Abstract
Osteoarthritis, the most prevalent form of joint disease, afflicts 9% of the U.S. population over the age of 30 and costs the economy nearly $100 billion annually in healthcare and socioeconomic costs. It is characterized by joint pain and dysfunction, though the pathophysiology remains largely unknown. Due to its avascular nature and limited cellularity, articular cartilage exhibits a poor intrinsic healing response following injury. As such, significant research efforts are aimed at producing engineered cartilage as a cell‐based approach for articular cartilage repair. However, the knee joint is mechanically demanding, and during injury, also a milieu of harsh inflammatory agents. The unforgiving mechano‐chemical environment requires tissue replacements that are capable of bearing such burdens. The use of mesenchymal stem cells (MSCs) for cartilage tissue engineering has emerged as a promising cell source due to their ease of isolation, capacity to readily expand in culture, and ability to undergo lineage‐specific differentiation into chondrocytes. However, to date, very few studies utilizing MSCs have successfully recapitulated the structural and functional properties of native cartilage, exposing the difficult process of uniformly differentiating stem cells into desired cell fates and maintaining the phenotype during in vitro culture and after in vivo implantation. To address these shortcomings, here, we present a concise review on modulating stem cell behavior, tissue development and function using well‐developed techniques from chondrocyte‐based cartilage tissue engineering. Stem Cells Translational Medicine 2017;6:1295–1303
Keywords: Chondrogenesis, Adult stem cells, Tissue regeneration, Mesenchymal stem cells
Significance Statement.
Using well‐developed protocols for mechanical and chemical stimulation of chondrocytes, we have consistently fabricated engineered cartilage reaching near native properties (mechanical stiffness and glycosaminoglycan content). In this concise review, we use the knowledge gained from these efforts and apply them for cartilage tissue engineering with mesenchymal stem cells to produce functional replacement tissue.
Introduction
Articular cartilage exhibits a poor response to injury due to the intrinsic avascular nature and limited cellularity of the tissue 1. When left untreated, a progressive loss of cartilage is followed by inadequate repair and remodeling of the underlying subchondral bone and ultimately leads to osteoarthritis (OA), the most prevalent form of joint disease 2, 3. The limitations of cartilage to repair itself, coupled with inadequate clinical strategies and rising incidence rates of OA, have compelled cell‐based therapies for sustained recovery of the functional properties of native tissue.
One distinct advantage for cartilage repair is the immunoprivileged nature of the tissue due to the lack of blood vessels as well as the dense extracellular matrix of cartilage 4. As such, cartilage allografts and allogeneic chondrocytes from cadaveric donors are used clinically in biologic repair of the diathrodial joint without need for immunosuppression measures 5, 6, 7. Our team, as well as others, has implanted engineered cartilage derived from allogeneic chondrocytes in preclinical models without immune reaction 8. However, while there has been considerable success using chondrocytes in engineered cartilage constructs, their use in clinical applications may be hampered by the limited availability of cells and limited expansion capacity. Juvenile human chondrocytes show increased matrix production and do not stimulate lymphocyte proliferation when compared to adult chondrocytes, suggesting that they are not immunogenic 9, though there are significant challenges in procuring juvenile cartilage. Differentiated adult chondrocytes may be harvested from a patient's own healthy, non load‐bearing cartilage, but associated complications include donor‐site morbidity and further tissue degeneration 10, 11. Additionally, these cells may produce tissues with inferior load‐bearing ability, as inherent stiffness is known to vary across joint surfaces 12. More often, cells are retrieved from patients undergoing treatment for OA. The aged, diseased phenotype of these cells may subsequently inhibit their ability to form functional constructs 13, 14, 15.
Mesenchymal stem cells (MSCs) are an attractive alternative to chondrocytes as they are readily available, exhibit a capacity for rapid expansion, and readily differentiate into cells from a number of mesenchyme‐derived tissues, including cartilage, fat, bone, and tendon upon the application of relevant external cues 16, 17, 18. Additionally, these cells can be isolated from a wide variety of tissues including bone marrow, muscle, adipose tissue, periosteum, and synovial membrane (summarized in 19), though differentiation potentials have been found to vary depending on the source of the cell. In particular, for cartilage tissue engineering, bone marrow‐, synovium‐, and periosteum‐derived cells have been found to possess the greatest ability for chondrogenesis 20, 21. Recently, more attention has been focused on the superior capacity of synovium derived MSCs (SDSCs) to produce extracellular matrix components similar to chondrocytes (i.e., collagen II and aggrecan) after the addition of an appropriate chondrogenesis‐promoting growth factor cocktail during expansion 20, 21, 22, 23, 24, 25, 26. In further support of the use of SDSCs, Sampat et al. showed that growth factor‐priming of cells during 2D expansion coupled with a transient application of transforming growth factor β3 resulted in mechanical stiffness approaching native immature bovine cartilage levels, with corresponding glycosaminoglycan (GAG) content 24.
The use of MSCs clinically for cell‐based therapies has already garnered some clinical success. Microfracture of the subchondral bone is a common surgical technique aimed at taking advantage of the reparative capacity of MSCs, with the idea that factors secreted in the local environment of damaged tissue recruit stem cells to the site and facilitate a repair response 27, 28. Alternative uses of MSCs in cell therapy have shown some early promise; intra‐articular injections of allogenic MSCs in patients with partial menisectomy have induced tissue regeneration and pain improvement 29. Similarly, percutaneous injections of autologous bone marrow derived MSCs into a diseased knee joint have resulted in significant cartilage growth, decreased pain, and increased joint mobility 30, 31. While promising, unfortunately, the resulting fibrous tissue formation lacks the mechanical properties and structure of articular cartilage needed to sustain the rigorous loading demands. As such, the tissue engineering paradigm of incorporating cells into biomaterial scaffolds coupled with in vitro manipulations may provide a more promising approach to repairing and restoring function of damaged cartilage.
Strategies for tissue development have largely looked at recapitulating natural cartilage development, basing the selection of exogeneous cues on those found in the native local environment. The sequence of cartilage formation begins with cellular aggregation, followed by the development of a three‐layered mesenchyme, and eventual differentiation of the outer layer cells into chondrocytes 32, 33, 34. During postnatal development, cartilage is then physiologically reorganized through tissue resorption and neoformation into a highly anisotropic structure of vertical columns and horizontal strata 35. With these developmental processes in mind, tissue engineering of cartilage offers unique opportunities for therapeutics in the repair of tissues damaged by injury or disease by combining cells, biomaterials, and exogenous stimuli (recently reviewed in 36, 37, 38) to promote tissue regeneration and functional restoration of the fledgling tissue to survive the harsh loading condition and often pro‐inflammatory milieu of the native joint 39, 40, 41, 42. These approaches are based on the premise that successful culture of engineered constructs in vitro that match the material properties of native cartilage will improve the long‐term success of the replacement tissue following implantation. Moreover, this success will be greatly influenced by the ability of the engineered cartilage to integrate with the surrounding host tissue, as poor adhesion strength can lead to potential failure sites 43.
In this concise review, we examine principles of cartilage tissue engineering that we have used with chondrocyte‐based tissues to consistently generate engineered cartilage with native functional properties and translate the knowledge acquired from those efforts to MSC‐based tissues to reproduce functional cartilage replacement.
Mechanical Stimulation
A variety of physical stimuli, including osmotic loading, hydrostatic pressure, electrokinetic phenomena, stress, and strain exist in the natural joint loading environment 44. During joint and cartilage development, too, mechanical forces play an important role in joint formation with the Indian hedgehog‐parathyroid hormone‐related protein (PTHrP) feedback loop regulating the maintenance and differentiation of articular chondrocytes 45, 46. These findings have long motivated the use of mechanical stimulation such as physiologic dynamic deformational loading or sliding contact loading in chondrocyte‐based cartilage tissue engineering, resulting in improved mechanical properties and biochemical content 39, 42, 47, 48 (Fig. 1). Such loading schemes both mimic the in vivo cyclical forces at physiological levels that have been suggested to be necessary to maintain chondrocyte structure and function 49 as well as enhance the convection of nutrients through the tissue 50, 51. Additionally, the complex interplay of collagen and proteoglycan content gives rise to the unique structure‐function relationship of articular cartilage. As such, the application of physico‐chemical stimuli can modulate the structural organization 52, amount and type of extracellular matrix that gives rise to the mechanical properties of engineered cartilage tissue.
Figure 1.
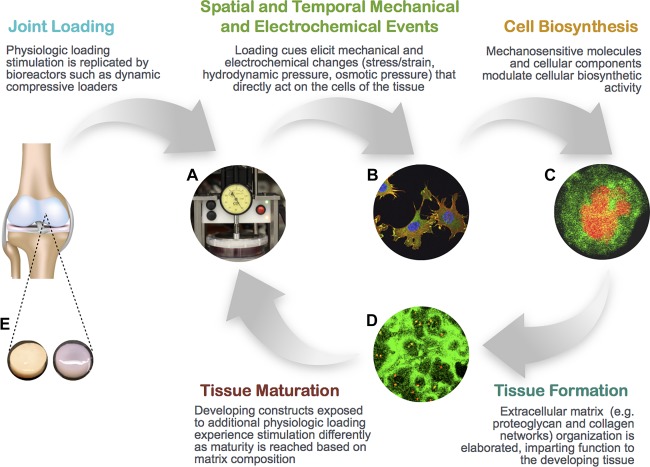
Schematic showing the role that bioreactors, which simulate aspects of the physiochemical environment of the chondrocyte, can play in modulating extracellular matrix composition and organization that gives rise to mechanical properties of the tissue. (A): Deformational loading bioreactor, (B): Cytoskeletal staining, (C): Type VI collagen (green) staining of pericellular matrix surrounding chondrocyte, (D): Extensive type II collagen network staining (green) in agarose construct, (E): Native explant (left), tissue engineered cartilage (right).
There has been evidence that similar mechanical stimulation may be beneficial for MSC‐based constructs. Results from several studies have found that physical forces can be used to modulate chondrogenesis of bone marrow derived MSCs 53, 54, 55, 56, though the timing of the application is important, suggesting a varying mechanosensitivity of the cells during chondrogenesis 57, 58, 59. For example, Huang et al. showed that loading of juvenile bovine bone marrow derived MSC constructs at early timepoints before chondrogenesis had occurred decreased functional maturation compared to non‐loaded samples even though chondrogenic gene expression increased 57. In contrast, after chondrogenesis and matrix elaboration had been initiated, loading improved the mechanical properties of the constructs. Some evidence exists that these responses, however, may be specific to stem cell source and donor. Luo et al. showed that while engineered tissues composed of either bone marrow derived MSCs or infrapatellar fat pad derived MSCs was capable of developing mechanical properties on the same order of magnitude as native juvenile tissue, dynamic compression was only beneficial to bone marrow derived MSCs 60. Further work is needed to characterize the mechanosensitivity of other stem cell sources to assess and optimize their use for functional cartilage tissue engineering.
Other relevant modes of mechanical stimulation such as hydrostatic pressure and osmotic loading have been successful at upregulating the expression of chondrogenic genes in MSC tissues 61, 62, 63, 64, 65. In particular, hypertonic loading has been found to provide a more physiologically relevant culture condition, mimicking the in vivo osmolarity of human articular cartilage (ranging from 350 to 450 mOsM depending on the zone 66, 67). In contrast, typical chondrogenic culture medium is hypotonic (330 mOsM). Indeed, physiologically relevant hyperosmotic loading during 2D expansion results in subsequently higher aggrecan expression when chondrocytes are later encapsulated in a 3D scaffold (Fig. 2A). Similarly, when hypertonic conditions are applied to encapsulated bovine chondrocytes in 3D culture, increases in mechanical properties and biochemical content are observed 63 (Fig. 2B). This beneficial effect was also observed for juvenile bovine SDSCs (Fig. 2B), producing tissues with mechanical functionality similar to native cartilage. Taken together, these studies support the use of osmotic loading regimes, either as a priming strategy or during 3D culture as a method for improvement of engineered cartilage tissue properties. Such growth factor priming strategies may also be optimized using high throughput screening measures including proteomics 68, 69.
Figure 2.
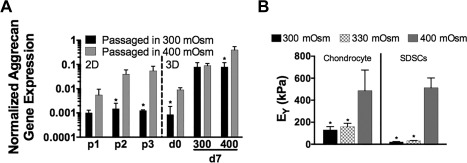
Effect of hypertonic (400 mOsm) loading on subsequent tissue development. (A): 2D Expansion of human chondrocytes in higher osmolarity medium increased aggrecan gene expression normalized to glyceraldehyde 3‐phosphate dehydrogenase expression that was paralleled during subsequent 3D tissue development. (B): Young's modulus (EY) was significantly increased when 3D juvenile bovine chondrocyte and SDSC constructs were cultured in 400 mOsm media. *p < .05 versus 400 mOsm condition, n = 5/group. Abbreviations: SDSC, synovium‐derived stem cells; EY, Young's modulus.
Recently, more advanced loading regimes incorporating combinations of tensile, shear, and compressive stresses have been investigated to more appropriately mimic the in vivo loading environment 70, 71, 72. For example, Schätti et al. observed that the synergistic effect of adding a shear component to compressive loading resulted in enhanced chondrogenic gene expression of MSCs 72. Further work is needed to elucidate optimal loading regimens and parameters to control and maintain differentiation.
Chemical Stimulation
In addition to mechanical loading to promote tissue growth, many groups have focused on the application of a range of chemical cues such as growth factors (TGF‐β3, TGF‐β1, insulin‐like growth factor (IGF), fibroblast growth factor 73, 74, 75, 76), corticosteroids 77, 78, and interleukins (IL) 40, 79, 80 for chondrocyte‐based tissue engineering as the exchange of chemical factors has been found to promote extracellular matrix (ECM) development. These same factors are potential regulators for MSCs; in fact, members of the TGFβ family as well as bone morphogenic proteins have been discovered to be the most potent inducers of chondrogenesis. Interestingly, the effects of the mechanical loading described above are amplified in, and in some cases, rely on, the presence of these growth factors 81. For example, while dynamic loading alone led to increases in GAG accumulation compared to unloaded controls, constructs exposed to TGF‐β3 alone led to much greater increase in GAG content, as well as an increase in collagen content 55. The effect of these growth factors on the transcription factor Sox9 may be the key to controlling chondrogenesis, as it has been linked to the commitment of a cell down the chondrogenic lineage. In support of the key role Sox9 plays during chondrogensis, when MSCs were manipulated to overexpress the Sox9 gene, chondrogenesis was enhanced, marked by increased proteoglycan and type II collagen deposition as well as prevention of terminal differentiation 82.
Recent studies have also examined the paracrine factors released by chondrocytes and their role in coculture systems with MSCs. Chondrocytes have been reported to secrete a range of soluble factors that promote chondrogenesis of MSCs during in vitro culture, regulating matrix remodeling, cell proliferation, and synthesis of extracellular matrix components by stem cells. For example, when combined in close proximity, mixed cell culture systems produced engineered cartilage with increased mechanical properties (Young's modulus and dynamic modulus), GAG levels, and collagen content 83, 84, 85. Interestingly, these chondrocytes were also able to decrease the deposition of collagen X 83, 86, a marker of MSC hypertrophy, perhaps by secreting PTHrP 87. Paracrine factors, therefore, may act as alternatives to growth factors that are typically supplemented in medium formulations.
It is important to note that a more thorough characterization of the influence of these two cell systems on one another is necessary to fully realize the potential of coculture systems. While a number of studies have found positive effects from chondrocytes on MSC chondrogenic differentiation 85, 88, 89, 90, other studies have found that the beneficial effects are due to the trophic role of MSCs in stimulating chondrocyte proliferation and matrix deposition 91, 92. Notably, using a xenogenic system and species‐specific gene expression analysis to determine the contribution of various cell populations to cartilage formation, Wu et al. showed that following coculture, micromass pellets contained predominantly DNA from the species of origin of the primary chondrocytes, indicating an overgrowth of chondrocytes or loss of MSCs during the culture period. Indeed, regardless of the tissue source, MSCs stimulated chondrocyte proliferation and GAG production, enhancing their potential for functional tissue repair.
Scaffold Choice
The choice of scaffold versus acellular constructs (e.g. self‐aggregating pellets) will greatly influence the cell–cell and cell‐ECM interactions that may modulate the response of MSCs to external cues (mechanotransduction and soluble factor delivery) and vary the differentiation potential. Ideal scaffolds for cartilage tissue engineering allow for spatial control over the distribution or placement of cells to enhance chondrogenesis and also offer a template for cells to anchor onto and elaborate extracellular matrix that mimics the in vivo biomechanical environment. Toward this end, careful optimization of the materials composition, 3D structure and porosity, and biocompatibility can influence chondrogenic tissue formation. Specifically, for cartilage tissue engineering, hydrogels are particularly relevant as they are capable of retaining a high water content, mimicking the chondrogenic environment and producing homogeneous cell distributions. Some common hydrogel materials used for cartilage tissue engineering include collagen type I or type II, fibrin, hyaluronic acid, chondroitin sulfate, polyethylene glycol (PEG), alginate, and agarose 93, 94, 95, 96, 97, 98, 99. Our lab has focused extensively on agarose, a clinically‐relevant scaffold that has been used widely for maintaining the phenotype of chondrocytes (i.e., maintain a rounded morphology due to a lack of epitopes for adhesion) for basic science studies of chondrocyte biology 100, 101, 102 as well as a copolymer to support autologous chondrocyte implantation for cartilage repair in Europe 103, 104, 105. This agarose environment has proved to be beneficial for both terminally differentiated cells (e.g. chondrocytes) and MSCs (e.g. synovium‐derived stem cells), encouraging robust extracellular matrix deposition and functional response to mechanochemical stimuli similar to native tissue 24, 39, 63, 106.
However, these base polymer scaffolds can also be modified in additional ways to increase their use and potential while influencing the response of cells embedded within. Scaffolds can act as a vehicle for locally delivering growth factors or genes and also help to increase access to chemical factors for cells at the center of developing constructs. Traditionally, these cells experience nutrient diffusion limitations as engineered tissues become denser with growth in culture 107. As such, scaffolds may be modified with surface‐immobilized growth factors to increase availability to neighboring cells. In studies by Chou et al., immobilization of the growth factor TGF‐β1 produced constructs with significantly higher GAG and type II collagen production compared to exogenous delivery of the growth factor 108. Similarly, Capito et al. showed that scaffolds that incorporate nonviral IGF‐1 DNA resulted in prolonged overexpression of the growth factor throughout culture 109. Even further, the kinetics of growth factor release can be modulated depending on the method of incorporation into the scaffold such as soaking or freeze‐drying.
Hydrogels can also be modified to alter a cell's affinity for its substrate or differentiation response. For example, PEG hydrogels modified with arginine‐glycine‐aspartyl (RGD) ligands, a cell‐adhesion moiety that promotes cell attachment and adhesion, exhibited increased viability 110, 111 and enhanced cartilage‐specific gene expression and matrix synthesis in the presence of mechanical stimulation 112. Other groups have investigated coupling selected cartilaginous ECM molecules to the base polymer structure to regulate cellular differentiation. In particular, despite its absence in healthy hyaline cartilage, scaffolds containing type I collagen has been favored for mesenchymal stem cell differentiation as it has been shown to maintain chondrogenic phenotype and promote cartilage repair 96, 113. Conversely, in the presence of chondroitin sulfate, type II collagen production has been promoted and hypertrophic mineralization reduced 98, 114. Toward this end, groups have modulated the presence of these matrix molecules in the scaffold design to control chondrogenesis. For example, Nguyen et al. showed that by manipulating different combinations of PEG‐based hydrogels with ECM molecules, unique niches could be created to direct differentiation of a single MSC population toward distinct phenotypically diverse chondrocytes (i.e., superficial, middle zone, and deep zone); that is, varying ratios of polymer to ECM molecules produced different quantities of proteoglycan and collagen type II deposition representative of the different zones 115.
For clinical translation, stimuli‐responsive injectable hydrogels have garnered particular interest recently due to their minimally‐invasive delivery and ability to fill small and irregular‐shaped defect sites. These initially fluid gels can be modified and gelled in place through the addition of heat or light that react with particular cross‐linkers. Groups have used such chemistry to their advantage, modulating scaffold architecture, mechanical properties, and cellular behavior 116, 117, 118, 119, 120. However, a significant limitation exists in that they may not be as compatible with traditional functional tissue engineering strategies that require significant de novo tissue development prior to clinical application (so as to survive joint loading forces).
Limitations With the Use of MSCs for Cartilage Tissue Engineering
While the use of MSCs for tissue engineering has shown potential, success has been variable and limited at recreating engineered tissues similar to native cartilage in terms of function or structure. These inconsistencies may point to the inherent heterogeneity associated with MSC populations in terms of cell proliferation capacity and differentiation potential 16, 121, 122, 123. For example, even when the cells uniformly express MSC markers such as CD29, CD44, CD73, CD90, CD105, and CD166, Mareddy et al. showed that there was a wide variation in cell doubling times, with fast‐growing and slow‐growing clones subsequently exhibiting altered differentiation 123. In contrast to clonal populations with rapid proliferative capacity that were mostly tripotential (capable of forming bone, fat, and cartilage phenotypes), slow‐growing clones were unipotential or bipotential. The large variation within a single population of cells and limited understanding of how to select the most potent cells for clinical application significantly challenge their use for tissue engineering.
Another challenge to cartilage tissue engineering with MSCs is how to regulate the differentiation progression, as cells may be pushed to hypertrophy, matrix mineralization, and ossification, similar to that which is in observed in the cartilage growth plate. This may be especially pertinent for clinical transplantation, as in vitro culture was found to prematurely upregulate hypertrophy‐related genes, including type X collagen, alkaline phosphatase, and matrix metalloproteinase 13 124. Subsequent implantation of these MSC pellets in ectopic sites in severe combined immunodeficient mice resulted in alterations associated with endochondral ossification rather than maintenance of a stable chondrogenic phenotype 124. Alternatively, even when chondroinductive factors induce the differentiation of MSCs into chondrocytes in vitro, the remarkable plasticity of stem cells may cause dedifferentiation following implantation or even transdifferentiation in the presence of other inductive extracellular cues 125.
Finally, the ability of MSCs to rapidly proliferate raises concerns as to whether MSCs can become tumorigenic after prolonged culture. Particularly, MSCs have been shown to be capable of secreting soluble factors to create a local immunosuppressive environment that favors tumor growth by promoting angiogenesis and preventing tumor recognition by the immune system 126, 127, 128. Though the use of MSCs in cell‐based tissue engineering and regenerative medicine is promising, further work will be needed to more fully understand the long‐term side effects of MSC‐laden constructs before clinical adoption.
Conclusion
In summary, the use of MSCs in biomimetic environments has begun to unlock the biological potential of these repair cells. However, successful treatment for cartilage repair remains challenging, requiring a greater understanding of developmental MSC chondrogenesis and new techniques that can recapitulate the structure and function of the native tissue. Adult MSCs are a promising candidate cell source as they are readily available from a number of different tissue sources and exhibit an ability to proliferate and differentiate into desired cell types in the presence of lineage‐specific cues. Recent efforts in tissue engineering MSC‐based constructs have explored using a combination of chondrogenic stimuli coupled with innovative scaffold biomaterials to influence differentiation and proliferation, but limited studies have yielded mechanical properties matching that of native cartilage 60, 129.
Genetically modified chondrocytes or MSCs have also shown promise in the treatment of arthritis by stimulation of anabolic pathways or inhibition of catabolic pathways (reviewed in 130). Following modification by nonviral and viral vectors, MSCs can be transplanted into articular cartilage defects in vivo for sustained transgene expression level that enhances the structural features of the repair tissue. In one example of a promising use for genetically modified cells, the transplantation of cells transduced to overexpress IL‐1 receptor antagonist onto osteoarthritic cartilage explants was successful at protecting cartilage from IL‐1 induced extracellular matrix degradation 131, 132.
While these systems have yielded encouraging data, further work is necessary to understand and characterize the duration of transgene expression and the potential benefit of genetically modified cells. It also remains to be explored the subsequent in vivo response after implantation: whether the native joint will promote further chondrogenesis or if supplementation of additional growth factors is necessary to prevent hypertrophy, dedifferentiation, or transdifferentiation. Toward this end, it remains to be elucidated what the role of the in vivo environment should be: maintenance of a terminal chondrogenic phenotype or promotion of further chondrogenesis at the risk of possible proliferation. However, as temporal gene expression may not accurately reflect the production of matrix molecules that impart mechanical functionality (reviewed in 133), it is likely that for in vivo success, the most important marker by which to evaluate engineered cartilage will be the development and maintenance of mechanical properties.
Another important consideration before clinical adoption will be understanding the inherent variation of MSCs and any confounding effects of sex, age, or disease state. For example, we have seen even with human chondrocytes that inherent donor‐to‐donor differences result in variations in mechanical properties and biochemical production when these cells are used in cartilage tissue engineering (Fig. 3). We posit that the individual variations in responses and behaviors of these cells to the differentiation cues necessary to create functional cartilage may hinder efforts at defining an optimal, set protocol for producing robust tissue. One potential area of improvement in this regard may be the development of a more comprehensive library of cell surface markers found on MSCs to improve the selection of desirable cells that are more capable of producing functional tissue and help to screen potential donors. While identification of MSCs has generally relied on the presence of a core set of surface markers, there is not yet a unique set of cell surface markers or differentiation molecules delineating a specific type of MSC or its lineage potential. Work is ongoing in these efforts; for example, recently, the combination of high CD105 (Endoglin) and CD166 (cell adhesion) expression has been attributed to the chondroprogenitor phenotype 134.
Figure 3.
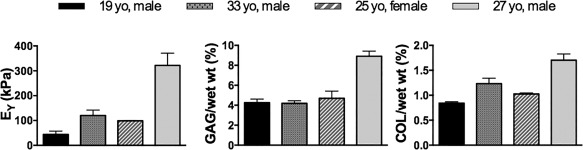
Representative data showing mechanical stiffness (EY) and biochemical content (GAG; COL) of human chondrocyte constructs vary by donor. Abbreviations: EY, Young's modulus; GAG, glycosaminoglycan; COL, collagen.
Taken together, while there are still significant hurdles, with further research and development the use of MSCs to yield a clinically relevant replacement cartilage remains promising for improving the quality of life for patients with joint disease.
Author Contributions
A.T.: developed plan for review, wrote manuscript, read and approved final manuscript; C.H.: developed plan for review, wrote manuscript, read and approved final manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Acknowledgments
Research findings from our laboratory appearing in this review were funded in part through financial support from the National Institutes of Health, The Coulter Foundation, and Columbia Technology Ventures.
References
- 1. Tew S, Redman S, Kwan A et al. Differences in repair responses between immature and mature cartilage. Clin Orthop Relat Res 2001;(suppl 391)S142–S152. [DOI] [PubMed] [Google Scholar]
- 2. Buckwalter JA, Martin JA. Osteoarthritis. Adv Drug Deliv Rev 2006;58:150–167. [DOI] [PubMed] [Google Scholar]
- 3. Buckwalter JA, Martin J, Mankin HJ. Synovial joint degeneration and the syndrome of osteoarthritis. Instr Course Lect 2000;49:481–489. [PubMed] [Google Scholar]
- 4. Langer F, Gross AE. Immunogenicity of allograft articular cartilage. J Bone Joint Surg Am 1974;56:297–304. [PubMed] [Google Scholar]
- 5. Bugbee WD. Fresh osteochondral allografts. J Knee Surg 2002;15:191–195. [PubMed] [Google Scholar]
- 6. Farr J, Cole BJ, Sherman S et al. Particulated articular cartilage: CAIS and DeNovo NT. J Knee Surg 2012;25:23–29. [DOI] [PubMed] [Google Scholar]
- 7. McCulloch PC, Kang RW, Sobhy MH et al. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: Minimum 2‐year follow‐up. Am J Sports Med 2007;35:411–420. [DOI] [PubMed] [Google Scholar]
- 8. Ng KW, Lima EG, Bian L et al. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: A canine model. Tissue Eng Part A 2010;16:1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adkisson HD, Martin JA, Amendola RL et al. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med 2010;38:1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilbert JE. Current treatment options for the restoration of articular cartilage. Am J Knee Surg. 1998;11:42–46. [PubMed] [Google Scholar]
- 11. Lee CR, Grodzinsky AJ, Hsu HP et al. Effects of harvest and selected cartilage repair procedures on the physical and biochemical properties of articular cartilage in the canine knee. J Orthop Res 2000;18:790–799. [DOI] [PubMed] [Google Scholar]
- 12. Mow VC, Ratcliffe A. Structure and function of articular cartilage and meniscus. In: Mow VC, Hayes WC, eds. Basic Orthopaedic Biomechanics. Philadelphia: Lippincott‐Raven, 1997:113–177.
- 13. Tran‐Khanh N, Hoemann CD, McKee MD et al. Aged bovine chondrocytes display a diminished capacity to produce a collagen‐rich, mechanically functional cartilage extracellular matrix. J Orthop Res 2005;23:1354–1362. [DOI] [PubMed] [Google Scholar]
- 14. Wang J, Verdonk P, Elewaut D et al. Homeostasis of the extracellular matrix of normal and osteoarthritic human articular cartilage chondrocytes in vitro. Osteoarthr Cartil 2003;11:801–809. [DOI] [PubMed] [Google Scholar]
- 15. Bulstra SK, Buurman WA, Walenkamp GH et al. Metabolic characteristics of in vitro cultured human chondrocytes in relation to the histopathologic grade of osteoarthritis. Clin Orthop Relat Res 1989;242:294–302. [PubMed] [Google Scholar]
- 16. Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 17. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 18. Chamberlain G, Fox J, Ashton B et al. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007;25:2739–2749. [DOI] [PubMed] [Google Scholar]
- 19. Alexander PG, Hofer HR, Clark KL et al. Mesenchymal Stem Cells in Musculoskeletal Tissue Engineering. Principles of Tissue Engineering. Fourth Edition. Elsevier, London, 2014:1171–1199. [Google Scholar]
- 20. Sakaguchi Y, Sekiya I, Yagishita K et al. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum 2005;52:2521–2529. [DOI] [PubMed] [Google Scholar]
- 21. Yoshimura H, Muneta T, Nimura A et al. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 2007;327:449–462. [DOI] [PubMed] [Google Scholar]
- 22. Pei M, He F, Kish VL et al. Engineering of functional cartilage tissue using stem cells from synovial lining: A preliminary study. Clin Orthop Relat Res 2008;466:1880–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koga H, Muneta T, Nagase T et al. Comparison of mesenchymal tissues‐derived stem cells for in vivo chondrogenesis: Suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res 2008;333:207–215. [DOI] [PubMed] [Google Scholar]
- 24. Sampat SR, O'Connell GD, Fong JV et al. Growth factor priming of synovium‐derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2011;17:2259–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fan J, Varshney RR, Ren L et al. Synovium‐derived mesenchymal stem cells: A new cell source for musculoskeletal regeneration. Tissue Eng Part B 2009;15:75–86. [DOI] [PubMed] [Google Scholar]
- 26. Kim JH, Lee MC, Seong SC et al. Enhanced proliferation and chondrogenic differentiation of human synovium‐derived stem cells expanded with basic fibroblast growth factor. Tissue Eng Part A 2011;17:991–1002. [DOI] [PubMed] [Google Scholar]
- 27. Mendelson A, Frank E, Allred C, Jones E, Chen M, Zhao W, et al. Chondrogenesis by chemotactic homing of synovium, bone marrow, and adipose stem cells in vitro. FASEB J 2011;25:3496–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dennis JE, Cohen N, Goldberg VM et al. Targeted delivery of progenitor cells for cartilage repair. J Orthop Res 2004;22:735–741. [DOI] [PubMed] [Google Scholar]
- 29. Vangsness CT, Farr J, Boyd J et al. Adult human mesenchymal stem cells delivered via intra‐articular injection to the knee following partial medial meniscectomy: A randomized, double‐blind, controlled study. J Bone Joint Surg Am 2014;96:90–98. [DOI] [PubMed] [Google Scholar]
- 30. Centeno CJ, Busse D, Kisiday J et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Phys 2008;11:343–353. [PubMed] [Google Scholar]
- 31. Davatchi F, Abdollahi BS, Mohyeddin M et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis 2011;14:211–215. [DOI] [PubMed] [Google Scholar]
- 32. Mitrovic D. Development of the diarthrodial joints in the rat embryo. Am J Anat 1978;151:475–485. [DOI] [PubMed] [Google Scholar]
- 33. Caldwell KL, Wang J. Cell‐based articular cartilage repair: The link between development and regeneration. Osteoarthr Cartil 2015;23:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Archer CW, Morrison H, Pitsillides AA. Cellular aspects of the development of diarthrodial joints and articular cartilage. J Anat 1994;184(Pt 3):447–456. [PMC free article] [PubMed] [Google Scholar]
- 35. Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthr Cartil 2007;15:403–413. [DOI] [PubMed] [Google Scholar]
- 36. Bernhard JC, Vunjak‐Novakovic G. Should we use cells, biomaterials, or tissue engineering for cartilage regeneration? Stem Cell Res Ther 2016;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nazempour A, Van Wie BJ. Chondrocytes, mesenchymal stem cells, and their combination in articular cartilage regenerative medicine. Ann Biomed Eng 2016;44:1325–1354. [DOI] [PubMed] [Google Scholar]
- 38. Vinatier C, Guicheux J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann Phys Rehabil Med 2016;59:139–144. [DOI] [PubMed] [Google Scholar]
- 39. Lima EG, Bian L, Ng KW et al. The beneficial effect of delayed compressive loading on tissue‐engineered cartilage constructs cultured with TGF‐beta3. Osteoarthr Cartil 2007;15:1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lima EG, Tan AR, Tai T et al. Differences in interleukin‐1 response between engineered and native cartilage. Tissue Eng Part A 2008;14:1721–1730. [DOI] [PubMed] [Google Scholar]
- 41. Hung CT, Mauck RL, Wang CCB et al. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng 2004;32:35–49. [DOI] [PubMed] [Google Scholar]
- 42. Mauck RL, Soltz MA, Wang CC et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte‐seeded agarose gels. J Biomech Eng. 2000;122:252–260. [DOI] [PubMed] [Google Scholar]
- 43. Reindel ES, Ayroso AM, Chen AC et al. Integrative repair of articular cartilage in vitro: Adhesive strength of the interface region. J Orthop Res 1995;13:751–760. [DOI] [PubMed] [Google Scholar]
- 44. Guilak F, Hung CT. Physical regulation of cartilage metabolism. In: Mow VC, Hayes WC, eds. Basic Orthopaedic Biomechanics. 3rd ed. Philadelphia: Lippincott‐Raven 2005:179–207.
- 45. Chen X, Macica CM, Nasiri A et al. Regulation of articular chondrocyte proliferation and differentiation by Indian hedgehog and parathyroid hormone‐related protein in mice. Arthritis Rheum 2008;58:3788–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vortkamp A, Lee K, Lanske B et al. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH‐related protein. Science 1996;273:613–622. [DOI] [PubMed] [Google Scholar]
- 47. Mauck RL, Seyhan SL, Ateshian GA et al. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte‐seeded agarose hydrogels. Ann Biomed Eng 2002;30:1046–1056. [DOI] [PubMed] [Google Scholar]
- 48. Bian L, Fong JV, Lima EG et al. Dynamic mechanical loading enhances functional properties of tissue‐engineered cartilage using mature canine chondrocytes. Tissue Eng Part A 2010;16:1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buckwalter JA, Mankin HJ. Articular cartilage: Tissue design and chondrocyte‐matrix interactions. Instr Course Lect 1998;47:477–486. [PubMed] [Google Scholar]
- 50. Mauck RL, Hung CT, Ateshian GA. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: Implications for articular cartilage biosynthesis and tissue engineering. J Biomech Eng 2003;125:602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Albro MB, Chahine NO, Li R et al. Dynamic loading of deformable porous media can induce active solute transport. J Biomech 2008;41:3152–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kelly TN, Ng KW, Wang CC‐B et al. Spatial and temporal development of chondroctye‐seeded agarose constructs in free‐swelling and dynamically loaded cultures. J Biomech 2006;39:1489–1497. [DOI] [PubMed] [Google Scholar]
- 53. Elder SH, Goldstein SA, Kimura JH et al. Chondrocyte differentiation is modulated by frequency and druation of cyclic compressive loading. Ann Biomed Eng 2001;29:476–482. [DOI] [PubMed] [Google Scholar]
- 54. Huang C‐YC, Hagar KL, Frost LE et al. Effects of cyclic compressive loading on chondrogenesis of rabbit bone‐marrow derived mesenchymal stem cells. Stem Cells 2004;22:313–323. [DOI] [PubMed] [Google Scholar]
- 55. Kisiday JD, Frisbie DD, McIlwraith CW et al. Dynamic compression stimulates proteoglycan synthesis by mesenchymal stem cells in the absence of chondrogenic cytokines. Tissue Eng Part A 2009;15:2817–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang C‐YC, Reuben PM, Cheung HS. Temporal expression patterns and corresponding protein inductions of early responsive genes in rabbit bone marrow‐derived mesenchymal stem cells under cyclic compressive loading. Stem Cells 2005;23:1113–1121. [DOI] [PubMed] [Google Scholar]
- 57. Huang AH, Farrell MJ, Kim M et al. Long‐term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell‐laden hydrogel. Eur Cells Mater 2010;19:72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mouw JK, Connelly JT, Wilson CG et al. Dynamic compression regulates the expression and synthesis of chondrocyte‐specific matrix molecules in bone marrow stromal cells. Stem Cells 2007;25:655–663. [DOI] [PubMed] [Google Scholar]
- 59. Thorpe SD, Buckley CT, Vinardell T et al. The response of bone marrow‐derived mesenchymal stem cells to dynamic compression following TGF‐beta3 induced chondrogenic differentiation. Ann Biomed Eng 2010;38:2896–2909. [DOI] [PubMed] [Google Scholar]
- 60. Luo L, Thorpe SD, Buckley CT et al. The effects of dynamic compression on the development of cartilage grafts engineered using bone marrow and infrapatellar fat pad derived stem cells. Biomed Mater 2015;10:055011. [DOI] [PubMed] [Google Scholar]
- 61. Miyanishi K, Trindade MC, Lindsey DP et al. Effects of hydrostatic pressure and transforming growth factor‐beta 3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue Eng. 2006;12:1419–1428. [DOI] [PubMed] [Google Scholar]
- 62. Angele P, Yoo JU, Smith C et al. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res 2003;21:451–457. [DOI] [PubMed] [Google Scholar]
- 63. Sampat SR, Dermksian MV, Oungoulian SR et al. Applied osmotic loading for promoting development of engineered cartilage. J Biomech 2013;46:2674–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Caron MMJ, van der Windt AE, Emans PJ et al. Osmolarity determines the in vitro chondrogenic differentiation capacity of progenitor cells via nuclear factor of activated T‐cells 5. Bone 2013;53:94–102. [DOI] [PubMed] [Google Scholar]
- 65. Ogawa R, Orgill DP, Murphy GF et al. Hydrostatic pressure‐driven three‐dimensional cartilage induction using human adipose‐derived stem cells and collagen gels. Tissue Eng Part A 2015;21:257–266. [DOI] [PubMed] [Google Scholar]
- 66. Koo J, Kim K‐I, Min B‐H et al. Controlling medium osmolality improves the expansion of human articular chondrocytes in serum‐free media. Tissue Eng Part C Methods 2010;16:957–963. [DOI] [PubMed] [Google Scholar]
- 67. Urban JP, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol 1993;154:262–270. [DOI] [PubMed] [Google Scholar]
- 68. Oswald ES, Brown LM, Bulinski JC et al. Label‐free protein profiling of adipose‐derived human stem cells under hyperosmotic treatment. J Proteome Res 2011;10:3050–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Alegre‐Aguarón E, Sampat SR, Xiong JC et al. Growth factor priming differentially modulates components of the extracellular matrix proteome in chondrocytes and synovium‐derived stem cells. PLoS One 2014;9:e88053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hosseini M‐S, Tafazzoli‐Shadpour M, Haghighipour N et al. The synergistic effects of shear stress and cyclic hydrostatic pressure modulate chondrogenic induction of human mesenchymal stem cells. Int J Artif Organs 2015;38:557–564. [DOI] [PubMed] [Google Scholar]
- 71. Li Z, Yao S‐J, Alini M et al. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin‐polyurethane composites is modulated by frequency and amplitude of dynamic compression and shear stress. Tissue Eng Part A 2010;16:575–584. [DOI] [PubMed] [Google Scholar]
- 72. Schätti O, Grad S, Goldhahn J et al. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur Cells Mater 2011;22:214–225. [DOI] [PubMed] [Google Scholar]
- 73. Byers BA, Mauck RL, Chiang I et al. Temporal exposure of TGF‐B3 under serum‐free conditions enhances biomechanical and biochemical maturation of tissue‐engineered cartilage. Trans Orthop Res Soc 2006;31:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mauck RL, Nicoll SB, Seyhan SL et al. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng 2003;9:597–611. [DOI] [PubMed] [Google Scholar]
- 75. Mauck RL, Seyhan SL, Nicoll SB et al. Transforming growth factor B1 increases the mechanical properties and matrix development of chondrocyte‐seeded agarose hydrogels. Adv Bioeng 2001;50:691–692. [Google Scholar]
- 76. Thorp BH, Anderson I, Jakowlew SB. Transforming growth factor‐beta1, ‐beta2 and ‐beta3 in cartilage and bone cells during endochondral ossification in the chick. Development 1992;114:907–911. [DOI] [PubMed] [Google Scholar]
- 77. Awad H, Halvorsen Y‐DC, Gimble JM et al. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose‐derived stromal cells. Tiss Eng 2003;9:1301–1312. [DOI] [PubMed] [Google Scholar]
- 78. Bian L, Stoker AM, Marberry KM et al. Effects of dexamethasone on the functional properties of cartilage explants during long‐term culture. Am J Sports Med 2010;38:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Aydelotte MB, Raiss RX, Caterson B et al. Influence of interleukin‐1 on the morphology and proteoglycan metabolism of cultured bovine articular chondrocytes. Connect Tissue Res. 1992;28:143–159. [DOI] [PubMed] [Google Scholar]
- 80. Ratcliffe A, Tyler JA, Hardingham TE. Articular cartilage cultured with interleukin 1. Increased release of link protein, hyaluronate‐binding region and other proteoglycan fragments. Biochem J 1986;238:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mauck RL, Byers BA, Yuan X et al. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol 2007;6:113–125. [DOI] [PubMed] [Google Scholar]
- 82. Tsuchiya H, Kitoh H, Sugiura F et al. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow‐derived mesenchymal stem cells. Biochem Biophys Res Commun 2003;301:338–343. [DOI] [PubMed] [Google Scholar]
- 83. Bian L, Zhai DY, Mauck RL et al. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A 2011;17:1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lettry V, Hosoya K, Takagi S et al. Coculture of equine mesenchymal stem cells and mature equine articular chondrocytes results in improved chondrogenic differentiation of the stem cells. Jpn J Vet Res 2010;58:5–15. [PubMed] [Google Scholar]
- 85. Kubosch EJ, Heidt E, Bernstein A et al. The trans‐well coculture of human synovial mesenchymal stem cells with chondrocytes leads to self‐organization, chondrogenic differentiation, and secretion of TGFβ. Stem Cell Res Ther 2016;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jikko A, Kato Y, Hiranuma H et al. Inhibition of chondrocyte terminal differentiation and matrix calcification by soluble factors released by articular chondrocytes. Calcif Tissue Int 1999;65:276–279. [DOI] [PubMed] [Google Scholar]
- 87. Fischer J, Dickhut A, Rickert M et al. Human articular chondrocytes secrete parathyroid hormone‐related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum 2010;62:2696–2706. [DOI] [PubMed] [Google Scholar]
- 88. Hwang NS, Varghese S, Puleo C et al. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol 2007;212:281–284. [DOI] [PubMed] [Google Scholar]
- 89. Alves da Silva ML, Costa‐Pinto AR, Martins A et al. Conditioned medium as a strategy for human stem cells chondrogenic differentiation. J Tissue Eng Regen Med 2015;9:714–723. [DOI] [PubMed] [Google Scholar]
- 90. Zhong J, Guo B, Xie J et al. Crosstalk between adipose‐derived stem cells and chondrocytes: When growth factors matter. Bone Res 2016;4:15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wu L, Leijten JCH, Georgi N et al. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A 2011;17:1425–1436. [DOI] [PubMed] [Google Scholar]
- 92. Wu L, Prins H‐J, Helder MN et al. Trophic effects of mesenchymal stem cells in chondrocyte co‐cultures are independent of culture conditions and cell sources. Tissue Eng Part A 2012;18:1542–1551. [DOI] [PubMed] [Google Scholar]
- 93. Kim IG, Ko J, Lee HR et al. Mesenchymal cells condensation‐inducible mesh scaffolds for cartilage tissue engineering. Biomaterials 2016;85:18–29. [DOI] [PubMed] [Google Scholar]
- 94. Freed LE, Vunjak‐Novakovic G, Biron RJ et al. Biodegradable polymer scaffolds for tissue engineering. Biotechnology 1994;12:689–693. [DOI] [PubMed] [Google Scholar]
- 95. Bryant SJ, Bender RJ, Durand KL et al. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: Engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol Bioeng 2004;86:747–755. [DOI] [PubMed] [Google Scholar]
- 96. Deponti D, Di Giancamillo A, Gervaso F et al. Collagen scaffold for cartilage tissue engineering: The benefit of fibrin glue and the proper culture time in an infant cartilage model. Tissue Eng Part A 2014;20:1113–1126. [DOI] [PubMed] [Google Scholar]
- 97. Zhu M, Feng Q, Sun Y et al. Effect of cartilaginous matrix components on the chondrogenesis and hypertrophy of mesenchymal stem cells in hyaluronic acid hydrogels. J Biomed Mater Res Part B Appl Biomater 2016. (In press). [DOI] [PubMed]
- 98. Varghese S, Hwang NS, Canver AC et al. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol 2008;27:12–21. [DOI] [PubMed] [Google Scholar]
- 99. Mouw JK, Case ND, Guldberg RE et al. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthr Cartil 2005;13:828–836. [DOI] [PubMed] [Google Scholar]
- 100. Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982;30:215–24. [DOI] [PubMed] [Google Scholar]
- 101. Buschmann MD, Gluzband YA, Grodzinsky AJ et al. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res 1992;10:745–758. [DOI] [PubMed] [Google Scholar]
- 102. Lee DA, Bader DL. The development and characterization of an in vitro system to study strain‐induced cell deformation in isolated chondrocytes. In Vitro Cell Dev Biol Anim 1995;31:828–835. [DOI] [PubMed] [Google Scholar]
- 103. Selmi TA, Verdonk PCM, Chambat P et al. Autologous chondrocyte implantation in a novel alginate‐agarose hydrogel: Outcome at two years. J Bone Joint Surg 2008;90:597–604. [DOI] [PubMed] [Google Scholar]
- 104. Selmi TA, Neyret P, Verdonk PCM et al. Autologous chondrocyte transplantation in combination with an alginate‐agarose based hydrogel (Cartipatch). Tech Knee Surg 2007;6:253–258. [Google Scholar]
- 105. Clavé A, Potel J‐F, Servien E et al. Third‐generation autologous chondrocyte implantation versus mosaicplasty for knee cartilage injury: 2‐year randomized trial. J Orthop Res 2016;34:658–665. [DOI] [PubMed] [Google Scholar]
- 106. Tan AR, VandenBerg CD, Attur M et al. Cytokine preconditioning of engineered cartilage provides protection against interleukin‐1 insult. Arthritis Res Ther 2015;17:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bian L, Angione SL, Ng KW et al. Influence of decreasing nutrient path length on the development of engineered cartilage. Osteoarthr Cartil 2009;17:677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chou C‐H, Cheng WTK, Lin C‐C et al. TGF‐beta1 immobilized tri‐co‐polymer for articular cartilage tissue engineering. J Biomed Mater Res Part B Appl Biomater 2006;77:338–348. [DOI] [PubMed] [Google Scholar]
- 109. Capito RM, Spector M. Collagen scaffolds for nonviral IGF‐1 gene delivery in articular cartilage tissue engineering. Gene Ther 2007;14:721–732. [DOI] [PubMed] [Google Scholar]
- 110. Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol 2005;24:208–218. [DOI] [PubMed] [Google Scholar]
- 111. Salinas CN, Anseth KS. The influence of the RGD peptide motif and its contextual presentation in PEG gels on human mesenchymal stem cell viability. J Tissue Eng Regen Med 2008;2:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Villanueva I, Weigel CA, Bryant SJ. Cell‐matrix interactions and dynamic mechanical loading influence chondrocyte gene expression and bioactivity in PEG‐RGD hydrogels. Acta Biomater 2009;5:2832–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kawamura S, Wakitani S, Kimura T et al. Articular cartilage repair. Rabbit experiments with a collagen gel‐biomatrix and chondrocytes cultured in it. Acta Orthop Scand 1998;69:56–62. [DOI] [PubMed] [Google Scholar]
- 114. Bryant SJ, Arthur JA, Anseth KS. Incorporation of tissue‐specific molecules alters chondrocyte metabolism and gene expression in photocrosslinked hydrogels. Acta Biomater 2005;1:243–252. [DOI] [PubMed] [Google Scholar]
- 115. Nguyen LH, Kudva AK, Guckert NL et al. Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage. Biomaterials 2011;32:1327–1338. [DOI] [PubMed] [Google Scholar]
- 116. Chen F, Yu S, Liu B et al. An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci Rep 2016;6:20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ren K, He C, Xiao C et al. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials 2015;51:238–249. [DOI] [PubMed] [Google Scholar]
- 118. Lin H, Cheng AW‐M, Alexander PG et al. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible‐light‐activated gelation capability in both air and aqueous solution. Tissue Eng Part A 2014;20:2402–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Moreira CDF, Carvalho SM, Mansur HS et al. Thermogelling chitosan‐collagen‐bioactive glass nanoparticle hybrids as potential injectable systems for tissue engineering. Mater Sci Eng C Mater Biol Appl 2016;58:1207–1216. [DOI] [PubMed] [Google Scholar]
- 120. Moreira Teixeira LS, Bijl S, Pully VV et al. Self‐attaching and cell‐attracting in‐situ forming dextran‐tyramine conjugates hydrogels for arthroscopic cartilage repair. Biomaterials 2012;33:3164–3174. [DOI] [PubMed] [Google Scholar]
- 121. Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci 2000;113(Pt 7):1161–1166. [DOI] [PubMed] [Google Scholar]
- 122. Guilak F, Lott KE, Awad HA et al. Clonal analysis of the differentiation potential of human adipose‐derived adult stem cells. J Cell Physiol 2006;206:229–237. [DOI] [PubMed] [Google Scholar]
- 123. Mareddy S, Crawford R, Brooke G et al. Clonal isolation and characterization of bone marrow stromal cells from patients with osteoarthritis. Tissue Eng 2007;13:819–829. [DOI] [PubMed] [Google Scholar]
- 124. Pelttari K, Winter A, Steck E et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 2006;54:3254–3266. [DOI] [PubMed] [Google Scholar]
- 125. Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J 2004;18:980–982. [DOI] [PubMed] [Google Scholar]
- 126. Djouad F, Plence P, Bony C et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003;102:3837–3844. [DOI] [PubMed] [Google Scholar]
- 127. Ramasamy R, Lam EW‐F, Soeiro I et al. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: Impact on in vivo tumor growth. Leukemia 2007;21:304–310. [DOI] [PubMed] [Google Scholar]
- 128. Tasso R, Augello A, Carida M et al. Development of sarcomas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis 2009;30:150–157. [DOI] [PubMed] [Google Scholar]
- 129. Bhumiratana S, Eton RE, Oungoulian SR et al. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci USA 2014;111:6940–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Madry H, Cucchiarini M. Clinical potential and challenges of using genetically modified cells for articular cartilage repair. Croat Med J 2011;52:245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Baragi VM, Renkiewicz RR, Jordan H et al. Transplantation of transduced chondrocytes protects articular cartilage from interleukin 1‐induced extracellular matrix degradation. J Clin Invest 1995;96:2454–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pelletier JP, Caron JP, Evans C et al. In vivo suppression of early experimental osteoarthritis by interleukin‐1 receptor antagonist using gene therapy. Arthritis Rheum 1997;40:1012–1019. [DOI] [PubMed] [Google Scholar]
- 133. Huang AH, Farrell MJ, Mauck RL. Mechanics and mechanobiology of mesenchymal stem cell‐based engineered cartilage. J Biomech 2010;43:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Shafiee A, Kabiri M, Langroudi L et al. Evaluation and comparison of the in vitro characteristics and chondrogenic capacity of four adult stem/progenitor cells for cartilage cell‐based repair. J Biomed Mater Res Part A 2015;104:600–610. [DOI] [PubMed] [Google Scholar]


