Abstract
8R-Lipoxygenase and allene oxide synthase (AOS) are parts of a naturally occurring fusion protein from the coral Plexaura homomalla. AOS catalyses the production of an unstable epoxide (an allene oxide) from the fatty acid hydroperoxide generated by the lipoxygenase activity. Here, we report the structure of the AOS domain and its striking structural homology to catalase. Whereas nominal sequence identity between the enzymes had been previously described, the extent of structural homology observed was not anticipated, given that this enzyme activity had been exclusively associated with the P450 superfamily, and conservation of a catalase fold without catalase activity is unprecedented. Whereas the heme environment is largely conserved, the AOS heme is planar and the distal histidine is flanked by two hydrogen-bonding residues. These critical differences likely facilitate the switch from a catalatic activity to that of a fatty acid hydroperoxidase.
Keywords: eicosanoids, heme
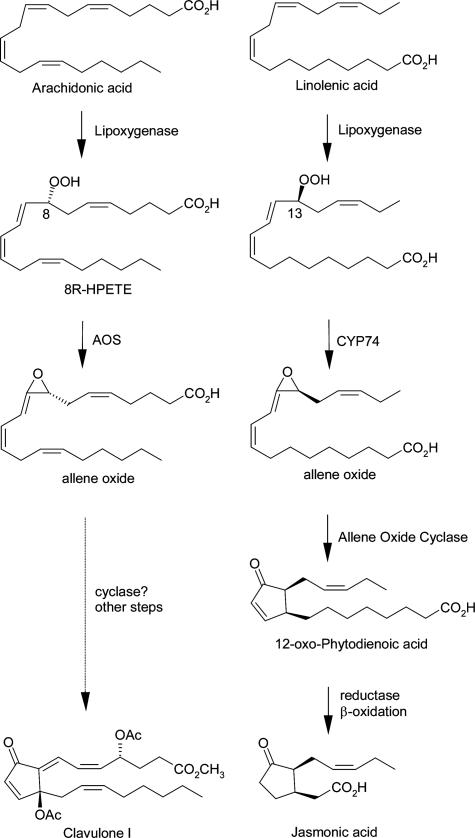
The occurrence of prostaglandins and other eicosanoids in soft corals such as the Caribbean gorgonian Plexaura homomalla has stimulated many investigations on the pathways of their biosynthesis. The prostaglandins are formed from arachidonic acid via a conventional cyclooxygenase route (1, 2), yet the overwhelming catalytic activity evident in coral extracts is lipoxygenase (LOX) pathway metabolism (3). Arachidonic acid is converted to an 8R-hydroperoxide that is further transformed to an allene oxide (epoxide) (3-5). This pathway bears many parallels with the route of jasmonic acid biosynthesis in plants (Fig. 1) (6). In corals, subsequent steps may lead to cyclic products such as the clavulones (4, 7), although this connection remains to be proven.
Fig. 1.
Comparison of allene oxide biosynthesis in coral and plants. The conversion of allene oxide to clavulone remains unproven.
In the course of their efforts to elucidate eicosanoid biosynthetic pathways in coral, Brash and coworkers (8) identified a gene coding for a 122-kDa fusion protein that includes both LOX and allene oxide synthase (AOS) domains. The C-terminal 79-kDa domain of the fusion protein, an 8R-LOX, is similar to mammalian LOXs in size, sequence, and substrate specificity; its closest homologue (≈40% sequence identity) in mammals is 5-LOX, an enzyme responsible for the synthesis of leukotrienes from arachidonic acid (9). The N-terminal 43-kDa AOS domain of the fusion protein uses the 8R-hydroperoxide as its preferred substrate (10). This hemoprotein domain has weak sequence identity to catalase (≈11% amino acid identity) with five regions of homology identified, all of which involve heme-binding or catalytic residues (8). Spectroscopic data (e.g., electron paramagnetic resonance, UV-vis, and magnetic CD) indicated that AOS, like catalase, is a heme protein with a tyrosine axial ligand (11). However, AOS is without catalase activity because it does not catalyze the dismutation of hydrogen peroxide to water (10).
The sequences of plant AOSs clearly establish the enzymes as members of the cytochrome P450 superfamily. Plant AOSs are members of a subfamily of the fatty acid hydroperoxide-metabolizing P450s designated as CYP74A (12). Other classes of P450 enzymes, such as mammalian prostacyclin synthase and thromboxane synthase, also catalyze similar chemistry on fatty acid peroxides (13). The coral AOS, with its sequence and spectral relationship to catalases, is, therefore, quite exceptional. Catalases are not recognized for an involvement in biosynthesis (14). Given that there is no precedent for a catalase-like fold in which catalatic activity is replaced completely by a biosynthetic activity, we determined the crystal structure of the AOS domain of the naturally occurring fusion protein to elucidate the features of this distinctive enzyme.
Methods
Expression and Purification. AOS fused to a C-terminal 4× His tag was overexpressed in BL21(DE3) cells. Cells were pelleted (at 5,000 × g for 20 min at 4°C), frozen at -80°C, and subsequently resuspended in Bugbuster lysis buffer (Novagen) in the presence of protease inhibitors (PMSF, leupeptin, and pepstatin A) and DNase I. After sonication of the cells and removal of debris by centrifugation (at 46,000 × g for 20 min at 4°C), the supernatant was applied to a nickel nitrilotriacetic acid agarose (Qiagen, Valencia, CA) preequilibrated with 20 mM imidazole and 500 mM NaCl. Protein was eluted with 200 mM imidazole. Further purification was accomplished with ion exchange and gel filtration chromatography [DE52, Sephacryl S300, and mono-Q (Pharmacia, Uppsala)] to yield pure protein (100 mg/liter of culture) that displayed the characteristic ratio of A406/A280 of 1.8, which is consistent with full heme occupancy as previously determined (11). All chromatographic procedures were buffered with 10 mM Tris·HCl, pH 8.0.
Crystallization. Initial screens were performed at 4°C and 22°C with commercial (Hampton Research and Emerald Biostructures) and in-house (ammonium sulfate, pentaerythritol propoxylates, and ethoxylates) screens. In addition, the publicly available microbatch screening services of the Hauptman-Woodward Institute in Buffalo, NY were used. Although crystals were obtained in four distinct space groups, only those that belong to the monoclinic space group C2 (a = 147.9 Å, b = 76.7 Å, c = 77.8 Å, β = 109.7°) proved useful for structure determination. These thin, plate-like crystals grow in hanging-drop setups from 100 mM BisTris·HCl, pH 5.7, 16% polyethylene glycol MME 5000 and diffract to 2-Å resolution on a rotating anode.
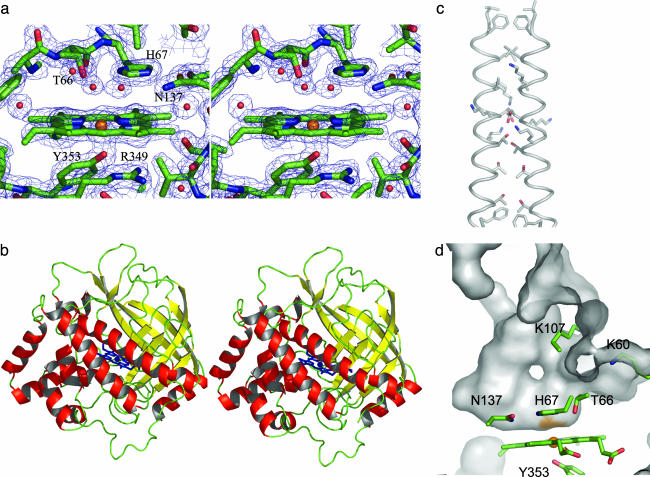
Data Collection and Phasing. Heavy-atom data were collected at 100 K on a Nonius FR591 rotating anode fitted with a Mar165 image plate detector. Fe-multiwavelength anomalous dispersion (Fe-MAD) data at 100 K were collected at the protein crystallography beamline at the Center for Advanced Microstructures and Devices at Louisiana State University, with a Mar charge-coupled device camera. Data processing and scaling were performed by using denzo (15). The structure was solved by combining phase information derived from MAD and multiple isomorphous replacement data sets (Tables 1 and 2). The 4.0-Å resolution anomalous difference Patterson calculated from Fe-MAD data revealed clear peaks attributable to the heme iron, and these positions were refined with 2.2-Å data in cns (16). The hand of the Fe positions was determined by calculation of heavy-atom difference Fourier maps with diffraction data from AuCl3-(1 mM for 1 h) and K2PtBr4 (1 mM for 24 h)-soaked crystals. For both heavy-atom data sets, obtained to resolutions of 3.6 and 3.8 Å, respectively, clear peaks were visible with only the original hand assigned to the Fe atom, and these positions were compatible with those determined from heavy-atom difference Patterson maps. Approximately 80% of the backbone was traceable in a 3.4-Å resolution solvent-flattened map calculated after combination of the heavy atom and MAD phases. The extent of the model and resolution of the maps were extended over several cycles of model building. The final model at 2.0-Å resolution (two molecules in the asymmetric unit, 5,964 protein atoms, 86 cofactor atoms, and 569 water molecules) has an Rwork/Rfree of 19.6/24.0, with no σ cutoffs applied. A representative 2 Fo - Fc electron density map contoured at 1.5σ is shown in Fig. 2a.
Table I. Data collection.
| Fe-MAD
|
MIR
|
|||||
|---|---|---|---|---|---|---|
| Data collection | Native | Peak | Inflection | Remote | AuCl3 | K2PtBr4 |
| Space group C2 | ||||||
| Cell | ||||||
| a, Å | 147.9 | 148.0 | 151.9 | 151.5 | ||
| b, Å | 76.7 | 76.8 | 79.8 | 79.3 | ||
| c, Å | 77.8 | 78.0 | 77.2 | 77.6 | ||
| β, ° | 109.7 | 109.8 | 110.6 | 111.0 | ||
| Resolution range, Å | 50–2.0 | 50–2.3 | 50–2.2 | 50–2.2 | 30–3.6 | 30–3.8 |
| Unique reflections | 55,410 | 71,477 | 81,796 | 80,984 | 9,115 | 9,970 |
| Completeness* | 100.0 (100.0) | 99.9 (99.6) | 99.9 (99.8) | 99.2 (97.4) | 97.5 (98.8) | |
| Redundancy | 5.8 | 3.4 | 3.4 | 3.6 | 4.8 | 5.9 |
| l/σ | 21.5(3.7) | 13.5 (3.1) | 13.3 (2.7) | 15.7 (4.1) | 16.8 (2.6) | 13.2 (3.6) |
| Rsym,† % | 8.2 (36.2) | 9.3 (38.5) | 9.4 (39.4) | 8.5 (29.5) | 8.2 (42.5) | 11.0 (43.1) |
MIR, multiple isomorphous replacement.
Values in parentheses are for the highest-resolution shell
Rsym = Σ Ii - 〈I〉|Σ Ii, where Ii is the intensity of the ith observation and <I> is the mean intensity of the reflection
Table 2. Refinement statistics.
| Rwork, % | 19.6 |
| Rfree,* % | 24.0 |
| No. of nonhydrogen atoms | |
| Protein | 5,964 |
| Water | 569 |
| Heme | 86 |
| rms deviation from ideality | |
| Bond lengths, Å | 0.0058 |
| Bond angles, ° | 1.35 |
| Estimated coordinate error (Luzzati), Å | 0.22 |
| Average B-factor | 20.4 |
| Protein, Å2 | 19.3 |
| Water | 25.2 |
| Heme | 14.9 |
| Disallowed, % | 0.3 |
R = Σ||Fo| – |Fc||/Σ|Fo|, where Fo and Fc are the observed and calculated structure factors amplitudes. Rfree is calculated by using 5% of the total reflections
Fig. 2.
The structure of the coral AOS. (a) Stereo image of representative 2 Fo- Fc electron density map of the active site contoured at 1.5σ. Distal-side residues (T66, H67, and N137) and proximal-side residues (R349 and Y353) are shown for reference. (This figure and all other structural renderings were created by using pymol, which can be accessed at www.pymol.org.) (b) Stereo image of coral AOS backbone trace: α-sheets, β-strands, coiled regions, and heme groups are red, yellow, green, and blue, respectively. (c) The dimer interface formed by α3 of the two molecules in the asymmetric unit. Residues that line the interface are drawn in a stick format. (d) Sectioned view of the solvent-accessible surface of the substrate-binding pocket. Basic residues at the opening of the cavity (K60 and K107) and active-site residues (T66, H67, N137, and Y353) are indicated.
Results
The monomer of AOS is a wedge-shaped protein with all three dimensions ≈45-50 Å. The enzyme has an α+β fold composed of an eight-stranded antiparallel β-barrel, located at one of the vertices of the wedge, and 13 α-helices (Fig. 2b). Of the 373 aa in the expressed protein, all but the first two (as well as the 4× His purification tag) and the last five are visible in the final electron density map. In the crystal, monomers associate by means of a parallel interaction of the α3 helices (Fig. 2c). This dimerization motif is observed in all four crystal forms (orthorhombic, triclinic, monoclinic, and C-centered monoclinic; data not shown). At the N termini of the associating helices, extensive contacts are made through hydrophobic amino acids (F37, L40, and L44), whereas at the C termini, intermolecular contacts involve the packing of threonines (T51, T54, and T58) in a fashion reminiscent of a leucine zipper. The oligomeric interface (1,070 Å2 per monomer) is localized on one edge of the wedge-shaped molecule, resulting in an overall bow-tie-shaped dimer.
In contrast to typical heme proteins, the prosthetic group in AOS is remarkably planar. The heme is centered in the monomer, positioned between one side of the β-barrel and helices α4 and α13. On the proximal side of the heme Y353, provided by α13, fills one of the axial sites for the Fe, whereas a large cavity flanked by loops 64-67, 105-108, and turn 156-158 allows access to the remaining axial position (the distal) where the tetrapyrrole ring forms the base of a U-shaped substrate-binding pocket (Fig. 2d). The outside edge of the pocket (≈13 Å from the heme Fe) is lined with basic residues (K107 and K60) that form a positively charged ridge flanked by α3. The interior region of the pocket is lined with hydrophobic residues (F153 and F241, V156, L106, L176, and L190). The propionic acid groups of the heme form salt bridges with three arginine residues (R64, R102, and R360). The penta-coordinated active-site Fe is positioned 2.0 Å from the pyrole nitrogens of the heme and 2.15 Å from the oxygen provided by Y353. Electron density attributed to water molecules is ≈2.85 Å from the heme Fe on the distal side. H67 is positioned slightly above this density, flanked by hydrogen-bonding residues N137 and T66 (Fig. 2a).
Discussion
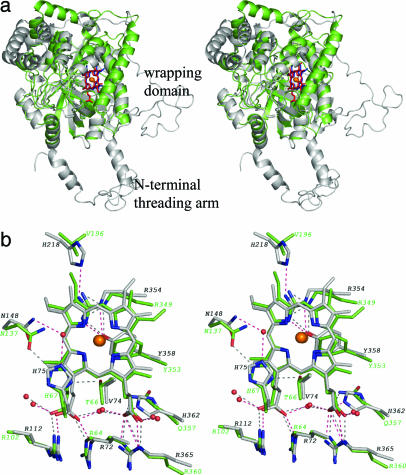
AOS Resembles a Catalase Core. Although a catalase-like structure had been predicted for AOS, the extent of the structural similarity is striking (rms deviation of 1.65 Å for 225 of 373 Cαs), because even with the aid of a structure-based sequence alignment the sequence identity between human erythrocyte catalase (HEC) and AOS is only 11% for ≈300 aa. The two enzymes share similar placement of 7 of the 10 β-strands and 7 of the 13 α-helices found in AOS (Fig. 3). AOS appears to be a catalase “core” in which the eight-stranded β-barrel and the majority of the helical domain are maintained, whereas the N-terminal threading arm and the C-terminal wrapping domain, parts of the catalase structure important for tetramerization, are discarded. AOS has no sequence similarity to catalases in its N terminus and ends before the formation of a wrapping domain. AOS and catalase do use a similarly positioned helix to form an oligomeric interface, although in AOS the helices remain associated with their respective monomers, and are not “domain-swapped,” as they are in catalase tetramers. It is noteworthy that the placement of the elements of secondary structures containing the three arginines that neutralize the heme carboxylates, and the residues adjacent to the proximal and distal sides of the heme is preserved. In a context of minimal sequence identity, the conservation of the heme environment and the heme protein hydrogen-bonding network is striking (Fig. 3b): the notable differences are the substitution of Val with Thr (T66 and AOS), displacement of N137 relative to its counterpart in catalase, and the substitution of His with Val (V196).
Fig. 3.
Comparison of AOS and catalase. (a) Superimposition of AOS (green; heme is red) with HEC (gray; heme is blue). The catalase N-terminal threading arm and wrapping domains are indicated. (b) Superposition of the heme environments of AOS (green carbons) and catalase (gray carbons). Dashed lines indicating active-site hydrogen bonds are black (AOS) and pink (HEC), respectively.
The AOS Structure Is Consistent with Spectroscopic Data. The AOS crystal structure clearly supports the results of earlier spectroscopic studies that compared AOS to HEC and indicated a tyrosine-ligated penta-coordinated ferric heme group with a vacant sixth position (11). In contrast, plant AOS as a P450 uses a cysteinate axial ligand. The mirror image Soret CD spectra of AOS and HEC are explained by the heme group being flipped in AOS with respect to its orientation in the catalase: AOS heme has a His-III and HEC a His-IV orientation. This terminology refers to which ring of the porphyrin lies below the distal histidine. The “flipped” orientation is observed in other catalases, e.g., CatF from Pseudomonas syringae (17), and there is some evidence to suggest that this His-IV orientation is associated with the evolutionary earliest catalases (14).
The Chemical Nature of the U-Shaped Cavity Is Complementary to the Substrate. AOS has converted the catalase H2O2 selectivity channel to a fatty acid binding pocket. In the catalase tetramer, funnel-shaped tunnels of 30 Å are lined with phenylalanine residues and end with a narrow constriction to aid in the selection of H2O2. In AOS, the entrance to the cavity is positively charged and the distance to the heme is significantly shorter (≈13 Å). The size of the binding pocket (≈1,150 Å3) as calculated with the castp server (18) is similar to those described for other eicosanoid binding proteins {e.g., rabbit reticulocyte 15S-LOX [Protein Data Bank (PDB) ID code 1LOX], PPARγ (PDB ID code 1PRG), and adipocyte lipid-binding protein (PDB ID code 1ADL)}. The dimensions and chemical nature of the U-shaped binding pocket are consistent with 8R-hydroperoxyeicosatetraenoic acid binding with its carboxylate tail at the mouth of the cavity, presumably neutralized with a salt bridge to either K107 or K60. Either residue could position C8 of the substrate in suitable proximity to H67, N137, and the penta-coordinated Fe. The hydrophobic residues lining the pocket provide for extensive protein-substrate van der Waals interaction.
The large substrate binding cavity of AOS appears to expose the heme to small pseudosubstrates and inhibitors. However, Abraham et al. (11) reported that both azide or cyanide, inhibitors of catalase because they are able to fill the open sixth coordination position of the heme Fe, have significantly reduced affinity (≥500-fold) for the heme in AOS. In catalase, these inhibitors are positioned to coordinate the heme Fe with hydrogen bonds to the distal His (azide, ref. 19), or a water molecule positioned by its hydrogen bond to the distal His (cyanide, ref. 20). In AOS, the distal His (H67) is flanked by T66 and N137 and fixed by hydrogen bonds to both of these amino acids, and is thus unable to mediate binding of the inhibitors. This arrangement of the active site appears to limit the approach to an otherwise accessible heme by potential pseudosubstrates.
AOS promotes 1,400 turnovers s-1, and the enzyme is deactivated in <1 min at room temperature (10). This deactivation has been attributed to off-pathway heterolytic cleavage of the peroxide bond (21). Catalases have turnover numbers from 54,000 to 833,000 s-1 (22), whereas no catalase activity is detected with AOS at enzyme concentrations 100 times those needed to produce a signal for the reaction with 8R-hydroperoxyeicosatetraenoic acid, and deactivation of AOS occurs with only with a 10-fold excess of H2O2 over the course of 1.5 h (21). Thus, despite a large substrate access cavity that cannot exclude the approach of H2O2 to the heme, AOS does not have catalatic activity, nor is it rapidly deactivated by hydrogen peroxide.
The Adaptation of a Catalase to an AOS. Whereas AOS and catalase both perform cleavage of a peroxide bond, in the former one of the oxygens remains associated with the substrate in an epoxide, whereas in the latter a two-step process that requires the production of a heme species known as compound I results in the formation of water and molecular oxygen. The two steps of the catalase reaction are as follows: (i) A hydrogen peroxide molecule oxidizes the heme to an oxyferryl species in which one oxidant equivalent is removed from the iron and one from the porphyrin ring to form the intermediate known as compound I, and (ii) a second hydrogen peroxide molecule is used as a reductant of compound I to regenerate the resting state of the enzyme. The first step of the reaction, the heterolytic cleavage of the peroxide bond that yields the compound I intermediate, can be trapped upon reaction with the substrate analog peracetic acid. In contrast, the reaction of AOS with peracetic acid produces compound I as a transient intermediate on a deactivation pathway: electron paramagnetic resonance studies suggest that the unpaired electron of compound I is rapidly (≈1 s) transferred to a Tyr residue (21). While in catalase, the enzyme-compound I intermediate can be maintained for 30 min (e.g., ref. 23).
How is the adaptation of catalase, an enzyme that catalyzes heterolysis of the peroxide bond and requires the formation of an oxyferryl intermediate, to an AOS that catalyzes homolytic cleavage of the peroxide without compound I formation, accomplished? There are three possible factors that may contribute to this fundamental difference in catalytic mechanisms: the heme environment, heme conformation, and the nature of the substrate and its fit into the binding site. As can be seen from Fig. 3b, the heme environments are strikingly conserved. Both catalase and AOS have similarly positioned arginines at the heme propionic acid groups (R64, R102, and R360 in AOS, and R72, R112, and R365 in HEC) and the proximal Tyr (R349 in AOS and R354 in HEC). Guallar et al. (24) have suggested that charged residues near the heme propionic acids attenuate the properties of the prosthetic group. However, the only difference here is that in HEC H362 is hydrogen bonded to a propionic acid group, whereas in AOS it is Q195. This difference is not likely to be significant because Gln is found at this position in catalases as well. Similarly, the Arg-proximal Tyr interaction has been proposed to be important for compound I formation because it “tunes” the heme so that oxidation occurs on the porphoryin ring (25). Whereas both enzymes have this interaction, in catalase the Arg participates in a hydrogen bond with a histidine residue that is replaced by a Val in AOS. Perhaps a deprotonated highly conserved histidine helps stabilize the compound I intermediate of catalase by dissipating the charge-charge repulsion between the Arg and the pi-cation radical.
An additional distinctive feature of AOS (vs. catalases and heme enzymes in general) is the planarity of its heme. It is the protein environment surrounding the heme that determines the extent of prosthetic group distortion that is conserved among members of a given superfamily (26). Quantitation of deviation from planarity of the heme by the method developed by Shellnut et al. (27) gives a value for the total out-of-plane distortion for the AOS heme of 0.11 Å, a number within the coordinate error for the structure determination. In contrast, the same value for HEC, a structure determined to 1.5-Å resolution, is 0.72 Å. Studies with synthetic hemes have shown that nonplanar heme groups are more easily oxidized than their planar counterparts (28), and the nonplanarity can affect not only the site of oxidation (metal or ring) but also the type of oxidation (two electrons vs. one electron) (29). Because distortion of heme planarity is energetically unfavorable (30), the difference in AOS and catalase heme shape is likely necessary for the difference in redox properties. In catalase, the nonplanar heme is oxidized with a two-electron oxidation removing electrons from the Fe and the porphyrin ring in the formation of compound I. As revealed by crystallographic studies, this transition involves the movement of the Fe out of the plane of the heme in the direction of the distal histidine, and the oxygen of the proximal Tyr to which it is bound shifts as well (23). The planarity of the AOS heme would make such a conformational change, the displacement of the Fe out of the plane of the heme unfavorable, and thus serve as a barrier for the formation of a catalase-like compound I species. This feature is of critical importance given that compound I formation is not productive in the ultimate formation of the allene oxide product, but also without the aid of an additional reductant (H2O2 in catalase) a compound I form of AOS leads to its inactivation (21). Another factor that may correlate with heme chemistry is the Fe-axial ligand distance, which is longer in AOS than it is in catalase structures: 2.15 (AOS) vs. 1.84 Å (HEC).
The fatty acid hydroperoxide substrate itself may play a role in the fact that cleavage of the peroxide bond proceeds through homolytic rather than heterolytic cleavage because (i) the conjugated diene structure can stabilize the free radical product of homolytic cleavage, and (ii) the large bulky substrate fills the pocket, and there is no room for water to promote heterolytic cleavage by protonation of the leaving group. In their studies with P450s, Correia et al. (31) found that with smaller substrates the ratio of heteroto homolytic cleavage products increased, an observation attributed to the fact that the smaller substrates do not exclude water from the enzyme active site.
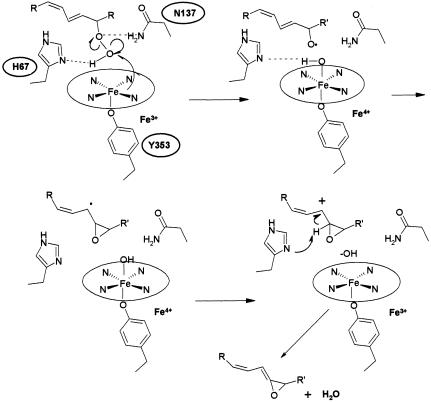
We propose that in AOS, minor modifications to the heme environment promote homolytic bond cleavage (Fig. 4), and thus the generation of a free radical intermediate, in a mechanism akin to that of plant AOSs that are P450 enzymes (12). As in catalase, the distal histidine (H67) hydrogen bonds to the peroxide group of the fatty acid substrate, whereas N137 (or T66) forms a hydrogen bond with the distal oxygen of the peroxide. Homolysis of the peroxide bond produces an alkoxyl radical that rearranges to a carbon radical, and ultimately a short-lived epoxy allylic carbocation that is deprotonated by H67 to form the allene oxide. The carbon radical formed reduces Fe-IV and the carbocation produced is eliminated by the removal of a proton by H67. The planarity of the heme, a barrier to the formation of a compound I intermediate, and the exclusion of water from the binding site facilitate the switch from a catalase to an AOS.
Fig. 4.
Proposed reaction scheme for coral AOS. H67 hydrogen bonds to the terminal peroxide oxygen, favoring homolytic cleavage. This process results in formation of an alkoxyl radical, then a carbon radical, and, ultimately, a short-lived epoxy allylic carbocation that is deprotonated to form the allene oxide. The carbon radical formed reduces Fe-IV and the carbocation produced is eliminated by the removal of a proton by H67.
AOS Is Found in a Naturally Occurring Fusion Protein. As noted earlier, the coral AOS occurs as the N-terminal domain of a fusion protein with a C-terminal domain of 8R-LOX. Covalent fusion of the AOS and LOX should greatly enhance any mutual affinity of the two domains by increasing their effective concentrations, facilitating rapid transfer of the labile 8R-hydroperoxide from the LOX to the AOS active site. In an evolutionary context, Eisenberg and coworkers (32) noted that when two interacting proteins are fused, successive mutations will eventually create an optimized protein-protein interface, which, accordingly is to be anticipated for the AOS-LOX fusion protein.
Marcotte et al. (32) suggest that the existence of a fusion protein in the genome of one species could be used to infer protein-protein interactions in homologues in other species where the two proteins are expressed separately. The fusion of AOS to LOX in coral, therefore, may be indicative of catalase-related AOS homologues interacting with LOX in other organisms. By analogy to the plant CYP74 P450 family that metabolize fatty acid hydroperoxides, such homologues could exhibit AOS, hydroperoxide lyase, epoxyalcohol synthase, or divinyl ether synthase activities (6). Whether residing in fusion proteins or as autonomous polypeptides, 8R-LOX and AOS activities are ubiquitous in corals (5, 33, 34), and their expression will likely be detected in other invertebrates (35). Other homologues of the AOS domain suggest expanded roles. One such homolog from cyanobacteria is a hypothetical protein of 773 residues (30% identity to coral AOS in 373 residues) that also contains a domain of ≈400 residues with significant identity to LOXs (36). Other potential matches are a domain in a 2,391-residue protein from the rice genome (28% identity for 301 of 373 residues) (37), and a short catalase-related protein of 378 residues in the wheat blight fungus Fusarium graminearum that resides in the genome next to a putative LOX. Thus, catalase-like AOS enzymes are likely to have diverse roles in eicosanoid biosynthesis.
Concluding Remarks. Coral AOS comprises a catalase-like core with minor active site alterations that confer AOS activity. Adaptation of catalase to a biosynthetic enzyme is accomplished primarily with surprisingly modest changes in the immediate heme environment in the context of only 11% sequence identity. Another eicosanoid biosynthetic enzyme has been described that represents similarly an adapted enzyme: leukotriene A4 hydrolase is a modified zinc peptidase with structural homology to thermolysin (38). Leukotriene A4 hydrolase retains the catalytic activity of the progenitor enzyme and is a peptidase as well as a hydrolase, whereas AOS represents a complete switch from a H2O2-metabolizing catalase to a biosynthetic fatty acid hydroperoxidase.
Acknowledgments
We thank Henry Bellamy for assistance at the Center for Advanced Microstructures and Devices PX beamline, William Boeglin for assistance in construct design and expression, and M. Graça Vicente for insights into heme chemistry. This work was supported in part by National Institutes of Health Grants GM 55420 (to M.E.N.) and GM 53638 (to A.R.B.) and the Louisiana Governor's Biotechnology Initiative.
Author contributions: M.L.O. and M.E.N. designed research; M.L.O. performed research; M.L.O., A.R.B., and M.E.N. analyzed data; and M.L.O., A.R.B., and M.E.N. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AOS, allene oxide synthase; HEC, human erythrocyte catalase; LOX, lipoxygenase; MAD, multiwavelength anomalous dispersion.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 1U5U).
References
- 1.Valmsen, K., Jarving, I., Boeglin, W. E., Varvas, K., Koljak, R., Pehk, T., Brash, A. R. & Samel, N. (2001) Proc. Natl. Acad. Sci. USA 98, 7700-7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valmsen, K., Boeglin, W. E., Jarving, I., Schneider, C., Varvas, K., Brash, A. R. & Samel, N. (2004) Eur. J. Biochem. 271, 3533-3538. [DOI] [PubMed] [Google Scholar]
- 3.Brash, A. R., Baertschi, S. W., Ingram, C. D. & Harris, T. M. (1987) J. Biol. Chem. 262, 15829-15839. [PubMed] [Google Scholar]
- 4.Corey, E. J., d'Alarcao, M., Matsuda, S. P. T., Lansbury, P. T. & Yamada, Y. (1987) J. Am. Chem. Soc. 109, 289-290. [Google Scholar]
- 5.Corey, E. J., Matsuda, S. P. T., Nagata, R. & Cleaver, M. B. (1988) Tetrahedron Lett. 29, 2555-2558. [Google Scholar]
- 6.Feussner, I. & Wasternack, C. (2002) Annu. Rev. Plant Biol. 53, 275-297. [DOI] [PubMed] [Google Scholar]
- 7.Grechkin, A. N. (1995) J. Lipid. Mediat. Cell Signal. 11, 205-218. [DOI] [PubMed] [Google Scholar]
- 8.Koljak, R., Boutaud, O., Shieh, B. H., Samel, N. & Brash, A. R. (1997) Science 277, 1994-1996. [DOI] [PubMed] [Google Scholar]
- 9.Werz, O. (2002) Curr. Drug Targets Inflamm. Allergy 1, 23-44. [DOI] [PubMed] [Google Scholar]
- 10.Boutaud, O. & Brash, A. R. (1999) J. Biol. Chem. 274, 33764-33770. [DOI] [PubMed] [Google Scholar]
- 11.Abraham, B. D., Sono, M., Boutaud, O., Shriner, A., Dawson, J. H., Brash, A. R. & Gaffney, B. J. (2001) Biochemistry 40, 2251-2259. [DOI] [PubMed] [Google Scholar]
- 12.Song, W. C., Funk, C. D. & Brash, A. R. (1993) Proc. Natl. Acad. Sci. USA 90, 8519-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanabe, T. & Ullrich, V. (1995) J. Lipid. Mediat. Cell Signal. 12, 243-255. [DOI] [PubMed] [Google Scholar]
- 14.Chelikani, P., Fita, I. & Loewen, P. C. (2004) Cell Mol. Life Sci. 61, 192-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otwinowski, Z. & Minor, W. (1997) Methods Enzymol. 276, 307-326. [DOI] [PubMed] [Google Scholar]
- 16.Brunger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J. S., Kuszewski, J., Nilges, M., Pannu, N. S., et al. (1998) Acta Crystallogr. D 54, 905-921. [DOI] [PubMed] [Google Scholar]
- 17.Carpena, X., Soriano, M., Klotz, M. G., Duckworth, H. W., Donald, L. J., Melik-Adamyan, W., Fita, I. & Loewen, P. C. (2003) Proteins 50, 423-436. [DOI] [PubMed] [Google Scholar]
- 18.Liang, J., Edelsbrunner, H. & Woodward, C. (1998) Protein Sci. 7, 1884-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mate, M. J., Zamocky, M., Nykyri, L. M., Herzog, C., Alzari, P. M., Betzel, C., Koller, F. & Fita, I. (1999) J. Mol. Biol. 286, 135-149. [DOI] [PubMed] [Google Scholar]
- 20.Putnam, C. D., Arvai, A. S., Bourne, Y. & Tainer, J. A. (2000) J. Mol. Biol. 296, 295-309. [DOI] [PubMed] [Google Scholar]
- 21.Wu, F., Katsir, L. J., Seavy, M. & Gaffney, B. J. (2003) Biochemistry 42, 6871-6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Switala, J. & Loewen, P. C. (2002) Arch. Biochem. Biophys. 401, 145-154. [DOI] [PubMed] [Google Scholar]
- 23.Gouet, P., Jouve, H. M., Williams, P. A., Andersson, I., Andreoletti, P., Nussaume, L. & Hajdu, J. (1996) Nat. Struct. Biol. 3, 951-956. [DOI] [PubMed] [Google Scholar]
- 24.Guallar, V., Baik, M. H., Lippard, S. J. & Friesner, R. A. (2003) Proc. Natl. Acad. Sci. USA 100, 6998-7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green, M. T. (2001) J. Am. Chem. Soc. 123, 9218-9219. [DOI] [PubMed] [Google Scholar]
- 26.Jentzen, W., Ma, J. G. & Shelnutt, J. A. (1998) Biophys. J. 74, 753-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shelnutt, J. A., Song, X.Z., Ma, J. G., Jia, S. L., Jentzen, W. & Medforth, C. J. (1998) Chem. Soc. Rev. 27, 31-41. [Google Scholar]
- 28.Ravikanth, M. & Chandrashekar, T. K. (1995) Coord. Chem. 82, 105-188. [Google Scholar]
- 29.Kadish, K. M., Vancaemelbecke, E., Dsouza, F., Medforth, C. J., Smith, K. M., Tabard, A. & Guilard, R. (1995) Inorg. Chem. 34, 2984-2989. [Google Scholar]
- 30.Anderson, K. K., Hobbs, J. D., Luo, L. A., Stanley, K. D., Quirke, J. M. E. & Shelnutt, J. A. (1993) J. Am. Chem. Soc. 115, 12346-12352. [Google Scholar]
- 31.Correia, M. A., Yao, K., Allentoff, A. J., Wrighton, S. A. & Thompson, J. A. (1995) Arch. Biochem. Biophys. 317, 471-478. [DOI] [PubMed] [Google Scholar]
- 32.Marcotte, E. M., Pellegrini, M., Ng, H. L., Rice, D. W., Yeates, T. O. & Eisenberg, D. (1999) Science 285, 751-753. [DOI] [PubMed] [Google Scholar]
- 33.Corey, E. J., Lansbury, P. T. & Yamada, Y. (1985) Tetrahedron Lett. 26, 4171-4174. [Google Scholar]
- 34.Varvas, K., Jarving, I., Koljak, R., Valmsen, K., Brash, A. R. & Samel, N. (1999) J. Biol. Chem. 274, 9923-9929. [DOI] [PubMed] [Google Scholar]
- 35.Brash, A. R., Hughes, M. A., Hawkins, D. J., Boeglin, W. E., Song, W. C. & Meijer, L. (1991) J. Biol. Chem. 266, 22926-22931. [PubMed] [Google Scholar]
- 36.Kaneko, T., Nakamura, Y., Wolk, C. P., Kuritz, T., Sasamoto, S., Watanabe, A., Iriguchi, M., Ishikawa, A., Kawashima, K., Kimura, T., et al. (2001) DNA Res. 8, 205-213, 227-253. [DOI] [PubMed] [Google Scholar]
- 37.Feng, Q., Zhang, Y. J., Hao, P., Wang, S. Y., Fu, G., Huang, Y. C., Li, Y., Zhu, J. J., Liu, Y., Hu, X., et. al. (2002) Nature 420, 316-320. [DOI] [PubMed] [Google Scholar]
- 38.Rudberg, P. C., Tholander, F., Thunnissen, M. M. & Haeggstrom, J. Z. (2002) J. Biol. Chem. 277, 1398-1404. [DOI] [PubMed] [Google Scholar]