Abstract
Human amnion epithelial cells (hAECs) have been shown to possess potent immunomodulatory properties across a number of disease models. Recently, we reported that hAECs influence macrophage polarization and activity, and that this step was dependent on regulatory T cells. In this study, we aimed to assess the effects of hAEC‐derived proresolution lipoxin‐A4 (LXA4) on T‐cell, macrophage, and neutrophil phenotype and function during the acute phase of bleomycin‐induced lung injury. Using C57Bl6 mice, we administered 4 million hAECs intraperitoneally 24 hours after bleomycin challenge. Outcomes were measured at days 3, 5, and 7. hAEC administration resulted in significant changes to T‐cell, macrophage, dendritic cell, and monocyte/macrophage infiltration and phenotypes. Endogenous levels of lipoxygenases, LXA4, and the lipoxin receptor FPR2 were elevated in hAEC‐treated animals. Furthermore, we showed that the effects of hAECs on macrophage phagocytic activity and T‐cell suppression are LXA4 dependent, whereas the inhibition of neutrophil‐derived myleoperoxidase by hAECs is independent of LXA4. This study provides the first evidence that lipid‐based mediators contribute to the immunomodulatory effects of hAECs and further supports the growing body of evidence that LXA4 is proresolutionary in lung injury. This discovery of LXA4‐dependent communication between hAECs, macrophages, T cells, and neutrophils is important to the understanding of hAEC biodynamics and would be expected to inform future clinical applications. Stem Cells Translational Medicine 2017;6:1085–1095
Keywords: Lipoxin A4, Lung fibrosis, Inflammation, Macrophages, Neutrophils
Significance Statement.
In this study, stem‐like cells derived from the human placenta, called human amnion epithelial cells (hAECs), were shown to prevent lung injury by producing a lipid‐based molecule called lipoxin A4. This molecule works by encouraging interactions between a variety of immune cells in the lungs to effect repair. Following hAEC administration, fewer proinflammatory immune cells were attracted to the lungs and their activity was suppressed to achieve resolution of lung injury. The findings from this study will help inform the design of safe and efficacious hAEC therapy for lung disease.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a debilitating chronic inflammatory disease that affects more than 300 million people worldwide. The disease is characterized by progressive chronic inflammation; patients usually have limited numbers of CD4+/CD28+/CD25+/Foxp3+ regulatory T‐regulatory cells and higher numbers of Th1 effector CD4+ T cells in the lung [1]. Patients with severe forms of IPF also have significantly higher numbers of neutrophils, macrophages, and dendritic cells present in their bronchoalveolar lavage fluid (BALF) [2]. Currently, there is no effective treatment; lung transplantation is the only therapeutic option for end‐stage disease. However, cell‐based therapies have been suggested as a promising treatment approach [3, 4, 5, 6, 7, 8, 9]. Studies with early administration of mesenchymal stromal cells (MSCs) after bleomycin challenge reported significant anti‐inflammatory effects [10].
Preclinical studies have shown that stem cells reduce T‐cell proliferation, myeloperoxidase production, and profibrotic chemokine CCL‐2 and collagen deposition to repair lung injury [8, 11, 12]. Specifically, MSCs home to sites of injury and regulate inflammatory processes by reducing macrophage and neutrophil infiltration. These promising experimental studies led to early‐phase clinical trials of MSCs in patients with IPF, and these confirmed the safety of MSCs [13, 14, 15]. A number of efficacy trials of MSCs as a treatment for IPF are now underway [14]. However, if successful, the future application of MSCs is likely to be limited by the low cell yields from MSC harvesting. In that regard, human amnion epithelial cells (hAECs), stem‐like cells with potent immunomodulatory properties, are a promising alternative cell therapy. These cells appear equally effective in experimental models of lung injury [3, 5, 6, 7] and are more readily sourced in much greater cell numbers without the need for in vitro manipulation or expansion [4, 16, 17]. Like MSCs [18, 19], it would appear that the principle mechanisms of action of hAECs are paracrine, modulating the host immune cell responses and supporting the recruitment and expansion of host niche lung progenitor cells [20, 21, 22].
Although it is not essential to clinical application of hAECs, a better understanding of the paracrine signaling involved in hAEC‐modulated injury repair would be expected to improve the design of future clinical trials and, potentially, lead to novel non‐cell‐based therapeutics. In that regard, we wondered whether hAECs were exerting their reparative effects via lipoxins. Lipoxins are a subset of lipid mediators, inclusive of resolvins, protectins, and maresins, collectively called specialized proresolving mediators. Lipoxins are eicosanoids derived from arachidonic acid via enzymatic steps by lipoxygenases‐5, ‐12, and ‐15. They are regulators of inflammation and act by limiting polymorphonuclear neutrophil (PMN) infiltration and by enhancing nonphlogistic phagocytosis of apoptotic PMNs. Preclinical studies have shown the potent anti‐inflammatory properties of LXA4 in diverse disease models, including microbial infection, Alzheimer disease, liver transplantation, and myocardial ischemia reperfusion injury [23, 24, 25, 26]. In the lung, LXA4 and aspirin‐triggered lipoxin analogs have been shown to improve alveolarization in a neonatal model of hyperoxia‐induced injury and to prevent inflammation and fibrosis in bleomycin‐induced lung injury [27, 28, 29]. It has been shown that hAECs administered 24 hours after bleomycin challenge was beneficial [4, 8, 9]; therefore, we sought to assess the role of lipoxins in hAEC‐mediated lung repair in the same mouse model of lung injury.
Materials and Methods
Experimental Animals
Animal experiments were carried out with approval by Monash University Ethics Committee and conducted in accordance to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. A total of 38 female mice, aged 8–12 weeks and weighing 18–21 g were administered 15 IU of bleomycin intranasally (Willow Pharmaceuticals, New South Wales, Australia http://willowpharma.com.au). Each mouse was randomly allocated to receive either 4 million hAECs in 0.2 ml of saline or saline alone via intraperitoneal injection 24 hours after receiving bleomycin. Mice were culled 1, 3, 5, or 7 days after bleomycin challenge. Bronchoalveolar lavage fluid and lung tissues were collected and processed as previously described [4].
Human Amnion Epithelial Cell Isolation
Amnion cells were collected from placentae of consenting women undergoing an elective cesarean section delivery at term. Isolation of hAECs was performed according to previously described studies [16] in accordance with guidelines and approval from Monash Health Human Research Ethics Committee. Mean gestational age at collection was 38 weeks and 4 days. A total of 10 amnions were used in these in vitro and 1 of the 10 was randomly selected and used for all in vivo experiments.
Tissue Collection
Animals were culled at days 1, 3, 5, and 7 by CO2 asphyxiation, and 2 ml of bronchoalveolar lavage fluid was collected with saline. The right lung was ligated at the right mainstem bronchus, then the trachea was exposed and the left lung was instilled with 4% paraformaldehyde. The right lung was excised for RNA analysis and the left lung for histological analysis. A separate cohort of animals was used for flow cytometric analysis, for which the entire lung (left and right) was processed (bleomycin and saline, n = 5, bleomycin and hAECs, n = 6).
Histological and Immunohistochemical Analysis
Immunofluorescent Staining for Lipoxin Receptor and Macrophages
To measure the effect of hAECs on endogenous lipoxin A4 receptor expression and macrophage number, we performed immunohistochemistry for the lipoxin receptor N‐formyl peptide receptor 2 (FPR2) and for the macrophage marker F4/80 on lung slices. Briefly, paraffin‐embedded slices (0.5‐μm thick) were dewaxed and rehydrated in water. Antigen retrieval was performed with 10 mM citrate buffer, pH 6.0, in a microwave oven for 20 minutes. Blocking was performed with a universal protein blocking solution before immunostaining with anti‐FPR2 antibody at 1:100 (NSL1878; Novus Biologicals, Littleton, CO, https://www.novusbio.com) and anti‐F4/80 antibody at 1:200 (MCA497; Bio‐Rad Laboratories, Oxford, U.K., https://www.bio-rad-antibodies.com) with an overnight incubation at 4°C. Secondary antibody incubation was performed at room temperature for 1 hour, followed by nuclear stain with 4′,6‐diamidino‐2‐phenylindole (DAPI) for 10 minutes at room temperature. For each section, five fields of view were taken using the Nikon C1 confocal microscope running the NIS Elements Software (Nikon, Tokyo, Japan, http://www.nikon.com), where dual positive staining was manually quantified and analyzed with FIJI ImageJ analysis software (version 1.480; http://imagej.net/).
Flow Cytometry
Whole lungs were perfused with saline and minced using a tissue chopper (Campden Instruments, Lafayette, IN, http://campdeninstruments.com). Lung tissues were digested in Dulbecco’s modified Eagle’s medium‐F12 media (11330‐057; Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com) containing 25 mg/ml collagenase IA (10103578001; Roche, NSW, Australia, http://www.roche.com), 2.5 mg/ml DNase I (AMPD1; Sigma‐Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), and 10% (volume per volume) heat‐inactivated fetal bovine serum (16110‐082; Thermo Fisher) for 15 minutes at 37°C. Lung lysates were passed through a 70‐μM cell strainer and red blood cells were lysed. Fc receptors were blocked with anti‐CD16/32 (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) before staining for CD45, CD4, CD11b, F4/80, Ly6C, and CD11c. Data were acquired using a BD LSR II analyzer (BD, Franklin Lakes, NJ, http://www.bd.com). Representative gating strategies are shown in supplemental online Figure 1.
Neutralization of Lipoxygenases With Nordihydroguaiaretic Acid in hAECs
Human amnion epithelial cells were cultured in a T75 flask at a density of 5 × 106 cells. Neutralization of lipoxygenases was performed by adding 2.5 µM or 10 µM of nordihydroguaiaretic acid (NDGA) and incubated at 37°C for 24 hours. After the 24‐hour incubation, hAECs were collected for quantitative polymerase chain reaction analysis of lipoxygenase‐5, ‐12, and ‐15 expression and supernatant was collected for enzyme‐linked immunosorbent assay (ELISA) of LXA4 before further coculture studies. Conditioned medium was obtained according to previous protocols after neutralization of NDGA [18].
Macrophage Phagocytic Assay
Macrophage phagocytosis was determined as previously described [18]. Briefly, macrophages were plated in 6‐well flat‐bottom culture plates at a density of 5 × 105 cells per well for 48 stimulated lipopolysaccharide (LPS; 10 ng/ml) with or without NDGA preprimed hAECs and with or without primary hAECs (1:1 ratio). Staphylococcus aureus particles labeled with pHrodo (Thermo Fisher) were added to each well (10 μg/ml) and incubated for 30 minutes. Incubation on ice inhibits membrane movement and was used as a negative control. Only cells that phagocytosed pHrodo‐labeled S. aureus were fluorescent and stained positive on fluorescence‐activated cell sorting (FACS).
Measuring T‐Cell Proliferation and Migration
Naïve T cells were isolated from spleens of C57Bl/6 mice using a CD4 magnetic‐bead isolation kit (130‐095‐248; Miltenyi Biotec, San Diego, CA, http://www.miltenyibiotec.com). CD4‐enriched T cells (0.5 × 106) labeled with carboxyfluorescein succinimidyl ester were stimulated with CD3ε at 10 μg/ml (MAB484; R&D systems, Minneapolis, MN, https://www.rndsystems.com) and 2 μg/ml CD28 (553294; BD Biosciences) in complete Roswell Park Memorial Institute (RPMI) medium with or without NDGA preprimed hAECs and with or without primary hAECs (ratios: 1:2, 1:5, 1:10). T cells were cocultured directly with hAECs for 72 hours before staining with SYTOXBlue dead‐cell dye (1 μM; S34857; Thermo Fisher) and analyzed by flow cytometry (LSRII analyzer; BD). For T‐cell migration, enriched CD4+ cells were added to the top chamber of a transwell insert in a 24‐well plate and stimulated with CCL17 in either complete RPMI medium or hAEC‐conditioned medium. T cells that migrated across the transwell membrane were counted after 4 hours.
Neutrophil Myeloperoxidase Activity Assay
Neutrophils were isolated from mouse femoral bone‐marrow exudates using a CD11b magnetic‐bead isolation kit (Miltenyi Biotec). Enriched neutrophils (0.5 × 106) were cultured in complete RPMI with or without NDGA‐preprimed hAECs and with or without primary hAECs (0.5 × 106), and activated with granulocyte‐colony stimulating factor (100 ng/ml; Amgen, Sydney, Australia, http://www.amgen.com) for 12 days. The medium was changed every other day. After 12 days, cells were lysed with cetyltrimethylammonium bromide at 500 μl per 1 × 106 cells. Then, 50 μl was added to 950 μl of 50 mM potassium phosphate buffer (pH 6.0) that contained 0.167 mg/ml o‐dianisidine (D9143; Sigma‐Aldrich) and 0.0005% H2O2 (Sigma‐Aldrich), and the absorbance at 460 nm (A460) was measured. One unit of myeloperoxidase (MPO) activity was defined as a change in A460 of 1.0 after 5 minutes; results were expressed as mU of MPO activity per milligram of lysate.
LXA4 ELISA or Th1/Th2/Th17 Cytokine Bead Array
The concentration of lipoxin A4 in BALF and Th1/Th2/Th17 cytokines in culture supernatants were measured by ELISA and bead array according to manufacturer’s guidelines. Data were collected using the FACS LSRII analyzer and analyzed with BD FRAP Array software (BD Biosciences).
Statistical Analysis
Data were expressed for each experimental group as mean ± SEM. Differences between two experimental groups were determined using an unpaired, one‐tailed t test. Differences across three or more experimental groups were determined using one‐way analysis of variance with the Bonferroni post hoc test. Confidence intervals of 95% were deemed significant. All analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA, http://www.graphpad.com).
Results
Effects of hAECs on Cytokines and Inflammatory Cells
hAEC administration significantly increased IL‐4 expression in the lung at day 3 (2.52 ± 0.20 pg/ml vs. 3.90 ± 0.42 pg/ml, p = .012; Fig. 1A) and significantly decreased levels of IL‐10 at day 5 (53.36 ± 4.20 pg/ml vs. 35.17 ± 5.67 pg/ml, p = .015; Fig. 1B) and of IL‐6 and IL‐2 at day 7 (IL‐6: 3.52 ± 0.95 pg/ml vs. 1.14 ± 0.46 pg/ml, p = .048; IL‐2: 4.58 ± 0.56 pg/ml vs. 2.08 ± 0.78 pg/ml, p = .026; Fig. 1C).
Figure 1.
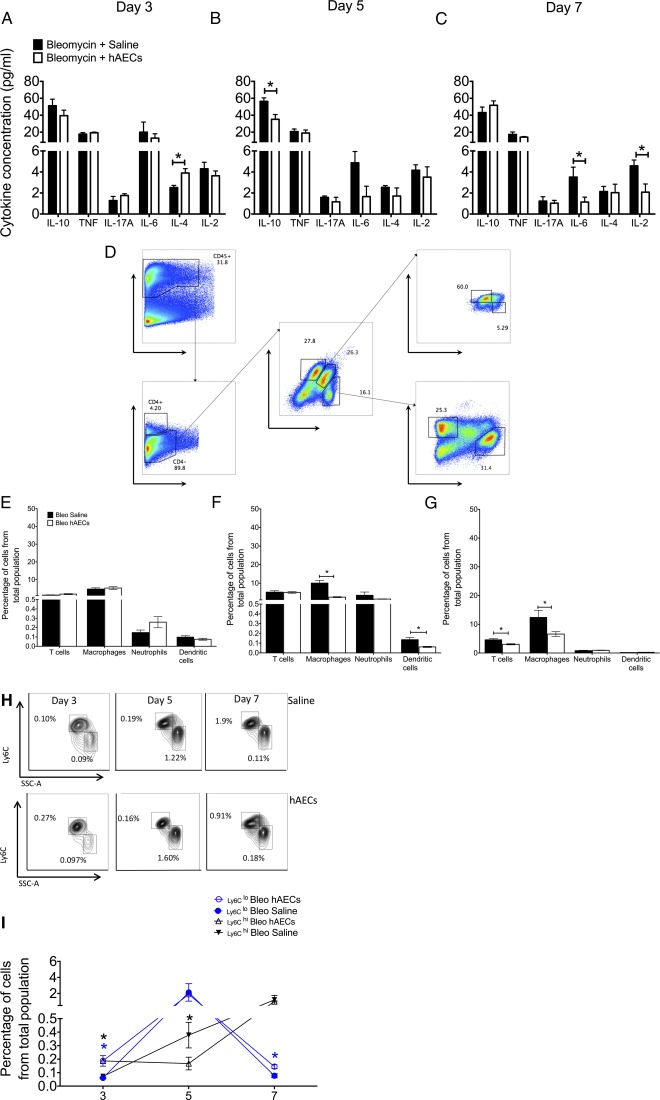
hAECs altered cytokine expression and immune cell infiltrate in vivo. (A): Administration of hAECs significantly increased anti‐inflammatory IL‐4 cytokine levels at the onset of inflammation (control group: 2.52% ± 0.20% vs. hAEC group: 3.90% ± 0.42%). Expression of IL‐10 (B) and inflammatory IL‐6 and IL‐2 levels (C) were reduced in animals that received hAECs (control vs. hAEC groups: IL‐10: 56.36% ± 4.199% vs. 35.17% ± 5.67%; IL‐6: 3.52% ± 0.94% vs. 1.14% ± 0.46%; IL‐2: 4.58% ± 0.55% vs. 2.08% ± 0.78%). (D): Immune cell populations were measured via flow cytometry with gating strategies. Percentages on flow cytometry plots represent the percentages from the parent population. Table 1 lists markers used for each population. There was no change in percentage in all immune cell types at day 3 (E). (F, G): F4/80‐positive macrophage numbers were reduced by day 5 (F) and day 7 (G) in mice given hAECs (day 5: 10.05% ± 1.48% vs. 2.49% ± 0.25%; day 7: 12.39% ± 2.45% vs. 6.59% ± 0.84%). (F): Dendritic CD11c+ cell numbers were significantly reduced at day 5 (control group: 0.13% ± 0.02% vs. hAEC group: 0.06% ± 0.01%). (G): Administration of hAECs slightly reduced CD4 T‐cell numbers in the lung at day 7 (control group: 4.60% ± 0.40% vs. hAEC group: 3.03% ± 0.20%). (H): Representative flow cytometry plots for monocytic subpopulations CD11b+/ Ly6C+ cells are shown. Percentages on flow cytometry plots represent the percentages from parent population. (I ): Changes in Ly6Clo and Ly6Chi cells across days 3, 5, and 7 are shown. Percentage of Ly6Chi cells peaked at day 5 and decreased by day 7 in both groups; hAEC administration resulted in significantly increased percentages of Ly6Clo monocytes at days 3 and 7 (day 3: 0.072% ± 0.012% vs. 0.187% ± 0.039%; day 7: 0.084% ± 0.013% vs. 0.145% ± 0.015%). in both groups. The percentage of Ly6Chi cells gradually increased from day 3 to day 7 in both groups. hAEC treatment resulted in significantly increased percentages of Ly6Chi cells on days 3 and 5 (day 3: 0.001% ± 0.0002% vs. 0.005% ± 0.001%; day 7: 0.006% ± 0.001% vs. 0.053% ± 0.011%). ∗, p < .05. Abbreviations: bleo, bleomycin; hAEC, human amnion epithelial cell; IL, interleukin; TNF, tumor necrosis factor; FSC, forward scatter; SSC, side scatter.
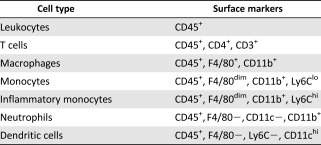
Next, we assessed changes to the immune‐cell population in the lung based on surface markers listed in Table 1 and gated according to the gating strategies seen in Figure 1D.
Table 1.
Characterization of immune cell types
|
There were no significant changes in percentages of all cell types at day 3 (Fig. 1E). At day 5, the percentage of macrophage and dendritic cells, compared with that of bleomycin controls, was significantly lower in hAEC‐treated animals (macrophages: 10.054% ± 1.483% vs. 2.496% ± 0.249%, p = .001; dendritic cells: 0.136% ± 0.022% vs. 0.061% ± 0.006%, p = .012; Fig. 1F). At day 7, compared with control mice, administration of hAECs decreased percentages of CD4+ T cells and macrophages (T cells: 4.604% ± 0.404% vs. 3.026% ± 0.204%, p = .008; macrophages: 12.394% ± 2.452% vs. 6.597% ± 0.838%, p = .039; Fig. 1G). Figure 1H is a representative flow cytometry plot of F4/80lo/CD11b+/Ly6Chi/SSC‐Alo cells. Taken together, these findings demonstrate that hAEC treatment 24 hours after bleomycin exposure suppressed the macrophage and dendritic cell milieu during onset of inflammation. Subsequently, inflammatory T‐cell infiltration is restricted either through hAEC signaling or from lack of macrophage and dendritic cell signaling.
No significant differences in percentage of T‐cell, neutrophil, macrophage, and dendritic cells between any groups were observed at day 3. However, when we looked closer into subpopulations of inflammatory monocytes/macrophages, compared with the bleomycin control group, animals treated with hAECs had a significantly higher percentage of Ly6Chi monocytes at days 3 and 7 (day 3: 0.072% ± 0.012% vs. 0.187% ± 0.039%; day 7: 0.084% ± 0.013% vs. 0.145% ± 0.015%; p < .05 for both; Fig. 1I).
hAECs’ Effect on Lipoxin A4 and its Receptor, FPR2
Given earlier reports that lipoxin A4 is highly expressed in placental tissues [20, 22] and plays a valuable role in resolving inflammation and fibrosis, we evaluated the role of lipoxin A4 in hAEC‐mediated lung repair. Protein analysis of bronchoalveolar lavage fluids revealed that, compared with the control group of animals treated with bleomycin plus saline, hAEC administration was associated with significantly higher lipoxin A4 levels at day 7 (0.13 ± 0.02 vs. 0.25 ± 0.07; p = .036; Fig. 2B) despite no changes in total protein content (supplemental online Fig. 1). This was accompanied by a significant increase in the gene expression of lipoxygenase‐5 on day 7, lipoxygenase‐12 and ‐15 on day 5 (ALOX‐5: 0.393 ± 0.114 vs. 3.78 ± 1.98, p = .013; ALOX‐12: 0.484 ± 0.248 vs. 1.52 ± 0.20, p = .018; ALOX‐15: 0.02 ± 0.01 vs. 1.99 ± 0.98, p = .019; Fig. 2A). Compared with the control group, hAEC treatment was associated with significantly elevated expression of F4/80 and FPR2 in the lungs of day 7 animals (Fig. 2C). Representative immunofluorescent staining showing colocalization positive for the macrophage marker F4/80, FPR2, and nuclear stain DAPI of hAEC‐treated lung is shown in Figure 2D.
Figure 2.
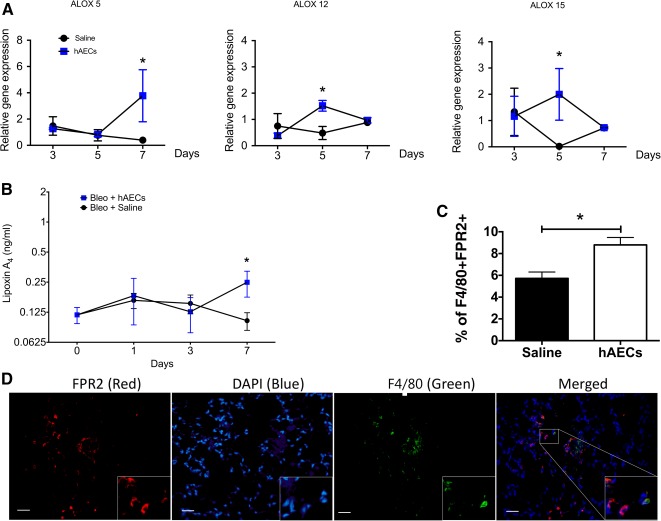
Lipoxin A4, along with its precursors and ligand, were altered by hAECs. (A): Expression of the lipoxin A4 precursor genes ALOX‐5, ‐12, and ‐15 was increased at days 5 and 7 compared with saline controls (ALOX‐5: 0.393 ± 0.114 vs. 3.777 ± 1.98; ALOX‐12: 0.484 ± 0.248 vs. 1.520 ± 0.203; ALOX‐15: 0.020 ± 0.007 vs. 1.998 ± 0.983). ∗, p < .05. (B): Lipoxin A4 protein levels were elevated in lung lysates at day 7 in animals treated with hAECs compared with controls (0.103 ± 0.021 ng/ml vs. 0.249 ± 0.072 ng/ml, respectively). ∗, p < .05. (C): At day 7, bleomycin challenge resulted in positively stained F4/80/FPR2 cells, which were was elevated in mice treated with hAECs compared with control mice (5.720% ± 0.587% vs. 8.795% ± 0.687%, respectively). ∗, p < .05. (D): Representative images of F4/80‐ and FPR2‐positive‐stained lung sections from hAEC‐treated animals. Magnification: ×200. Scale bar =100 μm. Abbreviations: DAPI, 4′,6‐diamidino‐2‐phenylindole; FPR2, N‐formyl peptide receptor 2; hAEC, human amnion epithelial cell.
Effect of hAECs on Macrophage and Neutrophil Function
Pulmonary inflammation induced by bleomycin is managed through a balance of neutrophil infiltration, apoptosis, and subsequent macrophage efferocytosis of apoptotic neutrophils. Lipid mediators, in particular, LXA4, are potent regulators of this process and, accordingly, we evaluated the direct effects of hAEC‐derived LXA4 on macrophage and neutrophil function in vitro. We first showed that we could induce and inhibit hAEC production of LXA4 and its precursor enzymes ALOX‐5, ‐12, ‐15. Proinflammatory cytokines interferon (IFN)‐γ and tumor necrosis factor (TNF)‐α significantly increased LXA4 production in hAECs (0.5309 ± 0.0359 ng/ml vs. 1.0450 ± 0.1393 ng/ml, p = .012; Fig. 3A).
Figure 3.
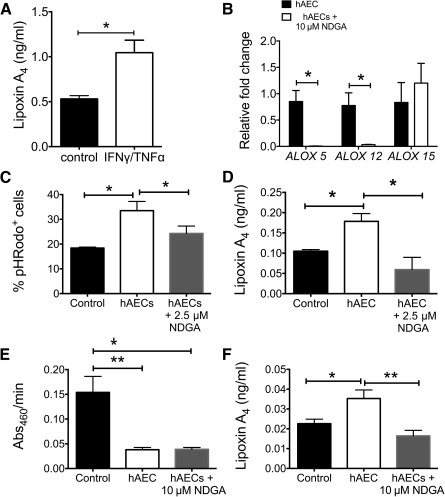
Lipoxin A4 is required for hAEC‐mediated upregulation of macrophage phagocytosis. (A): hAECs expressed elevated lipoxin A4 in the presence of inflammatory cytokines IFN‐γ and TNF‐α (control group: 0.531 ± 0.036 ng/ml vs. IFN‐γ/TNF‐α: 1.045 ± 0.139 ng/ml). ∗, p < .05. (B): Pretreating hAECs with NDGA significantly reduced its precursors ALOX‐5 and ‐12 compared with hAEC alone (ALOX‐5: 0.852 ± 0.209 vs. 0.004 ± 0.002, ALOX‐12: 0.776 ± 0.241 vs. 0.032 ± 0.005, respectively). (C): Bone‐marrow‐derived macrophages cultured with hAECs demonstrated increased uptake of pHrodo‐labeled bacterial particles in comparison with control medium. Preculturing hAECs with lipoxygenase inhibitor NDGA reversed macrophage phagocytic uptake of pHrodo particles (control group: 18.40% ± 0.374% vs. hAEC group: 33.52% ± 3.703% vs. hAEC plus NDGA group: 24.29% ± 2.985%). ∗, p < .05. (D): Concentration of LXA4 in the culture supernatant was measured. Protein level of LXA4 increased in the presence of hAECs and was reduced when hAECs were precultured with NDGA (control group: 0.105 ng/ml ± 0.004 ng/ml vs. hAEC group: 0.178 ng/ml ± 0.019 ng/ml vs. hAEC plus NDGA group: 0.059 ng/ml ± 0.030 ng/ml). ∗, p < .05; ∗∗∗, p < .001. (E): Neutrophil myeloperoxidase activity in the presence of hAECs was significantly reduced and, conversely, addition of NDGA did not alter neutrophil activity (absorbance at 460 nm/minute: control group: 0.154 ± 0.032 vs. hAEC group: 0.038 ± 0.005 vs. hAEC plus NDGA group: 0.038 ± 0.004). ∗∗, p < .01. (F): Culturing neutrophils with NDGA‐precultured hAECs significantly reduced LXA4 protein synthesis (control group: 0.02 ± 0.002 ng/ml vs. hAEC group: 0.04 ± 0.004 ng/ml vs. 0.01 ± 0.002 ng/ml). ∗, p < .01; ∗∗, p < .05. Abbreviations: Abs, absorbance; hAEC, human amnion epithelial cell; IFN, interferon; NDGA, nordihydroguaiaretic acid; TNF, tumor necrosis factor.
Next we showed that in the presence of nordihydroguaruretic acid (NDGA), gene expression of lipoxygenase‐5 and ‐12 was significantly reduced as well (ALOX‐5: 0.852 ± 0.209 vs. 0.004 ± 0.002, p = .004; ALOX‐12; 0.776 ± 0.241 vs. 0.032 ± 0.005, p = .015; ALOX‐15; 0.834 ± 1.200 ± 0.374; Fig. 3B), suggesting that biosynthesis of LXA4 may occur in hAECs in an inflammatory environment.
Using bone‐marrow‐derived macrophages, we showed that hAECs increased phagocytic activity as previously described [18], and this effect was diminished with the addition of 2.5 µM NDGA (data not shown for 10µM; respective data for control, hAEC, and hAEC plus NGDA groups: 18.40% ± 0.374% vs. 33.52% ± 3.703% vs. 24.29% ± 2.985%, p = .0313; Fig. 3C). We also confirmed that NDGA successfully inhibited LXA4 production by hAECs in macrophage cultures for the three groups (0.105 ± 0.004 ng/ml vs. 0.178 ± 0.019 ng/ml vs. 0.116 ± 0.043 ng/ml [p = .059 and p = .0317, respectively]; Fig. 3D).
Next, we assessed the effects of hAECs on myeloperoxidase (MPO) production by primary neutrophils. Whereas coculture of CD11b+ neutrophils with hAECs significantly reduced their production of MPO, the addition of 2.5 µM (data not shown) and 10 μM NDGA did not affect MPO production, as determined by absorbance measurements (0.154 ± 0.0323 A460/minute vs. 0.038 ± 0.005 A460/minute vs. 0.039 ± 0.004 A460/minute, p = .001; Fig. 3E). Successful inhibition of hAEC‐derived lipoxin A4 by NDGA is shown in Figure 3F (0.023 ± 0.002 ng/ml vs. 0.035 ± 0.004 ng/ml vs. 0.016 ± 0.003 ng/ml [p = .037 and p = .005, respectively]; Fig. 3F).
hAEC‐Mediated Change in T‐Cell Phenotype Is Not Mediated via Lipoxin A4
We evaluated the effect of hAECs and hAEC‐derived LXA4 on T‐cell phenotype, function, and behavior. In comparison with the control treatment (i.e., medium alone), hAECs significantly decreased IL‐2 and increased IL‐17A levels in vitro (IL‐2: 37.20 ± 0.00 vs. 21.60 ± 0.81, p = .031; IL‐17A: 6.670 ± 0.40 vs. 26.960 ± 8.76, p = .005; Fig. 4A). We showed that T‐cell chemotaxis toward CCL17 was unaltered by hAECs (Fig. 4B). However, coculture with hAECs significantly suppressed T‐cell proliferation, which diminished in the presence of 2.5 µM NDGA (data not shown for 10 µM; 1.508 ± 0.024 vs. 1.077 ± 0.018 vs. 1.570 ± 0.098 [p = .006 and p = .001, respectively]; Fig. 4C).
Figure 4.
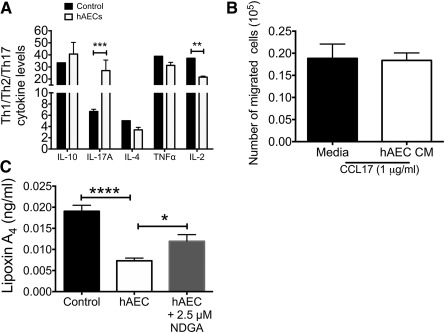
hAECs alter T‐cell proliferation through cytokines. (A): CD4+ T cells enriched from splenocytes were cultured with hAECs, and the supernatant Th1/Th2/Th2 cytokine profile was measured. In the presence of hAEC, naïve T cells expressed significantly more IL‐17A but less IL‐2 (IL‐17A: 6.67 ± 0.40 vs. 26.96 ± 8.76; IL‐2: 37.20 ± 0.00 vs. 21.60 ± 0.81). ∗∗, p < .01; ∗∗∗, p < .001. (B): We then evaluated the effect of hAECs on T‐cell behavior by supplementing cultures with either chemokine CCL17 or CD3ε/CD28 to assess migration and proliferation, respectively. In the presence of CCL17, T cells increased motility; however, this effect was not attenuated by hAECs (medium: 0.19 ± 0.033 cells vs. hAEC CM: 0.18 ± 0.017 cells). (C): T‐cell proliferation was significantly suppressed by presence of hAECs after 72 hours in culture (Proliferation Index score: control group: 1.508 ± 0.023 vs. hAEC group: 1.077 ± 0.018 vs. hAEC plus NDGA group: 1.57 ± 0.098). ∗, p < .05. Inhibiting lipoxygenases with NDGA significantly attenuated hAEC‐mediated suppression of T‐cell proliferation (C) but not LXA4 levels (data not shown). Abbreviations: CCL, chemokine (C‐C motif) ligand; CM, conditioned medium; hAEC, human amnion epithelial cell; IL, interleukin; NDGA, nordihydroguaiaretic acid; TNF, tumor necrosis factor.
Discussion
Owing to their extensive immunomodulatory properties, stem cells and stem‐like cells such as hAECs have been suggested as therapeutic avenues for treating IPF. This is supported by a study showing the role of lipoxins in cell therapy‐mediated lung repair, where human bone‐marrow‐derived MSCs induced production of LXA4 by alveolar epithelial cells in a mouse model of LPS‐induced acute lung injury [30]. Guilherme and colleagues showed that LXA4 analogs were potent anti‐inflammatory and antifibrotic compounds in a bleomycin‐induced model of lung injury [27]. We thus focused our attention on lipoxin A4 a key regulator of macrophage infiltration and function in hAEC‐mediated repair of lung fibrosis.
We observed that hAEC treatment was associated with increased IL‐4 cytokine expression at day 3 after bleomycin challenge, accompanied by a reduction in IL‐10 at day 5, and IL‐6 and IL‐2 at day 7. Levels of inflammatory cytokines may affect immune cell infiltration into the lung, as we observed increased inflammatory monocyte numbers at day 3 followed by fewer macrophages and dendritic cells by day 5. These changes progressed, and on day 7, CD4+‐naïve T‐cell and macrophage numbers were reduced in animals treated with hAECs. Next, we measured the effect of hAEC administration on endogenous production of lipoxin A4 and its receptor FPR2, and showed significant increase in LXA4 levels in BALF and increased macrophage FPR2 expression on lung tissue at day 7. We further characterized lipoxygenase‐mediated effects of hAECs on neutrophil, macrophage, and T‐cell behavior, using a lipoxygenase‐neutralizing compound, NDGA. We showed that hAECs increased macrophage phagocytosis and suppressed T‐cell proliferation in a lipoxygenase‐mediated fashion. However, hAECs’ ability to reduce primary neutrophil MPO activity appeared to be independent of LXA4.
Polarity of Th1 and Th2 cells is a stochastic process usually directed by proinflammatory (i.e., IL‐6, IL‐2, IL‐12) and anti‐inflammatory (i.e., IL‐10, IL‐4) cytokines, which provides positive feedback signaling for downstream inflammatory response. Bronchoepithelial cells express IL‐4 receptors, which, upon activation by IL‐4, results in the production of cytokines (i.e., monocyte‐chemotactic protein ‐1, IL‐8, and IL‐1Ra) and increased migration of human airway epithelial cells [31, 32]. IL‐4 is a known profibrotic cytokine in preclinical studies using bleomycin. Huaux and colleagues showed that IL‐4−/− mice have lower profibrotic factors such as OH‐proline, soluble collagen, fibronectin, and tumor growth factor (TGF)‐β1 from day 14 onward [33]. However, as our current study investigates changes during acute inflammation, we draw our attention toward the role of IL‐4 in inflammation. Interestingly, IL‐4 is a potent inducer of lipoxygenase ‐12 and ‐15. Chaitidis et al. showed that human peripheral blood monocytes strongly upregulated LOX‐15 and conversely downregulated classical proinflammatory cytokines TNFα, IL‐6, IL‐1, and IL‐8 when cultured with IL‐4 [34]. In our study, we observed that administration of hAECs modestly increased levels of IL‐4 at day 3. This suggests that application of hAECs can be detrimental in a fibrotic environment but beneficial in an inflammatory environment, and considerations toward clinical applications should be made with this in mind.
Conversely, we found significant reduction in Th1 drivers IL‐6 and IL‐2 by day 7, suggesting a repression of inflammatory responses. This is supported by an earlier report that showed blocking IL‐6 at days 8, 9, and 10 after bleomycin instillation can ameliorate lung fibrosis [35]. Surprisingly, we observed a transient reduction in IL‐10 levels on day 5, which contradicts a previous finding by Manuelpillai and colleagues that described an increase in IL‐10 levels following hAEC treatment in a mouse model of liver fibrosis [36]. CD11c dendritic cells [37, 38] and macrophages are potent producers of IL‐10. Sakamoto and colleagues showed that after bleomycin challenge, the levels of IL‐10 in bronchoalveolar mononuclear cells began increasing at day 3, then peaked at day 7 and persisted until day 14. It is, therefore, likely that the reduced levels of IL‐10 on day 5 are reflective of the reduced infiltration of dendritic cells and macrophages on day 3 [39].
Lipoxin A4 is a lipid mediator capable of anti‐inflammatory signaling by regulating neutrophil infiltration and proresolving signaling through macrophage polarization and nonphlogistic uptake of apoptotic PMNs. Their innate ability to perpetuate and limit inflammation is of particular importance in our study to define probable immune regulatory roles of hAECs. As our first step in identifying the importance of LXA4 in hAEC‐mediated repair, we evaluated endogenous levels of LXA4 and gene expression of its precursor enzymes ALOX‐5, ‐12, and ‐15 in vivo. Here, hAECs significantly increased levels of LXA4 in BALF at day 7 after bleomycin challenge. Gene expression of the enzymes ALOX‐5, ‐12, and ‐15 responsible for the biosynthesis of lipoxin was significantly increased in hAEC‐treated animals at day 7 (ALOX‐5) and day 5 (ALOX‐12 and ‐15), suggesting that hAEC treatment had an effect on endogenous LXA4 and lipoxygenase synthesis in bleomycin‐induced lung injury. However, changes to lipoxygenase expression varied between days 5 and 7 and may be a consequence of the behavior of lipoxygenase‐expressing immune cells [40]. Treatment with hAECs could indirectly contribute to lipoxygenase expression of endogenous immune cells.
In our study, we saw no change in neutrophil numbers across all days between injured and treated animals, suggesting that, in our study at least, hAECs did not alter neutrophil infiltration to the site of injury and may, instead, mediate activity of neutrophils to resolve injury. Neutrophils are cells recruited as the first line of inflammatory defense, hence behavioral changes to these cells will inevitably influence downstream inflammatory processes. Neutrophil release of MPOs contributes to alveolar damage and, subsequently, fibrosis in patients with IPF, and their activity can be regulated by aspirin‐triggered 15‐epi‐lipoxin A4 in vitro [41, 42, 43]. Our previous studies showed that hAECs significantly reduced proinflammatory MPO‐positive cells in vivo [4, 8]. In this study, we showed that hAECs reduced neutrophil MPO activity in vitro, but this was unlikely to be mediated through LXA4. Although these results are contrary to our initial hypothesis, it was unsurprising, because other lipid‐based mediators are involved in this process. Resolvin E1 administration inhibited PMN infiltration and reduced MPO levels in aspiration pneumonia in mice [44, 45]. Taken together, these studies suggest that hAEC inhibition of neutrophil MPO activity is driven by alternatives to the lipoxygenase pathway.
Next, we assessed the changes to pulmonary monocyte subpopulations where two distinct subtypes can be found: Ly6Chi monocytes, which secrete inflammatory cytokines TNF‐α and IL‐1β; and Ly6Clo monocytes, which patrol the endothelium. The percentage of CD11b+Ly6Chi monocytes was significantly higher in hAEC‐treated animals at days 3 and 5 of this study. This was unexpected because there was less inflammation and honeycomb injury in those mice. Although denoted as inflammatory monocytes, CD11b+Ly6Chi cells have the ability to differentiate into mature alternatively activated macrophages [46]. Gibbons and colleagues reported that the adoptive transfer of bone‐marrow‐derived Ly6Chi inflammatory monocytes during the progressive fibrotic phase led to increased numbers of M2 macrophages and exacerbation of fibrosis [47, 48, 49]. In a similar vein, M2 polarization during the peak of lung inflammation has been reported to have a protective effect in the bleomycin model [18], an outcome that was not achieved with later administration of hAECs, coinciding with the progressive fibrotic phase [45, 50]. Collectively, this suggests that the spatiotemporal infiltration of inflammatory monocytes could be supporting an M2‐driven proreparative response at day 7. Additionally, increase in LXA4 levels at day 7 in animals treated with hAECs could lead to the increase in inflammatory monocytes seen in our study. Studies have shown that LXA4 stimulates monocyte migration, adherence, and Ca2+ motility [51, 52]. This finding coincided with increased numbers of FPR2+ macrophages in lung tissues at day 7 in mice treated with hAECs; however, FPR expression was not wholly restricted to macrophages. This is unsurprising because FPR2 expression is not restricted to macrophages and is expressed by other phagocytic leukocytes such as neutrophils. Given that annexin A1 has been shown to bind strongly to FPR2 on the surface of activated PMNs [53] and is critical for leukocyte adhesion and rolling, as well as the attenuation of MPO activity in neutrophils [54], FPR2‐annexin A1 interaction could explain the nonmacrophage effects observed in our current study.
Our observations suggest that hAECs exert their effects, at least in part, through LXA4, which is a potent stimulus for macrophage phagocytosis [55, 56]. Macrophage phagocytosis of apoptotic PMNs is a rate‐limiting step in inflammation, which could contribute to the pathogenesis of IPF. Alveolar macrophages of patients with IPF have reportedly impaired efferocytosis activity compared with alveolar macrophages from patients affected by other forms of interstitial lung disease [57, 58]. It is plausible, therefore, that hAECs’ ability to promote phagocytosis may contribute toward lung repair. However, to determine if hAEC‐mediated increase in phagocytosis was driven by LXA4, we showed that neutralization of lipoxygenases negated the ability of hAECs to promote macrophage phagocytosis. Data reported here extend previous observations that hAECs polarized macrophages from an M1 to an M2 phenotype by increasing their mannose receptor CD206 expression [18]. As shown by Shirey and colleagues, neutralization of lipoxygenase pathways inhibits M2 macrophage differentiation in mice infected with respiratory syncytial virus [50, 59]. Moreover, Maderna and colleagues showed that bone‐marrow‐derived macrophages from fpr2−/− have defective phagocytic ability for apoptotic PMN [25, 60], further supporting the importance of LXA4‐FPR2 binding in regulating macrophage phagocytosis.
LXA4 is a potent regulator of inflammation and resolution; however, other specialized lipid mediators released by hAECs remain to be investigated [61, 62, 63]. Our study showed an increase in LXA4 levels in vivo and in vitro, albeit at low levels (<0.05 ng/ml). Notably, lipoxins are formed in picogram and nanogram amounts in humans, with their potency maintained. Guilherme and colleagues showed that treatment with relatively low concentrations of 1 μg and 0.1 μg of aspirin‐triggered lipoxin A4 per mouse at days 7 and 10 significantly reduced inflammatory cell infiltration and reversed established fibrosis [27]. Furthermore, it should be noted that although NDGA has been shown to preferentially inhibit lipoxygenase‐5 [64, 65, 66], its activity is not exclusive to lipoxygenases. Indeed, NDGA has also been shown to also block leukotriene B4 and it has potent antioxidative properties [67].
Dendritic cells (DCs) are antigen‐presenting cells known to influence T‐cell proliferation and plasticity. In this study, we showed that hAEC treatment significantly reduced DC infiltration (day 5) to the site of injury and this might subsequently have led to changes in T‐cell numbers that we saw on day 7. This study identified possible mechanisms of hAEC‐mediated regulation of T‐cell behavior and phenotype. Our coculture migration and proliferation assays found that T‐cell migration toward CCL17 was unaffected by the hAEC‐conditioned medium but was able to suppress T‐cell proliferation. To elucidate the consequence of hAEC‐derived LXA4 on T‐cell proliferation, we found that addition of NDGA mitigated hAEC suppression of T‐cell proliferation. This may be due to the inhibition of extracellular signal‐regulated kinase signaling, which is known to regulate T‐cell proliferation [59].
The bleomycin mouse model of pulmonary fibrosis has come under recent scrutiny [10]. IPF is a disease with a complex pathology, and simple anti‐inflammatory and antifibrotic approaches are not curative. Although the bleomycin model does not capture the full complexity of the disease, it does replicate the acute exacerbations of the disease [10] where proinflammatory cytokines predominate (e.g., TNF‐α, IL‐1β, IL‐6, TGF‐β, fibronectin, and procollagen‐1) [68]. Furthermore, at the early stages of inflammation, when PGE2 and LTB4 provoke neutrophil extravasation, LXA4 and its epimer 15‐epi‐LXA4 was shown to have a profound inhibitory effect on PMN infiltration in vivo [69]. Our focus on acute inflammatory events provided an opportunity to investigate the immune regulatory mechanism of hAECs during acute exacerbations. Immunological changes exerted by hAECs beyond this period were not examined and should be the subject of future research. Previous work showed 4 million hAECs given 24 hours after bleomycin challenge via intraperitoneal administration ameliorated lung fibrosis and restored lung function in bleomycin‐challenged mice irrespective of hAEC engraftment [4, 45]; thus, this model was selected for our study. Whether lower doses, repeated dosing, or varying the route of administration will improve acute inflammatory exacerbations and/or long‐term outcomes on fibrosis remains to be investigated. Assessing the efficacy of hAECs in a model of repeated bleomycin challenge will also likely be useful in terms of evaluating their therapeutic utility in intractable lung fibrosis.
Conclusion
Our current study provides evidence that hAECs can alter neutrophil, macrophage, and T‐cell migration, phenotype, and behavior in resolving bleomycin‐induced lung injury, in part through LXA4. In the process of lung repair, hAECs promote resolution by upregulating macrophage phagocytosis, partly through LXA. It was highlighted at the 2012 National Institutes of Health, National Heart, Lung, and Blood Institute workshop that understanding the mechanism of action of stem cells is paramount for the advancement of cell‐based therapies [70]. Acumen of the biodynamics of cell therapy candidates in various disease or injury settings, therefore, will expedite the development of cell‐based therapies for lung disease. Additional studies on how these exogenously delivered stem cells and stem‐like cells aid other endogenous repair processes, such as their effects on stem/progenitor cell populations and extracellular matrices, during different phases of injury will be needed to best exploit their regenerative properties.
Author Contributions
J.L.T.: study design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; Y.Z.T., R.M., S.T.C., and S.N.L.: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; J.C.M.: administrative support; final approval of manuscript; E.M.W.: study design, data analysis and interpretation, manuscript writing, final approval of manuscript; R.L.: study design, data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
S.T.C. has compensated employment and intellectual property rights. E.M.W. holds a patent for use of amnion cells as a lung repair therapy. The other authors indicated no potential conflicts of interest.
Supporting information
Supporting Information
Acknowledgments
We thank the facilities and scientific and technical assistance of Monash Micro Imaging and Monash Flow Core at Monash University. This study was supported by the Victorian Government’s Operational Infrastructure Support Program and funded by the National Health and Medical Research Council Project Grant 1048899. R.L. holds a Fielding Foundation Fellowship.
References
- 1. Gilani SR, Vuga LJ, Lindell KO et al. CD28 down‐regulation on circulating CD4 T‐cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS ONE 2010;5:e8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bringardner BD, Baran CP, Eubank TD et al. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redux Signal 2008;10:287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim R, Chan ST, Tan JL et al. Preterm human amnion epithelial cells have limited reparative potential. Placenta 2013;34:486–492. [DOI] [PubMed] [Google Scholar]
- 4. Murphy S, Lim R, Dickinson H et al. Human amnion epithelial cells prevent bleomycin‐induced lung injury and preserve lung function. Cell Transplant 2011;20:909–923. [DOI] [PubMed] [Google Scholar]
- 5. Murphy SV, Lim R, Heraud P et al. Human amnion epithelial cells induced to express functional cystic fibrosis transmembrane conductance regulator. PLoS One 2012;7:e46533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hodges RJ, Jenkin G, Hooper SB et al. Human amnion epithelial cells reduce ventilation‐induced preterm lung injury in fetal sheep. Am J Obstet Gynecol 2012;206:448.e8–448.e15. [DOI] [PubMed] [Google Scholar]
- 7. Vosdoganes P, Hodges RJ, Lim R et al. Human amnion epithelial cells as a treatment for inflammation‐induced fetal lung injury in sheep. Am J Obstet Gynecol 2011;205:156.e26–156.e33. [DOI] [PubMed] [Google Scholar]
- 8. Cargnoni A, Gibelli L, Tosini A et al. Transplantation of allogeneic and xenogeneic placenta‐derived cells reduces bleomycin‐induced lung fibrosis. Cell Transplant 2009;18:405–422. [DOI] [PubMed] [Google Scholar]
- 9. Moodley Y, Ilancheran S, Samuel C et al. Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. Am J Respir Crit Care Med 2010;182:643–651. [DOI] [PubMed] [Google Scholar]
- 10. Srour N, Thébaud B. Mesenchymal stromal cells in animal bleomycin pulmonary fibrosis models: A systematic review. Stem Cells Translational Medicine 2015;4:1500–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moodley Y, Atienza D, Manuelpillai U et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin‐induced lung injury. Am J Pathol 2009;175:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Usunier BX, Benderitter M, Tamarat R et al. Management of fibrosis: The mesenchymal stromal cells breakthrough. Stem Cells Int 2014;2014:340257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ortiz LA, Gambelli F, McBride C et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A 2003;100:8407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chambers DC, Enever D, Ilic N et al. A phase 1b study of placenta‐derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology 2014;19:1013–1018. [DOI] [PubMed] [Google Scholar]
- 15. Tzouvelekis A, Paspaliaris V, Koliakos G et al. A prospective, non‐randomized, no placebo‐controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells‐stromal vascular fraction in idiopathic pulmonary fibrosis. J Transl Med 2013;11:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy S, Rosli S, Acharya R et al. Amnion epithelial cell isolation and characterization for clinical use. Curr Protoc Stem Cell Biol 2010;Chapter 1:Unit 1E.6. [DOI] [PubMed] [Google Scholar]
- 17. Díaz‐Prado S, Muiños‐López E, Hermida‐Gómez T et al. Multilineage differentiation potential of cells isolated from the human amniotic membrane. J Cell Biochem 2010;111:846–857. [DOI] [PubMed] [Google Scholar]
- 18. Tan JL, Chan ST, Wallace EM et al. Human amnion epithelial cells mediate lung repair by directly modulating macrophage recruitment and polarization. Cell Transplant 2014;23:319–328. [DOI] [PubMed] [Google Scholar]
- 19. Díaz‐Prado S, Muiños‐López E, Hermida‐Gómez T et al. Human amniotic membrane as an alternative source of stem cells for regenerative medicine. Differentiation 2011;81:162–171. [DOI] [PubMed] [Google Scholar]
- 20. Macdonald LJ, Boddy SC, Denison FC et al. A role for lipoxin A₄ as an anti‐inflammatory mediator in the human endometrium. Reproduction 2011;142:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tropea KA, Leder E, Aslam M et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2012;302:L829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maldonado‐Pérez D, Golightly E, Denison FC et al. A role for lipoxin A4 as anti‐inflammatory and proresolution mediator in human parturition. FASEB J 2011;25:569–575. [DOI] [PubMed] [Google Scholar]
- 23. Fahel JS, de Souza MB, Gomes MTR et al. 5‐Lipoxygenase negatively regulates Th1 response during Brucella abortus infection in mice. Infect Immun 2015;83:1210–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dunn HC, Ager RR, Baglietto‐Vargas D et al. Restoration of lipoxin A4 signaling reduces Alzheimer’s disease‐like pathology in the 3xTg‐AD mouse model. J Alzheimers Dis 2015;43:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liao W, Zeng F, Kang K et al. Lipoxin A4 attenuates acute rejection via shifting TH1/TH2 cytokine balance in rat liver transplantation. Transplant Proc 2013;45:2451–2454. [DOI] [PubMed] [Google Scholar]
- 26. Zhao Q, Hu X, Shao L et al. LipoxinA4 attenuates myocardial ischemia reperfusion injury via a mechanism related to downregulation of GRP‐78 and caspase‐12 in rats. Heart Vessels 2014;29:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guilherme RF, Xisto DG, Kunkel SL et al. Pulmonary antifibrotic mechanisms aspirin‐triggered lipoxin A4 synthetic analog. Am J Respir Cell Mol Biol 2013;49:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martin CR, Zaman MM, Gilkey C et al. Resolvin D1 and lipoxin A4 improve alveolarization and normalize septal wall thickness in a neonatal murine model of hyperoxia‐induced lung injury. PLoS One 2014;9:e98773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martins V, Valença SS, Farias‐Filho FA et al. ATLa, an aspirin‐triggered lipoxin A4 synthetic analog, prevents the inflammatory and fibrotic effects of bleomycin‐induced pulmonary fibrosis. J Immunol 2009;182:5374–5381. [DOI] [PubMed] [Google Scholar]
- 30. Fang X, Abbott J, Cheng L et al. Human mesenchymal stem (stromal) cells promote the resolution of acute lung injury in part through lipoxin A4. J Immunol 2015;195:875–881. [DOI] [PubMed] [Google Scholar]
- 31. van der Velden VHJ, Naber BAE, Wierenga‐Wolf AF et al. Interleukin 4 receptors on human bronchial epithelial cells. An in vivo and in vitro analysis of expression and function. Cytokine 1998;10:803–813. [DOI] [PubMed] [Google Scholar]
- 32. White SR, Martin LD, Abe MK et al. Insulin receptor substrate‐1/2 mediates IL‐4‐induced migration of human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2009;297:L164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huaux F, Liu T, McGarry B et al. Dual roles of IL‐4 in lung injury and fibrosis. J Immunol 2003;170:2083–2092. [DOI] [PubMed] [Google Scholar]
- 34. Chaitidis P, O’Donnell V, Kuban RJ et al. Gene expression alterations of human peripheral blood monocytes induced by medium‐term treatment with the TH2‐cytokines interleukin‐4 and ‐13. Cytokine 2005;30:366–377. [DOI] [PubMed] [Google Scholar]
- 35. Kobayashi T, Tanaka K, Fujita T et al. Bidirectional role of IL‐6 signal in pathogenesis of lung fibrosis. Respir Res 2015;16:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Manuelpillai U, Lourensz D, Vaghjiani V et al. Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLoS ONE 2012;7:e38631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McGuirk P, McCann C, Mills KHG. Pathogen‐specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: A novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis . J Exp Med 2002;195:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL‐10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol 2001;2:725–731. [DOI] [PubMed] [Google Scholar]
- 39. Sakamoto H, Zhao LH, Jain F, Kradin R. IL‐12p40(−/−) mice treated with intratracheal bleomycin exhibit decreased pulmonary inflammation and increased fibrosis. Exp Mol Pathol 2002;72(1):1–9. [DOI] [PubMed] [Google Scholar]
- 40. Serhan CN. Lipoxins and aspirin‐triggered 15‐epi‐lipoxins are the first lipid mediators of endogenous anti‐inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids 2005;73:141–162. [DOI] [PubMed] [Google Scholar]
- 41. Obayashi Y, Yamadori I, Fujita J et al. The role of neutrophils in the pathogenesis of idiopathic pulmonary fibrosis. Chest 1997;112:1338–1343. [DOI] [PubMed] [Google Scholar]
- 42. Schaaf B, Wieghorst A, Aries SP et al. Neutrophil inflammation and activation in bronchiectasis: Comparison with pneumonia and idiopathic pulmonary fibrosis. Respiration 2000;67:52–59. [DOI] [PubMed] [Google Scholar]
- 43. El Kebir D, József L, Pan W et al. 15‐epi‐lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med 2009;180:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seki H, Fukunaga K, Arita M et al. The anti‐inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol 2010;184:836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vosdoganes P, Wallace EM, Chan ST et al. Human amnion epithelial cells repair established lung injury. Cell Transplant 2013;22:1337–1349. [DOI] [PubMed] [Google Scholar]
- 46. Osterholzer JJ, Olszewski MA, Murdock BJ et al. Implicating exudate macrophages and ly‐6chigh monocytes in CCR2‐dependent lung fibrosis following gene‐targeted alveolar injury. J Immunol 2013;190:3447–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Petasis NA, Keledjian R, Sun Y‐P et al. Design and synthesis of benzo‐lipoxin A4 analogs with enhanced stability and potent anti‐inflammatory properties. Bioorg Med Chem Lett 2008;18:1382–1387. [DOI] [PubMed] [Google Scholar]
- 48. Lee TH, Horton CE, Kyan‐Aung U et al. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N‐formyl‐l‐methionyl‐l‐leucyl‐l‐phenylalanine. Clin Sci (Lond) 1989;77:195–203. [DOI] [PubMed] [Google Scholar]
- 49. Gibbons MA, MacKinnon AC, Ramachandran P et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med 2011;184:569–581. [DOI] [PubMed] [Google Scholar]
- 50. Shirey KA, Lai W, Pletneva LM et al. Role of the lipoxygenase pathway in RSV‐induced alternatively activated macrophages leading to resolution of lung pathology. Mucosal Immunol 2014;7:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: Selective inactivation by dehydrogenation and reduction. J Exp Med 1996;183:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maddox JF, Hachicha M, Takano T et al. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP‐1 cells via a G‐protein‐linked lipoxin A4 receptor. J Biol Chem 1997;272:6972–6978. [DOI] [PubMed] [Google Scholar]
- 53. Perretti M, Chiang N, La M et al. Endogenous lipid‐ and peptide‐derived anti‐inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med 2002;8:1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pederzoli‐Ribeil M, Maione F, Cooper D et al. Design and characterization of a cleavage‐resistant Annexin A1 mutant to control inflammation in the microvasculature. Blood 2010;116:4288–4296. [DOI] [PubMed] [Google Scholar]
- 55. Godson C, Mitchell S, Harvey K et al. Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte‐derived macrophages. J Immunol 2000;164:1663–1667. [DOI] [PubMed] [Google Scholar]
- 56. Mitchell S, Thomas G, Harvey K et al. Lipoxins, aspirin‐triggered epi‐lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol 2002;13:2497–2507. [DOI] [PubMed] [Google Scholar]
- 57. Morimoto K, Janssen WJ, Terada M. Defective efferocytosis by alveolar macrophages in IPF patients. Respir Med 2012;106:1800–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Herold S. Acute lung injury: How macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol 2011;2:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ariel A, Chiang N, Arita M et al. Aspirin‐triggered lipoxin A4 and B4 analogs block extracellular signal‐regulated kinase‐dependent TNF‐alpha secretion from human T cells. J Immunol 2003;170:6266–6272. [DOI] [PubMed] [Google Scholar]
- 60. Maderna P, Cottell DC, Toivonen T et al. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin‐derived peptide‐stimulated phagocytosis. FASEB J 2010;24:4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Serhan CN. A search for endogenous mechanisms of anti‐inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol 2004;122:305–321. [DOI] [PubMed] [Google Scholar]
- 62. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti‐inflammatory and pro‐resolution lipid mediators. Nat Rev Immunol 2008;8:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bannenberg G, Serhan CN. Specialized pro‐resolving lipid mediators in the inflammatory response: An update. Biochim Biophys Acta 2010;1801:1260–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Salari H, Braquet P, Borgeat P. Comparative effects of indomethacin, acetylenic acids, 15‐HETE, nordihydroguaiaretic acid and BW755C on the metabolism of arachidonic acid in human leukocytes and platelets. Prostaglandins Leukot Med 1984;13:53–60. [DOI] [PubMed] [Google Scholar]
- 65. Lü J‐M, Nurko J, Weakley SM et al. Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives: An update. Med Sci Monit 2010;16:RA93–RA100. [PMC free article] [PubMed] [Google Scholar]
- 66. Bhattacherjee P, Boughton‐Smith NK, Follenfant RL et al. The effects of a novel series of selective inhibitors of arachidonate 5‐lipoxygenase on anaphylactic and inflammatory responses. Ann N Y Acad Sci 1988;524:307–320. [DOI] [PubMed] [Google Scholar]
- 67. Czapski GA, Czubowicz K, Strosznajder RP. Evaluation of the antioxidative properties of lipoxygenase inhibitors. Pharmacol Rep 2012;64:1179–1188. [DOI] [PubMed] [Google Scholar]
- 68. Chaudhary NI, Schnapp A, Park JE. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med 2006;173:769–776. [DOI] [PubMed] [Google Scholar]
- 69. Takano T, Clish CB, Gronert K et al. Neutrophil‐mediated changes in vascular permeability are inhibited by topical application of aspirin‐triggered 15‐epi‐lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest 1998;101:819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Matthay MA, Anversa P, Bhattacharya J et al. Cell therapy for lung diseases. Report from an NIH–NHLBI workshop, November 13–14, 2012. Am J Respir Crit Care Med 2013;188:370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


