Abstract
The ability to identify and stratify patients that will respond to specific therapies has been transformational in a number of disease areas, particularly oncology. It is anticipated that this will also be the case for cell‐based therapies, particularly in complex and heterogeneous diseases such as rheumatoid arthritis (RA). Recently, clinical results with expanded allogenic adipose‐derived mesenchymal stem cells (eASCs) have indicated clinical efficacy in highly refractory RA patients. In this study, we set out to determine if circulating microRNAs (miRNAs) could be identified as potential biomarkers associated with response to eASCs in these RA patients. The miRNA expression profiles of pre‐treatment plasma samples from responder and nonresponder patients were determined using microarrays. Ten miRNAs were identified that were differentially expressed in the responder group as compared to the nonresponder group. To confirm the differential expression of these 10 miRNA biomarkers, they were further assayed by quantitative reverse‐transcriptase polymerase chain reaction (QRT‐PCR). From this analysis, three miRNAs, miR‐26b‐5p, miR‐487b‐3p and miR‐495‐3p, were confirmed as being statistically significantly upregulated in the responder group as compared with the nonresponder group. Receiver operating characteristic analysis confirmed their diagnostic potential. These miRNAs could represent novel candidate stratification biomarkers associated with RA patient response to eASCs and are worthy of further clinical validation. Stem Cells Translational Medicine 2017;6:1202–1206
Keywords: MicroRNA, Plasma stratification biomarkers, Cell‐based therapy, Adipose mesenchymal stem cells, Rheumatoid arthritis
Significance Statement.
Identifying and stratifying patients that will respond to cell‐based therapies will be crucial to the success of these innovative therapeutic modalities. To our knowledge, this is the first report identifying circulating potential microRNA biomarkers associated with patient response to a cell‐based therapy.
Introduction
Rheumatoid arthritis (RA) is a common chronic, inflammatory, autoimmune disease which leads to progressive disability and morbidity with resulting significant socioeconomic costs 1. The cause of RA is unknown and it is a particularly complex and heterogeneous disease. Due to this, although advances in understanding the pathogenesis have resulted in the development of new therapeutic approaches, current conventional and biologic disease‐modifying therapies still fail and/or produce only partial responses in many patients 1, 2. Patient stratification in RA is limited or nonexistent, particularly for biologics 2. Therefore, identifying pretreatments predictors of response is likely to be highly valuable in RA 2. Certainly, the ability to identify and stratify patients that will respond to specific therapies has been transformational in a number of other disease areas, particularly oncology 3.
Given the clear unmet medical need in RA, cell‐based therapies such as adult mesenchymal stem cells offer much potential utility and early indications suggest that they could be promising new treatments in RA 4, 5. Indeed, recent clinical results from intravenous administration of expanded allogenic adipose‐derived mesenchymal stem cells (eASCs) indicated a trend for clinical efficacy in highly refractory RA patients 6. As with current conventional and biologic disease‐modifying therapies for RA, identifying and stratifying patients that will respond to cell‐based therapies will be crucial to the success of these innovative therapeutic modalities in RA.
MicroRNAs (miRNAs) are single‐stranded noncoding RNA molecules around 21 to 23 nucleotides in length. They regulate gene expression in cells by hybridization to mRNA transcripts, resulting in translational blockade or transcript degradation 7 and have important roles in multiple biological processes and pathological processes 7, 8. MiRNAs also have many attributes that make them ideal as biomarkers. MiRNAs can be readily sampled and assayed in a variety of biofluids 9 and unlike messenger mRNA and proteins, they are highly resistant to enzymatic degradation and are very stable in biofluids, even after long‐term storage and multiple freeze‐thaw cycles 10, 11. There is an increasing focus on assaying circulating miRNAs in biofluids as biomarkers for a wide variety of applications. Recently, several circulating serum miRNAs have been identified a potential biomarker predicting response to conventional and biologic disease‐modifying therapies 12, 13. To our knowledge, however, there have been no reports identifying stratification miRNA biomarkers predicting patient response to a cell‐based therapy.
The aim of this study, using clinical trial samples and miRNA microarray and quantitative reverse‐transcriptase polymerase chain reaction (QRT‐PCR) analyses, was to identify candidate miRNA stratification biomarkers associated with eASC therapy response in RA.
Materials and Methods
eASC treatment responder and nonresponder patients from a clinical trial of intravenous administration of eASCs in refractory RA patients were selected (Supporting Information data) for circulating miRNA biomarker analysis 6. RNA was extracted from 0.5 ml plasma taken 2 weeks prior to treatment (Exiqon, Denmark, http://www.exiqon.com) and analyzed on miRNA microarrays (Agilent Technologies, U.K., http://www.agilent.com). Data preprocessing and normalization was carried with the “AgiMicroRNA” 14 package in Bioconductor 15. miRNAs differentially expressed between the responder and nonresponder groups were identified by fold change analysis and the top 10 most highly differentially expressed were selected. Candidate miRNAs for normalizing QRT‐PCR data were identified using the Two One‐Sided Tests approach (pFDR < 0.1, fold‐change < 2.0) 16. Expression analysis of the differentially expressed miRNAs was carried out by QRT‐PCR (Exiqon) using a Roche LightCycler 480 (Roche Diagnostics Ltd, UK, http://www.roche.co.uk). Identification of miRNAs for data normalization and QRT‐PCR comparative Cq data analysis was carried out using Biogazelle qbase+ v 2.5 (Biogazelle, Belgium, https://www.biogazelle.com). Mean fold changes were determined between normalized relative expression values for responder and nonresponder groups and tested for statistical significance using Student's t test (p <.05). Receiver operating characteristic (ROC) analysis was performed using the “ROCR” package 17 in R.
Results and Discussion
Microarray Analysis
To identify candidate miRNA stratification biomarkers for response to eASC treatment in highly refractory RA patients, RNA from pretreatment plasma samples from responder and nonresponder patients (Supporting Information data) were analyzed on miRNA microarrays. Using fold change analysis, we selected the 10 most highly differentially expressed between responders and nonresponders as potential miRNA biomarkers for response to eASC treatment (Figs. 1 and 2). It should be noted that red blood cells contain high levels of miRNAs, and identification of circulating miRNA biomarkers in plasma and serum can be severely confounded by differing levels of hemolysis occurring in these samples during collection and processing 18. However, the potential miRNA biomarkers identified here were not amongst the miRNAs specifically reported to be elevated by hemolysis 19.
Figure 1.
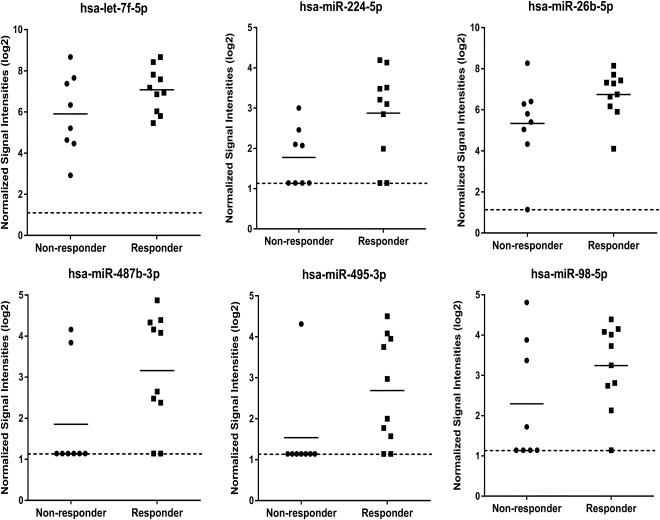
MicroRNA (miRNA) microarray expression data showing circulating miRNAs identified as being upregulated in plasma samples in the responder group as compared to the nonresponder group. The expression data is given as normalized signal intensities on a log 2 scale. Data is shown for the responder samples (n = 10) and nonresponder samples (n = 8) which generated microarray data from the 10 responder and 10 nonresponder samples analyzed (see Supporting Information data for details). Short solid lines on graphs indicates mean expression in each group. Dotted line on graphs indicates the lower limit of detection on the miRNA microarrays.
Figure 2.
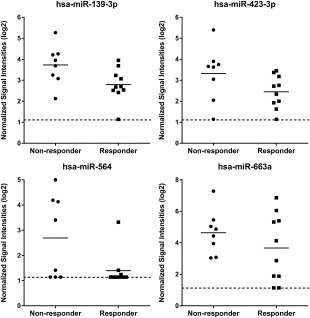
MicroRNA (miRNA) microarray expression data showing circulating miRNAs identified as being downregulated in plasma samples in the responder group as compared to the nonresponder group. The expression data is given as normalized signal intensities on a log 2 scale. Data is shown for the responder samples (n = 10) and nonresponder samples (n = 8) which generated microarray data from the 10 responder and 10 nonresponder samples analyzed (see Supporting Information data for details). Short solid lines on graphs indicates mean expression in each group. Dotted line on graphs indicates the lower limit of detection on the miRNA microarrays.
QRT‐PCR Analysis
The expression of these candidate miRNA biomarkers was then further investigated by QRT‐PCR. For normalization, we identified a set of 10 candidate normalizer miRNAs from the miRNA microarray data. There are no established universal normalizer miRNAs for plasma and case‐specific normalizers need to be identified and tested 20. This was therefore an important part of the analysis to allow normalization of QRT‐PCR data and accurate assessment of miRNA relative expression between the two responder groups. miR‐93‐5p and miR‐324‐3p were selected as normalizers and used to normalize the QRT‐PCR data. Of the selected miRNA biomarkers, miR‐26b‐5p and miR‐495‐3p were confirmed as being significantly upregulated (p = .01, .02; fold change = 1.3, 2.4, respectively), in the responder group, and miR‐487b‐3p was very close to being significantly upregulated (p = .05; fold change = 2.2; Fig. 3). The diagnostic potential of these miRNAs was further investigated by generating ROC curves 21 based on the QRT‐PCR data (Supporting Information data) and calculating the area under the curve (AUC). Though this analysis is likely to generate optimistic results in the absence of an independent sample set, the high AUC values of 0.9 (miR‐26b‐5p) and 0.8 (miR‐487b‐3p and miR‐495‐3p) support the ability of these miRNAs to allow discrimination between responders and nonresponders.
Figure 3.
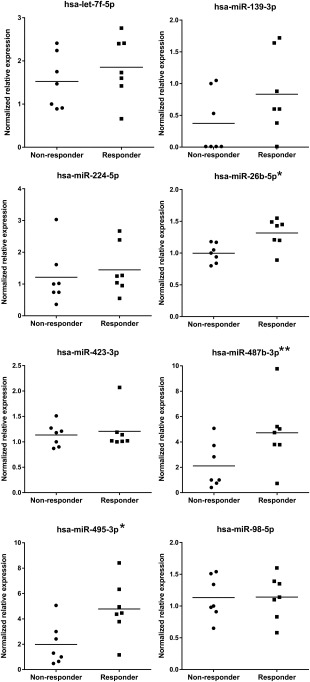
Quantitative reverse‐transcriptase PCR (QRT‐PCR) expression data showing expression levels of candidate circulating miRNA stratification biomarkers in plasma samples in the responder group and the nonresponder group. The expression data is given as relative normalized expression on a linear scale. Data is shown for the responder samples (n = 7) and nonresponder samples (n = 7) which generated QRT‐PCR data from the 11 responder and 11 nonresponder samples analyzed (see Supporting Information data for details). Short solid lines on graphs indicates mean expression in each group. *, miR‐26b‐5p, p = .01; miR‐495‐3p, p = .02. **, miR‐487b‐3p, p = .05.
All three of these miRNAs have also reported to be present in plasma/serum 9, 20, 22, although there appears to be no reports identifying these miRNAs being present in plasma/serum from RA patients. miR‐487b‐3p has been reported to be upregulated in RA synovial monocytes compared with normal peripheral blood monocytes 23. It is currently unclear what role(s) they may play in RA and the underlying mechanisms by which they are associated with response to cell‐based therapy. Nevertheless, these three miRNAs could represent potential candidate biomarkers associated with RA patient response to eASC‐based therapy.
Conclusion
In a highly refractory RA patient population treated with eASCs we identified three circulating miRNAs that were upregulated in the responder group compared with the nonresponder group and represent candidate biomarkers for success of eASC therapy treatment. The sample size in this study was relatively small and these candidates require validation in multiple, larger independent sample sets. However, this is an important first step, and to our knowledge, the first report identifying potential circulating miRNA biomarkers associated with patient response to a cell‐based therapy.
Author Contributions
D.J.M.: concept and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; D.R.D.: assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S.R.: collection and assembly of data, manuscript writing, final approval of manuscript; E.R.S.: data analysis and interpretation, manuscript writing, final approval of manuscript; O.D.L.R and E.L.: concept and design, provision of study material or patients, manuscript writing, final approval of manuscript; W.D.: concept and design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
D.J.M., D.R.D., S.R., and E.R.S. are or were compensated employees of Sistemic Ltd. O.D.L.R, W.D., and E.L. are compensated employees of TiGenix.
Supporting information
Supporting Information Figure 1.
Supporting Information Table 1.
Supporting Information.
Acknowledgments
This work was supported by the European Union's Seventh Programme for research, technological development and demonstration under grant agreement number 279174 to TiGenix and Sistemic.
References
- 1. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–2219. [DOI] [PubMed] [Google Scholar]
- 2. Astorri E, Nerviani A, Bombardieri M et al. Towards a stratified targeted approach with biologic treatments in rheumatoid arthritis: Role of synovial pathobiology. Curr Pharm Des 2015;21:2216–2224. [DOI] [PubMed] [Google Scholar]
- 3.The Academy of Medical Sciences. Realising the potential of stratified medicine. Available at http://www.acmedsci.ac.uk/policy/policy-projects/Stratified-medicine/. Accessed April 15, 2016.
- 4. El‐Jawhari JJ, El‐Sherbiny YM, Jones EA et al. Mesenchymal stem cells, autoimmunity and rheumatoid arthritis. Q J Med 2014;107:505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Bari C. Are mesenchymal stem cells in rheumatoid arthritis the good or bad guys? Arthritis Res Ther 2015;17:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Álvaro‐Gracia JM, Jover JA, García‐Vicuña R et al. Intravenous administration of expanded allogeneic adipose‐derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): Results of a multicentre, dose escalation, randomised, single‐blind, placebo‐controlled phase Ib/IIa clinical trial. Ann Rheumatic Dis 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7. Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol 2013;14:475–488. [DOI] [PubMed] [Google Scholar]
- 8. Huang Y, Shen XJ, Zou Q et al. Biological functions of microRNAs: A review. J Physiol Biochem 2011;67:129–139. [DOI] [PubMed] [Google Scholar]
- 9. Blondal T, Jensby Nielsen S, Baker A et al. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods 2013;59:S1–6. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell PS, Parkin RK, Kroh EM et al. Circulating microRNAs as stable blood‐based markers for cancer detection. Proc Natl Acad Sci USA 2008;105:10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jung M, Schaefer A, Steiner I et al. Robust microRNA stability in degraded RNA preparations from human tissue and cell samples. Clin Chem 2010;56:998–1006. [DOI] [PubMed] [Google Scholar]
- 12. Duroux‐Richard I, Pers YM, Fabre S et al. Circulating miRNA‐125b is a potential biomarker predicting response to rituximab in rheumatoid arthritis. Mediators Inflamm 2014;2014:342524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krintel SB, Dehlendorff C, Hetland ML et al. Prediction of treatment response to adalimumab: A double‐blind placebo‐controlled study of circulating microRNA in patients with early rheumatoid arthritis. Pharmacogenomics J 2016;16:141–146. [DOI] [PubMed] [Google Scholar]
- 14. López‐Romero, P. Pre‐processing and differential expression analysis of Agilent microRNA arrays using the AgiMicroRna Bioconductor library. BMC Genomics 2011;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gentleman RC, Carey VJ, Bates DM et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol 2004;5:R80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration . FDA guidance document statistical approaches to establishing bioequivalence. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070244.pdf. Accessed June 2011.
- 17. Sing T, Sander O, Beerenwinkel N et al. ROCR: Visualizing classifier performance in R. Bioinformatics. 2005;21:3940–3941. [DOI] [PubMed] [Google Scholar]
- 18. Shah JS, Soon PS, Marsh DJ. Comparison of methodologies to detect low levels of hemolysis in serum for accurate assessment of serum microRNAs. PLoS One 2016;11:e0153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirschner MB, Edelman JJ, Kao SC et al. The impact of hemolysis on cell‐free microRNA biomarkers. Front Genet 2013;4:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marabita F, de Candia P, Torri A et al. Normalization of circulating microRNA expression data obtained by quantitative real‐time RT‐PCR. Brief Bioinform 2016;17:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zweig MH, Campbell G. Receiver‐operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem 1993;39:561–577. Review. [PubMed] [Google Scholar]
- 22. Li D, Ji L, Liu L et al. Characterization of circulating microRNA expression in patients with a ventricular septal defect. PLoS One 2014;9:e106318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jong Dae Ji, Tae‐Hwan Kim, Bitnara Lee et al. Integrated analysis of microRNA and mRNA expression profiles in rheumatoid arthritis synovial monocytes. J Rheum Dis 2011;18:253–263. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.
Supporting Information Table 1.
Supporting Information.


