Abstract
Gene expression during oocyte maturation, fertilization, and early embryo development until zygotic gene activation is regulated mainly by translational activation of maternally derived mRNAs. This process requires the presence of a poly(A)-binding protein. However, the cytoplasmic somatic cell poly(A)-binding protein (PABP1) is not expressed until later in embryogenesis. We recently identified an embryonic poly(A)-binding protein (ePAB) in Xenopus. ePAB is the predominant cytoplasmic PABP in Xenopus oocytes and early embryos and prevents deadenylation of mRNAs, suggesting its importance in the regulation of gene expression during early Xenopus development. Here we report the identification of the mouse ortholog of Xenopus ePAB. The mouse ePAB gene on chromosome 2 contains 14 exons that specify an alternatively spliced mRNA encoding a protein of 608 or 561 aa with ≈65% identity to Xenopus ePAB. Mouse ePAB mRNA is expressed in ovaries and testis but not in somatic tissues. In situ hybridization localizes ePAB RNA to oocytes and confirms its absence from surrounding somatic cells in the mouse ovary. During early development, mouse ePAB is expressed in prophase I and metaphase II oocytes and one-cell and two-cell embryos and then becomes undetectable in four-or-more-cell embryos. In contrast, PABP1 mRNA expression is minimal in oocytes and early embryos until the eight-cell stage when it increases, becoming predominant at the blastocyst stage. The expression of mouse ePAB before zygotic gene activation argues for its importance in translational activation of maternally derived mRNAs during mammalian oocyte and early preimplantation embryo development.
Keywords: translational activation, embryogenesis, oogenesis
Mechanisms for establishing the germline and carrying out oogenesis in evolutionarily distant animals exhibit common themes. Gametes develop from primordial germ cells that are set aside during early embryogenesis (1). In most metazoans, primordial germ cells have an extragonadal origin and migrate to reach the somatic gonad, where they proliferate by mitosis to form oocytes (1). Oocytes, in turn, enter meiosis only to be arrested at the prophase of the first meiotic division (2, 3). This first meiotic arrest may last up to a few years in Xenopus and several decades in humans and is characterized by synthesis and storage of large quantities of dormant mRNA (4, 5). The resumption of meiosis is stimulated by progesterone in Xenopus (6, 7) or by gonadotropins in mouse and human (8, 9) and marks the onset of oocyte maturation.
Oocyte maturation is accompanied by a complex network of translational activation and repression of dormant maternal mRNAs (10–13), whereas transcription is limited at best. These maternal mRNAs drive the oocyte's reentry into meiosis and control the rate of mitosis during the early cleavage divisions of the embryo (13–16). The transcriptional silencing that begins with oocyte maturation persists during the initial mitotic divisions of the embryonic cells. In Xenopus, after 12 rapid synchronous cleavages generating >4,000 cells, the mid-blastula transition occurs and is characterized by the activation of zygotic transcription, also called zygotic gene activation (ZGA) (17, 18). In mouse and human, ZGA occurs at the two-cell and four- to eight-cell stages, respectively (19–21). Despite the earlier occurrence of ZGA in mammals compared with Xenopus, translational activation of maternally inherited mRNAs appears to use similar mechanisms (13, 16, 22).
The first step in translational activation of stored maternal mRNAs is cytoplasmic extension of their poly(A) tails. This process, called cytoplasmic polyadenylation, was initially thought to be confined to gametes and embryos, although recent evidence suggests that it is also important in neurons (23). The molecular mechanisms regulating cytoplasmic polyadenylation have been studied primarily in mouse (24, 25) and in Xenopus oocytes (26–29) and appear to be highly conserved. In addition to the nuclear cleavage and polyadenylation signal, AAUAAA, a second cis-acting sequence in the 3′ UTR of the mRNA, the cytoplasmic polyadenylation element (CPE) (consensus sequence UUUUUA1–2U), is necessary for cytoplasmic polyadenylation (30). The CPE is specific to mRNAs polyadenylated during meiotic maturation and binds the CPE-binding protein (CPEB) (12, 16). When Xenopus oocytes are stimulated by progesterone, the kinase aurora (Eg2) phosphorylates CPEB (11), an event that may help CPEB stabilize binding of the cleavage and polyadenylation specificity factor to the AAUAAA (31). However, simple extension of the poly(A) tail by a cytoplasmic form of poly(A) polymerase, which is recruited by cleavage and polyadenylation specificity factor binding to the AAUAAA sequence (32, 33), is not sufficient to explain maturation-induced translation of an oocyte mRNA because an inhibitory factor called maskin interacts simultaneously with CPEB and eIF4E (34) to inhibit the assembly of the eIF4G-mediated 43S initiation complex on CPE-containing mRNAs. Displacement of maskin requires that a poly(A)-binding protein (PABP) bind to the newly elongated poly(A) tail (35) and promote the association of eIF4G with eIF4E, allowing initiation of translation. Until recently, a cytoplasmic PABP present in significant amounts in vertebrate oocytes and embryos had not been identified.
Two structurally distinct groups of PABPs have been identified in vertebrates. PABP1 (36) [also called PABPC1 in human and mouse or PABC in Xenopus (37, 38)], the prototype of the first group, is ≈70 kDa and contains four RNA recognition motifs (RRMs) at its N terminus and a unique C-terminal PABP domain (37, 38). PABP1 is expressed in the cytoplasm of all cells in metazoans with the exception of oocytes and early embryonic cells (39) and is implicated in the control of mRNA stability and translation. An inducible PABP (iPAB or PABC4) described in human T cells (40) and a testis-specific PABP identified in human (41) and in mouse (42) belong to the same group as PABP1. PABPN1 [initially called PAB II (43)] is the prototype of the second group of PABPs. PABPN1 is smaller (49 kDa) (44), with only one RRM, and is present in all cells of the organism, including oocytes and embryos (45). PABPN1 is a nuclear protein required for processive elongation of the poly(A) tail and control of its length during pre-mRNA processing (46, 47). An embryonic, cytoplasmic form of this protein (ePABP2), which may be involved in cytoplasmic poly(A) tail elongation, has recently been described in Xenopus and mouse (45, 48).
An embryonic form of the first group of PABP was discovered in Xenopus oocytes (49). Named ePAB, it is a 629-aa cytoplasmic protein with 72% identity with Xenopus PABP1. ePAB is expressed during Xenopus oocyte and early embryo development and effectively suppresses mRNA deadenylation in Xenopus egg extracts (49). As the predominant PABP during Xenopus early development, ePAB most likely regulates unmasking/translation of maternal mRNAs and may influence poly(A) tail length as well. Therefore, ePAB may play a role in important reproductive problems, such as reproductive failure, aneuploidy, and embryo death, and it is of interest to determine whether a similar gene is expressed in mammals. In this study, we identified a mouse ortholog of Xenopus ePAB and characterized its expression pattern during mammalian oogenesis and preimplantation embryo development.
Materials and Methods
Identification and Sequencing of a Mouse ePAB Ortholog. Nucleotide and protein sequence databases were searched by using standard nucleotide–nucleotide blast (blastn), protein query versus translated database blast (tblastn), and translated query versus translated database blast (tblastx) at the National Center for Biotechnology Information blast server (www.ncbi.nlm.nih.gov/blast). The entire sequence of Xenopus ePAB (GenBank accession no. AAK29408) or the portion (amino acids 399–540) that is most divergent from Xenopus PABP1 were used (49).
EST BQ554130 was derived from the National Institute on Aging Mouse 7.4K cDNA clone set (50) assembled from cDNA libraries constructed mainly from early mouse embryos [embryonic day (e)3.5, e7.5, e12.5, and e13.5] in addition to unfertilized oocytes and several stem cell libraries (courtesy of M. Wang, California Institute of Technology, Pasadena). EST W41641 and EST AI893186 (Soares mouse embryo NbME e13.5–e14.5 library) were obtained from American Type Culture Collection (Manassas, VA). DNA was derived from two colonies for each EST by miniprep (Qiagen, Valencia, CA). DNA identities were confirmed by sequencing performed by the W. M. Keck Facility at Yale University. Computerized sequence comparisons were made by using the sequencher program (Gene Codes, Ann Arbor, MI).
Pairwise and multiple alignments of the mouse and Xenopus ePAB genes and proteins were performed by using the megalign program of the lasergene package (DNASTAR, Madison, WI). The prediction and assignment of the protein structures were performed by using pfam (http://pfam.wustl.edu).
Superovulation and Oocyte and Embryo Retrieval in Mouse. Mouse oocytes and preimplantation embryos were collected by using standard protocols (51). Briefly, 3-week-old CD1 female mice (Charles River Laboratories) were superovulated by i.p. injection of 5 units of pregnant mare serum gonadotropin (PMSG) (Folligon, Sigma–Aldrich). To collect oocytes arrested at prophase I (PI), mice were killed 20 h later by CO2 inhalation, the ovaries were removed, and the oocytes were isolated by puncturing the ovaries with a 23-gauge needle under the dissecting microscope (SZHILLK, Olympus, Melville, NY). For all other stages of oocyte and embryo development, an additional injection of 5 units of human chorionic gonadotrophin (hCG) (Chorulon, Sigma–Aldrich) was given 48 h after the PMSG injection. Unfertilized oocytes at metaphase of the second meiotic division (MII) were collected from oviducts 14 h after the hCG injection.
To obtain fertilized embryos, females were placed individually with 12-week-old CD1 males immediately after the hCG injection. The following morning, the effectiveness of mating was confirmed by the presence of a vaginal plug (day 1). One-cell embryos were collected at 21–24 h after hCG injection from the oviducts into Hepes-buffered human tubal fluid media (Irvine Scientific). Removal of the cumulus cells was achieved in Hepes-buffered media containing 1 mg/ml hyaluronidase (Sigma). Collection of embryos at the two-cell, four-cell, eight-cell, and blastocyst stages was performed at 42 h, 60 h, 68 h, and 96 h after hCG injection, respectively. MII oocytes and one-cell embryos were liberated by puncturing the ampullary portion of the oviduct with a needle under the dissecting microscope. Two- to eight-cell embryos were collected from the oviducts and blastocysts were collected from the uterine horns by flushing the oviducts and uterine horns with Hepes-buffered media under the dissecting microscope. Oocytes and embryos (100 each) were pooled and washed thoroughly in Hepes buffer.
RT-PCR of Oocytes, Preimplantation Embryos, and Somatic and Gonadal Tissues. Total RNA from oocytes or embryos was obtained by using a RNAqueous Microkit (Ambion, Austin, TX) according to the manufacturer's instructions and kept at -80°C until use. Amplification by RT-PCR was done in two steps. First, reverse-transcription (RT) reactions with oligo d(T) primers and the RETROscript kit (Ambion) were performed on total RNA from 100 oocytes or embryos according to the manufacturer's instructions. Then, PCR by using SuperTaq (Enzyme Technologies, Cambridge, U.K.) and primers for mouse ePAB or PABP1 was performed on cDNA from the equivalent of five oocytes or embryos. DNAs amplified from EST clones by using the same primers provided positive controls. Control PCR for β-actin was performed on the same RT samples. All primers are shown in Table 2, which is published as supporting information on the PNAS web site.
Amplifications were carried out by 30 cycles of PCR in which the initial 5-min denaturation at 94°C was followed by a “touch-down” program for 10 cycles of 92°C/30 s, 65°C/20 s (-1°C per cycle), and 72°C/1 min per kilobase and then 20 cycles of 92°C/30 s, 55°C/20 s, and 72°C/1 min per kilobase in a volume of 20 μl containing 1× PCR buffer (Roche), 0.125 mM of each dNTP, 0.5 μM of each primer, and 2 units of SuperTaq polymerase.
Total RNA was obtained from somatic and gonadal tissues of the same mice by using TRIzol (GIBCO/BRL) according to the manufacturer's instructions. RT reactions were performed by using the Omniscript kit (Qiagen) based on the manufacturer's instructions and 2 μg of total RNA from each tissue. PCR was performed as described above for oocytes and embryos.
In Situ Hybridization. Ovaries collected 20 h after stimulation with 5 units of PMSG were fixed in 4% paraformaldehyde overnight and dehydrated in increasing concentrations of ethanol. The ovaries were then cleared in xylene and embedded in paraffin. Paraffin blocks were stored at 4°C.
RNA hybridization probes were used to detect mouse ePAB RNA. The longer probe (ePAB 8–10.7) spans exons 8–10.7 (Table 3, which is published as supporting information on the PNAS web site) and is ≈550 nt long. The shorter probe (ePAB 11) detects only exon 11 (Table 3) and is ≈150 nt in length. Templates for synthesizing the sense and antisense ePAB 8–10.7 probe were prepared from EST-1 by PCR with primers containing the T7 promoter at either end. Templates for the ePAB 11 probe were amplified from mouse genomic DNA (Clontech). PCR products were purified by using the QIAquick PCR purification kit (Qiagen) according to the manufacturer's instructions. RNA probes were synthesized by in vitro transcription by using a digoxigenin RNA-labeling kit (SP6/T7), and the efficiency of synthesis was confirmed by comparison with a labeled control RNA in a dot blot according to the manufacturer's instructions (Roche Diagnostics). The labeled RNA probe was stored at -80°C until use.
Paraffin sections (5 μm) were incubated at 60°C for 1 h before in situ hybridization. Then, the sections were treated twice with fresh xylene and rehydrated in decreasing concentrations of ethanol followed by rinsing in water treated with 0.1% diethylpyrocarbonate (Sigma–Aldrich). Permeabilization with 1 μg/ml proteinase K in 1× PBS with 0.1% Tween 20 (PBST) at 37°C for 30 min was followed by postfixation in 4% paraformaldehyde for 5 min at room temperature. After being washed with PBST, sections were incubated in prehybridization buffer [50% formamide/5 mM EDTA (Sigma–Aldrich)/4× sodium chloride/sodium citrate (SSC)/0.1% Tween 20/1% blocking reagent (Roche Diagnostics)/100 μg/ml yeast tRNA (Roche Diagnostics)/50 μg/ml heparin (Sigma–Aldrich)/0.5% CHAPS (Sigma–Aldrich)] at 37°C for 1 h. Sections were then hybridized with 150–200 ng/ml digoxigenin-labeled sense or antisense RNA probe at 55°C overnight. After hybridization, slides were washed twice in 2× SSC and then in 1× SSC for 15 min each at 37°C. To degrade unbound RNA and reduce background, sections were RNase-A-treated (20 μg/ml) then given two 30-min washes in 0.1× SSC at 37°C and two 10-min washes in NT (100 mM Tris/150 mM NaCl) and MABT [0.1 M maleic acid (Sigma–Aldrich)/0.15 M NaCl/0.1% Tween 20] at room temperature. Blocking was performed in 1% (wt/vol) blocking reagent (Roche) in MAB [0.1 M maleic acid (Sigma–Aldrich)/0.15 M NaCl] for 30 min. Sections were incubated in anti-digoxigenin antibody conjugated to alkaline phosphatase (1:200; Roche Diagnostics) for 1 h at room temperature. After six 10-min washes with MABT, the sections were equilibrated in NTMT buffer [0.1 M NaCl/0.1 M Tris·HCl/50 mM MgCl2/2 mM Levamisole (Sigma–Aldrich)/0.1% Tween 20] and incubated in 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium in NTMT buffer color solution for ≈16 h [4.5 μl/ml 5-bromo-4-chloro-3-indolyl phosphate/3.5 μl/ml nitroblue tetrazolium (Roche Diagnostics)]. Color development was stopped with several washes in PBST, and slides were mounted (Immu-Mount, Thermo Shandon, Pittsburgh). Sections were observed by bright-field with an Olympus IX71 microscope.
Results
Identification and Characterization of the Mouse ePAB Gene. An initial database search to find the mouse ortholog of the Xenopus ePAB gene identified the database entry with GenBank accession no. AAK29408. The entry predicted 12 exons sharing 60% amino acid identity with the Xenopus gene.
The dbEST database of the National Center for Biotechnology Information was then searched for mouse ESTs with similarity to the predicted mouse ePAB gene. Three ESTs (GenBank accession nos. BQ554130, W41641, and AI893186) matching parts of the predicted nucleotide sequence were identified. All three ESTs were derived from cDNA libraries constructed mainly from early mouse embryos.
Sequencing of the mouse ePAB ESTs revealed two additional exons between exons 10 and 11 (Table 3), termed 10.5 and 10.7, whereas the predicted exon 11 was skipped. RT-PCR performed on RNA from mouse ovaries and oocytes, followed by sequencing, demonstrated the presence of the two additional exons and the skipping of exon 11. Data confirming the alternative splicing of exon 11 of mouse ePAB RNA is presented below.
The 3′ UTR (≈500 nt) was contained in all ESTs in its entirety, with the poly(A) tail initiating 9 nt downstream of the hexanucleotide (AUUAAA) (52). Exons 1 and 2 and part of exon 3 were not present in ESTs; these were amplified by using primers designed against the genomic sequence and oocyte cDNA as template and sequenced. Exact determination of the transcription start site was precluded by the paucity of oocyte RNA.
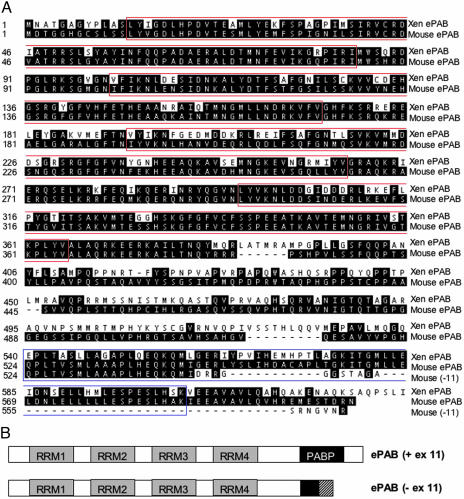
The predicted exon/intron boundaries of the ePAB gene were determined by aligning the nucleotide sequence with the mouse genome, identifying 14 exons on chromosome 2 (Table 3). Including all 14 exons, the ORF of mouse ePAB is 1,824 bp long encoding a 608-aa protein with 66% identity and 75% amino acid similarity to Xenopus ePAB (Fig. 1A). Like other PABPs, mouse ePAB contains four RRMs that bind RNA (53) and a C-terminal PABP domain, which functions in protein–protein interactions (53) (Fig. 1A and Table 1). The amino acid sequence is 76% identical to Xenopus ePAB in the N-terminal region, where the RRMs reside (amino acids 1–408), but only 44% identical in the C-terminal region (amino acids 409–608).
Fig. 1.
Amino acid sequences and domain structures of mouse ePAB alternatively spliced forms. (A) Pairwise alignment of the mouse and the Xenopus ePAB amino acid sequences. The sequences of both ePAB spliced variants, including and excluding exon 11, are shown. The four RRM motifs are boxed in red, and the PABP domain is boxed in blue. (B) Schematic representation of mouse ePAB alternatively spliced forms. The four RRMs are indicated by gray boxes. Because the PABP domain is encoded by exons 10.7 and 11, the shorter form (-ex 11) contains only part of the PABP motif (amino acids 524–543). The C termini of the two forms also differ because of shifting of the exon 12 reading frame.
Table 1. Pattern search and multiple alignment of mouse, human, and Xenopus PABPs.
| RRM1 | RRM2 | RRM3 | RRM4 | PABP domain | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | length, aa | e value | length, aa | e value | length, aa | e value | length, aa | e value | length, aa | e value |
| Mouse ePAB (+11) | 13–84 | 6.6 ± 10-27 | 101–170 | 4 × 10-23 | 193–263 | 2.2 × 10-27 | 296–365 | 4.7 × 10-23 | 524–595 | 1.3 × 10-20 |
| Mouse ePAB (–11) | 13–84 | 6.6 × 10-27 | 101–170 | 4 × 10-23 | 193–263 | 2.2 × 10-27 | 296–365 | 4.7 × 10-23 | Absent | Absent |
| Xenopus ePAB | 13–84 | 9.7 × 10-25 | 101–170 | 2.8 × 10-24 | 193–263 | 5.7 × 10-26 | 296–365 | 8.7 × 10-22 | 540–611 | 8.6 × 10-42 |
| Mouse PABP1 | 13–84 | 6.6 × 10-27 | 101–170 | 9 × 10-25 | 193–263 | 1.2 × 10-30 | 296–365 | 1.7 × 10-23 | 543–614 | 4.7 × 10-47 |
| Xenopus PABP1 | 13–84 | 5.4 × 10-27 | 101–170 | 8.8 × 10-25 | 193–263 | 6.1 × 10-27 | 296–365 | 4.1 × 10-22 | 541–612 | 1.3 × 10-47 |
| Human PABP1 | 13–84 | 3 × 10-27 | 101–170 | 9.2 × 10-25 | 193–263 | 7.5 × 10-31 | 296–365 | 1.8 × 10-23 | 543–614 | 9.5 × 10-48 |
The positions of each structrual motif and their similarity to the consensus are shown. The analysis was done with pfam (see Materials and Methods). Shown are expected values (e values) that describe the number of hits one can expect to see by chance when searching a database of a particular size. An e value of 1 assigned to a hit can be interpreted as meaning that, in a database of the current size, one might expect to see one match with a similar score simply by chance. The lower the e value is, the higher the significance of the match.
The alternatively spliced form of mouse ePAB mRNA without exon 11 has a 1,686-nt ORF encoding a 561-aa protein with 64% identity to Xenopus ePAB (Fig. 1A). In the C-terminal region (amino acid 409–561), the amino acid sequence shows 33% identity to Xenopus ePAB. In the absence of exon 11, the reading frame of exon 12 is shifted, producing a different C terminus (Fig. 1B). Specifically, exon 12 encodes 20 and 18 amino acids in the long and the short forms of ePAB, respectively.
Mouse ePAB Is Expressed Only in Gonadal Tissues. PABPs comprise a growing family of RNA-binding proteins, with multiple homologs of PABP1 identified in both mouse and human (40–42). To identify a gene as an embryonic PABP, it is therefore necessary to demonstrate that its expression is confined to gonads, gametes, and embryos. We first evaluated the expression pattern of the putative mouse ePAB in mouse somatic and gonadal tissues. RT reactions were performed by using 2 μg of total RNA from each tissue, followed by 30 cycles of PCR amplification with equal amounts of RT product for each reaction. Amplification with β-actin primers provided a control ensuring comparable loading and allowing semiquantitative analysis (54).
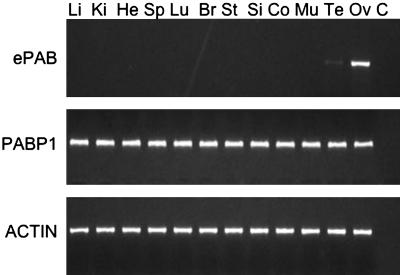
As expected, mouse ePAB mRNA was not detected in the somatic tissues tested but was present in mouse ovaries (Fig. 2). Expression of ePAB was also observed in testis although to a lesser extent. The same tissues were also analyzed for PABP1 mRNA, which was ubiquitously present.
Fig. 2.
Expression analysis of ePAB and PABP1 in mouse tissues. RT-PCR analysis of mRNA from several mouse tissues shows an ePAB-specific band in ovary and testis. PABP1 mRNA expression was detected in all tissues studied. β-Actin was used as an internal control. Li, liver; Ki, kidney; He, heart; Sp, spleen; Lu, lung; Br, brain; St, stomach; Si, small intestine; Co, colon; Mu, muscle; Te, testis; Ov, ovary; C, H2O.
Mouse ePAB and PABP1 Expression in Mouse Oocytes and Preimplantation Embryos. Next, we evaluated the temporal expression pattern of mouse ePAB and PABP1 mRNA in unfertilized oocytes (PI and MII) and preimplantation embryos (Fig. 3). Total RNA from 100 oocytes or embryos was used for each RT reaction; amplification by PCR was then performed by using cDNA from the equivalent of five oocytes or embryos. ZGA occurs at the two- to four-cell stage in the mouse. Therefore, we expected mouse ePAB to be present in oocytes and expressed until the two-cell stage, after which it would be replaced by newly synthesized PABP1.
Fig. 3.
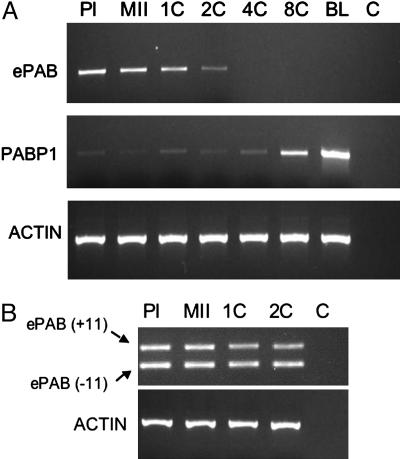
Expression analysis of mouse ePAB and PABP1 in oocytes and preimplantation embryos. (A) ePAB and PABP1 mRNA expression in mouse oocytes and preimplantation embryos at different stages of development. RNA was extracted from 100 oocytes or embryos and subjected to RT. PCR was performed on the resulting cDNA from five oocyte or embryo equivalents for each experiment by using specific primers for ePAB (primers 2F and 9R) or PABP1 (Table 2). β-Actin provided a loading control. PI, PI oocyte; MII, MII oocyte; 1C, one-cell embryo; 2C, two-cell embryo; 4C, four-cell embryo; 8C, eight-cell embryo; BL, blastocyst; C, H2O control. (B) Expression of alternatively spliced ePAB mRNA variants with and without exon 11 (+11 and -11; respectively) was evaluated in oocytes and one- and two-cell embryos by using ePAB-specific primers (10.5F–12R; see Table 2). Two ePAB-specific bands were detected at 318 nt (+exon 11) and 184 nt (-exon 11) by agarose gel fractionation. β-Actin provided a loading control.
As predicted, mouse ePAB mRNA was expressed in PI and MII oocytes and in one-cell and two-cell embryos but was not detected in four-cell or more advanced embryos (Fig. 3A). A decrease was observed in mouse ePAB expression at the two-cell stage compared with oocytes.
To investigate the skipping of exon 11 observed in the ESTs, we performed RT-PCR by using primers in exons 10.7 and 12 and RNA from oocytes and early embryos. Two bands were detected by agarose gel fractionation, and sequencing of these PCR products confirmed the inclusion of exon 11 in a subset of ePAB RNA (Fig. 3B). Both splice variants were present up to the two-cell stage.
In contrast to ePAB, PABP1 mRNA expression was minimal but detectable until the eight-cell stage of embryonic development, when it showed an increase (Fig. 3A). This increase was further pronounced at the blastocyst stage. The low expression of PABP1 in oocytes and in one- to four-cell embryos, as well as its later increase at the eight-cell and blastocyst stages, was confirmed in repeated experiments with different amplification cycle numbers and amounts of cDNA.
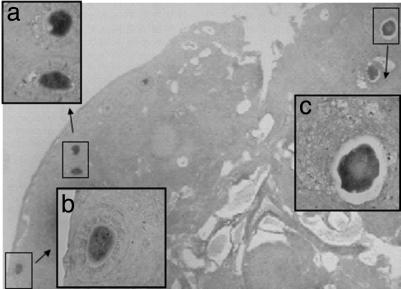
ePAB Is Differentially Expressed Within Mouse Ovaries. To determine the cellular localization of ePAB expression, we performed in situ hybridization on sections of mouse ovaries. Tissue was collected 20 h after stimulation with 5 units of PMSG and therefore contained follicles at different stages of development. Consistent with the RT-PCR results, hybridization with a probe spanning mouse ePAB exons 8–10.7 (data not shown) or exon 11 (Fig. 4) demonstrated the presence of ePAB RNA in oocytes. Signal was absent from surrounding somatic cells of the ovary. Oocytes showed specific staining independent of the stage of follicular development (55). Negative controls hybridized with sense probe or treated with RNase A before hybridization with the antisense probe remained unstained (data not shown). In situ hybridization with the ePAB 11 probe confirms that exon 11 is present in a fraction of ePAB transcripts in oocytes.
Fig. 4.
Localization of ePAB RNA in mouse ovaries. Expression of ePAB in ovaries of mice treated with PMSG was analyzed by in situ hybridization with the ePAB exon 11 antisense probe. A bright-field image of ovarian sections (×100) is presented. (Inset) Shown are higher magnification images (×600) of primary (Inset a and Inset b) and preantral (Inset c) follicles. The follicle classification is based on Pedersen and Peters (55).
Discussion
Here we report the mouse ortholog of Xenopus ePAB. We identify this gene as the mouse ePAB not only because it exhibits the highest identity of any mouse PABP to Xenopus ePAB but also because its expression pattern is consistent with that expected for a mammalian embryonic PABP. ePAB is present in ovaries and to a lesser extent in testis but absent from 10 different somatic tissues studied. At the cellular level, ePAB is expressed in both unovulated (PI) and ovulated (MII) oocytes, as well as in one-cell and two-cell embryos. It becomes undetectable after ZGA. Importantly, in situ hybridization demonstrates that mouse ePAB is expressed in oocytes but is not detected in the surrounding somatic cells of the ovary.
Mouse ePAB contains four RRMs at its N terminus, a characteristic of Xenopus ePAB and PABP1 from multiple species (Table 1). In Xenopus, the N-terminal regions containing the four RRMs are 82% conserved at the amino acid level between ePAB and PABP1, whereas the C-terminal regions are only 56% identical (49). Mouse ePAB shows a similar profile with 71% identity to mouse PABP1 in the N-terminal region (amino acid 1–408) and 45% at the C terminus of the longer splice variant (amino acid 409–608). Xenopus, mouse, and human PABP1 are 93% identical overall, whereas Xenopus and mouse ePAB are only 66% identical, mainly because of differences in the C-terminal region. The alternatively spliced variant of mouse ePAB lacking exon 11 contributes to further divergence between the C-terminal regions of ePAB in mouse compared to Xenopus. The overall low homology between Xenopus and mouse ePABs in the C-terminal region probably accounts for the inability of polyclonal antibodies raised against amino acids 386–629 of the Xenopus protein (49) to react with mouse ePAB protein (our unpublished observation).
ePAB orthologs in other mammals may be identified by using approaches similar to that we used in mouse. We identified one such gene in human (GenBank accession no. XM 114158) with a predicted sequence that encodes a 703-aa protein with 71% and 72% identity to Xenopus and mouse ePAB, respectively. However, RT-PCR analysis of several human tissues revealed that expression of this gene is not restricted to gonads (data not shown). Whether such findings reflect a different expression pattern for ePAB in human or indicate the existence of another homologous gene with an expression pattern similar to that of mouse ePAB remains to be investigated.
During Xenopus oocyte and early embryo development, ePAB is the predominant PABP expressed, as demonstrated by both Western blot analysis and UV-crosslinking to substrates with [α-32P]ATP-labeled poly(A) tails (49). In the same study, PABP1 was undetectable in Xenopus until after the early neurula stage (49). This observation is consistent with the previous findings of Zelus et al. (56) and suggested that PABP1 expression is induced only after ZGA, which occurs at the mid-blastula stage in Xenopus (18). More recently, Cosson et al. (57) confirmed ePAB as the predominant PABP during early Xenopus development although, by using Western blot analysis, they were able to detect a low level of PABP1 expression before ZGA, after which PABP1 expression dramatically increased.
In mouse, ZGA occurs at the two-cell stage. Therefore, we expected to observe PABP1 mRNA expression at an earlier stage of embryonic development in mouse than in Xenopus. While this study was ongoing, Zeng and Schultz (58) reported the results of suppression subtractive hybridization analysis of oocyte- and embryo-specific gene transcripts in mouse. PABP1 was one of the genes expressed at much higher levels in eight-cell embryos than in oocytes (26-fold difference). Our findings are consistent with theirs. We find low baseline expression of PABP1 mRNA in mouse oocytes (Fig. 3A), as well as in one-, two-, and four-cell embryos. We detect elevated expression of PABP1 at the eight-cell stage, which becomes more pronounced in blastocysts. Thus, although the increase in PABP1 expression occurs at an earlier developmental stage in mouse compared to Xenopus, it closely follows ZGA, suggesting similar regulatory mechanisms in the two species.
Although Xenopus (49) and mouse ePAB are structurally similar to PABP1, ePABs are expressed only in oocytes and early embryos, when PABP1 expression is minimal or absent. Xenopus and mouse ePAB are expected to be similar in their contributions to the control of gene expression during oocyte and early embryo development by regulating poly(A) tail length and unmasking/translation of maternal mRNAs. However, when Cao and Richter (35) tested human PABP1 and Xenopus ePAB, they found their activities to be equivalent in several in vitro assays assessing the dissociation of maskin from eIF4E. It could be that the differences in the C-terminal regions of ePAB versus PABP1 are functionally significant only in the context of the oocyte and early embryo development. In mouse, the two alternatively spliced forms of ePAB may play distinct roles because the C-terminal PABP domain, which is disrupted in the absence of exon 11 (Fig. 1B), mediates critical protein–protein interactions (53).
Very recently, two groups (45, 48) independently identified a PABP in Xenopus with 50% amino acid identity to Xenopus PABPN1, called ePABP2. Unlike PABPN1, ePABP2 is localized to the cytoplasm. ePABP2 is expressed in Xenopus oocytes and embryos at levels that are constant up to day 5 of development (tadpoles) but decreases in older embryos and becomes almost undetectable at day 15. Good et al. (45) also identified ePABP2 in mouse, where they showed RNA expression in oocytes but not in blastocysts. These observations suggest that ePABP2, like ePAB, may play a role in the cytoplasmic polyadenylation of mRNAs. However, it is noteworthy that Xenopus ePABP2 is present for a much longer period than ePAB (45, 48), suggesting that the control of its expression may be independent of ZGA. Moreover, Xenopus ePABP2, unlike Xenopus ePAB, is not able to interact with the cap-binding complex and is therefore unlikely to stimulate translation at the initiation step (48).
Based on these observations, we propose a model in which successive recruitment of specific embryonic PABPs mediate translation of maternal mRNAs before ZGA. According to this model, ePABP2 would first be recruited for processive cytoplasmic polyadenylation. Then the replacement of ePABP2 by ePAB would allow the initiation of mRNA translation. Such an exchange of PABN1 for PABP1 occurs in somatic cells upon transport into the cytoplasm of nuclear 3′ end-processed mRNA (48). Further studies are necessary to determine the exact role of the various cytoplasmic embryonic PABPs in the translational activation of maternally derived mRNAs. Elucidation of molecular mediators of gene expression during mammalian oocyte and early embryo development will have implications for important reproductive problems, such as reproductive failure, aneuploidy, and embryo death.
Supplementary Material
Acknowledgments
We thank Shobha Vasudevan for critical reading of the manuscript. This work was supported by National Institutes of Health Grants K08 HD046581 (to E.S.) and R01 GM26154 (to J.A.S.). M.D.L. is supported in part by Human Frontiers Science Program Long-Term Fellowship LT00606/2002. D.S. is supported in part by The Shulsky Foundation. J.A.S. is an investigator of the Howard Hughes Medical Institute.
Author contributions: E.S., M.D.L., S.M.F., D.S., N.T., and J.A.S. designed research; E.S., M.D.L., S.M.F., D.S., and N.T. performed research; E.S. contributed new reagents/analytic tools; E.S., M.D.L., S.M.F., N.T., and J.A.S. analyzed data; and E.S., M.D.L., S.M.F., D.S., N.T., and J.A.S. wrote the paper.
Abbreviations: PABP, poly(A)-binding protein; ZGA, zygotic gene activation; RRM, RNA recognition motif; PMSG, pregnant mare serum gonadotropin; hCG, human chorionic gonadotrophin; MII, metaphase II; PI, prophase I; RT, reverse transcription.
References
- 1.Matova, N. & Cooley, L. (2001) Dev. Biol. 231, 291-320. [DOI] [PubMed] [Google Scholar]
- 2.Sagata, N. (1996) Trends Cell Biol. 6, 22-28. [DOI] [PubMed] [Google Scholar]
- 3.Page, A. W. & Orr-Weaver, T. L. (1997) Curr. Opin. Genet. Dev. 7, 23-31. [DOI] [PubMed] [Google Scholar]
- 4.LaMarca, M. J., Smith, L. D. & Strobel, M. C. (1973) Dev. Biol. 34, 106-118. [DOI] [PubMed] [Google Scholar]
- 5.Rodman, T. C. & Bachvarova, R. (1976) J. Cell Biol. 70, 251-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian, J., Kim, S., Heilig, E. & Ruderman, J. V. (2000) Proc. Natl. Acad. Sci. USA 97, 14358-14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayaa, M., Booth, R. A., Sheng, Y. & Liu, X. J. (2000) Proc. Natl. Acad. Sci. USA 97, 12607-12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faiman, C. & Ryan, R. J. (1967) J. Clin. Endocrinol. Metab. 27, 1711-1716. [DOI] [PubMed] [Google Scholar]
- 9.Rao, A. J., Moudgal, N. R., Raj, H. G., Lipner, H. & Greep, R. O. (1974) J. Reprod. Fertil. 37, 323-330. [DOI] [PubMed] [Google Scholar]
- 10.Stebbins-Boaz, B., Hake, L. E. & Richter, J. D. (1996) EMBO J. 15, 2582-2592. [PMC free article] [PubMed] [Google Scholar]
- 11.Mendez, R., Hake, L. E., Andresson, T., Littlepage, L. E., Ruderman, J. V. & Richter, J. D. (2000) Nature 404, 302-307. [DOI] [PubMed] [Google Scholar]
- 12.Gebauer, F., Xu, W., Cooper, G. M. & Richter, J. D. (1994) EMBO J. 13, 5712-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh, B., Hwang, S., McLaughlin, J., Solter, D. & Knowles, B. B. (2000) Development (Cambridge, U. K.) 127, 3795-3803. [DOI] [PubMed] [Google Scholar]
- 14.Groisman, I., Huang, Y. S., Mendez, R., Cao, Q., Theurkauf, W. & Richter, J. D. (2000) Cell 103, 435-447. [DOI] [PubMed] [Google Scholar]
- 15.Uto, K. & Sagata, N. (2000) EMBO J. 19, 1816-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stutz, A., Conne, B., Huarte, J., Gubler, P., Volkel, V., Flandin, P. & Vassalli, J. D. (1998) Genes Dev. 12, 2535-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newport, J. & Kirschner, M. (1982) Cell 30, 687-696. [DOI] [PubMed] [Google Scholar]
- 18.Newport, J. & Kirschner, M. (1982) Cell 30, 675-686. [DOI] [PubMed] [Google Scholar]
- 19.Clegg, K. B. & Piko, L. (1982) Nature 295, 343-344. [DOI] [PubMed] [Google Scholar]
- 20.Flach, G., Johnson, M. H., Braude, P. R., Taylor, R. A. & Bolton, V. N. (1982) EMBO J. 1, 681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braude, P., Bolton, V. & Moore, S. (1988) Nature 332, 459-461. [DOI] [PubMed] [Google Scholar]
- 22.Richter, J. D. (1999) Microbiol. Mol. Biol. Rev. 63, 446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter, J. D. & Lorenz, L. J. (2002) Curr. Opin. Neurobiol. 12, 300-304. [DOI] [PubMed] [Google Scholar]
- 24.Bachvarova, R., De Leon, V., Johnson, A., Kaplan, G. & Paynton, B. V. (1985) Dev. Biol. 108, 325-331. [DOI] [PubMed] [Google Scholar]
- 25.Vassalli, J. D., Huarte, J., Belin, D., Gubler, P., Vassalli, A., O'Connell, M. L., Parton, L. A., Rickles, R. J. & Strickland, S. (1989) Genes Dev. 3, 2163-2171. [DOI] [PubMed] [Google Scholar]
- 26.McGrew, L. L. & Richter, J. D. (1989) Dev. Biol. 134, 267-270. [DOI] [PubMed] [Google Scholar]
- 27.McGrew, L. L. & Richter, J. D. (1990) EMBO J. 9, 3743-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paris, J. & Richter, J. D. (1990) Mol. Cell. Biol. 10, 5634-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paris, J., Swenson, K., Piwnica-Worms, H. & Richter, J. D. (1991) Genes Dev. 5, 1697-1708. [DOI] [PubMed] [Google Scholar]
- 30.Bilger, A., Fox, C. A., Wahle, E. & Wickens, M. (1994) Genes Dev. 8, 1106-1116. [DOI] [PubMed] [Google Scholar]
- 31.Mendez, R., Murthy, K. G., Ryan, K., Manley, J. L. & Richter, J. D. (2000) Mol. Cell. 6, 1253-1259. [DOI] [PubMed] [Google Scholar]
- 32.Dickson, K. S., Bilger, A., Ballantyne, S. & Wickens, M. (1999) Mol. Cell. Biol. 19, 5707-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickson, K. S., Thompson, S. R., Gray, N. K. & Wickens, M. (2001) J. Biol. Chem. 276, 41810-41816. [DOI] [PubMed] [Google Scholar]
- 34.Stebbins-Boaz, B., Cao, Q., de Moor, C. H., Mendez, R. & Richter, J. D. (1999) Mol. Cell 4, 1017-1027. [DOI] [PubMed] [Google Scholar]
- 35.Cao, Q. & Richter, J. D. (2002) EMBO J. 21, 3852-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blobel, G. (1973) Proc. Natl. Acad. Sci. USA 70, 924-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mangus, D. A., Evans, M. C. & Jacobson, A. (2003) Genome Biol. 4, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhn, U. & Wahle, E. (2004) Biochim. Biophys. Acta 1678, 67-84. [DOI] [PubMed] [Google Scholar]
- 39.Stambuk, R. A. & Moon, R. T. (1992) Biochem. J. 287, 761-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, H., Duckett, C. S. & Lindsten, T. (1995) Mol. Cell. Biol. 15, 6770-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feral, C., Guellaen, G. & Pawlak, A. (2001) Nucleic Acids Res. 29, 1872-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleene, K. C., Wang, M.-Y., Hall, C., Cutler, M. & Shih, D. (1994) Mol. Reprod. Dev. 39, 355-364. [DOI] [PubMed] [Google Scholar]
- 43.Wahle, E. (1991) Cell 66, 759-768. [DOI] [PubMed] [Google Scholar]
- 44.Wahle, E., Lustig, A., Jeno, P. & Maurer, P. (1993) J. Biol. Chem. 268, 2937-2945. [PubMed] [Google Scholar]
- 45.Good, P. J., Abler, L., Herring, D. & Sheets, M. D. (2004) Genesis 38, 166-175. [DOI] [PubMed] [Google Scholar]
- 46.Bienroth, S., Keller, W. & Wahle, E. (1993) EMBO J. 12, 585-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wahle, E. (1995) J. Biol. Chem. 270, 2800-2808. [DOI] [PubMed] [Google Scholar]
- 48.Cosson, B., Braun, F., Paillard, L., Blackshear, P. & Osborne, H. B. (2004) Biol. Cell 96, 519-527. [DOI] [PubMed] [Google Scholar]
- 49.Voeltz, G. K., Ongkasuwan, J., Standart, N. & Steitz, J. A. (2001) Genes Dev. 15, 774-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.VanBuren, V., Piao, Y., Dudekula, D. B., Qian, Y., Carter, M. G., Martin, P. R., Stagg, C. A., Bassey, U. C., Aiba, K., Hamatani, T., et al. (2002) Genome Res. 12, 1999-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wassarman, P. M. & De Pamphilis, M. L. (1993) in Methods in Enzymology (Academic, San Diego), Vol. 225.
- 52.Beaudoing, E., Freier, S., Claverie, J.-M. & Gauthere, D. (2000) Genome Res. 10, 1001-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kozlov, G., Tremple, J.-F., Khaleghpour, K., Kahvejian, A. & Ekiel, I. (2001) Proc. Natl. Acad. Sci. USA 98, 4409-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maaser, K., Sutter, A. P., Krahn, A., Hopfner, M., Grabowski, P. & Scherubl, H. (2004) Biochem. Biophys. Res. Comm. 324, 878-886. [DOI] [PubMed] [Google Scholar]
- 55.Pedersen, T. & Peters, H. (1968) J. Reprod. Fertil. 17, 555-557. [DOI] [PubMed] [Google Scholar]
- 56.Zelus, B. D., Giebelhaus, D. H., Eib, D. W., Kenner, K. A. & Moon, R. T. (1989) Mol. Cell. Biol. 9, 2756-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cosson, B., Couturier, A., Le Guellec, R., Moreau, J., Chabelskaya, S., Zhouravleva, G. & Philippe, M. (2002) Biol. Cell 94, 217-231. [DOI] [PubMed] [Google Scholar]
- 58.Zeng, F. & Schultz, R. (2003) Biol. Reprod. 68, 31-39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.