Abstract
Chronic ethanol abuse causes up-regulation of NMDA receptors, which underlies seizures and brain damage upon ethanol withdrawal (EW). Here we show that tissue-plasminogen activator (tPA), a protease implicated in neuronal plasticity and seizures, is induced in the limbic system by chronic ethanol consumption, temporally coinciding with up-regulation of NMDA receptors. tPA interacts with NR2B-containing NMDA receptors and is required for up-regulation of the NR2B subunit in response to ethanol. As a consequence, tPA-deficient mice have reduced NR2B, extracellular signal-regulated kinase 1/2 phosphorylation, and seizures after EW. tPA-mediated facilitation of EW seizures is abolished by NR2B-specific NMDA antagonist ifenprodil. These results indicate that tPA mediates the development of physical dependence on ethanol by regulating NR2B-containing NMDA receptors.
Keywords: proteases, excitotoxicity, alcoholism
Ethanol is one of the most commonly abused substances, and its consumption may lead to addiction (1). Characteristic features of alcoholism include development of tolerance to ethanol's effects and severe physical symptoms precipitated by the abrupt cessation of drinking. The latter, ethanol-withdrawal (EW) syndrome, is a life-threatening condition characterized by insomnia, tremor, muscle rigidity, hallucinations, and seizures (2).
Long-time ethanol consumption causes profound cognitive and motor deficits resulting from cortical and cerebellar atrophy and from neuronal death in the hippocampus (Hipp) (3). The mechanisms by which ethanol causes physical dependence and neurodegeneration are unclear, although NMDA and GABA(A) receptors are especially sensitive ethanol targets (1). Ethanol inhibits NMDA receptors in vitro (4) and in vivo (5), acting primarily on the NR2B subunit (6–8). Long-term ethanol abuse results in an adaptive increase in the number and sensitivity of NMDA-binding sites (9), which underlies seizures and neurotoxicity upon EW (10).
Mechanisms that govern the up-regulation of NMDA receptors and mediate physical dependence on ethanol may involve NMDA-receptor regulators in ethanol-sensitive brain regions. These may include the Hipp (11), the reward circuits (12), and/or the amygdala stress system (13), which contribute to drug craving and relapse. One candidate regulator is tissue plasminogen activator (tPA), a serine protease highly expressed in the Hipp and amygdala (14). tPA is involved in various forms of neuronal plasticity (15–18), including those underlying addiction (19) and stress-induced anxiety (20). Because tPA has been reported to potentiate NMDA receptor signaling (21), it could serve as an effector protease linking modulation of NMDA receptors with the development of ethanol dependence.
Here we show that tPA interacts with the NMDA receptor and regulates its ethanol-sensitive NR2B subunit. These events facilitate the development of physical dependence on ethanol. Consequently, tPA-mediated changes observed during ethanol administration promote seizures after EW.
Methods
Animals. Experiments were performed on 3-month old wild-type C57/Bl6 and tPA-/- (22) or plasminogen-/- (23, 24) mice, backcrossed to C57/Bl6 for at least nine generations. Animals were housed three to five per cage in a colony room with a 12-h light/dark cycle (lights on at 7:00 a.m.) with ad libitum access to commercial chow and tap water. All procedures were approved by the Institutional Animal Care and Use Committee of The Rockefeller University.
Induction of Physical Dependence. For chronic ethanol treatment, the mice were housed individually and given a measured amount of liquid diet (Bioserv, Frenchtown, NJ) containing 2.3–10% vol/vol ethanol and vitamin supplement as their sole nutrient source. The mice were gradually introduced to the ethanol diet as follows: days 1–3, 2.3% ethanol; days 4–6, 4.7% ethanol; days 7–10, 7% ethanol; and days 11–14, 10% ethanol. Every 24 h, the amount of diet consumed was measured and replaced with fresh ethanol-containing or control liquid diet. The pair-fed control mice were given the same volume of ethanol-free liquid diet (with sucrose substituted in isocaloric quantities for ethanol) as the ethanol-exposed mice had consumed the previous day (25). Every 24 h, the mice were rated for behavioral signs of ethanol intoxication by an observer who was unaware of the kind (ethanol-containing vs. -free) or amount of diet consumed as well as genotype of the animals, as described (25).
EW and Assessment of Seizure Severity. EW was initiated on day 15 at 8:00 a.m. by removing the ethanol-containing diet and replacing it with an ethanol-free diet. Handling-induced withdrawal seizures were rated on a scale of 0–7 (26). In brief, the mice were picked up by the tail and rated as follows: 0, no reaction; 1, no reaction when lifted by the tail and a slight jerkiness after gentle 360° spin; 2, no convulsion when lifted by the tail and slight tonic convulsion after gentle 360° spin; 3, slight tonic convulsion when lifted by the tail; 4, tonic-clonic convulsion when lifted by the tail with onset within 2 sec; 5, severe tonic-clonic convulsion when lifted by the tail with rapid onset and long duration, often continuing for several seconds after release; 6, spontaneous convulsions elicited by mild environmental stimuli (e.g., lifting the cage top); and 7, death due to seizure. Seizure severity was evaluated by an observer unaware of the animals' treatment and genotype.
In Situ Zymography. Mice were anesthetized and transcardially perfused with ice-cold PBS; their brains were removed, immediately frozen, and embedded in optimal cutting temperature medium (OCT, Tissue-Tek, Sakura USA, Torrance, CA). Fifteen-micrometer-thick sections were cut by using a cryostat, collected on silane-coated slides, immediately frozen, and stored at -80°C until analyzed. In situ zymography was performed according to Sappino et al. (14). In brief, an overlay mixture (10 mM Tris, pH 7.5/10 mg/ml low-melting-point agarose/2.5% commercial instant nonfat skim milk/25 μg/ml human plasminogen) was applied to prewarmed brain sections and spread evenly under glass coverslips. The slides were incubated at 37°C in a humid chamber for 2–4 h, and the developed zymograms were photographed under dark-field illumination. The optical density of the lytic zones and the area of lysis were quantified by using nih image.
Western Blotting. Mice were anesthetized, transcardially perfused with ice-cold PBS containing phosphatase inhibitors (10 mM NaF/10 mM β-glycerophosphate) and protease inhibitors (Complete Protease Inhibitor Cocktail, Roche Applied Sciences, Indianapolis) and their brains were removed. Hipp were dissected and homogenized in 0.1 M Tris, pH 7.4/0.1% Triton X-100 containing phosphatase and protease inhibitors, and the protein concentration was adjusted to 2 mg/ml. Samples (25 μg) were subjected to SDS/PAGE electrophoresis and transferred onto nitrocellulose membrane. The membrane was probed with goat anti-NR1 (Upstate Biotechnology, Lake Placid, NY; 1:1,000), rabbit anti-NR2A (Upstate Biotechnology, 1:1,000), goat anti-NR2B (Santa Cruz Biotechnology, 1:1,000), rabbit anti-NR2B phospho-Tyr 1472 (Chemicon, 1:1,000), rabbit anti-p44/42 MAPK (Cell Signaling Technology, Beverly, MA; 1:1,000) or rabbit anti-p44/42 mitogen-activated protein kinase phospho-Thr-202/Tyr-204 (Cell Signaling Technology, 1:1,000) antibodies, followed by peroxidase-labeled anti-goat or anti-rabbit IgG (Vector Laboratories, 1:2,500). To obtain loading control, the membrane was stripped and reblotted by using anti-β-actin antibody (Sigma, 1:1,000). Band intensity was quantified by nih image.
Coimmunoprecipitation. Hipp were dissected from wild-type mice, homogenized in RIPA buffer (50 mM Tris, pH 7.4/150 mM NaCl/1% deoxycholate/1% Triton X-100) with protease inhibitors, and protein concentration was adjusted to 2 mg/ml. Samples (400 μl) were precleared with nonspecific rabbit or goat IgG followed by adsorption on protein G-Sepharose (Amersham Pharmacia). Immunoprecipitation was performed overnight at 4°C by using 1 μg of rabbit anti-tPA (Molecular Innovations, Southfield, MI), goat anti-NR1 (Santa Cruz Biotechnology), rabbit anti-NR2A (Upstate Biotechnology), or goat anti-NR2B (Santa Cruz Biotechnology) antibodies, followed by adsorption to protein G-sepharose. In control experiments, the antibodies were replaced with nonspecific rabbit or goat IgG (Sigma). The proteins were eluted in a loading electrophoresis buffer containing DTT, separated by SDS/PAGE, and transferred onto nitrocellulose membrane. Western blotting was performed by using the same primary antibodies as for immunoprecipitation.
NR2B Subunit Cleavage. Hipp were dissected from wild-type mice, homogenized in 0.1 M Tris, pH 7.2/0.1% Triton X-100 without protease inhibitors, and protein concentration was adjusted to 2 mg/ml. The homogenate was incubated with human tPA (20 μg/ml) or tPA plus human plasminogen (50 μg/ml) for 30 min at 37°C, separated by SDS/PAGE electrophoresis, and probed with anti-NR2B antibody (Santa Cruz Biotechnology, 1:1,000) followed by horseradish peroxidase-conjugated secondary antibody (Vector Laboratories, 1:2,500). Band intensity was quantified by nih image.
Pharmacological Modification of Seizure Severity During EW. The experiments were performed on wild-type and tPA-/- mice in which cannulas had been implanted to allow for intracebroventricular (i.c.v.) drug delivery. Briefly, animals were injected i.p. with atropine (0.6 mg/kg), anesthetized with 2.5% avertin (0.02 ml per gram of body weight), and placed in a Kopf Instruments (Tujunga, CA) stereotaxic apparatus. The skull was exposed, a burr hole overlying the implantation coordinates was drilled, and a 26-gauge guide cannula (Plastics One, Roanoke, VA) was lowered into the right lateral ventricle. Stereotaxic coordinates of the cannula tip in relation to the bregma (anteroposterior, -0.3; dorsoventral, 2.5; and mediolateral, 1.0 mm) were selected according to ref. 27. The guide cannula was attached to the skull with dental cement, and a dummy cannula was inserted to maintain patency. i.c.v. injections were performed by using a 33-gauge injection cannula attached to PE-50 tubing fitted to a 10-μl Hamilton syringe.
After 3 days of recovery, the animals were subjected to induction of physical dependence on ethanol and EW, as described above. Based on the seizure scores during the first 4 h of EW, the animals were divided into two groups with comparable seizure severity. After 5.5 h of EW, one of the groups of tPA-/- mice received i.c.v. injection of tPA (Genentech, 0.1 μg in 1 μl), whereas the other (control) group was given the same volume of vehicle. Animals that had been injected with vehicle at the earlier time point were given tPA 7.5 h after EW and vice versa. Five minutes before i.c.v. injection, both groups were pretreated with the NMDA receptor antagonist, IFP (Sigma, 1 mg/kg, i.p). The above design allowed each experimental group to serve as a control, depending on the time point.
One group of wild-type mice received i.c.v. injection of tPA-STOP [American Diagnostica (Greenwich, CT), 1 μg in 2 μl] 5.5 h after EW, whereas the other (control) group was given the same volume of vehicle.
Statistical Analysis. The Mann–Whitney U test was used for statistical evaluation; P < 0.05 was considered statistically significant.
Results
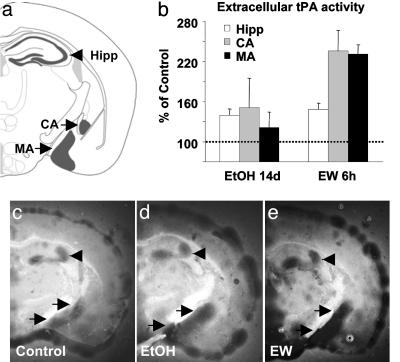
tPA Is Up-Regulated by Ethanol and EW. To investigate whether brain PA activity was affected by ethanol, we performed in situ zymography by using brain sections of wild-type mice during ethanol intoxication and/or EW. This assay measures extracellular PA activity on fresh-frozen sections through plasmin cleavage of casein in the overlay gel (14). The animals were given increasing percentages of ethanol in a liquid diet for 14 days. One group of animals was killed before EW, and ethanol was withdrawn from the remaining animals. EW precipitated profound physical symptoms, manifested by handling-induced seizures (25). We observed an increase in extracellular PA during ethanol treatment in the Hipp (by 35%; P = 0.05; Fig. 1 b–d) and even more robust up-regulation during EW in the Hipp and medial and central amygdala (by 40%, 230%, and 235%, P < 0.02, P < 0.03, and P < 0.01, respectively; Fig. 1 b and e). This increased PA activity was not observed in tPA-/- mice, indicating that it was due to tPA and not urokinase-type PA (not shown). tPA activity was also induced in the meninges (Fig. 1 d and e), which is consistent with its up-regulation in nonneuronal cells by ethanol (28, 29).
Fig. 1.
Ethanol treatment and EW up-regulate tPA activity in the limbic system. In situ zymography of brain sections from wild-type mice (the regions schematically shown in a) demonstrates that extracellular tPA activity (dark lytic zones) is slightly up-regulated in the Hipp (arrowheads) as well as the central amygdala (CA) and medial amygdala (MA) (upper and lower arrows, respectively) after 14 days of ethanol treatment (d), followed by robust up-regulation 6 h after EW (P < 0.02; P < 0.01; P < 0.03, respectively; e). The changes were compared with ethanol-naïve controls (c; dotted line in b). n = 4–6 for each value. The results are expressed as mean ± SEM.
To further confirm this result, we performed SDS/PAGE zymography, which distinguishes tPA and urokinase-type PA (uPA) by their relative molecular weights (14). tPA activity was up-regulated during both ethanol intoxication and EW in the Hipp, whereas uPA activity remained unchanged (not shown). These results indicate that tPA is regulated by ethanol and may have a role in promoting ethanol's effects.
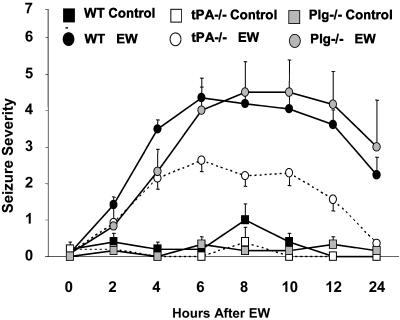
tPA Facilitates Physical Dependence on Ethanol in a Plasminogen-Independent Manner. tPA has been implicated in various forms of neuronal activity (17) and is induced by psychoactive drugs and mediates their rewarding properties (19, 30). To test whether tPA is involved in the development of ethanol dependence, we measured the severity of EW symptoms in wild-type and tPA-/- mice (26) (Fig. 2). Withdrawal symptoms peaked at 6 h, remained stable for the next 6 h, and decreased at 24 h. In wild-type mice, the withdrawal syndrome manifested itself as a decrease in spontaneous locomotor activity and resulted in handling-induced seizures. In contrast, EW-induced hypolocomotion was less pronounced in tPA-/- mice, and there was an overall 48% reduction of handling-induced seizures in tPA-/- compared with wild-type mice (P < 0.001; Fig. 2). There were no differences in the level of ethanol intoxication, consumption, brain ethanol levels, and weight loss between wild-type and tPA-/- mice during ethanol administration (not shown). Pairfed ethanol-naïve mice of the same genotypes did not have seizures (Fig. 2).
Fig. 2.
tPA promotes EW seizures in a plasminogen-independent manner. Wild-type, tPA-/-, and plasminogen-/- mice were fed an ethanol diet for 14 days. On day 15, ethanol was withdrawn, and the mice were tested for handling-induced seizures every 2 h after EW. tPA-/- mice had significantly less severe seizures (P < 0.001 at 4, 6, 8, 10, 12, and 24 h after EW, compared with wild-type mice), whereas plasminogen-/- mice displayed seizure severity similar to that of the wild-type animals. Pair-fed ethanol-naïve mice of the same genotypes did not show seizures. n = 5 for each ethanol-naïve group, and n = 6–25 for each ethanol-treated group. The results are expressed as mean ± SEM. The error bars in some data points are smaller than the symbols and therefore are not visible.
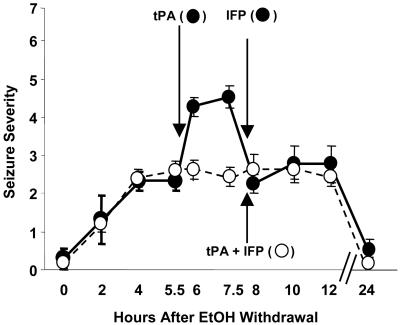
To ensure that the decrease in EW seizures in adult tPA-/- mice was not due to developmental abnormalities resulting from global deletion of the tPA gene, we injected tPA into the lateral ventricle of tPA-/- mice during EW. Injection of tPA resulted in an increase in EW seizures (Fig. 3). These results indicate that tPA promotes EW seizures in an acute and not developmentally related manner.
Fig. 3.
Injection of tPA into tPA-/- mice during EW increases seizure severity, and this effect is blocked by NR2B-specific NMDA receptor antagonist IFP. tPA-/- mice were implanted with cannulas into the lateral ventricle, fed an ethanol liquid diet for 14 days, and then subjected to EW. At 0, 2, and 4 h, seizure severity was assessed, and the animals were divided into two groups with equal average seizure severity. Each group received two i.c.v. injections. At 5.5 h, the first group (black circles) received recombinant tPA (100 ng in 1 μl), and the second group served as control (vehicle; white circles). The mice treated with tPA showed an enhanced seizure response within 30 min of injection (P < 0.01 vs. vehicle), which persisted for at least 90 min. At 7.5 h, the treatment regimen was reversed. Both groups were injected with IFP (1 mg/kg, i.p.) and then received i.c.v. injections of either vehicle (black circles) or tPA (white circles). The effect of tPA on seizures was prevented by IFP. n = 4–5 for each value. The results are expressed as mean ± SEM.
The best-characterized substrate for tPA is plasminogen, which is converted to plasmin (31). Because tPA in the CNS can act either in a plasminogen-dependent (32, 33) or -independent manner (34, 35), we examined whether EW seizures were plasminogen-dependent by subjecting plasminogen-/- mice to EW. Plasminogen-/- mice were equally susceptible to the hypnotic/sedative effect of ethanol and consumed similar amounts of diet as wild-type animals (not shown). However, in contrast to tPA-/- mice, plasminogen-/- animals showed seizure severity similar to wild-type (Fig. 2), indicating that the effect of tPA on EW seizures is plasminogen-independent.
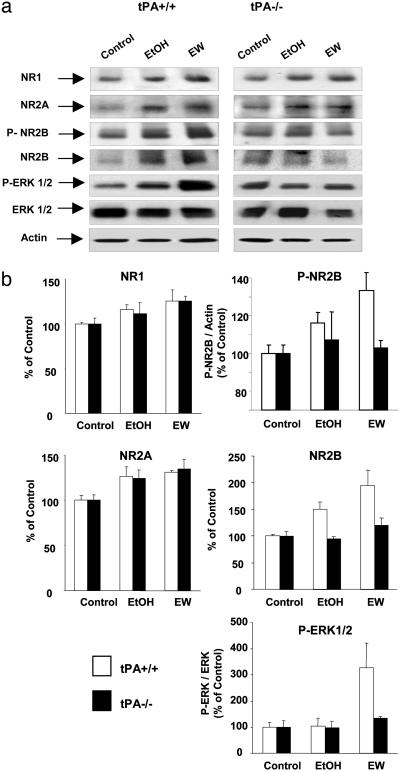
tPA Regulates NR2B-Containing NMDA Receptors. Chronic ethanol intoxication causes up-regulation of NMDA receptors (9, 36, 37), which facilitates seizures during EW (9, 36). To investigate whether NMDA receptors are regulated by tPA, we measured the expression of individual NMDA receptor subunits in the Hipp of wild-type and tPA-/- mice during ethanol intoxication and EW by Western blotting. NR1 and NR2A were slightly up-regulated during ethanol administration. These changes persisted for at least 6 h after EW (P < 0.01 and P < 0.005 for NR1 and NR2A, respectively, compared with no-ethanol treatment; Fig. 4) and were similar in wild-type and tPA-/- mice. NR2B was also up-regulated by ethanol and EW in wild-type mice, but this increase was absent in tPA-/- mice (P < 0.01; Fig. 4). This result shows that tPA is necessary for up-regulation of NR2B during ethanol treatment.
Fig. 4.
tPA regulates NR2B-containing NMDA receptors and its downstream signaling pathway. Wild-type and tPA-/- were treated with an ethanol diet for 14 days, and then ethanol was withdrawn. Hipp were collected after 14 days of ethanol administration (EtOH) or 6 h after EW. (a) Ethanol administration resulted in up-regulation of NR1 and NR2A subunits of NMDA receptors in both genotypes, which persisted 6 h after EW (P < 0.01 and P < 0.005 vs. control, respectively). Ethanol-induced up-regulation of NR2B subunit was significant in wild-type mice but was not observed in tPA-/- mice, indicating that tPA modulates the NR2B subunit. Deletion of the tPA gene prevented NR2B subunit phosphorylation at Tyr-1472 during EW (wild-type vs. tPA-/- mice, P < 0.05) as well as phosphorylation of its downstream signaling molecule ERK1/2 (wild-type vs. tPA-/- mice, P < 0.01). The changes in NR1, NR2A, NR2B, P-NR2B, and P-ERK1/2 are quantified in b. n = 4–6 for each value. The results are expressed as mean ± SEM.
To investigate how NR2B is regulated by tPA, we examined phosphorylation of NR2B at Tyr-1472, a site that is involved in modulating the properties of the NR1/NR2B receptor channel, and synaptic plasticity (38). The phosphorylation level of NR2B increased markedly during EW in wild-type mice (P < 0.05; Fig. 4) but remained unaffected in tPA-/- mice, suggesting that tPA-mediated changes permit the activation of NR2B-containing receptors by intracellular kinases (38). However, the contribution of activity-independent constitutive phosphorylation of NR2B receptors remains to be determined. If tPA facilitates the activation of NR2B, then during EW, NR2B-dependent signaling pathways should be disrupted in the absence of tPA. Activation of NR2B-containing NMDA receptors has been linked to phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) (39). We observed a 3-fold increase in phosphorylated ERK1/2 in the Hipp of wild-type mice during EW (P < 0.01; Fig. 4). This increase was not observed in tPA-/- animals, consistent with a role of tPA within this pathway. Altogether, these results show that tPA regulates the activity of NR2B-containing NMDA receptors and its downstream signaling cascade.
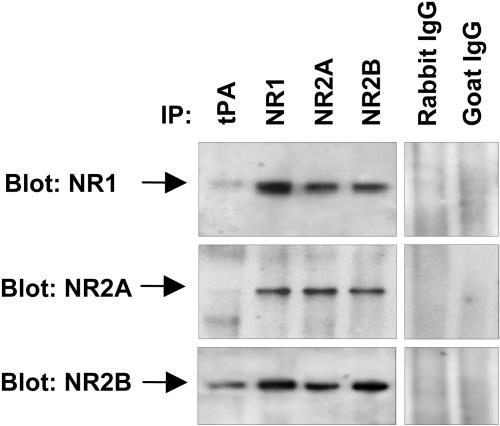
To examine whether tPA directly interacts with NMDA receptors, we performed coimmunoprecipitation by using antibodies against tPA and individual NMDA receptor subunits. Reliability of the immunoprecipitation was confirmed by immunoblotting the membranes with the antibodies used for immunoprecipitation (Fig. 5 and data not shown). We found that NR2B coimmunoprecipitated with tPA (Fig. 5). Consistent with the fact that NMDA receptor subunits coimmunoprecipitate with each other, we also found a weak band corresponding to NR1 and minute amounts of NR2A in the material immunoprecipitated with the anti-tPA antibody (Fig. 5).
Fig. 5.
tPA interacts with the NR2B-containing NMDA receptors. The interaction of tPA with the individual NMDA receptor subunits was examined by coimmunoprecipitation. Hippocampal homogenates from wild-type mice were incubated with anti-tPA, -NR1, -NR2A, or -NR2B antibodies followed by adsorption to protein G-sepharose. Immunoprecipitated proteins were eluted with electrophoresis-loading buffer, and the blots were developed by using antibodies against individual subunits of NMDA receptor. The strongest interaction was detected between tPA and NR2B. Weak bands corresponding to NR1 and NR2A detected in the anti-tPA-immunoprecipitated material may reflect indirect interaction through NR2B binding. In control experiments, the immunoprecipitating antibodies were replaced with irrelevant antibodies from the same species. The experiment was repeated on five different occasions with similar results.
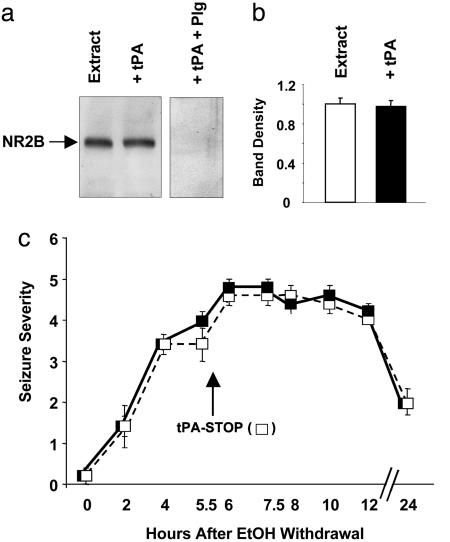
To investigate whether the action of tPA on NR2B was proteolytic, we incubated hippocampal extracts with tPA. tPA did not cleave NR2B, as shown by the lack of additional bands or a decrease in the native NR2B band (Fig. 6 a and b). Furthermore, i.c.v. injection of tPA inhibitor, tPA-STOP, did not affect EW seizure severity in wild-type animals (Fig. 6c). These results indicate that the effect of tPA on NR2B-containing NMDA receptors is nonproteolytic. However, addition of both plasminogen and tPA to the extract resulted in a complete disappearance of NR2B, indicating that plasmin can degrade NR2B subunits (Fig. 6a).
Fig. 6.
The effect of tPA on NR2B and EW seizures is nonproteolytic. (a) To examine whether tPA cleaves NR2B, hippocampal extracts were incubated with tPA (20 μg/ml) in the absence of protease inhibitors. No additional bands or decrease in full-length NR2B was detected by Western blotting (quantified in b; n = 4). The addition of plasminogen to the mixture resulted in the complete disappearance of NR2B, indicating that tPA converted plasminogen to plasmin, which then degraded NR2B. (c) Wild-type mice were implanted with cannulas into the lateral ventricle, fed an ethanol liquid diet for 14 days, and then subjected to EW. At 0, 2, and 4 h, seizure severity was assessed, and the animals were divided into two groups with equal average seizure severity. At 5.5 h, one group (white squares) received tPA-STOP (1 μgin2 μl), and the second group served as control (vehicle; black squares). tPA-STOP did not affect EW seizure severity in wild-type animals. These results indicate that the effect of tPA on NR2B-containing NMDA receptors and EW seizures is nonproteolytic. n = 4–6 for each value. The results are expressed as mean ± SEM.
To test whether NR2B was playing a role in the effects of tPA on EW in vivo, we injected tPA into tPA-/- mice during EW. The increase in seizure severity associated with tPA treatment was blocked by IFP, a specific inhibitor of NR2B subunits (P < 0.01 vs. tPA alone; Fig. 3).
These results indicate that the effect of tPA on EW seizures is mediated via NR2B-containing NMDA receptors.
Discussion
Ethanol dependence is a form of adaptive neuronal plasticity (13), and long-term administration of ethanol influences many neurotransmitter systems in the brain. However, the most specific and prominent changes are observed within several ligandgated ion channels, including excitatory NMDA and inhibitory GABA(A) receptors (4, 11, 40–43). Thus, the development of physical dependence on ethanol involves up-regulation of NMDA receptors, especially their NR2B subunits (6–8, 37). NR1/NR2B coassembly is present during development and adulthood and facilitates neuronal plasticity and learning (44). At present, little is known about the extracellular ligands and mechanism(s) that regulate the expression of NR2B subunits and modulate their channel-gating properties.
tPA, implicated in activity-dependent neuronal plasticity in the Hipp (15, 17) and amygdala (20), is a good candidate for such a modulator. It is released into the extracellular space upon neuronal activation (15, 45), stimulates NMDA receptors (21), and activates biochemical pathways required for dendritic plasticity and behavior modifications (20). Our present work shows that tPA is induced both in the Hipp and amygdala by long-term ethanol administration and even further by pathological neuronal activity observed during EW. Extracellular tPA physically interacts with NR2B-containing NMDA receptors. This interaction is required for the up-regulation of NR2B subunits during long-term ethanol administration, which promotes physical dependence on ethanol. tPA/NMDA–receptor interaction also enables phosphorylation of the NR2B subunit by intracellular kinases and triggers an ERK-dependent signaling cascade during EW. tPA-mediated facilitation of EW seizures can be blocked by an NR2B-selective NMDA receptor antagonist IFP.
Our results favor a model in which tPA interacts with the NMDA receptor to modulate the properties of the NR2B subunit. However, the precise tPA-binding site within the NMDA receptor is not known. Based on the fact that tPA coimmunoprecipitates with both NR1 and, to a lesser degree, NR2A subunits (also confirmed in this study), Nicole et al. (21) concluded that tPA interacts with the NR1 subunit of NMDA receptor. On the other hand, our coimmunoprecipitation experiments revealed the strongest interaction between tPA and the NR2B subunit. The presence of the NR1 and NR2A bands can reflect indirect binding through NR2B, because NMDA receptor subunits coassemble in vivo (and therefore can coimmunoprecipitate in vitro; see Results) to form a functional channel. However, we cannot exclude the possibility that tPA binds to the NR1 subunit to modulate the properties of NR2B.
Our experiments suggest that the effect of tPA on NMDA receptors is nonproteolytic. We did not observe any cleavage of NR1 (46) or NR2B (this study) even with large amounts of tPA (20 μg/ml). Similarly, cleavage of the NR1 subunit by tPA could not be observed in a recent study (47). This is not surprising, because tPA is a narrow-spectrum protease (31) and, unlike other serine proteases, has few known substrates. Thus, our results contrast with those of others (21, 48), who reported that tPA cleaves the NR1 subunit in membranes from cultured cortical neurons or in vitro. Nevertheless, it cannot be excluded that some factors protecting the NMDA receptor from cleavage are present in the brain but are absent in the cortical membrane preparation used by Nicole et al. (21).
It is important to stress that tPA–NMDA interactions are not solely responsible for the development of physical dependence on ethanol. The action of ethanol in the brain is complex, and multiple neurotransmitters and receptors participate in the development of physical dependence to ethanol, including GABA(A) receptors, opioid receptors, and L- and P-type calcium channels (1, 43). Modulation of these systems may explain why, in our experiments, tPA-/- mice, despite lacking adaptive up-regulation of NR2B subunit in response to ethanol, still developed moderate signs of EW. Also, the increased seizure severity observed in tPA-/- mice after tPA injection might be due to its action on basal levels of NR2B subunits.
Our findings, together with the work of others, suggest the following scenario for the effects of tPA in the development of physical dependence on ethanol and EW seizures. Ethanol inhibits NMDA receptors, acting preferably on NR2B subunits. tPA, up-regulated during ethanol intoxication, interacts with NR2B-containing NMDA receptors and is required for the up-regulation of NR2B in response to ethanol. High expression of NMDA receptors with slow-decay characteristics creates a potential state of hyperexcitability, uncovered by the rapid withdrawal of ethanol. NMDA receptor overstimulation causes neuronal depolarization, which further liberates tPA from neurons. This extracellular tPA promotes activation of NMDA receptors, leading to phosphorylation of NR2B and activation of ERK1/2.
These findings identify a tPA-dependent pathway of neuronal activation as a potential drug target against ethanol-related brain pathologies.
Acknowledgments
We thank Yuliya Keptsi for technical assistance and the members of the Strickland lab for discussions. This research was supported by a grant from the Alcoholic Beverage Medical Research Foundation (to R.P.) and by Grant NS35704 from the National Institute of Neurological Disorders and Stroke and Grant AA14630 from the National Institutes of Health (to S.S.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Hipp, hippocampus/hippocampi; EW, ethanol withdrawal; IFP, ifenprodil; tPA, tissue plasminogen activator; i.c.v., intracebroventricular.
References
- 1.Tabakoff, B. & Hoffman, P. L. (1996) Neuron 16, 909-912. [DOI] [PubMed] [Google Scholar]
- 2.Kosten, T. R. & O'Connor, P. G. (2003) N. Engl. J. Med. 348, 1786-1795. [DOI] [PubMed] [Google Scholar]
- 3.Charness, M. E. (1993) Alcohol. Clin. Exp. Res. 17, 2-11. [DOI] [PubMed] [Google Scholar]
- 4.Lovinger, D. M., White, G. & Weight, F. F. (1989) Science 243, 1721-1724. [DOI] [PubMed] [Google Scholar]
- 5.Simson, P. E., Criswell, H. E., Johnson, K. B., Hicks, R. E. & Breese, G. R. (1991) J. Pharmacol. Exp. Ther. 257, 225-231. [PubMed] [Google Scholar]
- 6.Fink, K. & Gothert, M. (1996) Naunyn–Schmiedeberg's Arch. Pharmacol. 354, 312-319. [DOI] [PubMed] [Google Scholar]
- 7.Lovinger, D. M. (1995) J. Pharmacol. Exp. Ther. 274, 164-172. [PubMed] [Google Scholar]
- 8.Yang, X., Criswell, H. E., Simson, P., Moy, S. & Breese, G. R. (1996) J. Pharmacol. Exp. Ther. 278, 114-124. [PubMed] [Google Scholar]
- 9.Grant, K. A., Valverius, P., Hudspith, M. & Tabakoff, B. (1990) Eur. J. Pharmacol. 176, 289-296. [DOI] [PubMed] [Google Scholar]
- 10.Iorio, K. R., Tabakoff, B. & Hoffman, P. L. (1993) Eur. J. Pharmacol. 248, 209-212. [DOI] [PubMed] [Google Scholar]
- 11.Miyakawa, T., Yagi, T., Kitazawa, H., Yasuda, M., Kawai, N., Tsuboi, K. & Niki, H. (1997) Science 278, 698-701. [DOI] [PubMed] [Google Scholar]
- 12.Littleton, J. & Little, H. (1994) Addiction 89, 1397-1412. [DOI] [PubMed] [Google Scholar]
- 13.Koob, G. F., Roberts, A. J., Schulteis, G., Parsons, L. H., Heyser, C. J., Hyytia, P., Merlo-Pich, E. & Weiss, F. (1998) Alcohol. Clin. Exp. Res. 22, 3-9. [PubMed] [Google Scholar]
- 14.Sappino, A. P., Madani, R., Huarte, J., Belin, D., Kiss, J. Z., Wohlwend, A. & Vassalli, J. D. (1993) J. Clin. Invest. 92, 679-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baranes, D., Lederfein, D., Huang, Y. Y., Chen, M., Bailey, C. H. & Kandel, E. R. (1998) Neuron 21, 813-825. [DOI] [PubMed] [Google Scholar]
- 16.Madani, R., Hulo, S., Toni, N., Madani, H., Steimer, T., Muller, D. & Vassalli, J. D. (1999) EMBO J. 18, 3007-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian, Z., Gilbert, M. E., Colicos, M. A., Kandel, E. R. & Kuhl, D. (1993) Nature 361, 453-457. [DOI] [PubMed] [Google Scholar]
- 18.Seeds, N. W., Williams, B. L. & Bickford, P. C. (1995) Science 270, 1992-1994. [DOI] [PubMed] [Google Scholar]
- 19.Nagai, T., Yamada, K., Yoshimura, M., Ishikawa, K., Miyamoto, Y., Hashimoto, K., Noda, Y., Nitta, A. & Nabeshima, T. (2004) Proc. Natl. Acad. Sci. USA 101, 3650-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawlak, R., Magarinos, A. M., Melchor, J., McEwen, B. & Strickland, S. (2003) Nat. Neurosci. 6, 168-174. [DOI] [PubMed] [Google Scholar]
- 21.Nicole, O., Docagne, F., Ali, C., Margaill, I., Carmeliet, P., MacKenzie, E. T., Vivien, D. & Buisson, A. (2001) Nat. Med. 7, 59-64. [DOI] [PubMed] [Google Scholar]
- 22.Carmeliet, P., Schoonjans, L., Kieckens, L., Ream, B., Degen, J., Bronson, R., De Vos, R., van den Oord, J. J., Collen, D. & Mulligan, R. C. (1994) Nature 368, 419-424. [DOI] [PubMed] [Google Scholar]
- 23.Bugge, T. H., Flick, M. J., Daugherty, C. C. & Degen, J. L. (1995) Genes Dev. 9, 794-807. [DOI] [PubMed] [Google Scholar]
- 24.Ploplis, V. A., Carmeliet, P., Vazirzadeh, S., Van Vlaenderen, I., Moons, L., Plow, E. F. & Collen, D. (1995) Circulation 92, 2585-2593. [DOI] [PubMed] [Google Scholar]
- 25.Malinowska, B., Napiorkowska-Pawlak, D., Pawlak, R., Buczko, W. & Gothert, M. (1999) Eur. J. Pharmacol. 377, 13-19. [DOI] [PubMed] [Google Scholar]
- 26.Olive, M. F., Mehmert, K. K., Nannini, M. A., Camarini, R., Messing, R. O. & Hodge, C. W. (2001) Neuroscience 103, 171-179. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos, G. & Franklin, K. (2001) The Mouse Brain in Stereotaxic Coordinates (Academic, San Diego), 2nd Ed.
- 28.Booyse, F. M., Aikens, M. L. & Grenett, H. E. (1999) Alcohol. Clin. Exp. Res. 23, 1119-1124. [DOI] [PubMed] [Google Scholar]
- 29.Tabengwa, E. M., Wheeler, C. G., Yancey, D. A., Grenett, H. E. & Booyse, F. M. (2002) Alcohol. Clin. Exp. Res. 26, 1121-1127. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto, T., Kajii, Y. & Nishikawa, T. (1998) Eur. J. Neurosci. 10, 3387-3399. [DOI] [PubMed] [Google Scholar]
- 31.Collen, D. (1999) Thromb. Haemostasis 82, 259-270. [PubMed] [Google Scholar]
- 32.Tsirka, S. E., Gualandris, A., Amaral, D. G. & Strickland, S. (1995) Nature 377, 340-344. [DOI] [PubMed] [Google Scholar]
- 33.Chen, Z. L. & Strickland, S. (1997) Cell 91, 917-925. [DOI] [PubMed] [Google Scholar]
- 34.Yepes, M., Sandkvist, M., Coleman, T. A., Moore, E., Wu, J. Y., Mitola, D., Bugge, T. H. & Lawrence, D. A. (2002) J. Clin. Invest. 109, 1571-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawlak, R. & Strickland, S. (2002) J. Clin. Invest. 109, 1529-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffman, P. L. & Tabakoff, B. (1994) EXS 71, 61-70. [DOI] [PubMed] [Google Scholar]
- 37.Kalluri, H. S., Mehta, A. K. & Ticku, M. K. (1998) Brain Res. Mol. Brain Res. 58, 221-224. [DOI] [PubMed] [Google Scholar]
- 38.Nakazawa, T., Komai, S., Tezuka, T., Hisatsune, C., Umemori, H., Semba, K., Mishina, M., Manabe, T. & Yamamoto, T. (2001) J. Biol. Chem. 276, 693-699. [DOI] [PubMed] [Google Scholar]
- 39.Krapivinsky, G., Krapivinsky, L., Manasian, Y., Ivanov, A., Tyzio, R., Pellegrino, C., Ben-Ari, Y., Clapham, D. E. & Medina, I. (2003) Neuron 40, 775-784. [DOI] [PubMed] [Google Scholar]
- 40.Follesa, P. & Ticku, M. K. (1996) J. Biol. Chem. 271, 13297-9. [DOI] [PubMed] [Google Scholar]
- 41.Ikonomidou, C., Bittigau, P., Ishimaru, M. J., Wozniak, D. F., Koch, C., Genz, K., Price, M. T., Stefovska, V., Horster, F., Tenkova, T., et al. (2000) Science 287, 1056-1060. [DOI] [PubMed] [Google Scholar]
- 42.Sanna, E., Serra, M., Cossu, A., Colombo, G., Follesa, P., Cuccheddu, T., Concas, A. & Biggio, G. (1993) Alcohol. Clin. Exp. Res. 17, 115-123. [DOI] [PubMed] [Google Scholar]
- 43.Lovinger, D. M. (1997) Naunyn–Schmiedeberg's Arch. Pharmacol. 356, 267-282. [DOI] [PubMed] [Google Scholar]
- 44.Tang, Y. P., Shimizu, E., Dube, G. R., Rampon, C., Kerchner, G. A., Zhuo, M., Liu, G. & Tsien, J. Z. (1999) Nature 401, 63-69. [DOI] [PubMed] [Google Scholar]
- 45.Gualandris, A., Jones, T. E., Strickland, S. & Tsirka, S. E. (1996) J. Neurosci. 16, 2220-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matys, T. & Strickland, S. (2003) Nat. Med. 9, 371-372. [DOI] [PubMed] [Google Scholar]
- 47.Liu, D., Cheng, T., Guo, H., Fernandez, J. A., Griffin, J. H., Song, X. & Zlokovic, B. V. (2004) Nat. Med. 10, 1379-1383. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Monreal, M., Lopez-Atalaya, J. P., Benchenane, K., Cacquevel, M., Dulin, F., Le Caer, J. P., Rossier, J., Jarrige, A. C., Mackenzie, E. T., Colloc'h, N., et al. (2004) J. Biol. Chem. 279, 50850-50856. [DOI] [PubMed] [Google Scholar]