Abstract
Neural differentiation of human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs) can produce a valuable and robust source of human neural cell subtypes, holding great promise for the study of neurogenesis and development, and for treating neurological diseases. However, current hESCs and hiPSCs neural differentiation protocols require either animal factors or embryoid body formation, which decreases efficiency and yield, and strongly limits medical applications. Here we develop a simple, animal‐free protocol for neural conversion of both hESCs and hiPSCs in adherent culture conditions. A simple medium formula including insulin induces the direct conversion of >98% of hESCs and hiPSCs into expandable, transplantable, and functional neural progenitors with neural rosette characteristics. Further differentiation of neural progenitors into dopaminergic and spinal motoneurons as well as astrocytes and oligodendrocytes indicates that these neural progenitors retain responsiveness to instructive cues revealing the robust applicability of the protocol in the treatment of different neurodegenerative diseases. The fact that this protocol includes animal‐free medium and human extracellular matrix components avoiding embryoid bodies makes this protocol suitable for the use in clinic. Stem Cells Translational Medicine 2017;6:1217–1226
Keywords: Cellular therapy, Clinical translation, Differentiation, Embryonic stem cells, Induced pluripotent stem cells, Neural differentiation, Pluripotent stem cells
Significance Statement.
Here we present the new simple, animal‐free, embryonic body free protocol for neural differentiation of pluripotent stem cells that does not require recombinant proteins. This simple media formulated protocol represents a clear advantage over similar protocols in the same field generating a high yield of rossettes‐like neural progenitors of clinical grade.
Introduction
Human pluripotent stem cells (hPSCs), which encompass human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), have broad appeal for numerous basic biology studies and for therapeutic applications due to their potential for renewal and for producing almost any cell type in the human body 1, 2. Derivation of neural progenitors from pluripotent stem cells holds promise for the study of human neurogenesis, central nervous system (CNS) development, and neural diseases, and has potential for cell therapy applications to treat neurodegenerative diseases such as Parkinson's disease 3 and spinal cord injury, which are currently mostly untreatable 4, 5.
To date, hPSC differentiation toward a defined neural lineage involves the formation of embryoid bodies (EBs) 6, 7 or uses undefined factors such as an animal extracellular matrix, to form neuroepithelial structures called “rosettes,” followed by retinoic acid (RA) exposure 8, 9. The majority of these cell lines are differentiated in the presence of animal feeder cell lines or animal components, which bears the risk of xenogenetic pathogen cross‐transfer, and as such they are unsuitable for medical applications. In addition, protocols involving EB formation yield only a small fraction of neural lineage cells due to the presence of other cell lineages of mesodermal or endodermal origin. Importantly, because of the many differences between hESCs and hiPSCs, particularly in their differentiation potential toward specific neural lineages, which is relevant for many applications, both cell types need to be considered when devising differentiation protocols 10, 11. In spite of recent advances in xeno‐free protocols 1, 12, 13, 14 to date, there is no protocol for the controlled conversion of hPSCs into homogeneous populations of defined neural progenitors avoiding the formation of EBs under animal‐free conditions 15.
In 2008, we developed a protocol for the high conversion of hESCs toward defined regional specific neural progenitors in adherent and chemically defined conditions 15. This protocol permitted the controlled differentiation toward regional specific types of neuronal cells by exposing the rosettes to different signaling factors. However, it was not possible to avoid culture medium containing animal components in order to obtain a pure neural cell population. Insulin, transferrin, and sodium selenite are commonly used to replace the fetal bovine serum in many culture mediums. We reasoned that optimizing different concentrations of these components with taurine combined with a human extracellular matrix could be used to develop an animal‐free adherent culture protocol for converting both hESCs and hiPSC into regional‐specific and transplantable neural progenitors specifically for clinical applications.
Materials and Methods
Cell Culture
In this study, we obtained the same results with both hESCs and both hiPSCs lines. Primary hESC colonies (H9 and H1 lines, WiCell Inc., Madison, WI) or human hiPSC (Clone 1 and 4) derived previously 16 were mechanically dispersed into several small clumps, which were cultured on fresh commercially available human foreskin fibroblasts (American Type Culture Collection, Manassas, VA), inactivated by irradiation (45Gy) in hESCs medium (ECM) containing Knockout‐DMEM (Invitrogen), 100 µM ß‐mercaptoethanol (Sigma), 1 mM l‐glutamine (Invitrogen), 100 mM nonessential amino acids, 20% serum replacement (SR; Invitrogen), 1% penicillin‐streptomycin (Invitrogen), and 8 ng/ml basic fibroblast growth factor (bFGF; Invitrogen). ECM was changed daily. Human pluripotent stem cells were passaged by incubation in 1 mg/ml collagenase IV (animal‐free, Invitrogen) for 5–8 minutes at 37°C or mechanically dissociated and then removed to freshly prepared human foreskin fibroblast layers.
Neural Differentiation
Undifferentiated hESCs or hiPSCs were maintained on feeders with ECM medium. On day 0, ECM medium was changed to ITS medium which contains: DMEM/F12, dextran (6%), human insulin 50 µg/ml, holotransferrin 5 ng/ml, sodium selenite (50 ng/ml), glutamax 1x, taurine (0.5M), and ascorbic acid (50 µg/ml) and cells were maintained for 7 days with daily medium changes. During this period, neural differentiation begins with the formation of small neural tube‐like structures called rosettes that subsequently form neural islands, which increase in size and grow three dimensionally. These structures were mechanically separated from surrounding feeder cells by needle and transferred to human defined matrix (Cellstart, Life Technologies, 1:50 prepared in 6% of Dextran) coated plates and maintained with ITS medium over the following 7 days. From D14 to D21, the cells were disaggregated by accutase and plated on human laminin/polyornithine precoated plates and maintained in ITS medium (Fig. 1A). In order to determine the role of insulin, additional experiments were performed in which insulin was omitted from the ITS medium during the first 7 days of the differentiation protocol. In order to determine the role of Akt signaling pathway in neural differentiation, Akt inhibitor VIII (Calbiochem, Darmstadt, Germany, http://www.emdbiosciences.com) was added to the ITS medium (1µM, D0‐D5).
Figure 1.
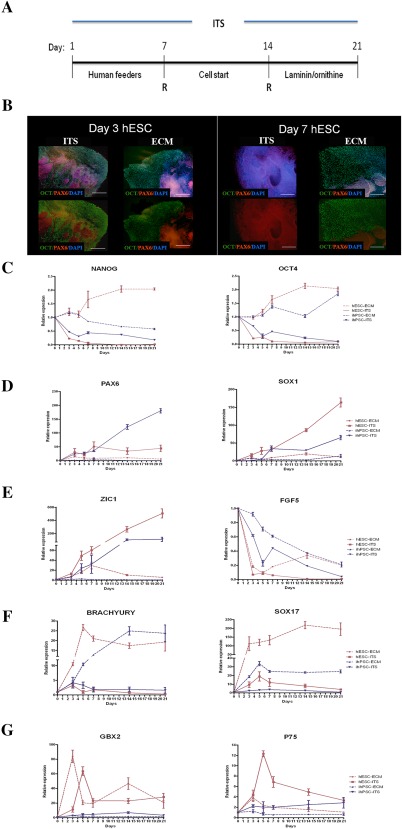
Initial differentiation of hPSCs to neural progenitors. (A): Schematic representation of the different steps in the feeder‐free, animal free and chemically defined medium conditions (see online methods). (B): Immunocytochemistry of hESCs colonies treated with ITS or ECM medium at day 3 and day 7 of the differentiation protocol. At day 7, all OCT4+ cells were converted to PAX6+ cells, while 60% of the ECM treated colony remained OCT4+. (C–G): Comparative RT‐PCR analysis of the hESCs and hiPSCs treated with ECM or ITS medium for: pluripotent genes NANOG and OCT4 (C), neurogenic markers PAX6 and SOX1 (D), ZIC1 and FGF5 (E), mesodermal (BRACHYURY) and endodermal marker (SOX17) (F) and GBX2 and P75 (G). Expression levels represent an average of at least 6 independent experiments ± SEM. Scale bar: (A) 100 μm. Abbreviations: ECM, embryonic stem medium; hESC, human embryonic stem cells; ITS, Insulin transferrin selenite.
The rosettes were propagated and expanded in ITS medium through more than 80 passages to analyze proliferation and telomerase activity. To study further differentiation and expansion, the neural progenitor cells were maintained in neural proliferation medium (NPM) for one week supplemented with 8 ng/ml human recombinant bFGF (Invitrogen), after which bFGF was withdrawn and cells maintained in NPM for 3 weeks. NPM medium consisted of DMEM:F‐12, xeno‐free B27 supplement (Invitrogen), 25 µg/ml human insulin (Sigma), 6.3 ng/ml progesterone, 10 µg/ml putrescine, 50 ng/ml sodium selenite, and 50 µg/ml human holotransferrin (Sigma).
For neuronal differentiation and induction of more posterior phenotypes the cells were maintained in NPM supplemented with 10 µM/ml all‐trans‐RA during the following 7 days, after which the RA was withdrawn and cells were maintained in NPM for 3 weeks. For oligodendrocyte differentiation, the cells were maintained for 4 weeks in NPM supplemented with 40 ng/ml triiodothyroidine (Sigma‐Aldrich) and 20 ng/ml of epidermal growth factor (EGF) (Sigma‐Aldrich) (Tit+EGF).
For differentiation of hESCs and hiPSCs toward dopaminergic neurons, after 21 days in ITS medium the cells were transferred to human laminin (L4544, Sigma‐Aldrich)/polyornithine precoated plates and maintained in neural induction medium: DMEM/F12 with N2 supplement supplemented with FGF8 (100 ng/ml) and sonic hedgehog (SHH; 200 ng/ml) for one week. Maturation was performed during the additional 2 weeks in neural maturation that includes: neurobasal medium, N2 supplement and cAMP 1 µM supplemented with brain‐derived neurotrophic factor (BDNF, 20 ng/ml), ascorbic acid (AA, 7 µl/10 ml) and glial cell‐derived neurotrophic factor (GDNF, 20 ng/ml).
Details about other methods used in this study such as RNA extraction and reverse transcription‐polymerase chain reaction (PCR) analysis, Immunocytochemistry, patch‐clamp, and animal surgery are available in Supporting Information methods.
Results
Undifferentiated hESCs and hiPSCs were maintained on a human foreskin fibroblast layer. To initiate controlled neural differentiation, the hESCs medium (ECM) was replaced by ITS medium (Fig. 1A). At day 3 (D3), the first sign of neural differentiation emerged as typical neuroepithelial structures or rosettes in the center of colonies, and at D5‐D7 the cells organized into neural tube‐like rosettes with lumens (Fig. 1B). After 7 days, the cell clusters were transferred to a human matrix (CellStart) and maintained in ITS medium for the following 7 days. For final neural differentiation, the clusters were dissociated and plated on a human laminin/polyornithine matrix and maintained in ITS medium for an additional 7 days (Fig. 1A). To confirm that the neural conversion of hESCs and hiPSCs was due to the medium conditions and not spontaneous differentiation, we performed immunocytochemical analysis of the cells at D3 and D7 of our protocol and compared it with the hESCs and hiPSCs maintained in ECM (Fig. 1B). This revealed that the columnar cells in rosettes (labeled with PAX6+) appeared in the center of colonies in both conditions. However, at D7 more that 95% of the cells cultured in ITS medium were PAX6+/OCT4−, which indicates a direct conversion of pluripotent stem cells. In contrast, 60% of hESCs and hiPSC colonies at D7 maintained in ECM were OCT4+. We next characterized the lineage progression of both hESCs and hiPSCs progeny grown in either ECM or ITS medium by real‐time (RT) PCR. Temporal analysis of gene expression in ITS treated cells showed a rapid loss of OCT4 and NANOG expression (Fig. 1C), and increased expression of the neuroectodermal markers PAX6, SOX1, and ZIC1 (Fig. 1D, 1E), further emphasizing the high level of direct neural conversion of these cells. Low expression of FGF5 in this early stage of the protocol (Fig. 1E) revealed the absence of intermediate cell types, in contrast to other protocols 17. Almost complete loss of Brachyury (a mesodermal marker) and SOX17 (endodermal marker) expression (Fig. 1F) indicated that ITS medium conditions mediate efficient conversion of hiPSCs and hESCs to neuroectoderm.
To determine whether insulin‐mediated induction of AKT signaling plays a crucial role in neural differentiation of hESCs and hiPSCs we tested the effect of either removing insulin from our medium or using an Akt inhibitor (VIII) during the first 5 days of the protocol 18. We observed a significant decrease of the neuroectodermal marker PAX6 and significant increase of the endodermal marker SOX17 in both conditions (Supporting Information Fig. 1). This indicates that insulin exerts a critical function likely through AKT signaling by redirecting differentiation from mesoderm and endoderm to neuroectoderm, consistent with a previous study on hESCs 18.
We next performed immunocytochemical analysis of the neural progenitors derived from both hESCs and hiPSCs for specific neural markers at D21. We found strong expression of neuroepithelial markers SOX1 and PAX6, as well as ZO1, a marker typically found in neural stem cells with asymmetric apical localization as a key feature of neural induction, as reported elsewhere 6, 19 (Fig. 2A). The proliferative nature of forming rosettes is confirmed by Ki67 and PHH3, as an evidence of interkinetic nuclear migration (PHH3) (Fig. 2D). Only a few cells (<0,5%) stained positive for pluripotent marker SSEA4 (Fig. 2A). Neural progenitors were also positive for other neural progenitor markers: Musashi, BF1, OTX2, A2B5, Nestin, SOX2, Tuj1, and Dach1 (Fig. 2B, 2C). Only a few cells (approximately 1.5%) expressed protein AP2 and P75 (neural crest marker) (Fig. 2D).
Figure 2.
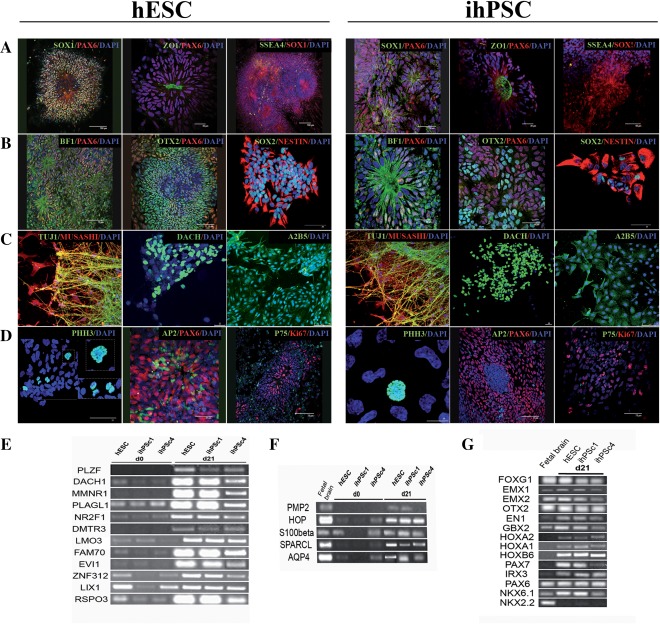
Immunocytochemical characterization of pluripotent stem cell derived neural precursors at D21. Neural progenitors derived from hESC (A–D) and hiPSC (E–H). Neural progenitors were analyzed with the following antibodies: SOX1/PAX6, ZO1/PAX6, and SSEA4/SOX1 (A), BF1/PAX6, OTX2/PAX6 and SOX2/NESTIN (B), TUJ1/MUSASHI, DACH, A2B5 (C), and PHH3, AP2/PAX6 and P75/Ki67 (D). RT‐PCR of neural progenitors at day 21 for neural rosettes markers (E), and genes proposed to be characteristic for fibroblast growth factor/epidermal growth factor‐expanded cells (F). RT‐PCR of neural progenitors for a wide range of anterior and posterior markers (G). Scale bars: (A) 100, 30 and 100 μm, 50, 30 and 50 μm (B) 75, 75 and 30 μm, 30, 50 and 25 μm (C) 75, 25 and 75 μm, 75, 25 and 75 μm, (D) 25, 25 and 75 μm, 10, 75 and 75 μm. Abbreviations: hESCs, human embryonic stem cells; hiPSC, human induced pluripotent stem cell.
Because the obtained progenitor cell populations had a uniform morphology with a neural tube rosette‐like pattern, we sought to identify rosette‐specific genes by RT‐PCR 6. At D21, the hESC‐ and hiPSC neural progenitors expressed PLZF, DACH1, MMNR1, PLAGL1, NR2F1, DMTR3, LMO3, FAM70, EVI, ZNF312, LIX1, and RSPO3 (Fig. 2E). In order to determine the subtype of neural stem cells generated, we first analyzed whether our neural rosette cells exhibited neural stem cell properties similar to those previously described as NSCFGF2/EGF 6, 19, 20. Indeed, the markers PMP2, HOP, S100β, SPARCL, and AQP4 were strongly expressed at D21 in all neural progenitors (Fig. 2F). To determine the positional identity and specification of the mature neuronal population, we next analyzed the expression of region‐specific transcription factors at D21. Strong expression of BF1 and OTX2 (anterior neural markers) revealed that culture conditions promote immediate rostral neuralization of primitive ectodermal cells (Fig. 2G). In addition, a very heterogeneous transcription factor profile was detected, with expression of the telencephalic markers (FOXG1, EMX1, EMX2, and OTX2), anterior hindbrain markers (GBX2, HOXA1, HOXA2, and HOXB6), and dorsal hindbrain markers (PAX7, IRX3, and PAX6), but no ventral hindbrain markers such as Nkx6.1 and NKX2.2 (Fig. 2G).
We next tested the stability of the derived neural progenitors in long‐term culture. After 21 days of neural differentiation, the progenitors were passaged in a 1:2 ratio and maintained in densely populated cultures in ITS medium. After extensive proliferation (>80 passages) the cell population maintained a uniform morphology, with stable proliferation capacity, as measured by the levels of telomerase reverse transcriptase (TERT) (Supporting Information Fig. 2a, 2b), and rosette‐like pattern, (data not shown) and neuroectodermal (PAX6 and SOX1) characteristics (Supporting Information Fig. 2c, 2d). There was no contamination by undifferentiated cells or cells with mesodermal (Brachyury) or endodermal (SOX17) origin (Supporting Information Fig. 2e, 2f). In addition, the cells could be frozen and thawed without detectable alterations in proliferation or differentiation properties. Immunocytochemical analysis after 2 passages in NPM revealed that 60% of the cells were Tuj1+, of which 45% (45% ± 12%; n = 4) were GABA‐ergic, 35% (35% ± 12%; n = 4) glutamatergic, and 2% (2% ± 0.2%; n = 4) serotoninergic neurons (Fig. 3A). At this stage, 34% of cells were immunoreactive to astrocytes glial fibrillary acidic protein (GFAP) (Fig. 3A).
Figure 3.
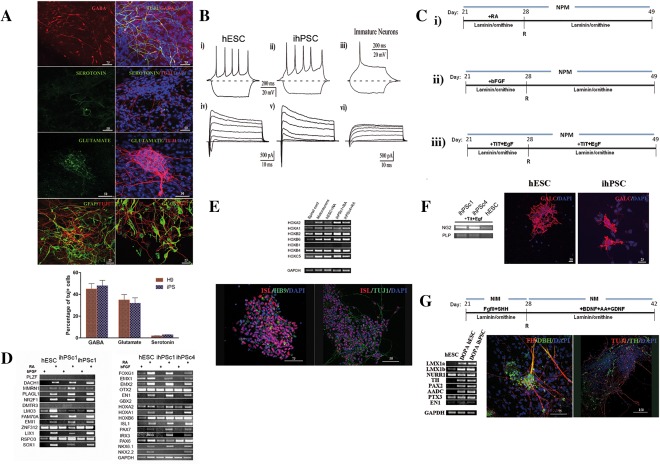
Neural progenitors behave as functional neurons and respond to regional specific cues. (A): Representative immunofluorescence images of differentiated neural progenitors derived from hESC labeled for GABA, glutamate and serotonin as well as immunocytochemistry of neural progenitors derived from hESC and hiPSC labeled with the neuronal specific marker β‐tubulin (tuj20) and the astrocyte marker GFAP (left image correspond to hESC and the right to hiPSC). (B): Functional characterization of hESCs, hiPSCs and immature neurons. (Bi–Biii): Current‐clamp recording of action potentials evoked by current injection. (Biv–Bvi): Whole‐cell voltage‐clamp recording of Na+ and K+ currents. Voltage pulses from −50 mV to +70 mV. (C): Schematic representation of the further differentiation steps using the feeder‐free, animal free and chemically defined medium conditions (see Material and Methods): (Ci): induction of posterior neural progenitors using RA, (Cii): induction of anterior neural progenitors using bFGF, (Ciii): induction of oligodendrocytes using TIT and EGF. (D): Real‐time (RT)‐PCR of RA treated neural progenitors confirmed rosettes specific markers and a caudal profile. (E): RT‐PCR and immunofluorescence analysis of RA treated progenitors for spinal phenotypes (HOX gene expression). The most of derived neurons were immature motoneurons expressing ISL. Only a few cells (1%, data not shown) were positive for mature motoneuron marker HB9. About 34% of generated cells were astrocytes expressing the GFAP marker. (F): Neural progenitors maintained for 3 weeks in NPM medium containing TIT and EGF were analyzed by RT‐PCR for expression of the NG2 and PLP markers and by immunochemistry the oligodendrocyte marker GALC. (G): Schematic representation of dopaminergic neural patterning using FGF8 and SHH from D21 to D28 followed by addition of BDNF, AA, and GDNF. Neural progenitors grown under these culture conditions were analyzed by RT‐PCR for the midbrain‐specific transcripts LIMX1a, LIMX1b, PAX2, TH, NURR1, PTX2, and AADC, and by immunofluorescence for TH and DBH. Data were averaged and represented as means ± S.E.M. Scale bars: (A) Scale bar: 50 and 25 μm, (E) 50 and 75 μm, (F) 50 and 75 μm, (G) 75 and 150 μm (K). Abbreviations: BDNF, brain‐derived neurotrophic factor; DBH, dopamine β‐hydroxylase; GDNF, glial cell‐derived neurotrophic factor; hESCs, human embryonic stem cells; ihPSC, induced human pluripotent stem cell; NIM, neural induction medium; NPM, neural proliferation medium; SHH, sonic hedgehog; TH, tyrosine hydroxylase.
We next analyzed whether hESCs and hiPSCs were capable of generating mature, electrically active neurons forming neuronal networks with functional chemical synapses. For this purpose, the electrophysiological properties of hESCs and hiPSCs were analyzed from 21 to 28 days of differentiation in NPM medium using the whole‐cell configuration of the patch clamp technique. In current‐clamp conditions, application of depolarizing current pulses (20–100 pA) evoked repetitive firing of action potentials in 80% of hESCs (n = 18) and 85% of hiPSC, (n = 20) (Fig. 3Bi, 3Bii). In contrast only a single action potential was generated in immature neurons (cultured without trophic factors) subjected to the same experimental protocol (Fig. 3Biii). This observation fits well with the high level of expression of voltage‐dependent Na+ (fast transient inward current) and K+ (delayed outward current) channels in mature neurons (Fig. 3Biv, 3Bv) in comparison with immature cells cultured only in ITS medium (Fig. 3Bvi). The properties of isolated Na+ and K+ currents recorded after application of specific ion channels blockers are shown in Supporting Information Figure 3. Inward Na+ currents in hESC‐ and hiPSC‐derived neurons were completely blocked by application of tetrodotoxin (1 microM (Supporting Information Fig. 3a, 3c). Outward K+ currents were fully abolished by application of 2 mM 4‐AP plus 20 mM TEA (Supporting Information Fig. 3b, 3d). Spontaneous postsynaptic currents were observed in mature neurons obtained from hESCs (n = 15) and hiPSC (n = 18) after 14 to 28 days of differentiation in vitro indicating functional synaptic transmission (Supporting Information Fig. 3e–3g). External application of CNQX (100 μM), a blocker of glutamate receptors, and bicuculline (2 μM), a blocker of GABA receptors, abolished the spontaneous synaptic activity (Supporting Information Fig. 3eii). Thus, both hESCs and hiPSCs are capable of generating electrophysiologically functional and synaptically connected neurons in vitro.
We next explored whether neural progenitors derived at D21 respond to anterior‐posterior and dorso‐ventral instructive regionalization by exposing them to morphogenic factors such as RA (Fig. 3C, 3I) and bFGF (Fig. 3Cii) during the 7 days of differentiation in animal free NPM. To determine whether these neural progenitors are capable of differentiating to a high yield of oligodendrocytes, they were also cultured in NPM supplemented with triiodothyronine hormone (TIT) 21, and EGF 22, 23, according to previous studies 24 (Fig. 3Ciii). The cells were analyzed by RT‐PCR at D49 of the differentiation protocol. While bFGF mostly suppressed rosette markers, maintaining the rostral pattern of differentiated cells, treatment with RA maintained the rosette expression profile (Fig. 3D) and enhanced the expression of caudal genes such as HOXA1, HOXA2, HOXB2, HOXB4, HOXB6, and HOXC1 (Fig. 3E). This profile of neural HOXC expression is indicative of spinal cord cells with a rostral cervical identity 25.
To determine whether the RA treated neural progenitors have a strictly caudal profile we next examined the expression of caudal markers such as class I (IRX3, PAX6, PAX7) and class II (NKX2.2, NKX6.1), which are homeodomain proteins important for motoneuron differentiation 26, and found high expression at D49 compared to bFGF treated progenitors (Fig. 3D). This suggests that our protocol, using RA (D21‐D28), efficiently supported differentiation towards caudal cells characteristic for the primary motoneuron (pMN) domain, giving rise to a significant number of ISL+ cells (Fig. 3E). Only a few cells were positive for HB9, a well‐known mature motoneuron marker. Thus, hPSCs, initially differentiated under chemically defined conditions to neural cells of forebrain‐like identity, can be caudalized to a motoneuron identity upon exposure to RA.
With the goal of restricting the multipotent nature of generated neural progenitors to oligodendroglial‐lineage cells, we exposed cultures to NPM supplemented with components influencing oligodendroglial‐lineage TIT and EGF (Fig. 3F). At D49, immunocytochemistry revealed that about 29% of total cells were positive for the oligodendrocyte marker GALC. RT‐PCR analysis also revealed high expression of the oligodendrocyte marker NG2 specifically in iPSC‐derived progenitors, but not PLP (Fig. 3F), revealing the high capacity of neural progenitors to differentiate towards mature oligodendrocytes.
Generation of dopamine neurons was observed when the neural progenitors were exposed to FGF8 and SHH from D21 to D29 and further maturated in medium containing BDNF, GDNF and AA for an additional 2 weeks (Fig. 3G). A midbrain dopaminergic profile of the generated cells was confirmed with the expression of transcription factors LMX1a, LMX1b 27 , NURR1, PAX2 28, AADC, PTX3, and EN1 involved in dopaminergic differentiation (Fig. 3G). Finally, the markers associated with the mature dopaminergic neuronal phenotype, tyrosine hydroxylase (TH) and dopamine β‐hydroxylase (DBH), were also expressed (Fig. 3G). These data demonstrate that our protocol generates neural progenitors capable of differentiating to any type of regional specific neural cell.
To explore whether hPSC‐derived neural progenitors can survive and integrate into the postnatal CNS and retain their differentiation potential, we implanted neural cells from D21 into adult mouse striatum. Immunohistochemistry analysis after 11 weeks using human specific nestin and neurofilament70 antibodies showed that the neural progenitors grafted, and the about 4%–8% (of total injected cells) survived and displayed typical neuronal morphology (Supporting Information Fig. 4a, 4b). The cells were localized in the striatum, cerebral cortex and hippocampus, consistent with previous studies 6. hESCs grafted in the brain of newborn SCID‐beige mice were previously shown to acquire predominantly GABAergic phenotypes 6. However, there was no detectable expression of calcium‐binding proteins in our grafted cells (such as calretinin or calbindin D28K, data not shown), perhaps because our hESCs were grafted into adults instead of newborns. Importantly, we did not observe teratomas or other signs of tumorigenicity in any of the animals grafted.
Discussion
Stem cell heterogeneity and a lack of efficient protocols for neural differentiation of hPSC represent significant obstacles to the clinical implementation of cell‐based therapies. In this study, we have demonstrated that neural progenitors can be efficiently generated from hPSC in a three‐step approach using animal‐free and feeder‐free conditions without formation of the EBs. Exposure of hESC and hiPSC to simply defined medium, including ITS and taurine, and human extracellular matrix leads to the generation of the highly enriched and proliferative rosette‐like neural progenitors without the presence of other cells with pluripotent, mesodermal, or endodermal characteristics.
Our protocol reveals advantages over a number of already existing protocols 9, 24, 29, 30, 31, 32, 33, 34, 35 describing the generation of neural progenitors. First, most of these protocols are based either on spontaneous hESC differentiation into a mixture of various cell types 31, 36 or generation of neural progenitors involving neurospheres 6, 37 or EB formation 38, 39 or using recombinant proteins to inhibit SMAD signaling 17. Our method is minimistic, less costly (according to actual prices; 88€vs. ∼300€per 500 ml compared to Erceg et al., 2008) and includes the initial differentiation of hPSC in chemically defined and animal‐free medium and adherent human substrate avoiding EB step resulting in morphological changes, including rosettes and neural tube‐like structures previously identified as typical neural progenitor cells 9, 31, 40. Additionally, the defined media is combined with the defined surface components (Cell start) vs Matrigel used in Chambers et al. 2009 17 or vitronectine, human collagen, and fibronectin used by Erceg et al., 2008 which all together create defined conditions for neural differentiation.
Second, the yield of obtained neural progenitors was higher than in previously published protocols where chemically defined medium and adherent conditions were used 6, 9, 17, 34, 35, 37. Comparing to spontaneous differentiation procedure we showed that high yield of neural progenitors (>98% at D7, compared to later stage (D11) of 82% of Chambers et al., 17) were achieved in specific medium conditions including defined combinations of insulin, transferrin, sodium selenite, and taurine applied in our protocol. The critical components in ITS medium that could contribute to this orchestrated and highly efficient conversion of hPSCs to neural progenitors are insulin and taurine. It has been shown that insulin can promote differentiation to the neuroectodermal lineage, which is dependent on PI3K/AKT signaling 18. In addition, while sodium selenite and human holotransferrin have mainly anti‐oxidant roles in the medium 41, taurine could have an important role in neural differentiation due to its neuroprotective function 42. That insulin‐mediated induction of AKT signaling plays a crucial role in neural differentiation of hESCs and hiPSCs, was confirmed in by removing insulin from the medium and using an Akt inhibitor (VIII) during the first 5 days of the protocol 18. This indicates that insulin exerts a critical function through AKT signaling by redirecting differentiation from mesoderm and endoderm to neuroectoderm, consistent with a previous study with hESCs 18. On the hand, recent studies have reported that taurine causes increased proliferation of neural stem/progenitor neural cells obtained from embryonic and adult rodent brain 43 that indicates the important role of taurine in our protocol possible related to proliferation of initially formed neuroepithelial cells.
Generated neural progenitors exhibit early neuroepithelial and neural rosette profile (expressing PLZF, DACH1, MMNR1, PLAGL1, NR2F1, DMTR3, LMO3, FAM70, EVI, ZNF312, LIX1, and RSPO3) independently of the hPSC source used, but the with notable variation in differentiation capacity between hiPSC and hESC. Although some studies have suggested that hESCs and hiPSCs have similar differentiation capacity toward neural cells 44, 45, our study is more compatible with other opposite studies in which have been demonstrated the differences between the neural differentiation propensities within hESC and hiPSC lines 44, 46, 47. In spite of variation in gene expression kinetics of some neuroepithelial markers (PAX6/SOX1), electrophysiological properties of generated neurons from both lines were similar, what is the essential aspect of the characterization of fully matured neuronal cells 48.
Furthermore, our neural rosette cells exhibited also neural stem cell properties similar to those previously described as NSCFGF2/EGF 6, 19, 20. Applying NPM medium that includes animal‐free B27 we showed that neuronal progenitor subtypes give rise to differentiated neurons that generate overshooting action potentials in response to depolarising current injection. The majority of neuron‐like cells responded to GABA and to a lesser extent to glutamate, which has been previously reported in neural progenitors derived from hPSC using neurospheres 6, 49 corresponding to neurons in developing and adult animals 15, 50, 51, 52.
Regarding the regional character of neural progenitors, it seems that our culture conditions promote immediate rostral neuralization of primitive ectodermal expressing telencephalic markers as well as anterior and dorsal hindbrain markers with tendency to mature predominantly into GABAergic neurons similar to our previously published study 15 and many others 6, 49.
We show here that neural progenitors retain responsiveness to instructive cues, in animal‐free conditions, enabling the derivation of ventral midbrain TH‐positive neurons, motoneurons or high yield of oligodendrocyte progenitors showing their potential for future use in the treatment specific neurodegenerative diseases.
During the development, RA and FGF signals act in opposing manner to impose rostrocaudal regional identity on hindbrain and spinal cord progenitor cells 15, 53. Interestingly, while bFGF mostly suppressed rosette markers, differentiation and maintenance of neural progenitors, in GRM/RA medium it maintained the rostral pattern of differentiated cells and enhanced the expression of caudal genes such as HOXA1, HOXA2, HOXB2, HOXB4, HOXB6, and HOXC1 profile indicative for spinal cord cells with a rostral cervical identity 15, 25. This medium efficiently supported further differentiation towards caudal cells characteristic for the pMN domain, giving rise to a significant number of ISL+ cells without more specific maturation procedure applied in other studies 6, 15, 19 where mostly sonic hedgehog as a ventralizing factor was used.
On the other hand, we have applied several factors can stimulate dopaminergic neuron development. Nurr1, Lmx1b, and Ptx3 transcription factors are mesencephalon specific, whereas the messenger molecules Shh and FGF8 seem to promote the dopaminergic phenotype irrespective of brain region 54, 55, 56. Our results clearly demonstrated that our neural progenitors respond to Shh and FGF8 recombination factors generating TH+ neurons expressing main midbrain dopaminergic markers.
In order to assess the multipotent nature of generated neural progenitors and their capacity to differentiate to oligodendroglial‐lineage cells, we exposed the cells to NPM supplemented with TIT and EGF. It has been described that these components play important role in proliferation and survival of oligodendrocyte preprogenitors 22 promoting differentiation in mature oligodendrocytes 57. Immunocytochemistry analysis revealed that about 29% of total cells were positive for the oligodendrocyte marker GALC revealing the higher capacity of neural progenitors to differentiate towards mature oligodendrocytes compared to similar protocols 34, 58, 59. This demonstrates that our differentiation protocol is advantageous over protocols where stromal cells 8, EBs 8, 31, 60 or adherent system were used 6, 15, 19. Further studies are required to conclusively determine whether other developing neural processes could be completely mimicked using hPSC and in vitro defined system.
Finally, in this study we answered the crucial question whether neural progenitor derived from hPSCs can be functionally grafted and survived in rodent host brain. We show that neural progenitors engrafted in rat striatum survive and exhibit mature neuronal phenotypes crucial for further application of these progenitors in cell therapy.
Conclusion
In summary, we have developed a simple, low cost protocol for highly efficient differentiation of hPSCs (both iPSCs and hESCs) toward neural progenitors using simple animal‐free and feeder‐free medium, which represents an important advantage over other published protocols 6, 17. This protocol also avoids generation of EBs, therefore reducing the mesoderm and endoderm derivates, and use of recombinant animal factors. In addition, it allows efficient further generation of regional specific neuronal subtypes in a much shorter time (3 weeks) compared to other similar approaches 6, 17. Together, this strategy could represent the standard differentiation procedure suitable for clinical applications including neurodegenerative diseases and spinal cord injury. Taking into account that these neural progenitors can be derived from patient‐specific hiPSC, thus could provide an attractive human in vitro cellular tool for disease modeling and pharmacological screening.
Author Contributions
D.L.: data analysis and interpretation, manuscript writing, final approval of manuscript, other (perform experiments); P.S.: concept and design, other (perform experiments); M.A.P.A., A.D.L., V.M.M., and J.K.: other (perform experiments); D.R.M.: other (in vivo experiments); S.S.B., M.S., E.S., and P.J.: final approval of manuscript; P.G.R. and J.L.B.: other (perform patch‐clamp), data analysis and interpretation; SE: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript, other (perform experiments).
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Supporting information
Supporting Information Figures.
Supporting Information Tables.
Acknowledgments
This work was supported by Wings for Life Foundation, funds for research from the “Miguel Servet” contract of Institute of Health Carlos III of Spanish Ministry of Science and Innovation (CP10/00579) (S.E.), Fund for Health of Spain PI14‐02209 (S.E.), Platform of Biomolecular and Bioinformatics Resources of the Institute of Health Carlos III PT13/0001/0042, Spain (D.L. and S.E.), Czech National Foundation GA CR P304/12/G069 (E.S.), and by the project „BIOCEV “(CZ.1.05/1.1.00/02.0109)” (P.J. and S.E.) and by the European infrastructure for translational medicine (EATRIS‐CZ LM2015064) (E.S.).
References
- 1. Lippmann ES, Estevez‐Silva MC, Ashton RS. Defined human pluripotent stem cell culture enables highly efficient neuroepithelium derivation without small molecule inhibitors. Stem Cells 2014;32:1032–1042. [DOI] [PubMed] [Google Scholar]
- 2. Thomson JA, Itskovitz‐Eldor J, Shapiro SS et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145–1147. [DOI] [PubMed] [Google Scholar]
- 3. Yang D, Zhang ZJ, Oldenburg M et al. Human embryonic stem cell‐derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells 2008;26:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nori S, Okada Y, Yasuda A et al. Grafted human‐induced pluripotent stem‐cell‐derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci USA 2011;108:16825–16830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsuji O, Miura K, Fujiyoshi K et al. Cell therapy for spinal cord injury by neural stem/progenitor cells derived from iPS/ES cells. Neurotherapeutics 2011;8:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koch P, Opitz T, Steinbeck JA et al. A rosette‐type, self‐renewing human ES cell‐derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci USA 2009;106:3225–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang SC. Neural subtype specification from embryonic stem cells. Brain Pathol 2006;16:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee H, Shamy GA, Elkabetz Y et al. Directed differentiation and transplantation of human embryonic stem cell‐derived motoneurons. Stem Cells 2007;25:1931–1939. [DOI] [PubMed] [Google Scholar]
- 9. Shin S, Mitalipova M, Noggle S et al. Long‐term proliferation of human embryonic stem cell‐derived neuroepithelial cells using defined adherent culture conditions. Stem Cells 2006;24:125–138. [DOI] [PubMed] [Google Scholar]
- 10. Erceg S, Ronaghi M, Stojkovic M. Human embryonic stem cell differentiation toward regional specific neural precursors. Stem Cells 2009;27:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lukovic D, Moreno Manzano V, Stojkovic M et al. Concise review: Human pluripotent stem cells in the treatment of spinal cord injury. Stem Cells 2012;30:1787–1792. [DOI] [PubMed] [Google Scholar]
- 12. Isoda M, Kohyama J, Iwanami A et al. Robust production of human neural cells by establishing neuroepithelial‐like stem cells from peripheral blood mononuclear cell‐derived feeder‐free iPSCs under xeno‐free conditions. Neurosci Res 2016;110:18–28. [DOI] [PubMed] [Google Scholar]
- 13. Li W, Sun W, Zhang Y et al. Rapid induction and long‐term self‐renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc Natl Acad Sci USA 2011;108:8299–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nguyen HX, Nekanti U, Haus DL et al. Induction of early neural precursors and derivation of tripotent neural stem cells from human pluripotent stem cells under xeno‐free conditions. J Comp Neurol 2014;522:2767–2783. [DOI] [PubMed] [Google Scholar]
- 15. Erceg S, Lainez S, Ronaghi M et al. Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions. PLoS One 2008;3:e2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Armstrong L, Tilgner K, Saretzki G et al. Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells 2010;28:661–673. [DOI] [PubMed] [Google Scholar]
- 17. Chambers SM, Fasano CA, Papapetrou EP et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 2009;27:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freund C, Ward‐van Oostwaard D, Monshouwer‐Kloots J et al. Insulin redirects differentiation from cardiogenic mesoderm and endoderm to neuroectoderm in differentiating human embryonic stem cells. Stem Cells 2008;26:724–733. [DOI] [PubMed] [Google Scholar]
- 19. Elkabetz Y, Panagiotakos G, Al Shamy G et al. Human ES cell‐derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev 2008;22:152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conti L, Pollard SM, Gorba T et al. Niche‐independent symmetrical self‐renewal of a mammalian tissue stem cell. PLoS Biol 2005;3:e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 1994;120:1097–1108. [DOI] [PubMed] [Google Scholar]
- 22. Ben‐Hur T, Rogister B, Murray K et al. Growth and fate of PSA‐NCAM+ precursors of the postnatal brain. J Neurosci 1998;18:5777–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakagaito Y, Satoh M, Kuno H et al. Establishment of an epidermal growth factor‐dependent, multipotent neural precursor cell line. In vitro Cell Dev Biol Anim 1998;34:585–592. [DOI] [PubMed] [Google Scholar]
- 24. Keirstead HS, Nistor G, Bernal G et al. Human embryonic stem cell‐derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci 2005;25:4694–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burke AC, Nelson CE, Morgan BA et al. Hox genes and the evolution of vertebrate axial morphology. Development 1995;121:333–346. [DOI] [PubMed] [Google Scholar]
- 26. Briscoe J, Ericson J. Specification of neuronal fates in the ventral neural tube. Curr Opin Neurobiol 2001;11:43–49. [DOI] [PubMed] [Google Scholar]
- 27. Cheng L, Chen CL, Luo P et al. Lmx1b, Pet‐1, and Nkx2.2 coordinately specify serotonergic neurotransmitter phenotype. J Neurosci 2003;23:9961–9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Broccoli V, Boncinelli E, Wurst W. The caudal limit of Otx2 expression positions the isthmic organizer. Nature 1999;401:164–168. [DOI] [PubMed] [Google Scholar]
- 29. Kawasaki H, Mizuseki K, Nishikawa S et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell‐derived inducing activity. Neuron 2000;28:31–40. [DOI] [PubMed] [Google Scholar]
- 30. Kawasaki H, Suemori H, Mizuseki K et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell‐derived inducing activity. Proc Natl Acad Sci USA 2002;99:1580–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li XJ, Du ZW, Zarnowska ED et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol 2005;23:215–221. [DOI] [PubMed] [Google Scholar]
- 32. Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells 2001;19:193–204. [DOI] [PubMed] [Google Scholar]
- 33. Roybon L, Brundin P, Li JY. Stromal cell‐derived inducing activity does not promote dopaminergic differentiation, but enhances differentiation and proliferation of neural stem cell‐derived astrocytes. Exp Neurol 2005;196:373–380. [DOI] [PubMed] [Google Scholar]
- 34. Joannides AJ, Fiore‐Heriche C, Battersby AA et al. A scaleable and defined system for generating neural stem cells from human embryonic stem cells. Stem Cells 2007;25:731–737. [DOI] [PubMed] [Google Scholar]
- 35. Nat R, Nilbratt M, Narkilahti S et al. Neurogenic neuroepithelial and radial glial cells generated from six human embryonic stem cell lines in serum‐free suspension and adherent cultures. Glia 2007;55:385–399. [DOI] [PubMed] [Google Scholar]
- 36. Carpenter MK, Inokuma MS, Denham J et al. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol 2001;172:383–397. [DOI] [PubMed] [Google Scholar]
- 37. Roese‐Koerner B, Stappert L, Koch P et al. Pluripotent stem cell‐derived somatic stem cells as tool to study the role of microRNAs in early human neural development. Curr Mol Med 2013;13:707–722. [DOI] [PubMed] [Google Scholar]
- 38. Brederlau A, Correia AS, Anisimov SV et al. Transplantation of human embryonic stem cell‐derived cells to a rat model of Parkinson's disease: Effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells 2006;24:1433–1440. [DOI] [PubMed] [Google Scholar]
- 39. Watanabe K, Kamiya D, Nishiyama A et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci 2005;8:288–296. [DOI] [PubMed] [Google Scholar]
- 40. Zhang SC, Wernig M, Duncan ID et al. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 2001;19:1129–1133. [DOI] [PubMed] [Google Scholar]
- 41. Yeo JE, Kang SK. Selenium effectively inhibits ROS‐mediated apoptotic neural precursor cell death in vitro and in vivo in traumatic brain injury. Biochim Biophys Acta 2007;1772:1199–1210. [DOI] [PubMed] [Google Scholar]
- 42. Leon R, Wu H, Jin Y et al. Protective function of taurine in glutamate‐induced apoptosis in cultured neurons. J Neurosci Res 2009;87:1185–1194. [DOI] [PubMed] [Google Scholar]
- 43. Hernandez‐Benitez R, Ramos‐Mandujano G, Pasantes‐Morales H. Taurine stimulates proliferation and promotes neurogenesis of mouse adult cultured neural stem/progenitor cells. Stem cell Res 2012;9:24–34. [DOI] [PubMed] [Google Scholar]
- 44. Boulting GL, Kiskinis E, Croft GF et al. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol 2011;29:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim K, Zhao R, Doi A et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol 2011;29:1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lappalainen RS, Salomaki M, Yla‐Outinen L et al. Similarly derived and cultured hESC lines show variation in their developmental potential towards neuronal cells in long‐term culture. Regen Med 2010;5:749–762. [DOI] [PubMed] [Google Scholar]
- 47. Osafune K, Caron L, Borowiak M et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol 2008;26:313–315. [DOI] [PubMed] [Google Scholar]
- 48. Toivonen S, Ojala M, Hyysalo A et al. Comparative analysis of targeted differentiation of human induced pluripotent stem cells (hiPSCs) and human embryonic stem cells reveals variability associated with incomplete transgene silencing in retrovirally derived hiPSC lines. Stem Cells Transl Med 2013;2:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Falk A, Koch P, Kesavan J et al. Capture of neuroepithelial‐like stem cells from pluripotent stem cells provides a versatile system for in vitro production of human neurons. PLoS One 2012;7:e29597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Belluzzi O, Benedusi M, Ackman J et al. Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci 2003;23:10411–10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pagani F, Lauro C, Fucile S et al. Functional properties of neurons derived from fetal mouse neurospheres are compatible with those of neuronal precursors in vivo. J Neurosci Res 2006;83:1494–1501. [DOI] [PubMed] [Google Scholar]
- 52. Tyzio R, Represa A, Jorquera I et al. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci 1999;19:10372–10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bel‐Vialar S, Itasaki N, Krumlauf R. Initiating Hox gene expression: In the early chick neural tube differential sensitivity to FGF and RA signaling subdivides the HoxB genes in two distinct groups. Development 2002;129:5103–5115. [DOI] [PubMed] [Google Scholar]
- 54. Sakurada K, Ohshima‐Sakurada M, Palmer TD et al. Nurr1, an orphan nuclear receptor, is a transcriptional activator of endogenous tyrosine hydroxylase in neural progenitor cells derived from the adult brain. Development 1999;126:4017–4026. [DOI] [PubMed] [Google Scholar]
- 55. Simon HH, Thuret S, Alberi L. Midbrain dopaminergic neurons: Control of their cell fate by the engrailed transcription factors. Cell Tissue Res 2004;318:53–61. [DOI] [PubMed] [Google Scholar]
- 56. Ye W, Shimamura K, Rubenstein JL et al. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell 1998;93:755–766. [DOI] [PubMed] [Google Scholar]
- 57. Ibarrola N, Mayer‐Proschel M, Rodriguez‐Pena A et al. Evidence for the existence of at least two timing mechanisms that contribute to oligodendrocyte generation in vitro. Dev Biol 1996;180:1–21. [DOI] [PubMed] [Google Scholar]
- 58. Itsykson P, Ilouz N, Turetsky T et al. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol Cell Neurosci 2005;30:24–36. [DOI] [PubMed] [Google Scholar]
- 59. Nistor GI, Totoiu MO, Haque N et al. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia 2005;49:385–396. [DOI] [PubMed] [Google Scholar]
- 60. Li XJ, Hu BY, Jones SA et al. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells 2008;26:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figures.
Supporting Information Tables.


