Abstract
Objective: To study the clinical characteristics, treatment, and prognosis of thyroid cancer in children and adolescents. Methods: We performed a retrospective analysis of clinical data from 83 cases of thyroid cancer in children and adolescents from January 1990 to December 2010. We compared extra-thyroid extension, lymph node metastasis, distant metastasis, and prognosis between pediatric patients ≤12 years of age (27 cases) and those >12 years of age (56 cases). All the patients agreed to undergo thyroidectomy and endocrine therapy, and the consent was obtained from parents or guardians. Results: Histopathology included papillary carcinoma in 67 cases, papillary carcinoma with partial follicular growth pattern in 1 case, papillary carcinoma with squamous metaplasia in 4 cases, follicular carcinoma in 7 cases, medullary carcinoma in 3 cases, and poorly differentiated carcinoma in 1 case. The total lymph node metastasis rate was 78.31%. Patients ≤12 years of age showed a higher rate of lymph node metastasis than the older group (92.59% vs. 71.43%, P=0.028). The incidence rate in females in the older group was higher than that in the younger group (80.36% vs. 59.26%, P=0.041). There were no significant differences in extra-thyroid extension, distant metastasis, survival rate, or recurrent disease between the two groups. Conclusions: The lymph node metastasis of thyroid cancer is higher in patients ≤12 years of age than in those >12 years of age; the incidence rate is higher in females than in males. Childhood thyroid cancer has a good prognosis, surgery being the most effective treatment. Choosing a reasonable surgery method and comprehensive postoperative treatment can achieve a cure and satisfactory survival rate.
Keywords: Children and adolescents, Thyroid cancer, Clinical characteristics, Surgical treatment
1. Introduction
Thyroid cancer is rare in children and adolescents, having a different clinical presentation and course from adults (Vaisman et al., 2011), in particular a high rate of malignancy. The incidence of malignant thyroid nodules in adults is about 5%, while in children and adolescents it is up to 25% (Baş et al., 2012; Osipoff and Wilson, 2012), with the risk being higher at a younger age (Alessandri et al., 2000). Despite this, the prognosis is acceptable but aspects of the treatment of thyroid cancer in children and adolescents are still controversial, for example whether children need to undergo aggressive therapy including total thyroidectomy and radioactive iodine (RAI) therapy after initial surgery. It is unclear whether younger patients are at greater risk for more extensive and recurrent disease and the American Thyroid Association (ATA) guidelines recommend that “prepubertal” and “pubertal/postpubertal” children should be studied to improve uniformity and accuracy (Francis et al., 2015).
In this study, we reviewed retrospective clinical information from 83 patients up to 18 years of age, who underwent surgery at our hospital for diagnosis and treatment of thyroid cancer. We analyzed the clinical features, effectiveness of our surgical approach, RAI therapy, thyroxin suppressive-substitutional treatment, and the long-term outcome of our patients.
2. Patients and methods
2.1. Patients
This study is a retrospective analysis of data from 83 pediatric patients suffering from thyroid cancer, aged from 6 to 18 years (mean (14.12±2.83) years). Patients’ general characteristics are presented in Table 1. All patients were operated on by the same surgeon at the Department of Head and Neck Surgery, Zhejiang Cancer Hospital (Hangzhou, China) from January 1990 to December 2010.
Table 1.
Patients’ basic characteristics
| Characteristics | Value*
|
||
| Total | ≤12 years | >12 years | |
| Sex | |||
| Male | 22 (26.51%) | 11 (40.74%) | 11 (19.64%) |
| Female | 61 (73.49%) | 16 (59.26%) | 45 (80.36%) |
| Age (year) | 14.12±2.83 | 11.67±2.04 | 16.80±1.12 |
| Primary symptom | |||
| Neck lump | 81 (97.60%) | 27 (100%) | 54 (96.42%) |
| Hoarseness | 1 (1.20%) | 0 (0%) | 1 (1.79%) |
| Dyspnea | 1 (1.20%) | 0 (0%) | 1 (1.79%) |
| Tumor diameter (cm) | 2.81±1.23 | 2.78±1.48 | 2.83±1.29 |
| Tumor location | |||
| Unilaterality | 57 (68.67%) | 19 (70.37%) | 38 (67.86%) |
| Bilaterality | 26 (31.33%) | 8 (29.63%) | 18 (32.14%) |
| Multifocality | |||
| Solitary | 50 (60.24%) | 16 (59.26%) | 34 (60.71%) |
| Mutifocal | 33 (39.76%) | 11 (40.74%) | 22 (39.29%) |
Values are expressed as number (percent) or mean±standard deviation (SD)
2.2. Group and follow-up
Patients were divided into two groups according to age: ≤12 and >12 years old. The younger group included 27 patients, 16 females and 11 males (1.45:1), while the older group included 56 patients, 45 females and 11 males (4.09:1). The differences in tumor location, extra-thyroid extension, lymph node metastasis, distant metastasis, and prognosis were compared between the two groups. Patients were followed up by telephone or letter and parents or guardians were surveyed in case patients were still minors. All of them were successfully followed up for 5–25 years (mean (11.48±5.46) years); however, 12 patients were lost to contact 10 years after initial surgery.
Preoperatively all patients received cervical and abdominal ultrasound examination, neck and chest computed tomography (CT) scan, indirect laryngoscopy, and complete thyroid hormone analysis including antibodies, thyroglobulin, calcitonin, and parathyroid hormone. Metastasis and node involvement were determined by clinical and pathological findings. Extra-thyroidal extension was evaluated through either clinical or pathological results. The postoperative pathological tumor-node-metastasis (pTNM) classification is according to the American Joint Committee on Cancer (AJCC) (Edge and Compton, 2010).
2.3. Statistical analysis
All results were analyzed by GraphPad Prism Version 6.0 (GraphPad Software for Science, San Diego, CA) statistical software. χ 2 test and Fisher exact probability analysis were used to test the difference between categorical variables. The statistical significance level was set at P<0.05.
3. Results
3.1. Clinical features
Sixty-one patients were females and 22 were males (2.77:1). Most presented with a neck lump: mean tumor diameter was (2.81±1.23) cm. Fifty-seven (68.67%) were unilateral lobe tumors and 50 (60.24%) were solitary tumors (Table 1). All patients underwent a surgical procedure shown in Table 2; 13 children underwent RAI therapy after initial surgery, 8 of them in the >12 years old group and 5 in the younger group. The incidence was higher in females than in males. The bilateral laryngeal recurrent nerve was invaded in 2 cases and the unilateral nerve in 4 cases. Five cases extended to the cervical anterior muscle, 3 cases involved vascular tumor thrombus and 2 involved the tracheal membrane, fatty tissue surrounding muscle, and the esophageal muscle layer excluding laryngeal recurrent nerve invasion. In 9 cases, cervical anterior muscle and surrounding tissues only were invaded (Table 3).
Table 2.
Surgical procedures of 83 cases
| Operation mode | Case number |
||
| Total | ≤12 years | >12 years | |
| UT+UniMRND | 40 | 14 | 26 |
| UT+UniCND | 9 | 1 | 8 |
| UT+Opp PT | 5 | 0 | 5 |
| UT+Opp PT+BilMRND | 7 | 2 | 5 |
| UT+BilMRND | 1 | 1 | 0 |
| Bil PT+UniMRND | 1 | 1 | 0 |
| TT+UniMRND | 5 | 2 | 3 |
| TT+BilMRND | 14 | 6 | 8 |
| TT+UniMRND+Opp CND | 1 | 0 | 1 |
UT: unilateral thyroidectomy; PT: partial thyroidectomy; TT: total thyroidectomy; CND: central neck dissection; MRND: modified radical neck dissection; Uni: unilateral; Bil: bilateral; Opp: opposite
Table 3.
Pathological type and encroachment scope of 83 cases
| Parameter | Case number |
||
| Total | ≤12 years | >12 years | |
| Pathological type | |||
| Papillary carcinoma | 67 | 19 | 48 |
| Papillary carcinoma with partial follicular growth pattern | 1 | 0 | 1 |
| Follicular carcinoma | 7 | 2 | 5 |
| Medullary carcinoma | 3 | 2 | 1 |
| Poorly differentiated carcinoma | 1 | 0 | 1 |
| Papillary carcinoma with squamous metaplasia | 4 | 4 | 0 |
| Encroachment scope | |||
| Double-side RLN | 2 | 2 | 0 |
| One-side RLN | 4 | 1 | 3 |
| Neck muscles and surrounding tissue | 9 | 3 | 6 |
| One-side RLN, neck muscles and surrounding tissue | 5 | 0 | 5 |
| Vessel tumor embolus | 3 | 2 | 1 |
| One-side RLN, muscles, trachea tunica adventitia, and tunica muscularis esophagi | 2 | 0 | 2 |
RLN: recurrent laryngeal nerve
3.2. Pathology and TNM classification
Pathological type is shown in Table 3. The majority of patients (67/83 or 80.72%) presented with papillary carcinoma, follicular and medullary carcinoma accounted for 7/83 (8.43%) and 3/83 (3.61%), respectively, and poorly differentiated carcinoma and papillary carcinoma with squamous metaplasia for 1/83 (1.20%) and 4/83 (4.82%), respectively. All postoperative patients’ pTNM was classified according to AJCC (Table 4). Eighty-one cases were at clinical stage I and two cases at stage II.
Table 4.
pTNM classification and clinical stage of the patients
| TNM and clinical stage | DTC/MTC/P-DTC |
|
| ≤12 years | >12 years | |
| T1–4N0M0 | ||
| T1a | 1/0/0 | 2/0/0 |
| T1b | 0/1/0 | 1/0/0 |
| T2 | 3/0/0 | 9/0/0 |
| T3 | 0/0/0 | 1/0/0 |
| T4a | 0/0/0 | 1/0/0 |
| T1–4N1aM0 | ||
| T2 | 0/0/0 | 4/0/0 |
| T3 | 0/0/0 | 2/0/0 |
| T1–4N1bM0 | ||
| T1a | 0/0/0 | 2/0/0 |
| T1b | 3/0/0 | 4/0/0 |
| T2 | 8/0/0 | 15/0/0 |
| T3 | 7/0/0 | 10/0/0 |
| T4a | 2/0/0 | 3/0/1 |
| T4b | 1/0/0 | 0/0/0 |
| T1–4N0–1M1 | ||
| T2N0M1 | 0/0/0 | 1/0/0 |
| T4bN1bM1 | 1/0/0 | 0/0/0 |
| Total | 27 | 56 |
| Clinical stage | ||
| I | 26/0/0 | 55/0/0 |
| II | 1/0/0 | 1/0/0 |
| Total | 27 | 56 |
DTC: differentiated thyroid carcinoma; MTC: medullary thyroid carcinoma; P-DTC: poorly differentiated thyroid carcinoma. The TNM and clinical stage were not listed above if there was no patient included
3.3. Metastasis and extra-thyroid extension
The aggressiveness of the two groups, 12 years old or younger and older than 12 years, was compared (Table 5 and Fig. 1). There was total lymph node metastasis in 65/83 (78.31%), unilateral central lymph node metastasis in 7/65 (10.77%), unilateral cervical lymph node metastasis in 35/65 (53.85%), and bilateral cervical lymph node metastases in 23/65 (35.38%). The occurrence of cervical lymph node metastasis in the younger group was significantly higher than that in the older group (92.59% vs. 71.43%, P=0.028), but there were no differences in tumor location, extra-thyroid extension, or distant metastasis (P=0.811, P=0.920, and P=0.548, respectively). The female incidence of thyroid cancer in the older group was significantly higher than that in the younger group (80.36% vs. 59.26%, P=0.041).
Table 5.
Comparison of the invasive capacity of thyroid carcinoma between two groups
| Group | Total number | Location |
Extra-thyroid extension |
Lymph node metastasis |
Distant metastasis |
||||
| Unilateral | Bilateral | Yes | No | Yes | No | Yes | No | ||
| ≤12 years | 27 | 19 (70.37%) | 8 (29.63%) | 8 (29.63%) | 19 (70.37%) | 25 (92.59%) | 2 (7.41%) | 1 (3.70%) | 26 (96.30%) |
| >12 years | 56 | 38 (67.86%) | 18 (32.14%) | 17 (30.36%) | 39 (69.64%) | 40 (71.43%) | 16 (28.57%) | 1 (1.79%) | 55 (98.21%) |
|
| |||||||||
| P value | 0.811 | 0.920 | 0.028 | 0.548 | |||||
Data are expressed as number (percent)
Fig. 1.
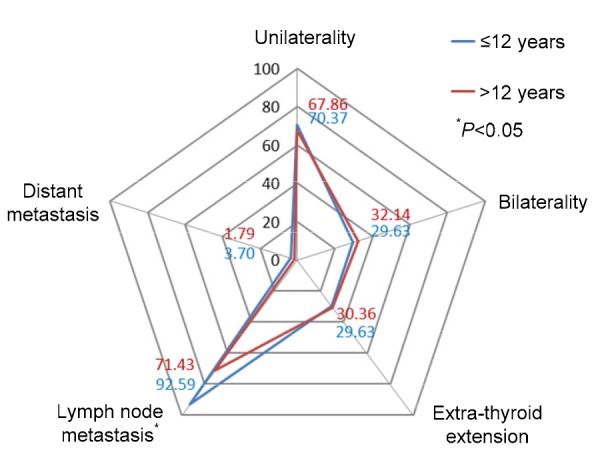
Comparison of location and invasion of thyroid cancer between the two groups of children
All data are expressed as percent (%)
3.4. Postoperative complications
There were relatively few postoperative complications. One case underwent permanent tracheal fistula because the bilateral laryngeal recurrent nerves were invaded and resected. One case underwent tracheotomy due to bilateral vocal cord paralysis and recovered three months later without dyspnea and hoarseness, while permanent hoarseness occurred in three cases on account of unilateral laryngeal recurrent nerve invasion and resection. In four cases transient hypoparathyroidism improved after calcium treatment, while in one case hypoparathyroidism was permanent. All patients underwent endocrine therapy with oral administration of levothyroxine.
3.5. Follow-up
All patients were followed up after initial therapy. Cervical lymph node metastasis was detected in 10 patients, in which 3 patients had local recurrence and 1 had lymph node metastasis. There were 6 patients with lung metastasis and 1 with lung metastasis accompanied by bone metastasis. Postoperative follow-up was at least 5 years on average, and at present, all patients were alive except the 12 patients who were lost to contact, of whom 10 with distant metastasis survived for more than 5 years. All patients received endocrine therapy with thyroid hormone tablets, and their growth and intelligence were normal.
3.6. Prognosis
All the children in the two groups survived for 5 years after initial treatment. There was no significant difference in recurrent disease between the two groups including local recurrence (0/27 or 0.0% vs. 3/56 or 5.4%, P=0.302), lymph node metastasis (3/27 or 11.1% vs. 7/56 or 12.5%, P=0.547), lung metastasis (2/27 or 7.4% vs. 5/56 or 8.9%, P=0.589), and bone metastasis (1/27 or 3.7% vs. 0/56 or 0.0%, P=0.325) , as shown in Fig. 2.
Fig. 2.
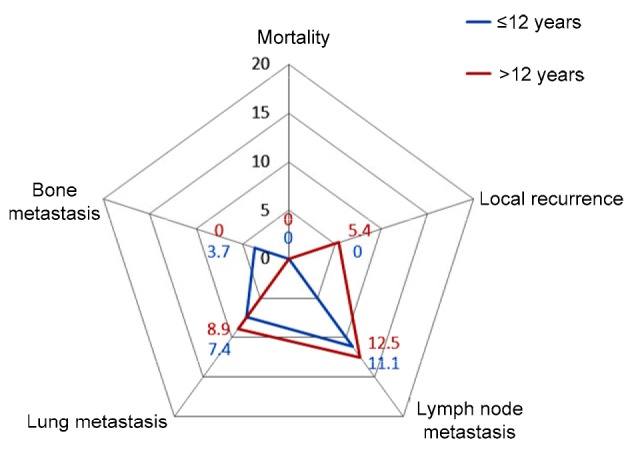
Mortality and recurrent disease of the two groups of children 5 years after initial therapy
All data are expressed as percent (%)
4. Discussion
Thyroid cancer in children and adolescents always manifests with a neck mass, which is too small to diagnose at the early stage. In this study, 97.59% of patients were treated for neck mass. The incidence of thyroid cancer is similar in females and males at age ≤12 years with no statistical difference, but with significantly higher incidence in females at age >12 years. This difference was thought to be due to the increase in estrogen at puberty to be close to the adult level.
The most pathological type of thyroid cancer was differentiated thyroid carcinoma. This group had 1 case of poorly differentiated cancer, with a high degree of malignancy. This patient underwent cervical lymph node dissection and isotope therapy in the third and fourth years, respectively, after surgery. Bone metastasis was found 9 years after surgery; nevertheless, this patient was still alive after more than 11 years with a tumor. The adult 5-year survival rate for this type of cancer is approximately 40%, with most patients dying in the first 3 years after diagnosis because of local and distant metastasis (Ward et al., 2006). However, it is uncertain whether the prognosis of poorly differentiated thyroid cancer in young patients is better than that in adults and further research on large samples is needed.
Thyroid cancer in young patients often shows higher rates of distant metastases and recurrent disease than that in adults (Vaisman et al., 2011; Mihailovic et al., 2014). Some studies have found that the rate of metastases in side neck lymph nodes in patients over 18 years old is only 23.6%, but up to 77% in patients 18 years old or younger (Dzepina, 2012). The lateral neck lymph node metastasis rate in this study was 69.88% (35 cases unilateral and 23 cases bilateral), which is compatible with reports in the literature (Dzepina, 2012; Jing et al., 2013). The rates of local lobe invasion and distant metastasis were 30.12% and 12.05%, respectively, both significantly higher than those in adults (Schlumberger and Sherman, 2012). Research has shown that younger patients have more local and distant extensions (Grigsby et al., 2002; Dzodic et al., 2014), and the neck lymph node metastasis rate at patients ≤12 years old was obviously higher than that at patients >12 years old. However, there was no significant difference in the extra-thyroid extension or distant metastasis between the two groups. Therefore, cervical lymph node metastasis should be noted in younger thyroid cancer patients, especially occult lymph node metastasis.
B-ultrasound is always used to define thyroid nodules and assign an ultrasound risk to a lesion. Fine needle aspiration (FNA) cytological diagnosis is also highly accurate and widely implemented in clinical practice for nodules more than 1 cm (Gupta et al., 2013). It is recommended that children who have a history of exposure to radiation, autoimmune thyroid disease, or a family history of thyroid cancer should be examined by FNA, even if the thyroid node is only a few centimeters in diameter, because thyroid cancer in children and adolescents is particularly invasive locally and at a distance.
Surgery is the primary treatment for thyroid cancer. The ATA and some studies have suggested that if FNA or intra-operative frozen pathology proved thyroid cancer, total or subtotal thyroidectomy should be done to decrease the recurrence rate significantly (Mihailovic et al., 2014; Francis et al., 2015). However, recent studies have shown that not all patients benefit, and it is only recommended for those with distant metastasis or partial extra-thyroid extension (O'Gorman et al., 2010; Atabek, 2011; Adam et al., 2014). Children and adolescents are still growing and the surgical method needs to be chosen with care. The perfect surgical therapy should not only remove the tumor, but also avoid the onset of complications including permanent hypothyroidism, hypoparathyroidism, and neurological dysfunction. Our suggestions are as follows: (1) Primary tumor treatment: unilateral thyroid lobectomy or union with lateral partial thyroidectomy is adopted for unilateral well-differentiated cancer without external invasion and metastasis; the RET gene mutation test is recommended for patients with medullary carcinoma or with a family history of multiple endocrine neoplasia type 2 (MEN 2). Unilateral lobe resection is recommended for unilateral carcinoma patients with no gene mutation, and total thyroidectomy for medullary carcinoma patients with gene mutation. Whether prophylactic total thyroidectomy can be done depends on the risk of MEN, according to the assessment of the RET gene mutation site (Kloos et al., 2009). Total thyroidectomy is recommended for thyroid cancer involving bilateral glands. (2) Treatment of lymph nodes: neck lymph node dissection is recommended for stage N1 patients. Some scholars suggest implementing prophylactic cervical lymph node dissection on N0 patients because they consider that the lymph node metastasis rate is higher in children and adolescents (Patron et al., 2011). However, we think that the prognosis of differentiated thyroid cancer is favorable because cervical lymph node metastasis is not an independent factor affecting the prognosis of thyroid cancer in children and adolescents, and more complications occurred after lymph node dissection. We suggest that differentiated thyroid cancer patients with N0 should be accepted only for central region lymph node dissection, and full-neck lymph node dissection should be considered when lateral cervical lymph node metastasis appears during postoperative follow-up. The rate of cervical lymph node metastasis is high in hereditary medullary carcinoma patients and neck lymph node dissection is recommended. Selective lymph node dissection is recommended for other undifferentiated thyroid cancer patients with N0.
Children and adolescents with differentiated thyroid cancer are offered endocrine therapy after surgery. ATA and some studies recommend that patients with distant metastases with a tumor diameter greater than 4 cm or 1–4 cm with lymph node metastasis or other high risks should receive isotope therapy after surgery (Mihailovic et al., 2007; 2009). Our study found that children and adolescents with big primary foci and cervical lymph node metastasis had a good prognosis. Isotope therapy requires total thyroidectomy, and increases the incidences of second primary tumors (Leboulleux et al., 2012), bone marrow suppression, and kidney injury. Therefore, we believe that patients without distant metastasis or serious local invasion, especially young children, should choose isotope therapy with care.
Patients without surgical indication and with refractory differentiated thyroid cancer not taking I131, medullary thyroid carcinoma, and undifferentiated carcinoma did not benefit from traditional treatment; molecular targeted therapy was the best treatment choice for them. A 9-year-old patient in this group with thyroid papillary cancer with recurrent bilateral neck lymph node and lung metastasis accepted two courses of isotope therapy, but the pulmonary lesion did not reduce and the iodine scan was negative. A subsequent targeted therapy achieved a good result.
This study showed that thyroid cancer in children and adolescents has a high rate of lymph node metastasis, especially in patients at age ≤12 years, but the prognosis is acceptable. Attaching great importance to its clinical features, improving the level of diagnosis, and selecting appropriate surgical procedures and comprehensive postoperative treatment can achieve a satisfactory cure and survival rate. Active treatment should be given to children who have poor pathological differentiation or distant metastasis because it can also lead to a satisfactory clinical effect and longer survival with the tumor.
Footnotes
Compliance with ethics guidelines: Xiao-chun MAO, Wen-qiao YU, Jin-biao SHANG, and Ke-jing WANG declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). The authors followed up with patients by telephone or letter, and the parents or guardians were surveyed if the patients were still minors at that time.
References
- 1.Adam MA, Pura J, Gu L, et al. Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61 775 patients. Ann Surg. 2014;260(4):601–607. doi: 10.1097/SLA.0000000000000925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessandri AJ, Goddard KJ, Blair GK, et al. Age is the major determinant of recurrence in pediatric differentiated thyroid carcinoma. Med Pediatr Oncol. 2000;35(1):41–46. doi: 10.1002/1096-911X(200007)35:1<41::AID-MPO7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Atabek ME. What is the safe and accurate procedure for thyroid nodules in childhood? Horm Res Paediatr. 2011;76(1):72. doi: 10.1159/000328721. [DOI] [PubMed] [Google Scholar]
- 4.Baş VN, Aycan Z, Çetinkaya S, et al. Thyroid nodules in children and adolescents: a single institution’s experience. J Pediatr Endocrinol Metab. 2012;25(7-8):633–638. doi: 10.1515/jpem-2012-0132. [DOI] [PubMed] [Google Scholar]
- 5.Dzepina D. Surgical and pathological characteristics of papillary thyroid cancer in children and adolescents. Int J Pediatr. 2012;2012:125389. doi: 10.1155/2012/125389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dzodic R, Buta M, Markovic I, et al. Surgical management of well-differentiated thyroid carcinoma in children and adolescents: 33 years of experience of a single institution in Serbia. Endocr J. 2014;61(11):1079–1086. doi: 10.1507/endocrj.ej14-0226. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC Cancer Staging Manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 8.Francis GL, Waguespack SG, Bauer AJ, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716–759. doi: 10.1089/thy.2014.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grigsby PW, Gal-or A, Michalski JM, et al. Childhood and adolescent thyroid carcinoma. Cancer. 2002;95(4):724–729. doi: 10.1002/cncr.10725. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Ly S, Castroneves LA, et al. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J Clin Endocrinol Metab. 2013;98(8):3238–3245. doi: 10.1210/jc.2013-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jing FJ, Liang ZY, Long W, et al. Invasive capacity of differentiated thyroid carcinoma in pediatric and adolescent patients. Acta Acad Med Sin. 2013;35(1):80–83. doi: 10.3881/j.issn.1000-503X.2013.01.015. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 12.Kloos RT, Eng C, Evans DB, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19(6):565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 13.Leboulleux S, Hartl D, Baudin E, et al. Differentiated thyroid cancer in childhood. Bull Cancer. 2012;99(11):1093–1099. doi: 10.1684/bdc.2012.1645. (in French) [DOI] [PubMed] [Google Scholar]
- 14.Mihailovic J, Stefanovic L, Malesevic M. Differentiated thyroid carcinoma with distant metastases: probability of survival and its predicting factors. Cancer Biother Radiopharm. 2007;22(2):250–255. doi: 10.1089/cbr.2006.313. [DOI] [PubMed] [Google Scholar]
- 15.Mihailovic J, Stefanovic L, Malesevic M, et al. The importance of age over radioiodine avidity as a prognostic factor in differentiated thyroid carcinoma with distant metastases. Thyroid. 2009;19(3):227–232. doi: 10.1089/thy.2008.0186. [DOI] [PubMed] [Google Scholar]
- 16.Mihailovic J, Nikoletic K, Srbovan D. Recurrent disease in juvenile differentiated thyroid carcinoma: prognostic factors, treatments, and outcomes. J Nucl Med. 2014;55(5):710–717. doi: 10.2967/jnumed.113.130450. [DOI] [PubMed] [Google Scholar]
- 17.O'Gorman CS, Hamilton J, Rachmiel M, et al. Thyroid cancer in childhood: a retrospective review of childhood course. Thyroid. 2010;20(4):375–380. doi: 10.1089/thy.2009.0386. [DOI] [PubMed] [Google Scholar]
- 18.Osipoff JN, Wilson TA. Consultation with the specialist: thyroid nodules. Pediatr Rev. 2012;33(2):75–81; quiz 82. doi: 10.1542/pir.33-2-75. [DOI] [PubMed] [Google Scholar]
- 19.Patron V, Bedfer C, le Clech G, et al. Pattern of lateral neck metastases in N0 papillary thyroid carcinoma. BMC Cancer. 2011;11:8. doi: 10.1186/1471-2407-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlumberger M, Sherman SI. Approach to the patient with advanced differentiated thyroid cancer. Eur J Endocrinol. 2012;166(1):5–11. doi: 10.1530/EJE-11-0631. [DOI] [PubMed] [Google Scholar]
- 21.Vaisman F, Corbo R, Vaisman M. Thyroid carcinoma in children and adolescents–systematic review of the literature. J Thyroid Res. 2011;2011:845362. doi: 10.4061/2011/845362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward LS, Marrone M, Camargo RY, et al. Low-risk differentiated thyroid carcinoma–literature review and management guidelines. Arq Bras Endocrinol Metabol. 2006;50(3):550–557. doi: 10.1590/s0004-27302006000300019. (in Portuguese) [DOI] [PubMed] [Google Scholar]


