Abstract
Implants of collagen-fibronectin gels containing Bcl-2-transduced human umbilical vein endothelial cells (Bcl-2-HUVECs) induce the formation of human endothelial cell (EC)/murine vascular smooth muscle cell (VSMC) chimeric vessels in immunodeficient mice. Microfil casting of the vasculature 60 d after implantation reveals highly branched microvascular networks within the implants that connect with and induce remodeling of conduit vessels arising from the abdominal wall circulation. Approximately 85% of vessels within the implants are lined by Bcl-2-positive human ECs expressing VEGFR1, VEGFR2, and Tie-2, but not integrin αvβ3. The human ECs are seated on a well formed human laminin/collagen IV-positive basement membrane, and are surrounded by mouse VSMCs expressing SM-α actin, SM myosin, SM22α, and calponin, all markers of contractile function. Transmission electron microscopy identified well formed EC–EC junctions, chimeric arterioles with concentric layers of contractile VSMC, chimeric capillaries surrounded by pericytes, and chimeric venules. Bcl-2-HUVEC-lined vessels retain 70-kDa FITC-dextran, but not 3-kDa dextran; local histamine rapidly induces leak of 70-kDa FITC-dextran or India ink. As in skin, TNF induces E-selectin and vascular cell adhesion molecule 1 only on venular ECs, whereas intercellular adhesion molecule-1 is up-regulated on all human ECs. Bcl-2-HUVEC implants are able to engraft within and increase perfusion of ischemic mouse gastrocnemius muscle after femoral artery ligation. These studies show that cultured Bcl-2-HUVECs can differentiate into arterial, venular, and capillary-like ECs when implanted in vivo, and induce arteriogenic remodeling of the local mouse vessels. Our results support the utility of differentiated EC transplantation to treat tissue ischemia.
Keywords: angiogenesis, cell transplantation, endothelium
Blood vessel formation begins with the generation of simple endothelial cell (EC)-lined tubes through either angiogenesis (generation of new vessels from differentiated ECs of existing vessels) or vasculogenesis (formation of vessels from circulating EC progenitors). These primitive endothelial tubes recruit supporting cells [pericytes or smooth muscle (SM) cells], and undergo enlargement and/or pruning as they develop into a mature vascular bed of interconnected arteries, capillaries, and veins (1). The ECs lining different vessel types acquire specialized functional characteristics, such as vasoregulation by arterial/arteriolar ECs, permselectivity by capillary ECs, or proinflammatory responses by postcapillary venular ECs to cytokines and autacoids (2). It is unknown to what extent these specialized ECs retain the capacity to redifferentiate into other types of ECs.
Most physiological angiogenesis and vasculogenesis ceases during fetal life. Reactivation of these processes has therapeutic potential to increase perfusion within ischemic tissues. Although attempts to induce angiogenesis with cytokines such as VEGF have shown benefit in some animal models (3), the resulting neovessels remain disorganized and immature (4). In controlled clinical trials, VEGF monotherapy fails to increase perfusion because it does not enlarge arterioles feeding the site, a process referred to as arteriogenesis (5). Infusion of stem cells to initiate vasculogenesis has been attempted experimentally, but little is yet known about how to effectively use this approach (6). An alternative to growth factor or stem cell-based therapy is in vivo implantation of differentiated ECs. ECs in gels spontaneously organize into networks of hollow vascular “tubes” that, after surgical implantation into a host, can inosculate into the circulation (7). Such EC-based neovascularization strategies may be enhanced by genetic manipulation of the cells before implantation, e.g., by expressing antiapoptotic proteins or factors that enhance vessel growth and maturation (8, 9). Although several techniques of EC implantation (using extracellular matrices of Matrigel or type I collagen) have been described (10–12), the resulting vessels have not been extensively characterized. We previously reported that implantation of collagen/fibronectin gels containing human umbilical vein endothelial cells (HUVECs) retrovirally transduced with the antiapoptotic gene Bcl-2 (but not control-transduced ECs) efficiently recruit host SM cells to form chimeric microvessels within the abdominal wall of immunodeficient mice. Here, we characterize these structures as mature vessels and further report that implantation of Bcl-2-HUVEC induces a host arteriogenic response that can increase perfusion of ischemic tissue.
Materials and Methods
Abdominal Wall Vascular Implants. Bcl-2-HUVECs, generated as described (8), were suspended in collagen/fibronectin gels and implanted into the abdominal walls of 8-week-old C.B-17 severe combined immunodeficient/beige mice (Taconic Farms) based on the method of Schechner et al. (11) under protocols approved by the Yale Human Investigations and Yale Animal Use and Care Committees (Details are available in Supporting Methods, which is published as supporting information on the PNAS web site). At the end of each experiment, gels were harvested from killed mice by removing the abdominal wall in the area immediately surrounding the gel for analyses as described below.
Microfil Perfusion. Sixty days after implantation, mice were anesthetized and perfused with 20 ml of 37°C PBS plus 10 units/ml heparin at a flow rate of 10–15 ml/min through the left ventricle. After PBS, mice received 20 ml of 4% paraformaldehyde, and 15 ml of Microfil [MV-112 (white), Flowtech, Carver, MA]. The Microfil polymerized overnight at 4°C, and the collagen gels and underlying abdominal musculature were harvested and clarified in graded glycerol solutions (40–100% glycerol in water, increased by 20% glycerol at 24-h intervals). The clarified specimens were viewed on an SMZ1000 dissecting microscope (Nikon).
Histology and Immunohistochemistry. Routine histology and immunohistochemistry were performed as described (11). Single and double staining was performed on 5-μm-thick frozen sections. (For antibody suppliers, see Supporting Methods.) To develop red-color staining, the ABC-horseradish peroxidase Elite reagent and 3-amino-9-ethylcarbazole substrate (both from Vector Laboratories) were used. For blue-color staining, alkaline phosphatase (AP)-linked secondary plus AP-linked anti-AP tertiary antibodies (both from Jackson ImmunoResearch) and the Vector blue substrate (Vector Laboratories) were used. Slides were coverslipped by using Aqua-Mount (Lerner Laboratories, Pittsburgh).
Electron Microscopy. Sixty days after abdominal wall implantation, cardiac perfusion with fixative (1% gluteraldehyde and 3% paraformaldehyde) was performed on anesthetized animals. The implants were dissected from surrounding tissue, cut into ≈2-mm3 cubes, and processed as described (13). Sections were viewed on an EM 910 electron microscope (Zeiss) at 80 kV.
Fluorescent Dextran and India Ink Injections. To assess permeability of implanted microvessels, 250 μl of either 70- or 3-kDa lysine-fixable FITC-dextran (Molecular Probes, both 25 mg/ml in PBS) was injected 30 or 3 min before harvest, respectively. In some cases, 200 μl of rhodamine-UEA-1 was also injected i.v. (5 min before harvest). To test autacoid responses, a 50-μl solution of histamine [3.7 μg/ml histamine, diluted in pyrogen-free saline from Histatrol stock solution (0.1 mg/ml histamine base, Center Laboratories, Port Washington, NY)] or saline alone was injected at the site of the implant after i.v. injection of 70-kDa FITC-dextran, and specimens were harvested 10 min later. All specimens were fixed overnight in 4% paraformaldehyde at 4°C, frozen in OCT compound, and 10-μm sections were photographed by using a fluorescence microscope (Microphot-FXA, Nikon; or Axiovert 200M, Zeiss). For colloidal carbon injections, 50 μl of PBS-dialyzed India ink (60 mg/ml, Art Products, Statesville, NC) was injected i.v. before local histamine or saline injections. Specimens were harvested 2 h later, snap-frozen, sectioned, stained with biotin-UEA-1, and analyzed by immunohistochemistry as described above.
TNF Treatment in Vitro and in Vivo. To evaluate EC responses to TNF in vitro, TNFα (R & D Systems) or saline was added to confluent Bcl-2-HUVEC monolayers at a final concentration of 10 ng/ml. Six hours later, cells were trypsinized, washed, and stained by using anti-human E-selectin, vascular cell adhesion molecule 1 (VCAM-1), or intercellular adhesion molecule-1 (ICAM-1) antibodies, and analyzed by indirect immunofluorescence flow cytometry as described (14). Alternatively, Bcl-2-HUVECs were suspended in collagen/fibronectin gels, and 18 h later, TNF was added to the inserts at a final concentration of 10 ng/ml. Six hours after addition of TNF, gels were snap-frozen for analysis by immunohistochemistry. To determine in vivo responses, animals received Bcl-2-HUVECs in collagen/fibronectin gel implants, and, in some cases, human skin grafts (as described in ref. 15). Thirty to 60 days after implantation, the mice were injected s.c. at a distal site with 300 ng TNF. Both skin and gels were harvested 6 h later and analyzed by immunohistochemistry as described above.
Hindlimb Ischemia. To induce ischemia, the femoral arteries were ligated bilaterally 3–5 mm distal to the profunda femoris by using 9/0 proline sutures (Ethicon, Somerville, NJ), and the reduced flow was verified by laser Doppler perfusion scanning (Periflux system 5000, Perimed AB, Järfälla, Sweden) with output analyzed by using powerlab software from AD Instruments (Colorado Springs, CO). Immediately after ligation, the fascia above the right or left gastrocnemius was opened, muscle fibers were bluntly separated by using forceps to form a 3-mm-long pocket, Bcl-2-HUVEC gels were inserted between muscle fibers, and the fascia was closed by using 7/0 proline sutures. The contralateral hindlimb received a mock construct containing collagen/fibronectin alone. Placement of Bcl-2-HUVEC or mock implant was blinded and randomized during surgery and subsequent perfusion measurements. Fifteen days after implantation, the mice were anesthetized, the skin opened over the gastrocnemius, and perfusion was assessed by using laser Doppler scanning over the muscle. Relative perfusion was calculated as a ratio of values acquired from the limb receiving the Bcl-2-HUVEC/limb containing the mock implant, providing an internal control for interanimal variation in anesthesia and heart rate. After perfusion measurements, the mice were killed, and tissues were fixed in formalin, sectioned, and stained as described above.
Results
Microfil Casting. To evaluate the vascular architecture of implanted gels containing Bcl-2-HUVECs, we prepared Microfil casts of mice implanted with either cellularized or acellular gels. Animals receiving ECs produced a dense, highly branched vascular network made up of heterogeneously sized vessels within the implanted gel that inosculated with the surrounding mouse vasculature at multiple locations. Unexpectedly, the host abdominal wall vasculature enlarged in response to the cellularized grafts, resulting in the development of multiple conduit (larger diameter) host vessels connecting the gel to the native epigastric vessels (Fig. 1 a, c, and d arrows). Gels devoid of ECs remained avascular and the abdominal wall vasculature of animals receiving these mock implants did not remodel, appearing identical to the epigastric vessels of mice that did not undergo surgery (Fig. 1b). Intravital microscopy of mice receiving Bcl-2-HUVEC implants and i.v.-injected with the human-specific lectin Ulex europaeus agglutinin (UEA)-1 demonstrated the human origin of the arborized vascular network within the gel (Fig. 7, which is published as supporting information on the PNAS web site).
Fig. 1.
Microfil casting. Glycerol-cleared casts of abdominal-wall vasculature of mice bearing either a collagen/fibronectin gel implant containing Bcl-2-HUVEC (a, c, and d) or a mock implant (b). (c and d) Higher magnifications of cellularized implants that reveal heterogeneously sized, branched vessels within the graft, and induced remodeling of conduit vessels connecting the implants to the local mouse circulation Results representative of casts from six animals. (Scale bar, 1 mm.)
Light Microscopy and Immunohistochemistry. We characterized microvessels within the gels after implantation of Bcl-2-HUVECs by light microscopy and immunohistochemistry. In hematoxylin/eosin-stained specimens, gel grafts harvested 60 d after implantation showed numerous microvessels of various diameters as described (11) (Fig. 8a, which is published as supporting information on the PNAS web site). The larger-sized vessels, ranging up to 50–75 μm in diameter, were either coated by multiple concentric layers of mural cells as found in arterioles, or by a more attenuated cell coating as found in venules. Numerous smaller (7–15 μm in diameter) vessels resembling simple capillaries were also observed. ECs that lined the vessels within the collagen-fibronectin gels were mostly positive for human CD31 and the Bcl-2 transgene (data not shown); ECs positive for murine CD31 were largely confined to the edges of the graft, accounting for 15 ± 3% (n = 6) of total vascular profiles within the implants. The human ECs were positive for human tie-2 (Fig. 8d) as well as VEGFR-1 and VEGFR-2 (data not shown), markers characteristic of vascular endothelium. The basement membranes of Bcl-2-HUVEC-lined vessels stained positively for human laminin and human type-IV collagen (using species-specific antibodies), resembling that seen in human skin (Fig. 8 e and f).
Cultured HUVECs require serum and growth factors for survival and proliferation in vitro. Consequently, cultured ECs enter cell cycle and express αvβ3, an integrin associated with angiogenic ECs in vivo (16). αVβ3 was uniformly expressed by Bcl-2-HUVECs in standard culture or in cells suspended in collagen gels for 18 h in vitro but was undetectable by immunohistochemistry in gels harvested at 60 d after implantation (Fig. 8b). ECs in 60-d implants were quiescent, as judged by absence of staining for Ki-67 or proliferating cell nuclear antigen (D.R.E., unpublished data).
Vascular SM cells (VSMCs) in normal vessels express several proteins that have been used to define a contractile state; many of these proteins are lost during intimal expansion or in cell culture (17). To assess the phenotype of cells investing the neovessels formed after EC implantation, we stained for markers of differentiated VSMCs. None of these investing cells express the human transgene (Bcl-2), which is consistent with their putative murine origin. Investing cells in chimeric arterioles were positive for SM α-actin (SMA), as well as calponin, SM22-α, and SM myosin (Fig. 2), all proteins associated with differentiated VSMC phenotypes (17).
Fig. 2.
Cellular phenotyping of investing VSMCs. Recruited mouse cells express SMA (a), calponin (b), SM22α (c), and SM myosin (d) molecules indicative of VSMC contractile function. All staining is red with blue hematoxylin counterstain. Similar results were obtained from four specimens. (Scale bar, 100 μm.)
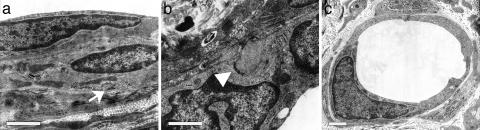
Electron Microscopy. Samples harvested at 60 d were analyzed by transmission electron microscopy. At the ultrastructural level, there were multiple vessels with characteristics of contractile arterioles, including investment by multiple layers of VSMCs containing microfilament networks loosely organized around peripheral dense bodies and subsarcolemmal dense plaques, consistent with the formation of an SMC contractile apparatus (Fig. 3a). These vessels displayed a well developed basement membrane and EC–EC interdigitations consistent with formation of junctional complexes (Fig. 3b). In addition, numerous simple, small vessels associated with occasional investing pericytes were present, which is consistent with the formation of true capillaries (Fig. 3c).
Fig. 3.
Transmission electron microscopy. Engrafted vessels have recruited multiple layers of investing cells (a) that contain networks of microfilaments organized around dense bodies and subsarcolemmal dense plaques (arrow), which is consistent with formation of an SMC contractile apparatus. EC junctional complexes (b, arrowhead) and basement membrane are also visible. Numerous simple capillaries (c) are also present in the implants. Images were collected from an analysis of four specimens. (Scale bars, 1 μm.)
Permselectivity. ECs regulate the passage of macromolecular solutes by controlling transcellular transport and the relative tightness of cell–cell junctions (18), restricting extravasation of plasma proteins such as albumin (66 kDa), while allowing smaller solutes to pass out of vessels into the surrounding tissues. Seventy kilodalton dextran (similar in size to albumin) is also retained within nonfenestrated, unstimulated vessels (19). In contrast, smaller 3-kDa dextran readily diffuses into the intersitium under all conditions. To test the permselectivity of the Bcl-2-HUVEC, 60 d after implantation, mice received i.v. injections of 70-kDa FITC-dextran, which was allowed to circulate for 30 min before harvest, and UEA-1, which was administered 5 min before harvest. In these specimens, the 70-kDa FITC-dextran was retained within the engrafted (UEA-1-positive) vessels and did not accumulate in the interstitial space, indicating the formation of a barrier to extravasation by the Bcl-2-HUVEC (Fig. 4a), whereas 3-kDa FITC-dextran readily leaked out of the bloodstream and was distributed throughout the collagen gel within 3 min of injection (Fig. 4b).
Fig. 4.
Analysis of vascular permeability and histamine response. (a) Bcl-2-HUVECs [stained with i.v.-injected, human EC-binding lectin rhodamine-UEA-1 (red)] retain 70-kDa FITC-dextran (a, green) that remains within vessel lumena 30 min after injection (n = 5). (b) In contrast, 3-kDa FITC-dextran (b, green) diffuses throughout the interstitum within 3 min of i.v. injection (n = 3). (c) Histamine injection induces leak of circulating 70-kDa FITC-dextran within gels containing Bcl-2-HUVECs (c, green) (n = 6). (d) Extravasation of circulating colloidal carbon (arrows) by a subset Bcl-2-HUVEC-lined vessels (identified by UEA-1 staining, red) after local injection of histamine, but not saline (Inset) (n = 3). (Scale bars, 100 μm.)
Histamine Response. Whereas normal, unstimulated vascular ECs retain large macromolecules under resting conditions, leakage of macromolecules may be triggered by stimuli such as histamine, VEGF, or TNF. Histamine rapidly induces a leak in the venular endothelium (20), which expresses higher levels of the H1 receptor (21). To test whether the Bcl-2-HUVECs were responsive to histamine, we administered 70-kDa FITC-dextran i.v., followed by histamine or saline, injected locally to the site of the implant 10 min before harvest. After histamine treatment, diffuse green fluorescence was observed within the implant, indicating a leak of 70-kDa FITC-dextran from graft vessels (Fig. 4c). This leak was not seen in saline-injected controls or mock implants containing collagen alone (data not shown). To identify the specific histamine-responsive vessels within the graft, we i.v.-injected colloidal carbon, a tracer that accumulates in the subendothelial spaces of histamine-responsive venules (20), followed immediately by local injection of histamine or saline. The mice were harvested 2 h later, allowing for clearance of the circulating colloid. In the histamine-treated samples, we observed extravasated carbon associated with a subset of UEA-1-positive vessels (Fig. 4d), which were only rarely observed in saline-treated gels (Fig. 4d Inset), confirming that only a subset of the vessels within the graft were histamine-responsive. Although we have not further characterized the responsive segments, they are larger than capillaries and are likely to be venules.
TNF Response. ECs lie at the interface between the blood and the tissues and are able to promote leukocyte recruitment into inflamed areas through TNF-induced adhesion molecule up-regulation. However, normal EC responses to TNF are not uniform across the vasculature: E-selectin and VCAM-1 up-regulation is limited to the postcapillary venules, whereas ICAM-1 is up-regulated in all ECs (2). These differences are lost in cultured ECs that uniformly up-regulate all three adhesion molecules. As expected, TNF-induced E-selectin expression uniformly in Bcl-2-HUVECs cultured on 2D plates and within 3D collagen gels (Fig. 5 a and b). A similar, uniform pattern of up-regulation was also observed for VCAM-1 and ICAM-1 (data not shown). However, 30–60 d after implantation, only a subset of Bcl-2-HUVECs up-regulated E-selectin (Fig. 5) and VCAM-1 (Fig. 5f), whereas all ECs continued to up-regulate ICAM-1 (Fig. 5h) (indicating all vessels were exposed to TNF). This pattern of TNF-response was similar to that seen in control human skin grafts present on mice receiving Bcl-2-HUVEC implants. In both sites, anti-SMA staining demonstrated that the subset of vessels that up-regulated E-selectin in vivo were generally those vessels of larger caliber which are surrounded by a less-developed VSMC coat (compared with E-selectin-negative vessels of a similar caliber), correlating venular behavior (E-selectin up-regulation) with venular morphology (lesser VSMC investment) (Fig. 5 i and j).
Fig. 5.
Analysis of TNF response in vitro and in vivo. In vitro, Bcl-2-HUVECs uniformly up-regulate E-selectin in response to TNF, as noted by flow cytometry of E-selectin expression on 2D-cultured ECs (a) or immunohistochemistry of Bcl-2-HUVECs suspended in TNF-treated gels (b Inset, with saline-treated control gel). Specimens in b were double-stained with anti-E-selectin (blue) and UEA-1 (red). (d, f, h, and j) In vivo, Bcl-2-HUVECs are compared with vessels in human skin grafts harvested from the same animal (shown in c, e, g, and i). (Insets) Identically stained, saline-treated controls are shown. Double staining with anti-E-selectin (c and d), anti-VCAM-1 (e and f), and anti-ICAM-1 (g and h, all in blue) with UEA-1 (red) demonstrates that all human ECs in both the skin and Bcl-2-HUVEC implants respond to TNF by up-regulating ICAM-1, in contrast to a restricted induction of E-selectin and VCAM-1. E-selectin expression is generally confined to vessels with venular, and not arteriolar, specialization of the vessel wall, as indicated by double-staining with anti-SMA (blue) and anti-E-selectin (red) in both human skin (i) and Bcl-2-HUVECs (j). Similar results obtained in gel implants from four experiments. (Scale bar, 100 μm.)
Engraftment in Ischemic Hindlimb. To test whether Bcl-2-HUVEC implants were capable of engrafting in and augmenting perfusion of ischemic tissue, we used a model of moderate hindlimb ischemia in which the femoral artery was ligated 3–5 mm distal to the profunda femoris, a site that (in severe combined immunodeficient/beige mice) produces clinical (mild foot clubbing and occasional gangrenous toe) and histological (myocyte injury and necrosis) signs of ischemia observable 2 weeks after ligation (Fig. 6). In six animals, we followed bilateral femoral ligation with implantation of gel containing Bcl-2-HUVECs into the right or left gastrocnemius muscle in a blinded, randomized fashion, with the contralateral limb receiving a mock gel of collagen/fibronectin alone. Vascular structures similar to those seen in the abdominal wall were observed in all animals receiving Bcl-2-HUVEC implants but not mock implants. Fifteen days after ligation/gel implantation, increased perfusion of the gastrocnemius receiving the Bcl-2-HUVEC implant was detected by laser Doppler perfusion monitoring in five of six animals, with an average perfusion ratio compared with mock implant of 1.60 ± 0.168 (P = 0.055, two-tailed paired Student's t test). In most animals, the presence of an EC-containing implant was associated with decreased foot clubbing and preservation of muscle mass. Quantitative assessment of the clinical benefit will require a larger trial.
Fig. 6.
Bcl-2-HUVEC implants engraft in ischemic hindlimb. After bilateral ligation of the femoral artery (a and b, arrowheads), implants containing Bcl-2-HUVECs (a) or a mock implant of collagen/fibronectin alone (b) were placed into the gastrocnemius muscle (a and b, black arrows). Hematoxylin/eosin staining demonstrates engraftment and formation of complex microvessels within the graft containing Bcl-2-HUVECs (c) but not mock (d). Images are representative of specimens harvested from six animals over two experiments. (White scale bar, 1 mm; black scale bar, 100 μm.)
Discussion
We previously reported a technique for implanting Bcl-2-transduced HUVECs into severe combined immunodeficient/beige mice, and demonstrated that the resulting microvascular networks contained heterogeneously sized microvessels variably invested by murine SMA-positive mesenchymal cells. In the present study, we characterized the 3D organization, cellular phenotypes, and functional properties of these neovessels. Our data indicate Bcl-2-HUVECs induce formation of a mature vascular bed lined by the implanted (human) ECs and recruited (mouse) VSMCs and pericytes, and acquire function associated with fully differentiated vessels. We also observed that microvessel implants induce a host arteriogenic response that can augment perfusion of ischemic tissues.
The ability of Bcl-2-HUVECs to develop into a complete microvascular bed composed of differentiated arterioles, capillaries, and venules suggests a surprising plasticity of endothelial fate. Classically, differences between arterial and venous ECs were attributed to physiological stimuli, but recent genetic evidence from model organisms has suggested that arterio/venous fate may be fixed during early embryonic vascular development and persist through adulthood (ref. 22; reviewed in ref. 23). However, the molecular mechanisms that maintain EC differentiation remain unclear, and studies of embryonic vascular patterning may not apply to the specialized and varied vasculature of adults. In cultured ECs, some characteristics of differentiated vessels become dysregulated (such as the TNF response), but other arterio/venous differences persist (24). In our study, we have taken ECs from a differentiated venous source (umbilical vein) and demonstrated these cells can acquire both arterial characteristics (investment by concentric layers of contractile VSMCs and reduced responsiveness to histamine and TNF) and venous characteristics (investment by fewer VSMCs and full responses to TNF and histamine). Other Bcl-2-HUVECs form narrow caliber vessels and associate with pericytes, which is indicative of capillary differentiation. These findings suggest that EC fate is not rigidly defined during embryonic development, and our model may be useful to study the mechanisms underlying arterial/venous differentiation.
Other techniques for producing microvessels from cultured ECs that have been described (10, 12) use Matrigel, a proangiogenic, incompletely defined tumor matrix with controversial effects on vessel formation (25). Such models are unlikely to be suitable for long-duration functional studies due to apoptosis of the human ECs and ingrowth of murine vessels. In contrast, we use a defined matrix composed only of type-I collagen and fibronectin that does not promote inflammation or significant ingrowth of murine ECs. This matrix proved suitable for use in long-term studies lasting 90–120 d, and should, through its simple composition, facilitate adaptation of this technique for use in artificial tissues. The utility of collagen/fibronectin gel-based models to study long-term vascular development has recently been confirmed by a study in which coculture of HUVECs with an immortalized murine mesenchymal line results in the formation of persistent microvascular beds in vivo (26).
Vessel maturation in our model depends on the recruitment and differentiation of mouse mesenchymal cells. Interestingly, when grown in a collagen/fibronectin matrix, Bcl-2 transduction is required for HUVECs to recruit VSMCs, and implants containing control-transduced ECs remain as simple endothelial-lined tubes. Although the precise molecular mechanism behind this Bcl-2-mediated effect is unknown, the changes do not result from increased vascular density alone (due to decreased apoptosis), but instead represent a qualitative change in the responses of the implanted HUVECs (11). Bcl-2 overexpression is not absolutely required for in vivo VSMC/pericyte recruitment when ECs are suspended in matrices other than collagen/fibronectin. For example, SMA-positive cells have been seen investing HUVECs suspended in Matrigel (12), decellularized human dermis (27), or even in collagen/fibronectin gels that also contain FBS (28). However, when ECs are seeded into decellularized dermis, Bcl-2 transduction promotes further mesenchymal cell recruitment, demonstrating that Bcl-2 continues to have a qualitative effect (27). In fact, Bcl-2 may play a more general role in physiologic angiogenesis as a mediator of the ECs response to VEGF (9, 29).
The effects of Bcl-2 on vessel development in vivo were not anticipated, and inhibition of apoptosis had been proposed to have negative effects on vessel maturation by blocking the normal pruning and remodeling required for nascent tubes to reorganize (30). This seemingly contradictory finding may result from the use of a different methods of apoptosis inhibition (in vivo N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone administration versus Bcl-2 overexpression), which may more completely block cell death and lack proangiogenic actions of Bcl-2. Regardless of the precise molecular mechanisms behind Bcl-2's effects, transduction of ECs with Bcl-2 appears to be a safe manipulation that does not transform the ECs or induce formation of vascular tumors (31). Furthermore, Bcl-2 transduction has been shown to confer resistance to immune-mediated graft rejection, a property that will be critical if allogeneic synthetic microvessel grafts are to be implanted into immunocompetent hosts.
The chimeric microvascular bed formed by Bcl-2-HUVECs is capable of inducing remodeling/growth of the feeding arterioles and increasing overall tissue perfusion. This arteriogenic effect is demonstrated by the formation of large-diameter conduit vessels seen in microfil vascular casts and the augmentation of blood flow by Bcl-2-HUVEC implants in the ischemic hindlimb. Such an increase in the arterial supply is critical to treatment of ischemic vascular diseases. This finding was unexpected because it is not observed in microvessels induced by overexpression of growth factors such as VEGF-165 (5). Further studies will be needed to elucidate the mechanism responsible for this effect that may result from flow-induced changes or factors produced by the implanted Bcl-2-HUVECs.
Supplementary Material
Acknowledgments
We thank Tom Ardito for assistance with electron microscopy. This work was supported by National Institutes of Health Grants HL-36003, HL-51014, AR-41942, and AR-02134, Medical Scientist Training Program Grant GM-07205, the Roche Organ Transplantation Research Foundation, a Medical Research Council planning grant to the Joint Cambridge–Yale Cardiovascular Research Program, and the British Heart Foundation.
Author contributions: D.R.E., B.R.S., A.Q., C.M.S., P.L.W., M.K., J.S.P., and J.S.S. designed research; D.R.E., B.R.S., Y.W., A.Q., and J.S.S. performed research; C.M.S., P.L.W., and M.K. contributed new reagents/analytic tools; D.R.E., B.R.S., A.Q., C.M.S., P.L.W., M.K., J.S.P., and J.S.S. analyzed data; and D.R.E., J.S.S., and J.S.P. wrote the paper.
Abbreviations: SM, smooth muscle; EC, endothelial cell; SMA, SM α-actin; HUVEC, human umbilical vein EC; VSMC, vascular SM cell; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule 1; UEA, Ulex europaeus agglutinin.
References
- 1.Jain, R. K. (2003) Nat. Med. 9, 685-693. [DOI] [PubMed] [Google Scholar]
- 2.Choi, J., Enis, D., Koh, K., Shiao, S. & Pober, J. S. (2004) Annu. Rev. Immunol. 22, 683-709. [DOI] [PubMed] [Google Scholar]
- 3.Tsurumi, Y., Takeshita, S., Chen, D., Kearney, M., Rossow, S. T., Passeri, J., Horowitz, J. R., Symes, J. F. & Isner, J. M. (1996) Circulation 94, 3281-3290. [DOI] [PubMed] [Google Scholar]
- 4.Dor, Y., Djonov, V. & Keshet, E. (2003) Ann. N.Y. Acad. Sci. 995, 208-216. [DOI] [PubMed] [Google Scholar]
- 5.de Muinck, E. D. & Simons, M. (2004) J. Mol. Cell. Cardiol. 36, 25-32. [DOI] [PubMed] [Google Scholar]
- 6.Kawamoto, A., Asahara, T. & Losordo, D. W. (2002) Cardiovasc. Radiat. Med. 3, 221-225. [DOI] [PubMed] [Google Scholar]
- 7.Vailhe, B., Vittet, D. & Feige, J. J. (2001) Lab. Invest. 81, 439-452. [DOI] [PubMed] [Google Scholar]
- 8.Zheng, L., Dengler, T. J., Kluger, M. S., Madge, L. A., Schechner, J. S., Maher, S. E., Pober, J. S. & Bothwell, A. L. (2000) J. Immunol. 164, 4665-4671. [DOI] [PubMed] [Google Scholar]
- 9.Nor, J. E., Christensen, J., Mooney, D. J. & Polverini, P. J. (1999) Am. J. Pathol. 154, 375-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nor, J. E., Peters, M. C., Christensen, J. B., Sutorik, M. M., Linn, S., Khan, M. K., Addison, C. L., Mooney, D. J. & Polverini, P. J. (2001) Lab. Invest. 81, 453-463. [DOI] [PubMed] [Google Scholar]
- 11.Schechner, J. S., Nath, A. K., Zheng, L., Kluger, M. S., Hughes, C. C., Sierra-Honigmann, M. R., Lorber, M. I., Tellides, G., Kashgarian, M., Bothwell, A. L. & Pober, J. S. (2000) Proc. Natl. Acad. Sci. USA 97, 9191-9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skovseth, D. K., Yamanaka, T., Brandtzaeg, P., Butcher, E. C. & Haraldsen, G. (2002) Am. J. Pathol. 160, 1629-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slowik, M. R., De Luca, L. G., Min, W. & Pober, J. S. (1996) Circ. Res. 79, 736-747. [DOI] [PubMed] [Google Scholar]
- 14.Kluger, M. S., Johnson, D. R. & Pober, J. S. (1997) J. Immunol. 158, 887-896. [PubMed] [Google Scholar]
- 15.Murray, A. G., Petzelbauer, P., Hughes, C. C., Costa, J., Askenase, P. & Pober, J. S. (1994) Proc. Natl. Acad. Sci. USA 91, 9146-9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sipkins, D. A., Cheresh, D. A., Kazemi, M. R., Nevin, L. M., Bednarski, M. D. & Li, K. C. (1998) Nat. Med. 4, 623-626. [DOI] [PubMed] [Google Scholar]
- 17.Shanahan, C. M. & Weissberg, P. L. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 333-338. [DOI] [PubMed] [Google Scholar]
- 18.Michel, C. C. & Curry, F. E. (1999) Physiol. Rev. 79, 703-761. [DOI] [PubMed] [Google Scholar]
- 19.Dvorak, H. F., Nagy, J. A., Dvorak, J. T. & Dvorak, A. M. (1988) Am. J. Pathol. 133, 95-109. [PMC free article] [PubMed] [Google Scholar]
- 20.Majno, G., Palade, G. E. & Schoefl, G. I. (1961) J. Biophys. Biochem. Cytol. 11, 607-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heltianu, C., Simionescu, M. & Simionescu, N. (1982) J. Cell Biol. 93, 357-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin, D., Garcia-Cardena, G., Hayashi, S., Gerety, S., Asahara, T., Stavrakis, G., Isner, J., Folkman, J., Gimbrone, M. A., Jr., & Anderson, D. J. (2001) Dev. Biol. 230, 139-150. [DOI] [PubMed] [Google Scholar]
- 23.Lawson, N. D. & Weinstein, B. M. (2002) Nat. Rev. Genet. 3, 674-682. [DOI] [PubMed] [Google Scholar]
- 24.Chi, J. T., Chang, H. Y., Haraldsen, G., Jahnsen, F. L., Troyanskaya, O. G., Chang, D. S., Wang, Z., Rockson, S. G., van de Rijn, M., Botstein, D. & Brown, P. O. (2003) Proc. Natl. Acad. Sci. USA 100, 10623-10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vernon, R. B., Angello, J. C., Iruela-Arispe, M. L., Lane, T. F. & Sage, E. H. (1992) Lab. Invest. 66, 536-547. [PubMed] [Google Scholar]
- 26.Koike, N., Fukumura, D., Gralla, O., Au, P., Schechner, J. S. & Jain, R. K. (2004) Nature 428, 138-139. [DOI] [PubMed] [Google Scholar]
- 27.Schechner, J. S., Crane, S. K., Wang, F., Szeglin, A. M., Tellides, G., Lorber, M. I., Bothwell, A. L. & Pober, J. S. (2003) FASEB J. 17, 2250-2256. [DOI] [PubMed] [Google Scholar]
- 28.Zheng, L., Gibson, T. F., Schechner, J. S., Pober, J. S. & Bothwell, A. L. (2004) J. Immunol. 173, 3020-3026. [DOI] [PubMed] [Google Scholar]
- 29.Gerber, H. P., Dixit, V. & Ferrara, N. (1998) J. Biol. Chem. 273, 13313-13316. [DOI] [PubMed] [Google Scholar]
- 30.Segura, I., Serrano, A., De Buitrago, G. G., Gonzalez, M. A., Abad, J. L., Claveria, C., Gomez, L., Bernad, A., Martinez, A. C. & Riese, H. H. (2002) FASEB J. 16, 833-841. [DOI] [PubMed] [Google Scholar]
- 31.Schechner, J. S., Pober, J. S., Bothwell, A. L. M., Wang, F. & Arbiser, J. (2001) in 62nd Annual Meeting of the Society for Investigative Dermatology (Blackwell Science, Oxford), Vol. 117, pp. 492. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.