Abstract
Mesenchymal stromal cell (MSC) application in Coxsackievirus B3 (CVB3)‐induced myocarditis reduces myocardial inflammation and fibrosis, exerts prominent extra‐cardiac immunomodulation, and improves heart function. Although the abovementioned findings demonstrate the benefit of MSC application, the mechanism of the MSC immunomodulatory effects leading to a final cardioprotective outcome in viral myocarditis remains poorly understood. Monocytes are known to be a trigger of myocardial tissue inflammation. The present study aims at investigating the direct effect of MSC on the mobilization and trafficking of monocytes to the heart in CVB3‐induced myocarditis. One day post CVB3 infection, C57BL/6 mice were intravenously injected with 1 x 106 MSC and sacrificed 6 days later for molecular biology and flow cytometry analysis. MSC application reduced the severity of myocarditis, and heart and blood pro‐inflammatory Ly6Chigh and Ly6Cmiddle monocytes, while those were retained in the spleen. Anti‐inflammatory Ly6Clow monocytes increased in the blood, heart, and spleen of MSC‐treated CVB3 mice. CVB3 infection induced splenic myelopoiesis, while MSC application slightly diminished the spleen myelopoietic activity in CVB3 mice. Left ventricular (LV) mRNA expression of the chemokines monocyte chemotactic protein‐1 (MCP)−1, MCP‐3, CCL5, the adhesion molecules intercellular adhesion molecule‐1, vascular cell adhesion molecule‐1, the pro‐inflammatory cytokines interleukin‐6, interleukin‐12, tumor necrosis factor‐α, the pro‐fibrotic transforming growth factorβ1, and circulating MCP‐1 and MCP‐3 levels decreased in CVB3 MSC mice, while LV stromal cell‐derived factor‐1α RNA expression and systemic levels of fractalkine were increased in CVB3 MSC mice. MSC application in CVB3‐induced myocarditis modulates monocytes trafficking to the heart and could be a promising strategy for the resolution of cardiac inflammation and prevention of the disease progression. Stem Cells Translational Medicine 2017;6:1249–1261
Keywords: Myocarditis, Mesenchymal stromal cells, Monocytes trafficking
Significance Statement.
Mesenchymal stromal cell (MSC) are leading candidates for cardiac cellular therapy due to their cardioprotective and immunomodulatory properties. Despite their well‐recognized immunomodulatory features, the effect of MSC on cardiac trafficking of monocytes has still not been investigated. The present study reveals that MSC attenuate myocardial inflammation via suppressing the cardiac infiltration of pro‐inflammatory monocytes while promoting cardiac influx of anti‐inflammatory monocytes. The present findings are dedicated to extent the knowledge of the immunomodulatory effects of MSC, advancing their potential use for cardiac therapies.
Introduction
Inflammation is a major trigger and a dominant mechanism in the pathogenesis of inflammatory cardiomyopathy and heart failure, evident by the finding that extensive inflammation in patients with acute myocarditis is an independent predictor of a poor outcome 1.
The immune and inflammatory cell network is emerging as a major inducer of immune cell‐mediated cardiac injury, which is associated with extreme changes of the mononuclear phagocyte network of the failing heart, spleen, peripheral blood, and bone marrow 2. In heart failure, the splenic microenvironment plays a major role in the activation and trafficking of innate immune cells to the heart where they induce immune cell‐mediated injury. Monocytes and macrophages have been recognized as key effector cells after myocardial infarction and in myocarditis 3, comprising a major proportion of the heart infiltrating cells and playing a leading role in the pathogenesis 4, 5. Monocytes are a heterogeneous multifunctional cellular population, of which CD115+CD11b+Ly6ChighCCR2highCXC3CR1low and CD115+CD11b+ Ly6CmiddleCCR2highCXC3CR1low cells infiltrate to sites of inflammation in response to chemokine signals and differentiate into inflammatory M1 macrophages secreting pro‐inflammatory cytokine, such as tumor necrosis factor‐α (TNF‐α) and interleukin‐6 (IL‐6) and contribute to tissue degradation and T cell activation 6. In contrast, the CD115+ CD11b+Ly6ClowCCR2lowCXC3CR1high monocytes are recruited to the inflamed tissue and are more likely to differentiate into M2 macrophages, which secrete anti‐inflammatory cytokines and contribute to tissue repair 6.
The chemokine system represents the main regulator of leukocyte recruitment 7, while the spleen, in addition to the blood, functions as a reservoir of monocytes outside the bone marrow. Monocytes are released from splenic reservoir, and Ly6Chigh monocytes are selectively recruited to the injured heart 8. Monocyte chemotactic protein‐1 (MCP‐1/CCL2) and its receptor chemokine (C‐C motif) receptor‐2 (CCR2) play a leading role in the recruitment of monocytes/macrophages during inflammatory processes in the cardiovascular system 9. Furthermore, MCP‐1 and MCP‐3 (also known as CCL7) are CC‐chemokines that bind to CCR2 and mediate Ly6Chigh monocyte recruitment 8, 10, 11. Ly6Clow monocytes respond to CX3C‐chemokine ligand 1 (CX3CL1; also known as fractalkine), which governs Ly6Clow monocyte infiltration into the myocardium 11.
There is a growing body of preclinical and early clinical evidence showing great promise of mesenchymal stromal cell (MSC) therapy in the repair of damaged cardiac tissue 12, 13, 14. We have demonstrated that MSC have the potential to treat acute coxsackievirus B3 (CVB3)‐induced inflammatory cardiomyopathy since MSC cannot be infected with CVB3 and exert anti‐viral effects 14. Moreover, intravenous (i.v.) MSC application in CVB3‐infected mice reduced CVB3‐associated cardiomyocyte apoptosis, myocardial fibrosis and inflammation, and induced prominent systemic immunomodulatory effects as indicated by the induction of IL‐10 secreting regulatory T cells and increased CD4+CD25+FoxP3+ cells in the spleen as well as in the circulation of CVB3 MSC mice 6, 7, 8, 15, 16. The abovementioned findings clearly demonstrate that MSC application is not only safe and cardioprotective, but also associated with prominent extra‐cardiac immunomodulatory effects in a model of CVB3‐induced inflammatory cardiomyopathy.
However, the exact mechanism of the MSC‐protective effects in viral myocarditis is still not completely understood. Since monocytes are known to be a marker and trigger of myocardial tissue inflammation 17, the aim of the present study is to evaluate the effect of i.v. MSC application on cardiac trafficking of monocytes in murine CVB3‐induced myocarditis. This study could further give a deeper understanding of the immunomodulatory effects of MSC leading to a final cardioprotective outcome in viral myocarditis.
Materials and Methods
Murine CVB3‐Induced Myocarditis and Cell Application
Eight‐week‐old male C57BL/6 (Charles Rivers, Wilmington, MA, USA) mice were infected by intraperitoneal injection of 1x105 plaque forming units (PFU) of CVB3 virus (Nancy strain). Control mice received PBS instead of CVB3. One day after CVB3 virus infection, 1 x 106 MSC of passage 5 were i.v. administrated via the tail vein into C57BL/6 mice. All mice were sacrificed on day seven post‐CVB3 infection. The left ventricle (LV) was harvested and snap‐frozen for molecular biology. For flow cytometry analysis, heart, blood, and spleen mononuclear cells (MNCs) were isolated. The investigation was approved by the ethical committee for the use of experimental animals of Charité‐Medical University, Berlin (Nr:G0094/11) and was performed, in accordance with the principles of laboratory animal care and German animal protection law.
MSC Isolation
Human adult MSC were isolated from iliac crest bone marrow aspirates of eight healthy donors after their written approval according to Binger et al. 18. MSC were cultured and expanded for injection in Dulbecco's modified Eagle's medium (Biochrom, Berlin, Germany) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 1% glutamine, 2% HEPES, and 2 ng/ml of basic fibroblast growth factor (Tebu‐bio, Offenbach, Germany). Cultivated MSC were triple negative for the markers CD45, CD34, and CD11b, but stained positively for the markers CD73, CD29, CD105, CD106, CD90, and CD44.
MNCs Isolation From Mouse Heart, Blood, and Spleen
Cardiac MNCs were isolated from control PBS, control MSC, CVB3 and CVB3 MSC mice, 7 days postinfection using the Neonatal Heart Dissociation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and gentleMACS Octo Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions. Splenocytes were isolated from control PBS, control MSC, CVB3, and CVB3 MSC mice according to Van Linthout et al. 16. Blood MNCs were isolated from whole blood by density gradient centrifugation using Histopaque‐1083 (Sigma, Steinheim, Germany). Heart, blood and spleen MNCs were used for the analysis of CD115+CD11b+Ly6ChighCCR2highCXC3CR1low, CD115+CD11b+Ly6CmiddleCCR2high CXC3CR1low, and CD115+ CD11b+Ly6ClowCCR2lowCXC3CR1high monocytes.
Flow Cytometry Analysis
Flow cytometry analysis of heart, blood, and spleen MNCs was performed using the directly conjugated monoclonal mouse antibodies anti‐CD115 Alexa488, anti‐CD11b PerCP/Cy5.5, anti‐Ly6C Brilliant Violet 421, anti‐CXC3CR1 PE and anti‐CCR2 Alexa647 antibody. Spleen MNCs were stained with anti‐mouse CD11c PercP Cy5.5, anti‐mouse F4/80 APC, anti‐mouse vascular cell adhesion molecule (VCAM) PE, anti‐mouse GM‐CSF PE (Biolegend, San Diego, CA), anti‐mouse CD4 vio Blue and anti‐mouse IL‐3 APC (Miltenyi, Bergisch Gladbach, Germany). Surface staining was performed according to the manufacturer's instructions. Sample analysis was performed on a MACSQuant Analyzer (Miltenyi Biotec, Bergisch Gladbach, Germany) and flow cytometry data were analyzed with FlowJo 8.7. software (FlowJo, LLC, RO). Supporting Information Figure 1 shows representative flow cytometry analysis of monocytes subsets according to Yang et al. 6.
Gene Expression Analysis
Frozen heart tissue was homogenized with an IKA T25D ULTRA TURRAY homogenisator (Laboratory equipment, Germany) in Trizol, followed by chloroform extraction and isopropanol precipitation. Next, RNA was DNase treated with the NucleoSpin RNA II Kit (Macherey‐Nagel, Düren, Germany) and subsequently reverse transcribed via the High Capacity cDNA Reverse Transcription Kit from Applied Biosystems by Thermo Fisher Scientific (Carlsbad, CA, USA). To assess the mRNA expression of the target genes MCP‐1, MCP‐3, CCL5, stromal cell‐derived factor‐1α (SDF‐1α), IL‐6, IL‐12, TNF‐α, transforming growth factor‐ß (TGF‐ß), VCAM‐1, and intercellular adhesion molecule‐1 (ICAM‐1) mRNA expression was analyzed via real‐time PCR using gene expression assays for MCP‐1 Mm00438270_m1, MCP‐3 Mm00443113_m1, CCL5 Mm01302428_m1, SDF‐1α Mm00445552_m1, IL‐6 Mm00446190_m1, IL‐12 Mm00434165_m1, TNF‐α Mm00443258_m1, TGF‐ß1 Mm00441724_m1, VCAM‐1 Mm01320970_m1, and ICAM‐1 Mm00516023_m1 from Applied Biosystems by Thermo Fisher Scientific (Carlsbad, CA), respectively. mRNA expression was normalized to the housekeeping gene CDKN1b Mm00438167_g1 and relatively expressed with the control group set as 1.
ELISA
Plasma from control phosphate buffered saline (PBS), control MSC, CVB3 and CVB3 MSC mice was collected using EDTA and centrifuged at 4°C. Circulating chemokine MCP‐1, MCP‐3, and SDF‐1α levels were measured via MCP‐1, MCP‐3, and SDF‐1α Enzyme‐linked Immunosorbent Assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. Mouse CX3CL1 levels were determined via the CX3CL1/Fractalkine ELISA kit (Abcam, Cambridge, U.K.) according to the manufacturer's protocol.
Spreading Assay
Spleen MNCs isolated from all experimental groups were resuspended in RPMI 1640 medium containing 10% FBS and 1% penicillin/streptomycin and seeded on CellCarrier 96‐well plate coated with 5 μg/ml recombinant mouse (rm) VCAM‐1 (Peprotech, Rocky Hill, NJ) for 2 hours at 37°C. Cells were fixed with BD Cytofix/Cytoperm solution (BD Biosciences, Franklin Lakes, NJ) and stained with Alexa Fluor 546 Phalloidin (Invitrogen Thermo Fisher Scientific, Waltham, MA). The spreading assay was performed on a rm VCAM‐1 matrix. Phase contrast pictures were taken on Zeiss Axio Observer Z1 microscope (Carl Zeiss, Oberkochen, Germany), using AxioVision Rel 4.7 imaging software (Carl Zeiss MicroImaging) and immunofluorescence images were acquired on Operetta High Content Imaging System with Harmony High Content Imaging and Analysis Software (PerkinElmer, MA, USA). Cells with flat morphology or lamellipodia were scored positive for spreading.
Myocarditis Score
Hematoxylin and eosin staining was performed on 5 μm thick cryosections. We used a semiquantitative scale with severity scores from 0 to 4 to quantify myocardial damage comprising cardiac cell necrosis, inflammation, and scarring according to Savvatis et al. 15 (score: 0, no inflammatory infiltrates; 1, small foci of inflammatory cells between myocytes; 2, foci >100 inflammatory cells; 3, larger foci with an area ≤5% of cross‐section involved; 4, >5% of a cross‐section involved). Analysis was made in a blinded manner by digital image analysis on a Leica DMRB microscope (Leica Microsystems, Wetzlar, Germany) at ×100 magnification.
Statistical Analysis
Statistical analysis was performed using Prism 6 for Mac OS X (GraphPad Software, Inc., La Jolla). Ordinary one‐way ANOVA was used for statistical analysis of the data with correction for multiple comparisons via the Tukey test. Data are presented as mean ± SEM. Differences were considered to be significant when the two‐sided p value was lower than .05.
Results
MSCs Decrease the CVB3‐Induced Pro‐Inflammatory Monocyte Subsets, While Promoting Anti‐Inflammatory Monocytes in the Heart
To evaluate the impact of MSC on the cardiac infiltrating monocyte subsets in CVB3‐induced myocarditis, flow cytometry analysis of the pro‐ and anti‐inflammatory monocyte subsets was performed. The analysis revealed a 3.4‐fold (p < .0001) and 2.4‐fold (p < .0001) increase in the percentage of pro‐inflammatory Ly6Chigh and Ly6Cmiddle monocytes defined as CD115+CD11b+Ly6ChighCCR2highCXC3CR1low and CD115+CD11b+Ly6CmiddleCCR2highCXC3CRlow, respectively, in CVB3 infected versus control mice. Supporting Information Figure 1 illustrates the gating strategy in detail. In contrast, MSC application reduced the percentage of Ly6Chigh and Ly6Cmiddle monocytes in the heart by 2.4‐fold (p < .0001) and 2‐fold (p < .0001) compared to CVB3 mice, respectively, suggesting a decreased influx of pro‐inflammatory monocytes to the heart after i.v. injection of MSC (Fig. 1 A, 1 B). With respect to the anti‐inflammatory monocyte‐subset CD115+CD11b+Ly6ClowCCR2lowCXC3CRhigh, MSC‐treated CVB3 mice exhibited a 2.5‐fold (p < .05) higher percentage of anti‐inflammatory Ly6Clow monocytes in the heart versus CVB3 mice (Fig. 1 C, 1 D).
Figure 1.
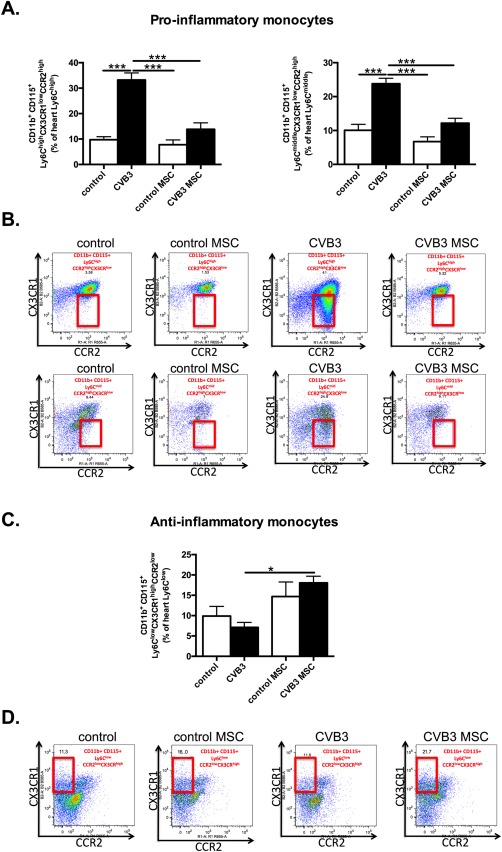
(See prior page) MSCs decrease pro‐inflammatory monocytes subsets and promote anti‐inflammatory monocytes in the heart of coxsackievirus B3‐infected mice. (A): Bar graphs represent the mean ± SEM of CD115+CD11b+Ly6ChighCCR2highCXC3CR1low and CD115+CD11b+Ly6CmiddleCCR2highCXC3CRlow positive cells in the heart of control mice (open bar) and CVB3‐infected mice (closed bar) injected with PBS or MSC, expressed as the percentage of Ly6Chigh and Ly6Cmiddle positive cells, respectively, with n = 8–9 per group and ***, p < .001. (B): The panel represents dot plots of cardiac CD115+CD11b+Ly6ChighCCR2highCXC3CR1low and CD115+CD11b+Ly6CmiddleCCR2highCXC3CRlow positive cells as percentage of Ly6Chigh or Ly6Cmiddle cells, as indicated. (C): Bar graphs represent the mean ± SEM of CD115+CD11b+Ly6ClowCCR2low CXC3CRhigh positive cells in the heart of control mice (open bar) and CVB3‐infected mice (closed bar) injected with PBS or MSC, expressed as the percentage of Ly6Clow positive cells with n = 8–9 per group and *, p < .05. (D): The panel represents dot plots of cardiac anti‐inflammatory CD115+CD11b+Ly6ClowCCR2low CXC3CRhigh positive cells as the percentage of Ly6Clow cells, as indicated. Abbreviations: CCR2, chemokine (C‐C motif) receptor‐2; CVB3, coxsackievirus B3; CX3CL1, chemokine (C‐X3‐C motif) ligand‐1; MSC, mesenchymal stromal cell.
MSCs Modulate the Monocyte Subsets in the Blood of CVB3‐Infected Mice
To understand how MSC affect the pro‐ and anti‐inflammatory monocytes trafficking to the heart, monocyte subsets were analyzed in the blood. In accordance with the observed induction of pro‐inflammatory monocyte subsets in the heart of CVB3‐infected mice, the percentages of pro‐inflammatory Ly6Chigh and Ly6Cmiddle monocytes were elevated by 13.2‐fold (p < .0001) and 2.4‐fold (p < .01) in CVB3‐infected mice in comparison to control mice, respectively (Fig. 2 A, 2 B). Consistent with the observations in the heart of CVB3 MSC treated mice, the percentages of the pro‐inflammatory Ly6Chigh and Ly6Cmiddle monocytes declined by 5.4‐fold (p < .0001) and 2.6‐fold (p < .01), respectively, while the percentage of circulating anti‐inflammatory monocytes increased by 2.4‐fold (p < .01), compared to CVB3 mice (Fig. 2 C, 2 D).
Figure 2.
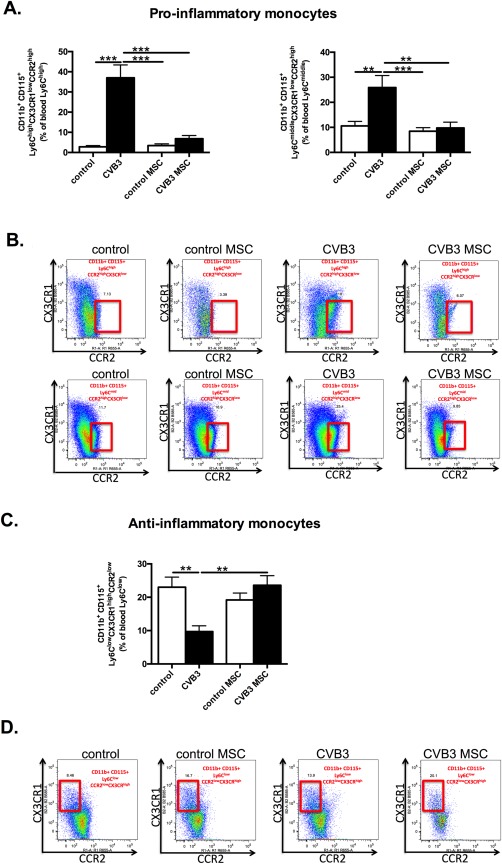
(See prior page) MSCs modulate monocyte subsets in the blood of coxsackievirus B3‐infected mice. (A): Bar graphs represent the mean ± SEM of CD115+CD11b+Ly6ChighCCR2highCXC3CR1low and CD115+CD11b+Ly6CmiddleCCR2highCXC3CRlow positive cells in the blood of control mice (open bar) and CVB3‐infected mice (closed bar) injected with PBS or MSC, expressed as the percentage of Ly6Chigh and Ly6Cmiddle positive cells, respectively with n = 8–9 per group and **, p < .01; ***, p < .001. (B): The panel represents dot plots of blood CD115+CD11b+Ly6ChighCCR2high CXC3CR1low and CD115+CD11b+Ly6CmiddleCCR2highCXC3CRlow positive cells as percentage of Ly6Chigh or Ly6Cmiddle cells, as indicated. (C): Bar graphs represent the mean ± SEM of CD115+CD11b+LyC6lowCCR2low CXC3CRhigh positive cells in the blood of control mice (open bar) and CVB3‐infected mice (closed bar) injected with PBS or MSC, expressed as the percentage of Ly6Clow positive cells with n = 8–9 per group and **, p < .01. (D): The panel represents dot plots of blood anti‐inflammatory CD115+CD11b+Ly6ClowCCR2lowCXC3CRhigh positive cells as the percentage of Ly6Clow cells, as indicated. Abbreviations: CCR2, chemokine (C‐C motif) receptor‐2; CVB3, coxsackievirus B3; CX3CL1, chemokine (C‐X3‐C motif) ligand‐1; MSC, mesenchymal stromal cell.
MSCs Retain Pro‐Inflammatory Monocyte Subset in the Spleen, While Inducing Anti‐Inflammatory Ly6Clow Monocytes
Given the importance of the spleen as a monocytes reservoir, by which splenic monocytes are recruited to the heart upon cardiac injury 8, we next evaluated the percentage of pro‐inflammatory and anti‐inflammatory monocytes in the spleen of CVB3 mice and the impact of MSC administration on splenic monocyte subsets 2. The percentage of pro‐inflammatory Ly6Chigh and Ly6Cmiddle monocytes in CVB3‐infected mice was augmented by 1.9‐fold (p < .01) and 2.1‐fold (p < .05), respectively, in comparison to control mice (Fig. 3A). Interestingly, the presence of pro‐inflammatory Ly6Chigh and Ly6Cmiddle monocytes was 1.5‐fold (p < .05) higher in the spleen of MSC‐treated CVB3 mice compared to CVB3 mice and 1.4‐fold (p < .05) higher versus control mice. In addition, a higher migration potential of splenic MNCs derived from CVB3 mice compared to MNCs derived from CVB3 MSC mice was evident in a spreading assay performed on a rmVCAM‐1 matrix (Fig. 3C, 3D). Cells, which exhibited flattened morphology and lamellipodia were considered positive for spreading. With respect to Ly6Clow monocytes, CVB3 MSC mice exhibited a 1.9‐fold (p < .01) higher percentage of anti‐inflammatory Ly6Clow monocytes in the spleen in comparison to CVB3 mice (Fig. 3B).
Figure 3.
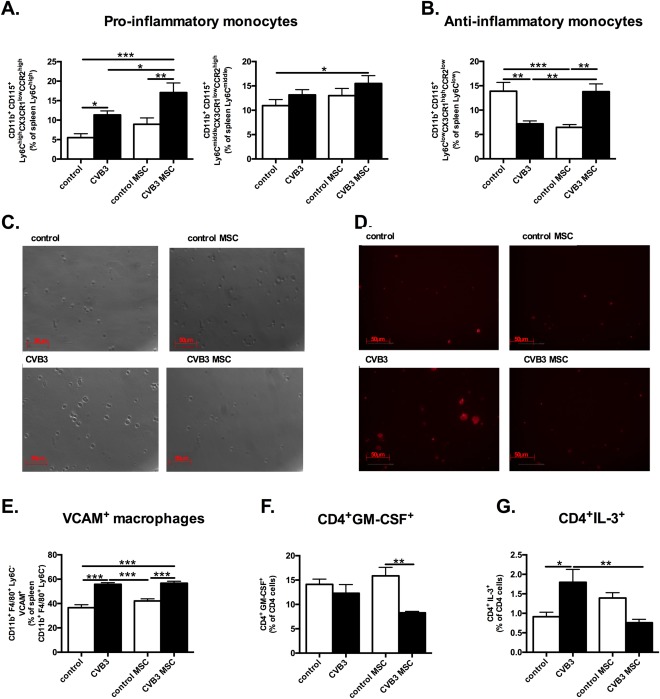
MSCs retain pro‐inflammatory monocytes subsets in the spleen, induce anti‐inflammatory monocytes and slightly affect the spleen extramedullary myelopoiesis in coxsackievirus B3‐infected myocarditis. (A): Bar graphs represent the mean ± SEM of CD115+CD11b+Ly6ChighCCR2highCXC3CR1low and CD115+CD11b+Ly6CmiddleCCR2highCXC3CRlow positive cells in the spleen of control mice (open bar) and CVB3‐infected mice (closed bar) injected with PBS or MSC, expressed as the percentage of Ly6Chigh and Ly6Cmiddle positive cells, respectively with n = 8–9 per group and *, p < .05; **, p < .01; ***, p < .001. (B): Bar graphs represent the mean ± SEM of CD115+CD11b+Ly6Clow CCR2lowCXC3CRhigh positive cells in the spleen of control mice (open bar) and CVB3‐infected mice (closed bar) injected with PBS or MSC, expressed as percentage of Ly6Clow positive cells with n = 8–9 per group and **, p < .01; ***, p < .001. (C): Phase contrast pictures of spleen mononuclear cell (MNCs) derived from control mice and CVB3‐infected mice injected with PBS or MSC, at ×100 magnification. Cells with flat morphology were considered positive for spreading. (D): Phalloiding staining of spleen MNCs derived from control mice and CVB3‐infected mice injected with PBS or MSCs, at ×100 magnification. Cells showing lamellipodia were considered positive for spreading. (E): Bar graphs represent the mean ± SEM of VCAM+ macrophages in the spleen of control mice (open bar) and CVB3‐infected mice (closed bar) injected with PBS or MSC, expressed as the percentage of CD11b+F4/80+Ly6C− cells, with n = 8–9 per group and *, p < .05; **, p < .01; ***, p < .001. (F): Bar graphs represent the mean ± SEM of CD4+GM‐CSF+ in the spleen of control mice (open bar) and CVB3‐infected mice (closed bar) injected with PBS or MSC, expressed as the percentage of total mononuclear cells (MNCs), with n = 5–6 per group and **, p < .01 (G): Bar graphs represent the mean ± SEM of CD4+IL‐3+ in the spleen of control mice (open bar) and CVB3‐infected mice (closed bar) injected with PBS or MSC, expressed as the percentage of total MNCs, with n = 5–6 per group and *, p < .05; **, p < .01. Abbreviations: CCR2, chemokine (C‐C motif) receptor‐2; CVB3, coxsackievirus B3; CX3CL1, chemokine (C‐X3‐C motif) ligand‐1; MSC, mesenchymal stromal cell; VCAM‐1, vascular cell adhesion molecule‐1.
MSCs Slightly Modulate Splenic Extramedullary Myelopoiesis
VCAM‐1+ macrophages in the splenic red pulp are essential for the extramedullary myelopoiesis and increase the systemic levels of inflammatory leukocytes 19. To investigate the role of splenic extramedullary myelopoiesis in CVB3‐induced myocarditis mice and to further understand the mechanism of the MSC‐mediated effects in the spleen, the percentage of splenic CD11b+F4/80+Ly6C‐VCAM+ macrophages was evaluated. CVB3 infection resulted in a 1.5‐fold (p < .0001) higher percentage of splenic VCAM+ macrophages, while MSC application in CVB3 mice did not have any effect on the percentage of splenic CD11b+F4/80+Ly6C−VCAM+ macrophages (Fig. 3E). Myelopoiesis is also positively regulated by splenic CD4+ T cells that produce myelopoietic cytokines GM‐CSF and IL‐3 20. Therefore, we next examined the frequencies of CD4+GM‐CSF+ and CD4+ IL‐3+ cells in the spleen of control, control MSC, CVB3, and CVB3 MSC mice. CVB3 infection or MSC treatment of CVB3 mice did not affect CD4+ cells producing the myelopoietic cytokine GM‐CSF+ (Fig. 3F). However, the frequency of CD4+IL‐3+ cells increased by 2‐fold (p < .05) (Fig. 3G) in CVB3‐infected mice versus control mice, while CVB3 MSC mice exhibited a 2.4‐fold (p < .01) lower percentage of CD4+IL‐3+ cells.
MSCs Regulate the Trafficking of Monocyte Subsets Through Chemokines Modulation
Considering the importance of chemokines for the recruitment of monocytes 10, 21, mRNA expression of the chemokines MCP‐1, MCP‐3, and CCR5, associated with the infiltration of pro‐inflammatory monocytes, was analyzed in the heart. CVB3‐infection led to a 90.5‐fold (p < .0001), 124.3‐fold (p < .0001) and 99.5‐fold (p < .001) higher LV mRNA expression of MCP‐1 (Fig. 4A), MCP‐3 (Fig. 4B), as well as of CCR5 (Fig. 4C) versus control mice, respectively. In contrast, CVB3‐infected mice treated with MSC showed prominent reduction by 51.4‐fold (p < .0001), 225‐fold (p < .001), and 71.8‐fold (p < .001) of LV MCP‐1, MCP‐3, and CCL5 chemokine expression, respectively, whereas, MSC application in CVB3 mice induced a 3.4‐fold (p < .001 vs. CVB3 mice) higher expression of SDF‐1α (Fig. 4 D), known to attract cardiac‐reparative monocytes. To investigate whether the cardiac chemokines expression pattern corresponds with the systemic chemokine levels, MCP‐1, MCP‐3, SDF‐1α, and CX3CR1 were evaluated in the serum. A similar trend of chemokine pattern was observed in the serum, CVB3 infection augmented the serum levels of MCP‐1 and MCP‐3 by 3.9‐fold (p < .0001) and 3.2‐fold (p < .0001) respectively, in comparison to control mice, whereas CVB3 MSC mice manifested 3.2‐fold (p < .0001) and 2.6‐fold (p < .01) lower MCP‐1 and MCP‐3 serum levels, respectively, compared to CVB3 mice (Fig. 5 A, 5 B). The detected SDF‐1α did not differ among the groups, but importantly, MSC application in CVB3 mice resulted in 1.4‐fold (p < .01 vs. CVB3 mice) higher serum levels of CX3CL1/Fractalkine (Fig. 5 C, 5 D).
Figure 4.
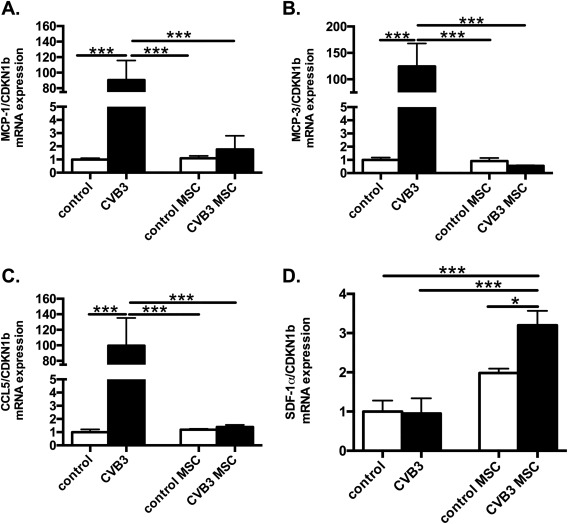
Mesenchymal stromal cells modulate cardiac chemokines expression in coxsackievirus B3‐infected myocarditis mice. Bar graphs represent the mean ± SEM of (A) MCP‐1, (B) MCP‐3, (C) CCL5, and (D) SDF‐1α mRNA expression in the LV of control, CVB3, control MSC, and CVB3 MSC mice, as indicated, with n = 5–6 per group and *, p < .05; ***, p < .001. Abbreviations: CVB3, Coxsackievirus B3; MCP, monocyte chemotactic protein; MSC, mesenchymal stromal cell; SDF‐1α, stromal cell‐derived factor‐1α.
Figure 5.
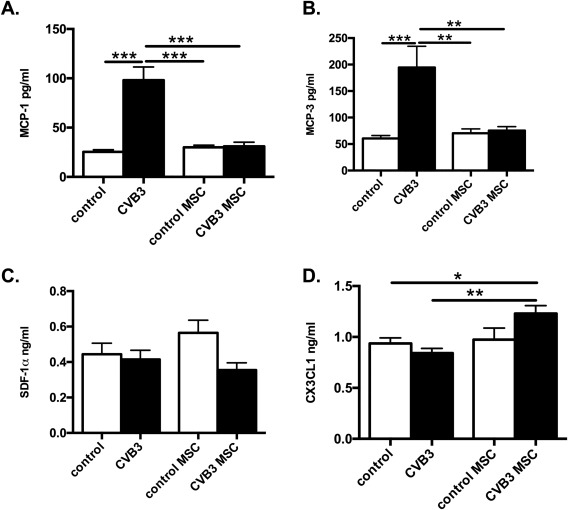
Mesenchymal stromal cells regulate systemic chemokines levels in Coxsackievirus B3‐infected myocarditis mice. Bar graphs represent the mean ± SEM of serum (A) MCP‐1 (pg/ml), (B) MCP‐3 (pg/ml), (C) SDF‐1α (ng/ml) and (D) CX3CL1/Fractalkine (ng/ml) levels of control, CVB3, control MSC, and CVB3 MSC mice, with n = 4–6 per group and *, p < .05; **, p < .01; ***, p < .001. Abbreviations: CVB3, Coxsackievirus B3; CX3CL1, chemokine (C‐X3‐C motif) ligand‐1; MCP, monocyte chemotactic protein; MSC, mesenchymal stromal cell; SDF‐1α, stromal cell‐derived factor‐1α.
MSCs Abrogate Cardiac Adhesion Molecules Expression in CVB3‐Infected Mice
Expression of adhesion molecules such as ICAM‐1 and VCAM‐1 on endothelial cells promotes leucocyte migration via direct binding to leucocyte cell surface receptors, firm adhesion and guidance of monocyte homing into the inflamed tissue 6, 22, 23. Taking the importance of adhesion molecules expression for monocytes adhesion and infiltration into account, LV VCAM‐1 and ICAM‐1 expression was evaluated. As expected, CVB3 infection magnified the LV VCAM‐1 and ICAM‐1 expression by 6.3‐fold (p < .0001) and 6.8‐fold (p <.0001), respectively, in comparison to control mice (Fig. 6A, 6B), while CVB3 MSC mice exhibited 11.7‐fold (p < .0001) and 5.1‐fold (p < .0001) lower expression of VCAM‐1 and ICAM‐1 versus CVB3 mice, respectively.
Figure 6.
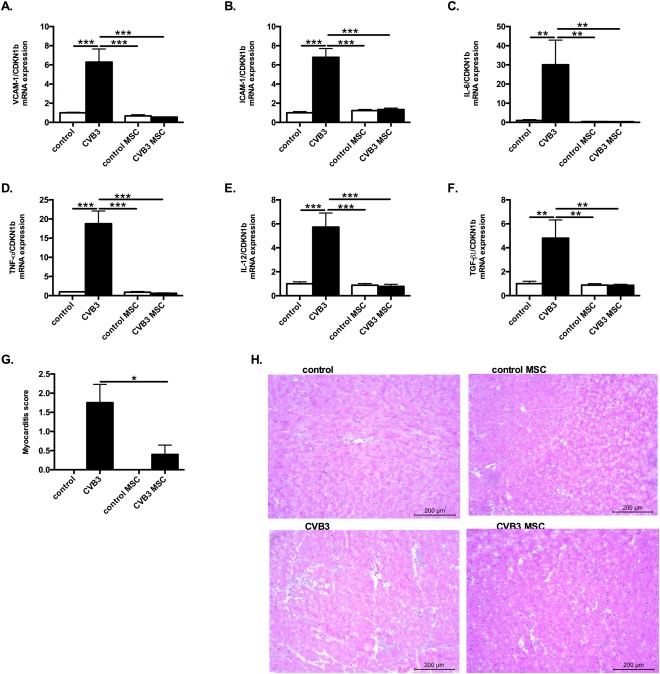
MSCs application abrogates left ventricular expression of adhesion molecules, pro‐inflammatory cytokines and the pro‐fibrotic cytokine TGF‐β1 in coxsackievirus B3‐infected mice and improves the pathologic myocarditis score. Bar graphs represent the mean ± SEM of (A) VCAM‐1 and (B) ICAM‐1, (C) IL‐6, (D) TNF‐α, (E) IL‐12, and (F) TGF‐β1 mRNA expression in the LV of control, CVB3, control MSC, and CVB3 MSC mice, as indicated, with n = 5–6 per group and **, p < .01 and ***, p < .001 (G) Bar graphs represent the mean ± SEM of the pathologic score of myocarditis assessed by hematoxylin/eosin staining of control, CVB3, control MSC, and CVB3 MSC mice, as indicated, with n = 4–5 per group and *, p < .05. (H): Hematoxylin/eosin‐stained heart sections of control mice receiving PBS (upper left panel) or MSC (upper right panel), or of CVB3 infected mice receiving PBS (lower left panel) or MSC (lower right panel), at a magnification of ×100. Abbreviations: CVB3, coxsackievirus B3; CX3CL1, chemokine (C‐X3‐C motif) ligand‐1; ICAM‐1, intercellular adhesion molecule‐1; IL, interleukin; MSC, mesenchymal stromal cell; TGF‐β1, transforming growth factor‐β1; TNF‐α, tumor necrosis factor‐α; VCAM‐1, vascular cell adhesion molecule‐1.
MSCs Diminish the Expression of Cardiac Pro‐Inflammatory Cytokines and the Pro‐Fibrotic Cytokine TGF‐β1 in CVB3‐Infected Mice
To evaluate whether the observed modulation of pro‐inflammatory monocytes in the heart of CVB3 mice upon MSC application corresponds with a modulation in cytokine expression, LV mRNA expression of IL‐6, IL‐12, TNF‐α known to be released by Ly6Chigh monocytes 24, 25, 26, 27 and by inflammatory M1 macrophages was analyzed. CVB3 mice exhibited 30.1‐fold (p < .005), 18.8‐fold (p < .0001), 5.7‐fold (p < .0001) and 4.8‐fold (p < .01) upregulated LV mRNA expression of IL‐6, TNF‐α, IL‐12 and TGF‐β1 (Fig. 6 C‐ 6 F), in comparison to control mice, respectively. MSC treatment of CVB3‐infected mice downregulated the LV mRNA expression of the pro‐inflammatory cytokines IL‐6, TNF‐α, IL‐12, and the pro‐fibrotic cytokine TGF‐β1 by 86.8‐fold (p < .01), 30.4‐fold (p < .0001), 7.4‐fold (p < .0001) and 5.6‐fold (p < .01), respectively, compared to CVB3 mice.
MSCs Reduce the Severity of Myocarditis
Histological sections were stained with hematoxylin/eosin to estimate the extent of myocardial damage. The pathological score of myocardial damage was significantly higher in the PBS‐ injected CVB3 mice compared to the MSC‐treated CVB3 mice, which displayed a reduced size of inflammatory foci (Fig. 6G, 6H).
Discussion
The salient finding of the present study is that i.v. MSC application diminishes trafficking of pro‐inflammatory monocyte subsets, promotes the migration of anti‐inflammatory monocytes toward the heart in CVB3 myocarditis mice via modulation of the local and systemic chemokine pattern, and only mildly affects the splenic myelopoiesis.
Myocarditis is characterized by a complex manifestation and pathogenesis associated with a profound inflammatory destruction of the myocardium 25. However, several clinical trials applying immunosuppressive treatment strategies have been largely disappointing 28, 29, 30 emphasizing the need for immunomodulatory therapies which could more selectively target the disease. Our previous studies demonstrated that MSC application in CVB3‐infected mice is safe, leads to anti‐apoptotic and anti‐fibrotic effects, reduces myocardial inflammation, and induces prominent systemic immunomodulation without compromising the virus clearance, which is all translated into improved heart function 15, 16, 31. MSC migrate to injured tissues and rapidly disappear after systemic infusion 32, 33 and mediate protective effects without a permanent engraftment 15, 34. Despite the limited engraftment, long‐lasting effects of MSC have thus been proposed to be mediated via paracrine secretion of soluble factors or microvesicles, such as exosomes 35. Factors present at the inflammatory site are critical determinants of the immunosuppressive activity of MSCs 16, 36, which includes inhibition of immune cells activation, suppression of pro‐inflammatory cytokines secretion by activated immune cells, and impairment of the immune cells migratory potential through inhibition of the adhesion molecules and receptors that are responsible for immune cell trafficking 37, 38. MSC mediate immunosuppression via secretion of soluble molecules and cytokines like indoleamine 2,3‐dioxygenase, prostaglandin E2, TGF‐β, hepatocyte growth factor, interleukin‐10, and human leukocyte antigen‐G 39. Moreover, MSC have been shown to directly act on monocytes and induce an immunomodulatory phenotype through the secretion of HGF and potentially factors downstream of the COX2 pathway 40, 41.
Considering the role of monocytes as a marker of myocardial tissue inflammation 17, and a trigger of disease progression on the one hand, and the immunomodulatory properties of MSC on the other hand, we investigated the potential of MSC to specifically regulate the pro‐ as well as anti‐inflammatory monocyte subsets in a model of CVB3‐induced myocarditis.
Systemic monocytosis occurs as a result of an acute or a chronic inflammation, where monocytes are recruited to the inflammatory site to exacerbate the immune activation and inflict tissue damage or to support inflammation resolution 42. Ly6Chigh monocytes are rapidly recruited to the heart and initially exert beneficial effects 6, 42, 43. However, their persistence is deleterious 44, 45, while Ly6Clow monocytes have anti‐inflammatory properties supporting healing 6, 46. Monocytes/macrophages are known to be specifically involved in CVB3‐induced myocarditis by maintaining a chronic inflammatory response 47. In agreement, the present study demonstrates the induction of pro‐inflammatory monocyte subsets in the heart, blood, and spleen upon CVB3‐infection, which was associated with a profound cardiac expression of inflammatory cytokines and chemokines, and cardiac damage. MSC application in CVB3‐infected mice diminished the infiltration of pro‐inflammatory monocyte subsets toward the heart and induced Ly6Clow monocytes associated with tissue repair, leading to less severe myocarditis 6, 46. In contrast to the decreased Ly6Chigh monocytes in the heart and blood after MSC application, the percentage of Ly6Chigh and Ly6Cmiddle monocytes was higher in the spleen of CVB3 MSC compared to CVB3 mice. The following observation suggests that MSC retained the pro‐inflammatory monocytes in the spleen and used it as a site for storage, decreasing the mobilization of inflammatory monocytes from the spleen, their emigration to the blood and subsequent accumulation into the heart, limiting further immune‐mediated cardiac injury. In line with this hypothesis, the spreading and lamellipodia of CVB3 MSC‐derived splenocytes was decreased in comparison to splenocytes from CVB3 mice, indicating that MSC abrogate the migration potential of splenic MNCs.
With respect to the increased presence of pro‐inflammatory monocytes in the spleen upon CVB3 infection, we speculate that this is due to the still active generation of monocytes in the spleen at this stage of the inflammatory process or due to postviral sensitization of the immune system, knowing that the spleen is a target of CVB3 infection 48. In this regard, we have indeed found that CVB3‐infected mice had a high hematopoietic stem cell activity in the spleen, as shown by an increase in VCAM‐1+ macrophages and CD4+IL‐3+ cells, which are essential for splenic myelopoiesis, retaining the hematopoietic stem cell activity in the spleen 19. MSC application did not affect CVB3‐increased VCAM‐1+ macrophages or CD4+GM‐CSF+ cells in the spleen, but diminished the percentage of CVB3‐induced CD4+IL‐3+ cells, indicating that MSC only mildly affect spleen myelopoiesis. We speculate that the increase of pro‐inflammatory monocytes in the spleen upon MSC application is due to retention and is not associated with MSC‐mediated effects on the spleen myelopoiesis.
Leukocytes trafficking is mainly regulated by the chemokine system 7. CVB3 infection stimulates the expression of MCP‐1 in myocardial cells, which subsequently leads to the migration of MNCs 21. CCR2 and MCP‐1/MCP‐3 are critical for monocyte mobilization and particularly Ly6Chigh monocyte recruitment to the injured heart 8, 10, while Ly6Clow monocytes respond to CX3CL1 guiding their infiltration into the myocardium 11. CCL5 (RANTES) expression is highly upregulated during infection/inflammation and is also important for the trafficking of Ly6Chigh monocytes expressing its ligand CCR5 [49]. SDF‐1α is known to mediate cardioprotection through mobilization of stem cells, cardiac myocyte survival, heart regeneration 50 and through the recruitment of anti‐inflammatory monocytes enhancing the remodeling of the microvascular network 51. MSC application suppressed the viral‐induced cardiac expression of the chemokines MCP‐1, MCP‐3, and CCL5 recruiting inflammatory monocytes within inflamed tissues 10, 49. In parallel, MSC induced SDF‐1α expression in the heart of CVB3‐infected mice.
Besides modulating the cardiac chemokine pattern, MSC modulated the systemic chemokine levels, reducing the CVB3‐increased circulating levels of MCP‐1 and MCP‐3, while promoting the increase in circulating CX3CL1 levels, which regulate the trafficking and mobilization of Ly6Clow monocytes into the myocardium 11.
The inflammatory response is dependent on the activation of adhesive interactions between endothelial cells and leukocytes. In this regard, our data reveal that MSC abrogated the CVB3‐induced expression of the adhesion molecules ICAM‐1 and VCAM‐1, resulting in a blocked adhesion and transmigration into the inflamed tissue of Ly6Chigh monocytes 22, 23 and subsequently, weakened expression of pro‐inflammatory cytokines in the failing myocardium. Prolonged exposure to inflammatory cytokines exacerbates adverse heart remodeling and enhances myocardial damage 52. MSC abrogated the CVB3‐induced expression of heart pro‐inflammatory cytokines, which corresponded with a diminished presence of pro‐inflammatory monocytes subsets in the heart of CVB3 MSC mice Furthermore, LV expression of the pro‐fibrotic cytokine TGF‐β1 was significantly downregulated in CVB3 MSC mice in contrast to CVB3 mice. This finding is in line with our previous finding revealing the anti‐fibrotic effect of MSC in a model of CVB3‐induced viral myocarditis 15.
Conclusion
The present study demonstrates that MSC effectively attenuated myocardial inflammation via suppressing the cardiac infiltration of pro‐inflammatory monocytes while promoting cardiac influx of anti‐inflammatory monocytes. We suggest that MSC modulate monocytes trafficking via regulation of the cardiac and systemic chemokine expression pattern (Fig. 7). Hence, the present study gives deeper insights in how MSC regulate the mobilization and adhesion of monocyte subsets in a model of viral myocarditis. This provides new perspectives for the development of therapeutic strategies for inflammatory heart diseases via targeting the monocyte response to improve cardiac repair and healing.
Figure 7.
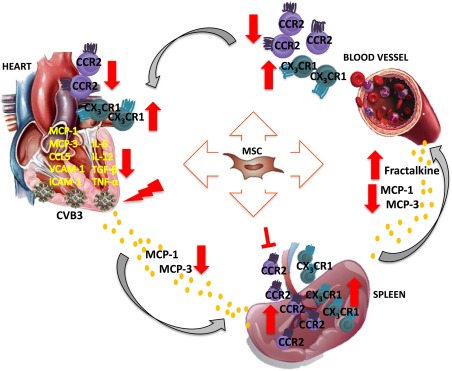
Hypothetical scheme how intravenous MSC application modulates monocytes trafficking to the heart in viral myocarditis. MSC application abrogated the cardiac expression of the chemokines MCP‐1, MCP‐3 and CCL5, which attract pro‐inflammatory monocytes, and lowered the levels of MCP‐1 and MCP‐3 in the circulation of CVB3‐infected mice. In the heart, MSC application further reduced the expression of the adhesion molecules VCAM‐1 and ICAM‐1 in CVB3 mice. These effects led to diminished trafficking and homing of pro‐inflammatory monocytes to the heart, as indicated by the reduced pro‐inflammatory monocytes in the blood and heart, as well as the downregulated expression of the pro‐inflammatory cytokines IL‐6, IL‐12, and TNF‐α and pro‐fibrotic TGF‐β in the heart of CVB3‐infected mice. In parallel, MSC injection increased systemic levels of fractalkine, raising the deployment of anti‐inflammatory monocytes to the heart. Finally, MSC retained the pro‐inflammatory monocyte subsets in the spleen, preventing their mobilization, emigration to the blood and accumulation in the heart, limiting further immune‐mediated cardiac injury and promoted spleen anti‐inflammatory monocytes. Abbreviations: CCR2, chemokine (C‐C motif) receptor‐2; CVB3, coxsackievirus B3; CX3CL1, chemokine (C‐X3‐C motif) ligand‐1; ICAM‐1, intercellular adhesion molecule‐1; IL, interleukin; MCP, monocyte chemotactic protein; MSC, mesenchymal stromal cell; TGF‐β1, transforming growth factor‐β1; TNF‐α, tumor necrosis factor‐α; VCAM‐1, vascular cell adhesion molecule‐1.
Author Contributions
K.M.: conception and design, administrative support, collection and/or assembly of data, data analysis and interpretation, manuscript writing; K.P., M.E.‐S., and F.D.: collection and/or assembly of data; J.R.: provision of study material or patients; C.T.: financial support, provision of study material or patients, final approval of manuscript; S.V.L.: conception and design, administrative support, collection and/or assembly of data; data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Supporting information
Supporting Information.
Acknowledgments
We like to acknowledge the assistance of the BCRT Flow Cytometry Lab and Annika Koschel, Kerstin Puhl, and Marzena Sosnowski (in alphabetical order) for excellent technical assistance. This study was supported by the Charité via a Lydia Rabinowitsch Stipendium to K.M.
References
- 1. Kindermann I, Kindermann M, Kandolf R et al. Predictors of outcome in patients with suspected myocarditis. Circulation 2008;118:639–648. [DOI] [PubMed] [Google Scholar]
- 2. Ismahil MA, Hamid T, Bansal SS et al. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: Critical importance of the cardiosplenic axis. Circ Res 2014;114:266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res 2013;112:1624–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: Protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010;121:2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Apostolakis S, Lip GY, Shantsila E. Monocytes in heart failure: Relationship to a deteriorating immune overreaction or a desperate attempt for tissue repair? Cardiovasc Res 2010;85:649–660. [DOI] [PubMed] [Google Scholar]
- 6. Yang J, Zhang L, Yu C et al. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res 2014;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 2006;354:610–621. [DOI] [PubMed] [Google Scholar]
- 8. Swirski FK, Nahrendorf M, Etzrodt M et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009;325:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leuschner F, Courties G, Dutta P et al. Silencing of CCR2 in myocarditis. Eur Heart J 2015;36:1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsou CL, Peters W, Si Y et al. Critical roles for CCR2 and MCP‐3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 2007;117:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nahrendorf M, Swirski FK, Aikawa E et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007;204:3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res 2015;116:1413–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karantalis V, DiFede DL, Gerstenblith G et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res 2014;114:1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mushtaq M, DiFede DL, Golpanian S et al. Rationale and design of the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis in Dilated Cardiomyopathy (the POSEIDON‐DCM study): A phase I/II, randomized pilot study of the comparative safety and efficacy of transendocardial injection of autologous mesenchymal stem cell vs. allogeneic mesenchymal stem cells in patients with non‐ischemic dilated cardiomyopathy. J Cardiovasc Transl Res 2014;7:769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Savvatis K, van Linthout S, Miteva K et al. Mesenchymal stromal cells but not cardiac fibroblasts exert beneficial systemic immunomodulatory effects in experimental myocarditis. PLoS One 2012;7:e41047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Linthout S, Savvatis K, Miteva K et al. Mesenchymal stem cells improve murine acute coxsackievirus B3‐induced myocarditis. Eur Heart J 2011;32:2168–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maekawa Y, Anzai T, Yoshikawa T et al. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction: A possible role for left ventricular remodeling. J Am Coll Cardiol 2002;39:241–246. [DOI] [PubMed] [Google Scholar]
- 18. Binger T, Stich S, Andreas K et al. Migration potential and gene expression profile of human mesenchymal stem cells induced by CCL25. Exp Cell Res 2009;315:1468–1479. [DOI] [PubMed] [Google Scholar]
- 19. Dutta P, Hoyer FF, Grigoryeva LS et al. Macrophages retain hematopoietic stem cells in the spleen via VCAM‐1. J Exp Med 2015;212:497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee JH, Wang C, Kim CH. FoxP3+ regulatory T cells restrain splenic extramedullary myelopoiesis via suppression of hemopoietic cytokine‐producing T cells. J Immunol 2009;183:6377–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen Y, Xu W, Chu YW et al. Coxsackievirus group B type 3 infection upregulates expression of monocyte chemoattractant protein 1 in cardiac myocytes, which leads to enhanced migration of mononuclear cells in viral myocarditis. J Virol 2004;78:12548–12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghigo A, Franco I, Morello F et al. Myocyte signalling in leucocyte recruitment to the heart. Cardiovasc Res 2014;102:270–280. [DOI] [PubMed] [Google Scholar]
- 23. Mestas J, Ley K. Monocyte‐endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med 2008;18:228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Serbina NV, Jia T, Hohl TM et al. Monocyte‐mediated defense against microbial pathogens. Annu Rev Immunol 2008;26:421–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barbalat R, Lau L, Locksley RM et al. Toll‐like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol 2009;10:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grainger JR, Wohlfert EA, Fuss IJ, et al. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med 2013;19:713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dunay IR, Damatta RA, Fux B et al. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii . Immunity 2008;29:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mason JW, O'Connell JB, Herskowitz A et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med 1995;333:269–275. [DOI] [PubMed] [Google Scholar]
- 29. Hahn EA, Hartz VL, Moon TE et al. The Myocarditis Treatment Trial: Design, methods and patients enrollment. Eur Heart J 1995;16(suppl O):162–167. [DOI] [PubMed] [Google Scholar]
- 30. Parrillo JE, Cunnion RE, Epstein SE et al. A prospective, randomized, controlled trial of prednisone for dilated cardiomyopathy. N Engl J Med 1989;321:1061–1068. [DOI] [PubMed] [Google Scholar]
- 31. Tschope C, Miteva K, Schultheiss HP et al. Mesenchymal stromal cells: A promising cell source for the treatment of inflammatory cardiomyopathy. Curr Pharm Des 2011;17:3295–3307. [DOI] [PubMed] [Google Scholar]
- 32. Zhao F, Zhang YF, Liu YG et al. Therapeutic effects of bone marrow‐derived mesenchymal stem cells engraftment on bleomycin‐induced lung injury in rats. Transplant Proc 2008;40:1700–1705. [DOI] [PubMed] [Google Scholar]
- 33. von Bahr L, Batsis I, Moll G et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long‐term engraftment and no ectopic tissue formation. Stem Cells 2012;30:1575–1578. [DOI] [PubMed] [Google Scholar]
- 34. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat Biotechnol 2014;32:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maumus M, Jorgensen C, Noel D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: Role of secretome and exosomes. Biochimie 2013;95:2229–2234. [DOI] [PubMed] [Google Scholar]
- 36. Ren G, Zhang G, Zhao X et al. Mesenchymal stem cell‐mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008;2:141–150. [DOI] [PubMed] [Google Scholar]
- 37. Ma S, Xie N, Li W et al. Immunobiology of mesenchymal stem cells. Cell Death Differ 2014;21:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benvenuto F, Voci V, Carminati E et al. Human mesenchymal stem cells target adhesion molecules and receptors involved in T cell extravasation. Stem Cell Res Ther 2015;6:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Castro‐Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: Biological aspects and clinical applications. J Immunol Res 2015. ;2015:394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Du Rocher B, Mencalha AL, Gomes BE et al. Mesenchymal stromal cells impair the differentiation of CD14(++) CD16(‐) CD64(+) classical monocytes into CD14(++) CD16(+) CD64(++) activate monocytes. Cytotherapy 2012;14:12–25. [DOI] [PubMed] [Google Scholar]
- 41. Chen PM, Liu KJ, Hsu PJ et al. Induction of immunomodulatory monocytes by human mesenchymal stem cell‐derived hepatocyte growth factor through ERK1/2. J Leukoc Biol 2014;96:295–303. [DOI] [PubMed] [Google Scholar]
- 42. Dutta P, Nahrendorf M. Regulation and consequences of monocytosis. Immunol Rev 2014;262:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frantz S, Hofmann U, Fraccarollo D et al. Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J 2013;27:871–881. [DOI] [PubMed] [Google Scholar]
- 44. Panizzi P, Swirski FK, Figueiredo JL et al. Impaired infarct healing in atherosclerotic mice with Ly‐6C(hi) monocytosis. J Am Coll Cardiol 2010;55:1629–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Swirski FK, Libby P, Aikawa E et al. Ly‐6Chi monocytes dominate hypercholesterolemia‐associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 2007;117:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Auffray C, Fogg D, Garfa M et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007;317:666–670. [DOI] [PubMed] [Google Scholar]
- 47. Henke A, Spengler HP, Stelzner A et al. Lipopolysaccharide suppresses cytokine release from coxsackie virus‐infected human monocytes. Res Immunol 1992;143:65–70. [DOI] [PubMed] [Google Scholar]
- 48. Yu JZ, Wilson JE, Wood SM et al. Secondary heterotypic versus homotypic infection by Coxsackie B group viruses: Impact on early and late histopathological lesions and virus genome prominence. Cardiovasc Pathol 1999;8:93–102. [DOI] [PubMed] [Google Scholar]
- 49. Terry RL, Getts DR, Deffrasnes C et al. Inflammatory monocytes and the pathogenesis of viral encephalitis. J Neuroinflammation 2012;9:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Penn MS, Pastore J, Miller T et al. SDF‐1 in myocardial repair. Gene Ther 2012;19:583–587. [DOI] [PubMed] [Google Scholar]
- 51. Krieger JR, Ogle ME, McFaline‐Figueroa J et al. Spatially localized recruitment of anti‐inflammatory monocytes by SDF‐1alpha‐releasing hydrogels enhances microvascular network remodeling. Biomaterials 2016;77:280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marchant DJ, Boyd JH, Lin DC et al. Inflammation in myocardial diseases. Circ Res 2012;110:126–144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.


