Abstract
Objective: To investigate the effects of resuscitation with normal saline (NS), hypertonic saline (HTS), and hydroxyethyl starch (HES) on regulatory T cells (Tregs), helper T 1 (Th1)/Th2 and cytotoxic T 1 (Tc1)/Tc2 profiles in the treatment of hemorrhagic shock. Methods: Rats subjected to severe hemorrhagic shock were resuscitated for 30 min with NS (n=8), HTS (n=8), or HES (n=8); sham (n=8) and naive control (n=8) groups were used for comparison. Following fluid resuscitation, the whole shed blood was reinfused for 30 min, and the rats were observed with continuous hemodynamic monitoring for 120 min. CD4+CD25+Foxp3+ Treg proportions, Th1/Th2 and Tc1/Tc2 profiles in spleen were analyzed by three-color flow cytometry. Results: The proportion of CD4+CD25+Foxp3+ Tregs and ratios of Th1/Th2 and Tc1/Tc2 did not differ among control, sham, and HTS groups, but were significantly lower in NS and HES groups (both P<0.05 vs. sham); NS and HES levels were similar. The level of Tc1 was significantly increased in HTS (P<0.05 vs. sham), and levels of Tc2 were increased in NS, HES, and HTS groups compared to sham (all P<0.05), but did not differ from each other. Conclusions: HTS resuscitation has a greater impact on immune system recovery than NS or HES by preserving the proportion of Tregs and maintaining the balance between Th1/Th2 and Tc1/Tc2 cells in the spleen. Thus, HTS resuscitation provides potential immunomodulatory activity in the early stage after hemorrhagic shock.
Keywords: Regulatory T cells, Helper T cells, Cytotoxic T cells, Hemorrhagic shock
1. Introduction
Hemorrhagic shock is an emergency event primarily caused by low organ perfusion, which results in marked and widespread oxidative tissue damage. Crucial treatment of this condition involves timely control of hemorrhage and adequate intravascular volume replacement. Even with successful fluid resuscitation, acute respiratory distress syndrome, systemic inflammatory response syndrome, or sepsis and multiple organ dysfunction syndrome can develop. These are the leading causes of death in middle and late phases of hemorrhagic shock. These conditions are thought to result from hyperinflammation or immunodepression (Menger and Vollmar, 2004; Moore et al., 2004; 2006; Brøchner and Toft, 2009).
Fluids for resuscitation include normal saline (NS), hypertonic saline (HTS), and hydroxyethyl starch (HES), although controversy remains on which strategy is superior (Angele et al., 2008). Recently, the immunologic effects of resuscitation with HTS solutions have been examined (Yip et al., 2007; Murao et al., 2009; Vincenzi et al., 2009; Isayama et al., 2012). HTS stimulates the rapid release of adenosine triphosphate (ATP) in a time-and concentration-dependent manner, which enhances T-cell activation by increasing Ca2+ influx (Yip et al., 2007). In addition, HTS resuscitation suppresses the release of proinflammatory cytokines (Isayama et al., 2012), and thus might improve immunosuppressive reactions after hemorrhagic shock. Indeed, our previous studies indicate that HTS alleviates immune disorders and limits tissue injury after hemorrhagic shock (Lu et al., 2007; 2008; 2010).
Although serious immune dysfunction often appears in the middle or late stage, immune and inflammation systems are involved from the onset of hemorrhagic shock. Hence, it is essential to reveal the initial impacts of resuscitation with different fluids on the immune system to provide advice for early recovery and immune intervention strategies. The purpose of this study was to compare the effects of three resuscitation fluids on immune cell regulation in the early stage of hemorrhagic shock. Specifically, we evaluated the proportion of regulatory T cells (Tregs), which help maintain immune balance, and can limit inflammatory tissue damage caused by infection, autoimmunity, allogeneic immune responses, and antitumor immunity (Belkaid and Rouse, 2005; Zhao et al., 2013); we also evaluated the ratios of helper T (Th) and cytotoxic T (Tc), which can execute many crucial immune functions (Marcu et al., 2007; Zhang et al., 2008; Chen et al., 2014).
2. Materials and methods
2.1. Ethics statement
This study was approved by the Ethics Committee of the School of Medicine, Zhejiang University, Hangzhou, China. Animals were handled according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
2.2. Animals
Forty male Sprague-Dawley rats, weighing 280–330 g, were purchased from the Laboratory Animal Center of the Medical Institute of Zhejiang Province, China and were maintained on standard rat diet and tap water ad libitum before the experiment. Animals were housed under controlled light/dark conditions for at least 3 d; the light period was from 8:00 a.m. to 5:00 p.m. in specific pathogen-free conditions.
2.3. Experimental protocol
Rats were anesthetized with 40 mg/kg pentobarbital intraperitoneally (IP) and placed in a supine position on a warm pad (25 °C). After sterilization with 10% (0.1 g/ml) povidone-iodine solution, the right carotid artery was isolated and cannulated with a polyethylene catheter, which was used for blood withdrawal and connected to a pressure transducer and computerized physiograph system (model 1290C; Hewlett-Packard Co., Palo Alto, CA, USA) for continuous hemodynamic monitoring. In the same way, the left femoral vein was cannulated for fluid infusion, and rats were heparinized (500 U/kg). Rats were observed for 10 min post-cannulation to ensure stable blood hemodynamics and spontaneous breathing. Blood loss during cannulation was collected by gauze for measurement by weight; only rats with <0.22 g blood loss were used for fluid resuscitation experiments.
Severe and controlled hemorrhagic shock was induced according to our previous studies (Lu et al., 2007; 2008; 2010). Briefly, while under the mild anesthesia (40 mg/kg pentobarbital IP), acute hemorrhage was initiated (time=0 min) with four controlled blood withdrawals via the right carotid arterial cannula: 10 ml/kg per 5 min for the first two withdrawals, then 5 ml/kg per 5 min for the second two, to yield a total hemorrhage volume of 30 ml/kg. The shed blood was collected in glass syringes with heparin for later reinfusion. Fluid resuscitation was administered after 30 min. In the third stage, at 60 min, rats were reinfused with the whole shed blood to represent in-hospital emergency treatment. An observation stage then ensued (time=90–210 min) via continuous hemodynamic monitoring. Rats were then sacrificed by heart puncture and the spleens were removed for preparation of cell suspensions used in subsequent analyses.
2.4. Grouping of animals
Forty rats were randomly assigned to one of five groups (n=8 each group). In the control group, rats received no operative intervention. In the sham group, rats only received anesthesia, cannulation, heparinization, and observation. In the fluid resuscitation phase, rats in the NS resuscitation group received 0.9% (9 g/L) NaCl infusion for a 3:1 ratio against the blood loss volume (Gurfinkel et al., 2003; Alam et al., 2004; Barros et al., 2011; Maier and Alam, 2011). The HTS group received 6.0 ml/kg body weight of 7.5% (0.075 g/ml) NaCl solution for a 1:5 ratio against blood loss volume (Murao et al., 2003a; 2003b). The HES group received 30 ml/kg body weight of 6% HES (200/0.5; Fresenius Kabi, Baden Humboldt, Germany) solution for a 1:1 ratio against blood loss volume (Barros et al., 2011).
2.5. Flow cytometry
RBC Lysis Buffer was used to remove the red blood cells from the sample. The monoplast suspension of spleen was prepared according to our previous protocol (Lu et al., 2013). Spleen cell suspensions were cultured for 4 h at 37 °C at 5% CO2 in RPMI-1640 media (Sigma-Aldrich, St. Louis, MO, USA) containing phorbol myristate acetate, ionomycin, monensin (Beyotime, China), and fetal bovine serum.
Three-color flow cytometer (FC500, FACSCalibur; Beckman-Coulter, Brea, CA, USA) was then used to detect the various cell types. The software FACSCalibur (Beckman-Coulter, USA) was used for flow cytometric analysis. Anti-rat CD4 FITC (fluorescein isothiocyanate), CD25 PE (phycoerythrin), and Foxp3 PerCP-Cy5 (peridinin chlorophyll protein-cyanine 5) antibodies, and their isotype controls (eBioscience, San Diego, CA, USA) were used for detecting Tregs (CD4+CD25+Foxp3+). Anti-rat CD3 FITC, CD8a PerCP, interleukin (IL)-4 PE, and interferon (IFN)-γ PE antibodies, and their isotype controls (BD Biosciences, San Jose, CA, USA) were used for detecting Th1 (CD3+IFN-γ +CD8–), Th2 (CD3+IL-4+CD8–), Tc1 (CD3+IFN-γ +CD8+), and Tc2 (CD3+IL-4+CD8+) cells.
2.6. Statistical analysis
Data analyses were performed using SPSS Version 13.0 software (SPSS Inc., Chicago, IL, USA). Cell type percentages and ratios were compared by the homogeneity test, and then one-way analysis of variance (ANOVA) and the least significant difference t-test were applied for comparisons among the five groups. A two-way repeated-measures ANOVA was performed to compare arterial pressures among the groups. Data are presented as mean±standard deviation (SD) with a P<0.05 considered statistically significant.
3. Results
3.1. Mean arterial pressure
All rats undergoing acute hemorrhagic shock survived the experiments, with about 50% loss of blood volume based on weight. Mean arterial pressures (MAPs) measured throughout the experiment are presented in Fig. 1. Blood pressure levels for rats in the control group were not obtained, as they did not receive polyethylene catheters for monitoring. Acute hemorrhage initially induced a dramatic decrease in MAP, to about 20 mmHg at 20 min, which increased to about 30 mmHg at 30 min by self-compensation. Fluid resuscitation restored pressures within 15 min, with MAPs approaching 90 mmHg at the 45 min time-point. The MAPs then remained stable throughout the emergency treatment (60–90 min) and observation (90–210 min) stages. MAP levels remained unchanged throughout the entire experiment for rats in the sham group.
Fig. 1.
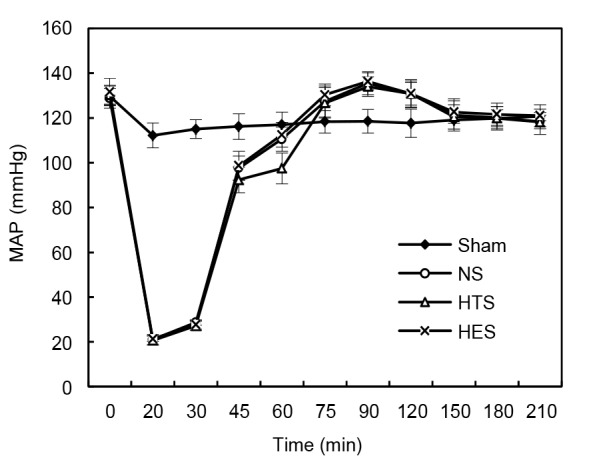
Mean arterial pressure (MAP) monitoring
Sham: sham group (n=8); NS: normal saline group (n=8); HTS: hypertonic saline group (n=8); HES: hydroxyethyl starch group (n=8). Data are represented as mean±SD
A two-way repeated-measures ANOVA was performed with fluids as the between-subjects factors and MAPs obtained at different time-points as within-subject variables. As a Mauchly’s test of sphericity indicated that variances in differences among the groups were not equal (df=54, P<0.05), degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε=0.34). As a result, no significant difference in MAP was found among NS, HTS, and HES groups during the experiment (F[6.78, 50.82]=1.943, P>0.05).
3.2. Changes of CD4+CD25+Foxp3+ Tregs in spleen
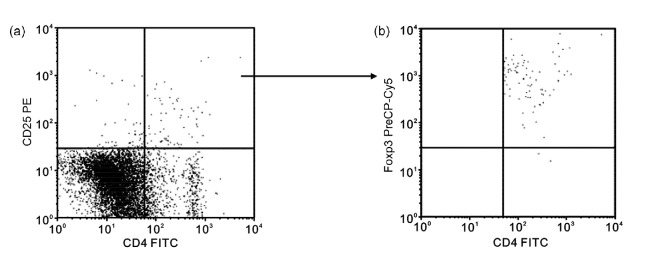
The representative illustration of flow cytometry for Tregs is shown in Fig. 2. The percentage of CD4+CD25+Foxp3+ Tregs in rats receiving HTS fluid resuscitation did not differ from those in sham and control rats (Table 1). However, the percentages of Tregs in NS and HES groups were similar and significantly lower than those in the control and sham groups (P<0.05).
Fig. 2.
Representative illustration of flow cytometry for Tregs
(a) Upper right quadrant represents CD4+CD25+ cells. (b) Upper right quadrant represents CD4+CD25+Foxp3+ Tregs
Table 1.
Percentages of CD4+CD25+Foxp3+ Tregs
| Group | n | Tregs (%) |
| Control | 8 | 2.21±0.35 |
| Sham | 8 | 1.96±0.30 |
| NS | 8 | 1.36±0.19*∆ |
| HTS | 8 | 2.10±0.31#§ |
| HES | 8 | 1.24±0.34*∆ |
|
| ||
| F | 17.072 | |
| P | 0.000 | |
HES: hydroxyethyl starch; HTS: hypertonic saline; NS: normal saline. Data are represented as mean±SD.
P<0.05, vs. control;
P<0.05, vs. sham;
P<0.05, vs. NS;
P<0.05, vs. HES
3.3. Changes of Th1/Th2 and Tc1/Tc2 ratios in spleen
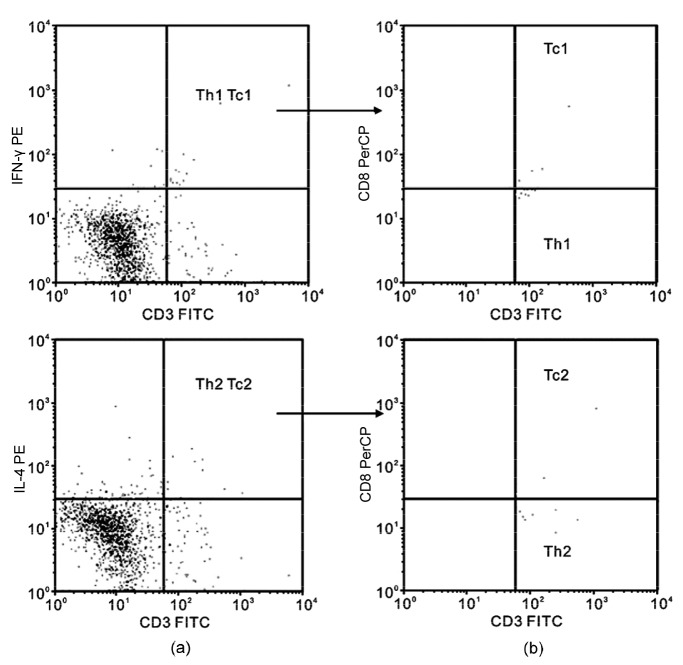
The representative illustration of flow cytometry for Th and Tc is shown in Fig. 3. Ratios of Th1/Th2 and Tc1/Tc2 in rats receiving HTS resuscitation did not differ from those in the control and sham rats (Table 2). However, both ratios were significantly lower in rats receiving NS and HES than in the control and sham groups (P<0.05). Interestingly, the percentage of Tc1 cells was significantly higher only in the HTS group, whereas the levels of Tc2 in all three groups receiving fluid resuscitation were equally and significantly improved (P<0.05 vs. control and sham; Fig. 4).
Fig. 3.
Representative illustration of flow cytometry for Th and Tc cells
(a) Upper right quadrants represent CD3+interferon (IFN)-γ+ (top) and CD3+interleukin (IL)-4+ (bottom) cells, respectively. (b) Upper right quadrant represents CD3+IFN-γ+ CD8+ Tc1 cells and lower right quadrant represents CD3+IFN-γ+ CD8− Th1 cells (top); upper right quadrant represents CD3+IL-4+CD8+ Tc2 cells and lower right quadrant represents CD3+IL-4+CD8− Th2 cells (bottom)
Table 2.
Th1/Th2 and Tc1/Tc2 ratios
| Group | n | Th1/Th2 | Tc1/Tc2 |
| Control | 8 | 0.37±0.08 | 0.46±0.10 |
| Sham | 8 | 0.35±0.08 | 0.47±0.11 |
| NS | 8 | 0.22±0.06*∆ | 0.33±0.04*∆ |
| HTS | 8 | 0.32±0.07#§ | 0.42±0.08#§ |
| HES | 8 | 0.23±0.07*∆ | 0.32±0.08*∆ |
|
| |||
| F | 7.051 | 5.395 | |
| P | 0.000 | 0.002 | |
HES: hydroxyethyl starch; HTS: hypertonic saline; NS: normal saline. Data are represented as mean±SD.
P<0.05, vs. control;
P<0.05, vs. sham;
P<0.05, vs. NS;
P<0.05, vs. HES
Fig. 4.
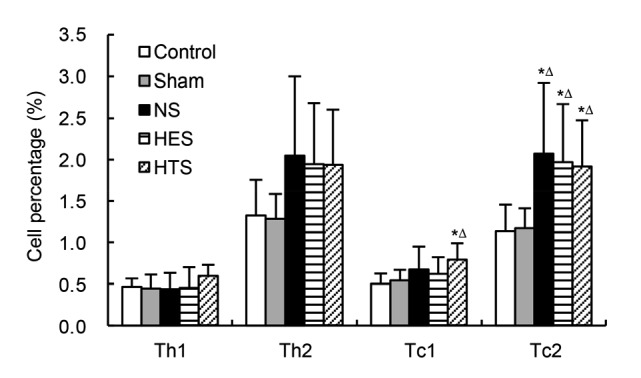
Percentages of Th and Tc in spleen
Control: control group (n=8); Sham: sham group (n=8); NS: normal saline group (n=8); HTS: hypertonic saline group (n=8); HES: hydroxyethyl starch group (n=8). Data are represented as mean±SD. * P<0.05, vs. control; ∆ P<0.05, vs. sham by one-way ANOVA and the least significant difference t-test
4. Discussion
Tregs mediate immune and inflammatory responses in many pathophysiologic processes. A sufficient quantity of Tregs is critical in keeping inflammation balance and reducing tissue injury (Li et al., 2009; Fogle et al., 2010b; Tang et al., 2014; Zhao et al., 2015; Zhang et al., 2016). Excessive activity or quantity of Tregs can promote infectious deterioration, sepsis, or tumor immune escape, whereas an insufficient amount can result in overactive inflammatory responses (Burgents et al., 2010; Fogle et al., 2010a; Carambia et al., 2014). Tregs have been shown to be differentially influenced by various resuscitation fluids after hemorrhagic shock (Murao et al., 2009; Isayama et al., 2012). In the present study, resuscitation with HTS did not alter the percentage of Tregs in the spleen, whereas NS and HES significantly lowered it. Thus, resuscitation with a small volume (1:5 ratio against blood loss volume) of HTS can stabilize spleen Tregs in the early phase, and may play a beneficial role in reducing immunologic stress after hemorrhagic shock. Indeed previous studies have shown that HTS resuscitation can reduce the injury to lungs and intestinal mucosa after hemorrhagic shock (Fernandes et al., 2009; Gao et al., 2009; Lu et al., 2010). The proposed mechanism by which HTS can mediate these effects is by shrinking cells and mechanically altering membranes, resulting in the release of ATP and promotion of T-cell function (Loomis et al., 2003; Woehrle et al., 2010; Ledderose et al., 2016). Whether a similar mechanism is involved in the effect on Tregs in our rat hemorrhagic shock model requires further research.
The ratios of Th1/Th2 and Tc1/Tc2 can reflect the balance of immune stability to some degree. Several studies have shown that hemorrhagic shock disturbs this balance by enhancing the differentiations of Th2 and Tc2 cells and suppressing the differentiations of Th1 and Tc1 cells, which increases the risks of sepsis and multiple organ failure (Marcu et al., 2007; Miller et al., 2007; Chen et al., 2014). In the present study, resuscitation with HES and NS significantly lowered the ratios of Th1/Th2 and Tc1/Tc2. However, the quantities of Tc1 and Tc2 cells were increased with HTS, whereas only Tc2 cells increased with NS and HES. It is possible that HTS resuscitation preserves Th1 and Tc1 cell differentiation, and may have more effective immunomodulation activity than NS and HES in the early stage after hemorrhagic shock. Consistent with this, Zhang et al. (2008) showed that the differentiation of Th1 cells was enhanced after resuscitation with HES.
Peripheral blood was not analyzed in the present study in consideration of the effect of blood reinfusion on immune function of peripheral blood mononuclear cells. However, the results of the present study indicated that resuscitation with all three fluids equally restored MAP levels in the early stage of hemorrhagic shock. Although the MAP in rats receiving HTS was slightly lower than those in the other groups at the end of the fluid resuscitation stage, it was still high enough ((97.50±6.83) mmHg) to maintain blood supply to vital organs, based on previous reports (Lu et al., 2010; Barros et al., 2011; Vallet, 2011; Cui et al., 2014). Fluid resuscitation with NS or HES may result in hypoosmolar dehydration, thus stimulating an immune system response and inflammatory injury, whereas HTS may promote a more beneficial state of immunologic stress. The effect of resuscitation with these fluids on coagulation and liver/kidney function still needs to be explored to provide more useful information for future clinical application.
In summary, this study shows that resuscitation with a small volume of HTS has a stronger impact on the spleen immune system than HES or NS. Specifically, HTS preserves Treg proportions in the spleen, while increasing the numbers of Th1 and Tc1 cells and maintaining the balance between Th1/Th2 and Tc1/Tc2. Additional studies are needed to further investigate the related mechanisms.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 81272075)
Compliance with ethics guidelines: Feng YAO, Yuan-qiang LU, Jiu-kun JIANG, Lin-hui GU, and Han-zhou MOU declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Alam HB, Stanton K, Koustova E, et al. Effect of different resuscitation strategies on neutrophil activation in a swine model of hemorrhagic shock. Resuscitation. 2004;60(1):91–99. doi: 10.1016/j.resuscitation.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Angele MK, Schneider CP, Chaudry IH. Bench-to-bedside review: latest results in hemorrhagic shock. Crit Care. 2008;12(4):218. doi: 10.1186/cc6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barros JM, do Nascimento PJr, Marinello JL, et al. The effects of 6% hydroxyethyl starch-hypertonic saline in resuscitation of dogs with hemorrhagic shock. Anesth Analg. 2011;112(2):395–404. doi: 10.1213/ANE.0b013e3181f2e9b2. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6(4):353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 5.Brøchner AC, Toft P. Pathophysiology of the systemic inflammatory response after major accidental trauma. Scand J Trauma Resusc Emerg Med. 2009;17:43. doi: 10.1186/1757-7241-17-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgents JE, Moran TP, West ML, et al. The immunosuppressive tumor environment is the major impediment to successful therapeutic vaccination in Neu transgenic mice. J Immunother. 2010;33(5):482–491. doi: 10.1097/CJI.0b013e3181d756bb. [DOI] [PubMed] [Google Scholar]
- 7.Carambia A, Freund B, Schwinge D, et al. TGF-β-dependent induction of CD4+CD25+Foxp3+ Tregs by liver sinusoidal endothelial cells. J Hepatol. 2014;61(3):594–599. doi: 10.1016/j.jhep.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Chen JM, Lv J, Ma K, et al. Assessment of internal mammary artery injury after blunt chest trauma: a literature review. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2014;15(10):864–869. doi: 10.1631/jzus.B1400098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui Y, Sun B, Wang C, et al. Effects of different types of hydroxyethyl starch (HES) on microcirculation perfusion and tissue oxygenation in patients undergoing liver surgery. Int J Clin Exp Med. 2014;7(3):631–639. [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandes CI, Llimona F, Godoy LC, et al. Treatment of hemorrhagic shock with hypertonic saline solution modulates the inflammatory response to live bacteria in lungs. Braz J Med Biol Res. 2009;42(10):892–901. doi: 10.1590/S0100-879X2009005000024. [DOI] [PubMed] [Google Scholar]
- 11.Fogle JE, Mexas AM, Tompkins WA, et al. CD4+CD25+ T regulatory cells inhibit CD8+ IFN-γ production during acute and chronic FIV infection utilizing a membrane TGF-β-dependent mechanism. AIDS Res Hum Retroviruses. 2010;26(2):201–216. doi: 10.1089/aid.2009.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogle JE, Tompkins WA, Tompkins MB. CD4+CD25+ T regulatory cells from FIV+ cats induce a unique anergic profile in CD8+ lymphocyte targets. Retrovirology. 2010;7:97. doi: 10.1186/1742-4690-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J, Zhao WX, Xue FS, et al. Effects of different resuscitation fluids on acute lung injury in a rat model of uncontrolled hemorrhagic shock and infection. J Trauma. 2009;67(6):1213–1219. doi: 10.1097/TA.0b013e31818cc1e4. [DOI] [PubMed] [Google Scholar]
- 14.Gurfinkel V, Poggetti RS, Fontes B, et al. Hypertonic saline improves tissue oxygenation and reduces systemic and pulmonary inflammatory response caused by hemorrhagic shock. J Trauma. 2003;54(6):1137–1145. doi: 10.1097/01.TA.0000064452.37534.29. [DOI] [PubMed] [Google Scholar]
- 15.Isayama K, Murao Y, Saito F, et al. Effects of hypertonic saline on CD4+CD25+Foxp3+ regulatory T cells after hemorrhagic shock in relation to iNOS and cytokines. J Surg Res. 2012;172(1):137–145. doi: 10.1016/j.jss.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 16.Ledderose C, Bao Y, Kondo Y, et al. Purinergic signaling and the immune response in sepsis: a review. Clin Ther. 2016;38(5):1054–1065. doi: 10.1016/j.clinthera.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Qian CN, Zeng YX. Regulatory T cells and EBV associated malignancies. Int Immunopharmacol. 2009;9(5):590–592. doi: 10.1016/j.intimp.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Loomis WH, Namiki S, Ostrom RS, et al. Hypertonic stress increases T cell interleukin-2 expression through a mechanism that involves ATP release, P2 receptor, and p38 MAPK activation. J Biol Chem. 2003;278(7):4590–4596. doi: 10.1074/jbc.M207868200. [DOI] [PubMed] [Google Scholar]
- 19.Lu YQ, Cai XJ, Gu LH, et al. Hypertonic saline resuscitation maintains a more balanced profile of T-lymphocyte subpopulations in a rat model of hemorrhagic shock. J Zhejiang Univ-Sci B. 2007;8(1):70–75. doi: 10.1631/jzus.2007.B0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu YQ, Huang WD, Cai XJ, et al. Hypertonic saline resuscitation reduces apoptosis of intestinal mucosa in a rat model of hemorrhagic shock. J Zhejiang Univ-Sci B. 2008;9(11):879–884. doi: 10.1631/jzus.B0820116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu YQ, Gu LH, Huang WD, et al. Effect of hypertonic saline resuscitation on heme oxygenase-1 mRNA expression and apoptosis of the intestinal mucosa in a rat model of hemorrhagic shock. Chin Med J (Engl) 2010;123(11):1453–1458. doi: 10.3760/cma.j.issn.0366-6999.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Lu YQ, Gu LH, Zhang Q, et al. Hypertonic saline resuscitation contributes to early accumulation of circulating myeloid-derived suppressor cells in a rat model of hemorrhagic shock. Chin Med J (Engl) 2013;126(7):1317–1322. [PubMed] [Google Scholar]
- 23.Maier RV, Alam HB. Hemostatic and pharmacologic resuscitation: results of a long-term survival study in a swine polytrauma model. J Trauma. 2011;70(3):636–645. doi: 10.1097/TA.0b013e31820d0dcc. [DOI] [PubMed] [Google Scholar]
- 24.Marcu AC, Paccione KE, Barbee RW, et al. Androstenetriol immunomodulation improves survival in a severe trauma hemorrhage shock model. J Trauma. 2007;63(3):662–669. doi: 10.1097/TA.0b013e31802e70d9. [DOI] [PubMed] [Google Scholar]
- 25.Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg. 2004;389(6):475–484. doi: 10.1007/s00423-004-0472-0. [DOI] [PubMed] [Google Scholar]
- 26.Miller AC, Rashid RM, Elamin EM. The “T” in trauma: the helper T-cell response and the role of immunomodulation in trauma and burn patients. J Trauma. 2007;63(6):1407–1417. doi: 10.1097/TA.0b013e31815b839e. [DOI] [PubMed] [Google Scholar]
- 27.Moore FA, McKinley BA, Moore EE. The next generation in shock resuscitation. Lancet. 2004;363(9425):1988–1996. doi: 10.1016/S0140-6736(04)16415-5. [DOI] [PubMed] [Google Scholar]
- 28.Moore FA, McKinley BA, Moore EE, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core–standard operating procedures for clinical care. III. Guidelines for shock resuscitation. J Trauma. 2006;61(1):82–89. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- 29.Murao Y, Loomis W, Wolf P, et al. Effect of dose of hypertonic saline on its potential to prevent lung tissue damage in a mouse model of hemorrhagic shock. Shock. 2003;20(1):29–34. doi: 10.1097/01.shk.0000071060.78689.f1. [DOI] [PubMed] [Google Scholar]
- 30.Murao Y, Hata M, Ohnishi K, et al. Hypertonic saline resuscitation reduces apoptosis and tissue damage of the small intestine in a mouse model of hemorrhagic shock. Shock. 2003;20(1):23–28. doi: 10.1097/01.shk.0000078832.57685.6c. [DOI] [PubMed] [Google Scholar]
- 31.Murao Y, Isayama K, Saito F, et al. Effect of hypertonic saline resuscitation on CD4+CD25+ regulatory T cells and γδ T cells after hemorrhagic shock and resuscitation in relation to apoptosis and iNOS. J Trauma. 2009;67(5):975–982. doi: 10.1097/TA.0b013e3181b83b7a. [DOI] [PubMed] [Google Scholar]
- 32.National Research Council. Guide for the Care and Use of Laboratory Animals. 8th Ed. Washington, DC, USA: The National Academies Press; 2011. ( http://grants.nih.gov/grants/)olaw/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf [Accessed on Apr. 10, 2016. [PubMed] [Google Scholar]
- 33.Tang L, Bai J, Chung CS, et al. Active players in resolution of shock/sepsis induced indirect lung injury: immunomodulatory effects of Tregs and PD-1. J Leukoc Biol. 2014;96(5):809–820. doi: 10.1189/jlb.4MA1213-647RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallet B. Intravascular volume expansion: which surrogate markers could help the clinician to assess improved tissue perfusion? Anesth Analg. 2011;112(2):258–259. doi: 10.1213/ANE.0b013e3182066299. [DOI] [PubMed] [Google Scholar]
- 35.Vincenzi R, Cepeda LA, Pirani WM, et al. Small volume resuscitation with 3% hypertonic saline solution decrease inflammatory response and attenuates end organ damage after controlled hemorrhagic shock. Am J Surg. 2009;198(3):407–414. doi: 10.1016/j.amjsurg.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Woehrle T, Yip L, Manohar M, et al. Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J Leukoc Biol. 2010;88(6):1181–1189. doi: 10.1189/jlb.0410211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yip L, Cheung CW, Corriden R, et al. Hypertonic stress regulates T-cell function by the opposing actions of extracellular adenosine triphosphate and adenosine. Shock. 2007;27(3):242–250. doi: 10.1097/01.shk.0000245014.96419.3a. [DOI] [PubMed] [Google Scholar]
- 38.Zhang AB, Qian YG, Zheng SS. Prognostic significance of regulatory T lymphocytes in patients with hepatocellular carcinoma. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2016;17(12):984–991. doi: 10.1631/jzus.B1600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Liang L, Wu W, et al. Resuscitation with hydroxyethyl starch solution prevents CD4+ T-lymphocyte apoptosis and modulates the balance of T helper type 1 and T helper type 2 responses in the rat with traumatic virgule/shill hemorrhagic shock. Shock. 2008;30(6):692–698. doi: 10.1097/SHK.0b013e31816f260d. [DOI] [PubMed] [Google Scholar]
- 40.Zhao XH, Jiang JK, Lu YQ. Evaluation of efficacy of resin hemoperfusion in patients with acute 2,4-dinitrophenol poisoning by dynamic monitoring of plasma toxin concentration. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(8):720–726. doi: 10.1631/jzus.B1500101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao XJ, Pen Z, Li P, et al. β-receptor blocker influences return of spontaneous circulation and chemical examination in rats during cardiopulmonary resuscitation. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2013;14(6):505–510. doi: 10.1631/jzus.B1200293. [DOI] [PMC free article] [PubMed] [Google Scholar]