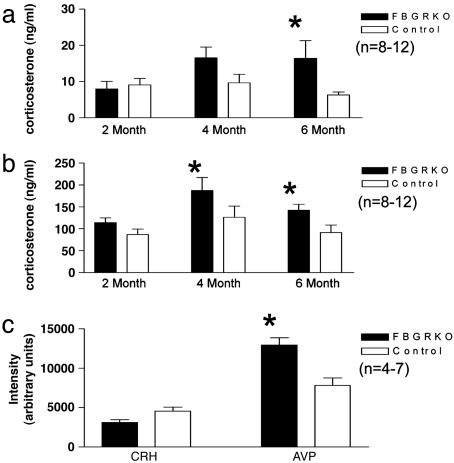
Circadian HPA Axis Activity Is Altered by Deletion of Forebrain GR. To address the hypothesis that forebrain GR regulates the activity of the HPA axis, we collected plasma at circadian nadir (8 a.m.) and peak (6 p.m.) from FBGRKO and control mice at 2, 4, and 6 months of age and. At 2 months of age, when only ≈60% of forebrain neurons have lost GR immunoreactivity, no significant differences in plasma corticosterone concentrations were found between genotypes. However, at 4 months of age, the FBGRKO mice showed a significant increase in peak corticosterone and a trend toward increased basal corticosterone (P = 0.07) release compared to controls. At 6 months of age, the FBGRKO mice showed a significant increase in both basal and peak corticosterone release (Fig. 3 a and b). Similarly. FBGRKO mice >4 months of age tended to have increased basal plasma ACTH (FBGRKO, 252 ± 27 pg/ml; control, 190 ± 18 pg/ml; P = 0.12, n = 9–15) and exhibited significantly increased peak ACTH (FBGRKO, 333 ± 34 pg/ml; control, 212 ± 14 pg/ml; P = 0.01, n = 8). These data suggest that forebrain GR is required for normal regulation of the HPA axis.
Fig. 3.
HPA axis regulation is altered in the FBGRKO mice. (a and b) Basal (a) and peak circadian (b) corticosterone release in FBGRKO and control mice at 2, 4, and 6 months of age. FBGRKO mice show a significant increase in basal corticosterone at 6 months of age, and in peak corticosterone at 4 and 6 months of age relative to control mice of the same age (*, P < 0.05). (c) Quantification of CRH and AVP mRNA expression by in situ hybridization. Densitometric analysis revealed a significant increase in AVP (*, P = 0.0035) but not CRH mRNA at baseline in the PVN of FBGRKO mice compared to controls.
Basal Expression of AVP, but not CRH, Is Altered in the PVN of FBGRKO Mice. We used in situ hybridization to evaluate the expression of CRH and AVP mRNA in the PVN of FBGRKO and control mice. At 6 months of age, FBGRKO mice showed a 65% increase in mean signal intensity of AVP mRNA in the PVN. Although it is difficult to distinguish between the magnocellular and parvocellular regions of the PVN in mice, AVP mRNA expression was not significantly different in the supraoptic nuclei (data not shown). This finding suggests that the increase observed in the PVN reflects changes in the parvocellular region, which directly influences HPA axis activity. No baseline differences were observed between groups in PVN expression of CRH mRNA (Fig. 3c).
FBGRKO Mice Show Increased Depression-Like Behaviors. MDD is associated with a variety of behavioral changes including hopelessness (despair), loss of energy, sleep difficulties, changes in appetite, learning and memory impairments, and anhedonia. To test the hypothesis that forebrain GR activity and HPA axis regulation influence depression-related behaviors, FBGRKO and control mice were tested for measures of despair and anhedonia. No significant differences were observed between genotypes for sensorimotor capabilities and for differences in general locomotor activity levels (data not shown).
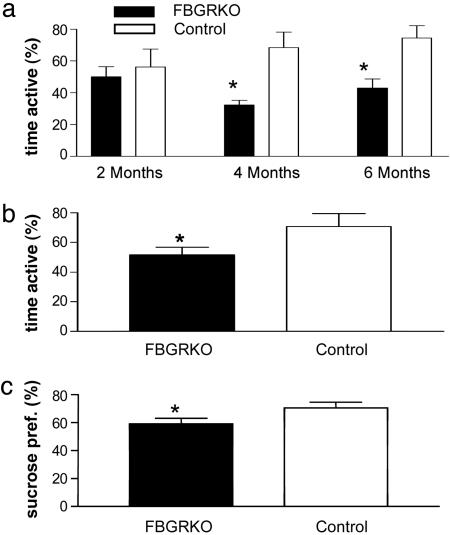
The forced swim and tail suspension tests are both standard measures of behavioral despair routinely used as a screen for rodent models of depression. Decreased activity in these tests is believed to indicate increased despair. In the forced swim test, we observed a significant decrease in activity in the FBGRKO mice at 4 and 6, but not at 2, months of age (Fig. 4a). We next tested 6-month-old FBGRKO and control mice on the tail suspension test to verify that this phenotype reflects an increase in despair-related behaviors and not a more subtle change specifically associated with the swim task. We again found a significant decrease in activity in the FBGRKO mice (Fig. 4b), demonstrating that deletion of forebrain GR leads to increased despair-related behaviors.
Fig. 4.
FBGRKO mice show a time-dependent increase in depression-like behaviors. (a) FBGRKO mice show significantly less activity in the forced swim test (n = 6–9) at 4 (*, P = 0.01) and 6 (**, P = 0.01) months, but not at 2 months of age. (b) In the tail suspension test (n = 6–8), 6-month-old FBGRKO mice again showed significantly less activity (*, P = 0.04). (c) In the two-bottle sucrose preference test (n = 5), FBGRKO mice show significantly decreased sucrose preference (pref.) (*, P < 0.04).
To determine the role of forebrain GR in mediation of anhedonia, we used the sucrose preference test, a commonly used measure of anhedonia in rodents. When given a choice between a bottle containing water and a bottle containing a sucrose solution, the FBGRKO mice consumed significantly less of the sucrose solution than controls, suggesting an increase in anhedonia in these mice (Fig. 4c).
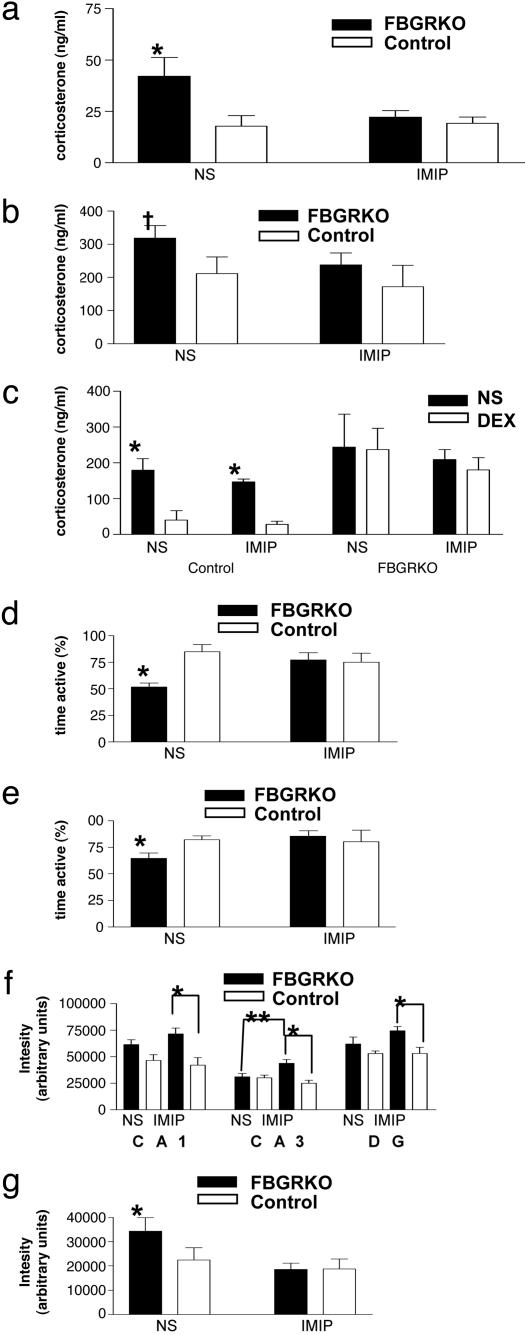
Chronic Treatment with the Tricyclic Antidepressant (TCA) Imipramine Reverses the Behavioral Despair Phenotype and Influences the HPA Axis Abnormalities in the FBGRKO Mice. To determine whether the depression-like phenotype of FBGRKO mice could be reversed with antidepressants, mice were treated daily, for 4 weeks, with the TCA, imipramine, or vehicle. After 3 weeks, FBGRKO mice treated with normal saline showed an increase in both nadir and peak corticosterone release. However, FBGRKO mice treated with imipramine showed corticosterone levels comparable to control mice (Fig. 5 a and b). These mice were also tested for impairments in the DST. FBGRKO mice treated with imipramine or NS showed no suppression of corticosterone release in response to DEX treatment (Fig. 5c). These findings suggest that imipramine can modulate circadian HPA axis activity without directly changing glucocorticoid-mediated feedback inhibition. FBGRKO mice treated with vehicle were again significantly less active in both the forced swim and tail suspension tests, indicating an increase in despair behavior. However, treatment with imipramine reversed this phenotype in both paradigms (Fig. 5 d and e). FBGRKO mice treated with imipramine showed no significant difference in activity compared to control mice receiving either treatment. These data indicate that both the HPA axis abnormalities and the behavioral phenotype are reversible with chronic imipramine treatment despite loss of GR action.
Fig. 5.
Chronic treatment with imipramine reverses HPA axis hyperactivity and the behavioral despair phenotype of FBGRKO mice. (a and b) FBGRKO mice treated with vehicle show an increase in basal circadian corticosterone release (P < 0.05, n = 4–6) (a) and a trend toward increased peak corticosterone release (†, P = 0.08, n = 4–6) (b). After chronic treatment with imipramine, FBGRKO mice show no significant differences in basal or peak corticosterone release from control mice treated with either imipramine or normal saline (NS). (c) In the DST, imipramine does not reverse the impairment in negative feedback suppression of the HPA axis in the FBGRKO mice. (d and e) FBGRKO mice treated with NS showed decreased activity in both the tail suspension (P < 0.0001, n = 4–6) (d) and forced swim tests (P < 0.001, n = 4–6) (e) compared with controls. However, FBGRKO mice treated with imipramine showed no significant difference compared with controls (n = 3–6). (f) NS administration tended to increase MR mRNA expression in areas CA1 and DG of the FBGRKO, and imipramine treatment further augmented the differences between genotypes (*,P < 0.004, n = 4–6). In region CA3, increased MR mRNA was specific for imipramine treatment in FBGRKO mice (**, P = 0.02). (g) FBGRKO mice treated with NS express significantly more CRH mRNA than vehicle-treated controls (P = 0.015, n = 4–6); imipramine treatment decreased PVN CRH mRNA to control levels.
Treatment with Imipramine Differentially Regulates Expression of MR and CRH mRNAs in FBGRKO and Control Mice. To address the mechanisms through which imipramine reverses the HPA axis and behavioral phenotype of the FBGRKO mice, we analyzed MR, CRH, and AVP mRNA expression after chronic vehicle or imipramine treatment. The handling stress associated with chronic vehicle (NS) administration led to an increase in MR mRNA expression in FBGRKO compared with control hippocampi, and impramine treatment further augmented the differences between genotypes (Fig. 5f). Next, we analyzed PVN CRH expression and found that vehicle-treated FBGRKO mice express significantly more CRH mRNA than vehicle-treated controls, suggesting greater stress reactivity in the FBGRKO mice. Imipramine treatment decreased PVN CRH mRNA to control levels (Fig. 5g). Imipramine treatment produced no significant changes in AVP expression in the PVN of FBGRKO or control mice (data not shown).
Discussion
In this report, we demonstrate that a primary deficit in forebrain GR leads to increased circadian glucocorticoid release, impaired negative feedback inhibition of the HPA axis, and increased depression-related behaviors. Moreover, the alterations in stress-associated hypothalamic neuropeptides in FBGRKO mice, AVP under baseline conditions, and CRH after chronic handling stress, are reminiscent of findings in human depression (21). In addition, we show that the depression-like phenotype of FBGRKO mice can be reversed with chronic antidepressant treatment. These studies both define the involvement of forebrain GR in regulation of the HPA axis and show that a primary defect in forebrain GR causes physiological and behavioral phenotypes mimicking those seen in MDD.
Forebrain GR Provides Negative Feedback Inhibition to the HPA Axis. Clinical depression is associated with hyperactivity and impaired negative feedback inhibition of the HPA axis (2). Various observations suggest that these changes may be central to the pathogenesis of depression. Impairments in negative feedback inhibition are believed to be diagnostic of depression, with between 40 and 80% of patients with MDD showing impaired glucocorticoid suppression in the DST (4, 22). Normalization of these HPA abnormalities is associated with successful antidepressant treatment (23, 24). Patients whose HPA abnormalities do not normalize are significantly more likely to relapse (25). Furthermore, nondepressed siblings of depressed patients show an intermediate HPA axis phenotype between that of depressed patients and controls, and those with more severe HPA alterations are more likely to later develop depression (23, 24, 26). These observations have led to the idea that a primary component in the development of depression may involve factors which influence negative feedback inhibition of the HPA axis, such as forebrain GR. This is supported by studies finding decreased GR mRNA expression in the prefrontal cortex of patients with depression as well as in postmortem tissue from suicide victims (4, 6, 27). Here we demonstrate that a primary deficiency of forebrain GR leads to increased nadir and peak circadian corticosterone release and impaired negative feedback inhibition of the HPA axis, paralleling what is seen in glucocorticoid feedback-resistant human depressive patients. These results suggest that the HPA axis abnormalities observed in depression may be related to decreased forebrain GR function and factors that alter GR expression and/or function may influence disease susceptibility. Interestingly, there is evidence that polymorphisms of the GR allele in humans influence HPA axis regulation (28). Our studies suggest that these may serve as a mechanism for genetic transmission of an increased susceptibility to depression.
Deletion of Forebrain GR Leads to a Depression-Like Behavioral Phenotype. The FBGRKO mice show time- and deletion-dependent increases in despair and decreased pleasure seeking behavior (anhedonia). At 2 months of age, a time at which about half of forebrain neurons still express GR, we found no differences in circadian corticosterone release or depression-related behaviors. This finding suggests that there is a threshold effect and these systems are not compromised until GR expression falls below a specific level. These results have potentially interesting implications for the etiology of depression. An underlying genetic predisposition for the disease could be related to mutations in GR or other loci which influence GR expression or function. Exposure to environmental factors such as stress or infection are known to decrease GR expression as well as influence the risk for depression (29, 30). These triggers may then initiate a pathway that leads to the development of depression by down-regulating GR action below a requisite threshold amount. This notion is supported by the observation that exposure to physical and psychological stressors have been shown to be temporally correlated with the onset of depression (30).
Imipramine Reverses the Depression-Like Phenotype in the FBGRKO Mice. To develop targeted therapies for depression that work on a more efficient time scale, it is necessary to better understand the molecular cascade that leads to depression. It has been hypothesized that forebrain GR may be an important component in this pathway. Imipramine has been shown to cause an increase in hippocampal GR expression (31). This has been suggested to be a mechanism through which antidepressants reverse the HPA axis phenotype associated with depression and lead to recovery of both the HPA axis and normal behavior. The up-regulation of GR with imipramine is not likely to be essential for efficacy, as chronic treatment with imipramine reversed both the circadian HPA axis abnormalities and the behavioral despair phenotype in the FBGRKO mice without a change in negative feedback inhibition of the HPA axis as measured by the DST.
MR is also an important regulator of HPA axis function (23). In addition to increasing GR expression, a number of antidepressants, including imipramine, have also been shown to increase MR expression in the hippocampus (32–34). It has been suggested that MR expression changes precede the changes in GR expression (34). Although MR expression in unmanipulated FBGRKO mice did not differ from controls, the handling associated with vehicle administration led to increased MR expression relative to controls. This difference in MR expression was further increased with imipramine administration. Because the despair behavior and HPA axis phenotypes of the vehicle-treated FBGRKO mice do not differ from unmanipulated controls, the data suggest that, if MR is involved in the therapeutic response to impramine, either the induction of MR must exceed a specific threshold to compensate for GR deficiency or MR is induced in a region of the brain specific for imipramine treatment (e.g., the hippocampal CA3 region), or that imipramine interacts with MR to exert its effects.
Depression is known to be associated with alterations in neurotransmitter systems including serotonin, dopamine, and norepinephrine. In addition to its effects on GR, imipramine also influences the reuptake of both serotonin and norepinephrine (35), and these actions may be central to its efficacy. Imipramine has also been shown to down-regulate serotonin 1A (5-HT1A) receptor expression in the hippocampus (36). Preliminary data from our laboratory suggests that FBGRKO mice express more 5-HT1A mRNA in the hippocampus (M.P.B. and L.J.M., unpublished data). This is another potential GR-independent mechanism through which imipramine could be acting to reverse the observed phenotype. Numerous studies have demonstrated a reciprocal interaction between serotonin systems and glucocorticoids (37). Further studies should address the mechanisms through which disruption of forebrain GR influences serotonin systems and determine how these changes contribute to the observed phenotype.
In summary, we present a system for studying MDD and find that alterations in forebrain GR activity could be a primary etiologic factor in the development of MDD. This notion is supported by a recent study demonstrating that GR overexpression in the forebrain results in increased emotional lability and a phenotype suggestive of bipolar disorder that is responsive to antidepressant administration (38), in accord with the model that increased allostatic load from both over- and underresponsive stress pathways contributes to human stress-related disorders (39). FBGRKO mice should prove to be valuable for identifying GR target genes in MDD and testing pharmacological agents for efficacy in this disorder.
Supplementary Material
Acknowledgments
We thank Jim Maas and Dr. James Herman for manuscript review and Sherri Vogt for expert technical assistance. This work was supported by National Institutes of Health grants (to L.J.M. and M.P.B.).
Author contributions: M.P.B. and L.J.M. designed research; M.P.B., J.A.B., M.F., D.F.W., Y.I., and L.J.M. performed research; J.Z.T. contributed new reagents/analytic tools; M.P.B., D.F.W., Y.I., and L.J.M. analyzed data; and M.P.B. and L.J.M. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HPA, hypothalamic–pituitary–adrenal; GR, glucocorticoid receptor; MDD, major depressive disorder; DEX, dexamethasone; DST, DEX suppression test; CRH, corticotropin-releasing hormone; PVN, paraventricular nucleus; ACTH, adrenocorticotropic hormone; FBGRKO, forebrain-specific GR knockout; CaMKIIα, calcium-stimulated calmodulin kinase IIα; LTD, long-term depression; MR, mineralocorticoid receptor; AVP, arginine vasopressin.
References
- 1.Herman, J. P., Figueiredo, H., Mueller, N. K., Ulrich-Lai, Y., Ostrander, M. M., Choi, D. C. & Cullinan, W. E. (2003) Front. Neuroendocrinol. 24, 151-180. [DOI] [PubMed] [Google Scholar]
- 2.Barden, N. (2004) J. Psychiatry Neurosci. 29, 185-193. [PMC free article] [PubMed] [Google Scholar]
- 3.O'Toole, S. M., Sekula, L. K. & Rubin, R. T. (1997) Biol. Psychiatry 42, 85-89. [DOI] [PubMed] [Google Scholar]
- 4.Calfa, G., Kademian, S., Ceschin, D., Vega, G., Rabinovich, G. A. & Volosin, M. (2003) Psychoneuroendocrinology 28, 687-701. [DOI] [PubMed] [Google Scholar]
- 5.Gass, P., Reichardt, H. M., Strekalova, T., Henn, F. & Tronche, F. (2001) Physiol. Behav. 73, 811-825. [DOI] [PubMed] [Google Scholar]
- 6.Modell, S., Yassouridis, A., Huber, J. & Holsboer, F. (1997) Neuroendocrinology 65, 216-222. [DOI] [PubMed] [Google Scholar]
- 7.Pariante, C. M. & Miller, A. H. (2001) Biol. Psychiatry 49, 391-404. [DOI] [PubMed] [Google Scholar]
- 8.Caldji, C., Diorio, J. & Meaney, M. J. (2000) Biol. Psychiatry 48, 1164-1174. [DOI] [PubMed] [Google Scholar]
- 9.Meaney, M. J., Diorio, J., Francis, D., Widdowson, J., LaPlante, P., Caldji, C., Sharma, S., Seckl, J. R. & Plotsky, P. M. (1996) Dev. Neurosci. 18, 49-72. [DOI] [PubMed] [Google Scholar]
- 10.Anisman, H., Zaharia, M. D., Meaney, M. J. & Merali, Z. (1998) Int. J. Dev. Neurosci. 16, 149-164. [DOI] [PubMed] [Google Scholar]
- 11.Schmid, W., Cole, T. J., Blendy, J. A. & Schutz, G. (1995) J. Steroid Biochem. Mol. Biol. 53, 33-35. [DOI] [PubMed] [Google Scholar]
- 12.Barden, N., Stec, I. S., Montkowski, A., Holsboer, F. & Reul, J. M. (1997) Neuroendocrinology 66, 212-220. [DOI] [PubMed] [Google Scholar]
- 13.Karanth, S., Linthorst, A. C., Stalla, G. K., Barden, N., Holsboer, F. & Reul, J. M. (1997) Endocrinology 138, 3476-3485. [DOI] [PubMed] [Google Scholar]
- 14.Tronche, F., Kellendonk, C., Kretz, O., Gass, P., Anlag, K., Orban, P. C., Bock, R., Klein, R. & Schutz, G. (1999) Nat. Genet. 23, 99-103. [DOI] [PubMed] [Google Scholar]
- 15.Tsien, J. Z., Chen, D. F., Gerber, D., Tom, C., Mercer, E. H., Anderson, D. J., Mayford, M., Kandel, E. R. & Tonegawa, S. (1996) Cell 87, 1317-1326. [DOI] [PubMed] [Google Scholar]
- 16.Frank, E. & Thase, M. E. (1999) Annu. Rev. Med. 50, 453-468. [DOI] [PubMed] [Google Scholar]
- 17.Brewer, J. A., Khor, B., Vogt, S. K., Muglia, L. M., Fujiwara, H., Haegele, K. E., Sleckman, B. P. & Muglia, L. J. (2003) Nat. Med. 9, 1318-1322. [DOI] [PubMed] [Google Scholar]
- 18.Brewer, J. A., Bethin, K. E., Schaefer, M. L., Muglia, L. M., Vogt, S. K., Weninger, S. C., Majzoub, J. A. & Muglia, L. J. (2003) Stress 6, 121-125. [DOI] [PubMed] [Google Scholar]
- 19.Ho, N., Liauw, J. A., Blaeser, F., Wei, F., Hanissian, S., Muglia, L. M., Wozniak, D. F., Nardi, A., Arvin, K. L., Holtzman, D. M., et al. (2000) J. Neurosci. 20, 6459-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McEwen, B. S. (1999) Annu. Rev. Neurosci. 22, 105-122. [DOI] [PubMed] [Google Scholar]
- 21.Holsboer, F. (2003) Ann. N.Y. Acad. Sci. 1007, 394-404. [DOI] [PubMed] [Google Scholar]
- 22.The APA Task Force on Laboratory Tests in Psychiatry (1987) Am. J. Psychiatry 144, 1253-1262. [DOI] [PubMed] [Google Scholar]
- 23.Holsboer, F. (2000) Neuropsychopharmacology 23, 477-501. [DOI] [PubMed] [Google Scholar]
- 24.De Kloet, E. R., Vreugdenhil, E., Oitzl, M. S. & Joels, M. (1998) Endocr. Rev. 19, 269-301. [DOI] [PubMed] [Google Scholar]
- 25.Steckler, T., Holsboer, F. & Reul, J. M. (1999) Baillieres Best Pract. Res. Clin. Endocrinol. Metab. 13, 597-614. [DOI] [PubMed] [Google Scholar]
- 26.Holsboer, F., Lauer, C. J., Schreiber, W. & Krieg, J. C. (1995) Neuroendocrinology 62, 340-347. [DOI] [PubMed] [Google Scholar]
- 27.Webster, M. J., Knable, M. B., O'Grady, J., Orthmann, J. & Weickert, C. S. (2002) Mol. Psychiatry 7, 985-994, 924. [DOI] [PubMed] [Google Scholar]
- 28.DeRijk, R. H., Schaaf, M. & de Kloet, E. R. (2002) J. Steroid Biochem. Mol. Biol. 81, 103-122. [DOI] [PubMed] [Google Scholar]
- 29.Agid, O., Kohn, Y. & Lerer, B. (2000) Biomed. Pharmacother. 54, 135-141. [DOI] [PubMed] [Google Scholar]
- 30.Dorian, B. & Garfinkel, P. E. (1987) Psychol. Med. 17, 393-407. [DOI] [PubMed] [Google Scholar]
- 31.Budziszewska, B., Jaworska-Feil, L., Kajta, M. & Lason, W. (2000) Br. J. Pharmacol. 130, 1385-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brady, L. S., Whitfield, H. J., Jr., Fox, R. J., Gold, P. W. & Herkenham, M. (1991) J. Clin. Invest. 87, 831-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reul, J. M., Stec, I., Soder, M. & Holsboer, F. (1993) Endocrinology 133, 312-320. [DOI] [PubMed] [Google Scholar]
- 34.Reul, J. M., Labeur, M. S., Grigoriadis, D. E., De Souza, E. B. & Holsboer, F. (1994) Neuroendocrinology 60, 509-519. [DOI] [PubMed] [Google Scholar]
- 35.Langer, S. Z. & Schoemaker, H. (1988) Prog. Neuropsychopharmacol. Biol. Psychiatry 12, 193-216. [DOI] [PubMed] [Google Scholar]
- 36.Shen, C., Li, H. & Meller, E. (2002) Neuropharmacology 42, 1031-1038. [DOI] [PubMed] [Google Scholar]
- 37.Porter, R. J., Gallagher, P., Watson, S. & Young, A. H. (2004) Psychopharmacology (Berlin) 173, 1-17. [DOI] [PubMed] [Google Scholar]
- 38.Wei, Q., Lu, X. Y., Liu, L., Schafer, G., Shieh, K. R., Burke, S., Robinson, T. E., Watson, S. J., Seasholtz, A. F. & Akil, H. (2004) Proc. Natl. Acad. Sci. USA 101, 11851-11856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McEwen, B. S. (1998) N. Engl. J. Med. 338, 171-179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.