Abstract
We have explored the role of ginsenoside Rg1 in promoting the differentiation of mouse adipose-derived stem cells (mADSC) towards the neuronal lineage. The central nervous system has long been regarded as incapable of self-repair; therefore neuronal differentiation from stem cells is of great interest. However, the use of embryonic stem cells is limited due to their inaccessibility and for ethical reasons, so the search is on for alternative pluripotent cells capable of differentiating into neuronal cells. adipose-derived stem cells (ADSC) can differentiate into different cell types, including neuronal cells: their accessibility, low risk, and capacity for long-term growth and self-renewal have made them the preferred stem cell type for clinical applications. Several methods have been indicated for promoting the neuronal differentiation of ADSC, but the mechanism of this process has not been clearly identified. As our previous study showed that microRNA-124 (miRNA-124) plays a positive role in promoting the neural differentiation of ADSC, we wanted to find reagents that can upregulate miRNA-124 expression during neural differentiation.
Keywords: Adipose-derived stem cells (ADSC), Ginsenoside Rg1, Surgical treatment
We have explored the role of ginsenoside Rg1 in promoting the differentiation of mouse adipose-derived stem cells (mADSC) towards the neuronal lineage. The central nervous system has long been regarded as incapable of self-repair; therefore neuronal differentiation from stem cells is of great interest. However, the use of embryonic stem cells is limited due to their inaccessibility and for ethical reasons, so the search is on for alternative pluripotent cells capable of differentiating into neuronal cells. Adipose-derived stem cells (ADSC) can differentiate into different cell types, including neuronal cells: their accessibility, low risk, and capacity for long-term growth and self-renewal have made them the preferred stem cell type for clinical applications. Several methods have been indicated for promoting the neuronal differentiation of ADSC, but the mechanism of this process has not been clearly identified. As our previous study showed that microRNA-124 (miRNA-124) plays a positive role in promoting the neural differentiation of ADSC, we wanted to find reagents that can upregulate miRNA-124 expression during neural differentiation.
Recent studies have indicated that ginsenoside Rg1, one of the most active and abundant components of ginseng, plays an important role in neural cell proliferation and differentiation (Attele et al., 1999). Lu et al. (2009) found that it has a neuroprotective role, and that it can regulate the proliferation of neural progenitor cells. Rg1 also plays an important role in cell differentiation: for example, it stimulates odontogenic/osteogenic differentiation of human dental pulp stem cells (Wang et al., 2014) and facilitates neural differentiation of mouse embryonic stem cells (Wu et al., 2013). To understand the role of ginsenoside Rg1 in neural differentiation, we investigated its effects on the neural differentiation of mouse ADSC. Our findings suggest that it promotes ADSC neural differentiation through the miRNA-124 signaling pathway.
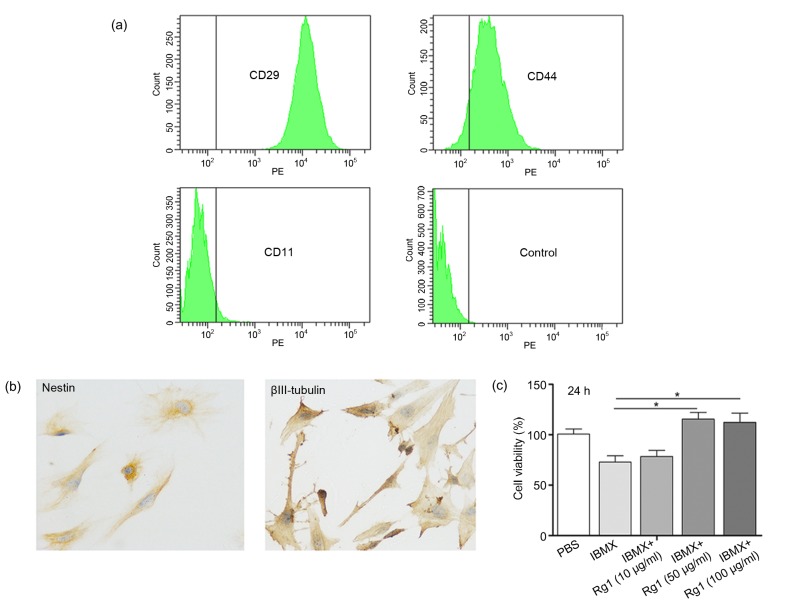
First we demonstrated that ginsenoside Rg1 promotes cell proliferation during ADSC neural differentiation. As shown in Fig. 1a, the isolated mouse ADSC at passage 3 had strong positive cell surface markers for expressions of CD29 and CD44 and low expression of the negative marker for CD11b. Previous studies reported that ADSC could be induced by isobutylmethylxanthine (IBMX) to differentiate into neural cells (Ning et al., 2006; 2008). After IBMX induction, ADSC showed positive neural staining (Fig. 1b). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was used to investigate the effect of ginsenoside Rg1 on cell proliferation of ADSC during neural differentiation. The ginsenoside Rg1-treated group had a significantly higher proliferation rate after 24 h compared with the control group (Fig. 1c), suggesting that ginsenoside Rg1 has a positive effect on cell proliferation during neural differentiation.
Fig. 1.
Effect of ginsenoside Rg1 on cell proliferation during ADSC neural differentiation
(a) Isolated ADSC were analyzed by flow cytometry. Cells were positive for ADSC markers CD44 and CD29, and negative for CD11b, a hematopoietic cell surface marker. PE: phycoerythrin. (b) Immunohistochemical staining of ADSC after neural induction. (c) ADSC were treated with different concentrations of ginsenoside Rg1 during IBMX induction, and cell viability was determined by MTT assay. Values are expressed as mean±standard deviation (SD) of triplicate experiments. * P<0.05
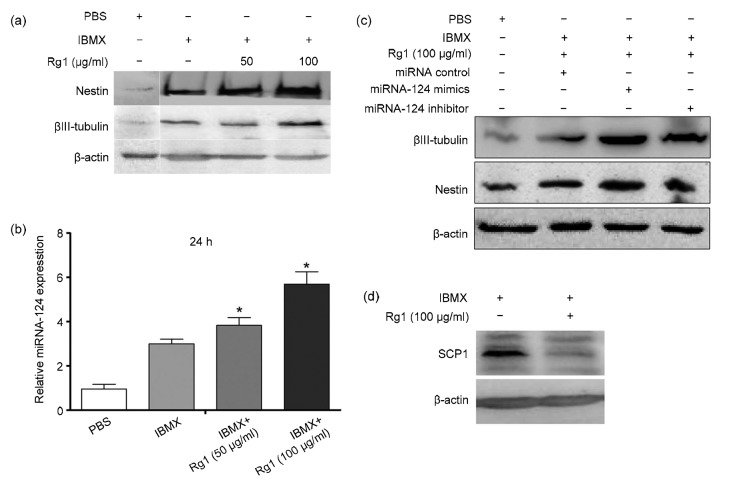
Second we found that ginsenoside Rg1 can promote the neural differentiation of mADSC. mADSC were exposed to IBMX plus 50 or 100 μg/ml ginsenoside Rg1 for 24 h; cytoplasmic proteins were then extracted to examine the neural markers nestin and βIII-tubulin. As shown in Fig. 2a, IBMX induced high levels of nestin and βIII-tubulin. It has been reported that miRNA-124 plays an important role in the development of the nervous system, where it is expressed abundantly (Lagos-Quintana et al., 2002). To determine the effect of ginsenoside Rg1 on miRNA-124 expression, quantitative polymerase chain reaction (qPCR) was performed. Ginsenoside Rg1 induced higher expression of miRNA-124 compared with the IBMX and control groups (Fig. 2b). To further elucidate the effect of ginsenoside R1 on miRNA-124 expression during ADSC neural differentiation, we transfected ADSC with miRNA-124 mimics or inhibitors during ginsenoside Rg1 treatment, and then examined the expression of neural markers. As shown in Fig. 2c, miRNA-124-down-regulation abrogated the neural differentiation of ADSC during ginsenoside Rg1 (100 μg/ml) treatment.
Fig. 2.
Effect of ginsenoside Rg1 on neural differentiation of ADSC
Ginsenoside Rg1 promotes neural differentiation of ADSC by down-regulation of anti-neural factor SCP1 expression. (a) Mouse ADSC were induced by IBMX supplemented with different concentrations of ginsenoside Rg1 for 24 h. The neural markers of nestin and βIII-tubulin were detected by Western blot. (b) miRNA-124 expression of ADSC during neural differentiation supplemented with ginsenoside Rg1. (c) Western blot analysis of the effect of miRNA-124 on the neuronal markers nestin and βIII-tubulin expression during neural differentiation. (d) Effect of ginsenoside Rg1 on SCP1 expression during neural differentiation of ADSC. Values are expressed as mean±SD of triplicate experiments. * P<0.05, vs. IBMX
Lastly, we detected the downstream molecular event involved in the process of mADSC neural differentiation. Our previous study had shown that miRNA-124 can decrease anti-neural factor small C-terminal domain phosphatase 1 (SCP1) expression by targeting its 3'-untranslated region (UTR) promoter (Shi et al., 2016). Because ginsenoside Rg1 treatment induced miRNA-124 up-regulation, we further examined SCP1 expression during neural differentiation. Unsurprisingly, ginsenoside Rg1 treatment significantly decreased anti-neural factor SCP1 expression (Fig. 2d), suggesting that ginsenoside Rg1 could promote ADSC neural differentiation through down-regulation of anti-neural factor SCP1 expression.
In conclusion, our study demonstrated that ginsenoside Rg1 promotes cell proliferation and neural differentiation of mouse ADSC in vitro, but further work is needed to identify the molecular mechanisms involved, particularly in ginsenoside Rg1-mediated miRNA-124 expression.
We found that ginsenoside Rg1 promoted mouse ADSC proliferation and neural differentiation in a dose-dependent manner. A high concentration of ginsenoside Rg1 (100 μg/ml) enhanced the expression of neural specific markers when mouse ADSC were maintained in a neural inductive medium for 24 h (Fig. 2a). Despite the fact that nestin and β-tubulin III represent just one marker of ADSC neural differentiation, these findings, together with previous reports demonstrate the potential use of Rg1 in nerve regeneration, and highlight the potential application of ginsenoside Rg1 to promote cell proliferation and differentiation. More studies are needed to elucidate the mechanisms of Rg1-induced neural differentiation.
During the process of neural differentiation, ginsenoside Rg1 increased the level of miRNA-124 in a dose-dependent manner. miRNA-124 plays an important role in CNS development, and is expressed abundantly in neural cells. miRNA-124 over-expression induces neural differentiation of mouse neuroblastoma cell lines Neuro2a and CAD and embryonal cell line P19 (Makeyev et al., 2007), and manipulation of miRNA-124 expression affects neuronal lineage differentiation in ES cell-derived cultures (Krichevsky et al., 2006). Our results indicate that ginsenoside Rg1 facilitated neural differentiation of ADSC through miRNA-124-mediated down-regulation of anti-neural factor SCP1.
The process of neurogenesis is accompanied by down-regulation of nonneuronal genes and up-regulation of neuronal genes. Repressor element 1 (RE1)-silencing transcription factor (REST), also known as neuron-restrictive silencer factor (NRSF), acts as a transcriptional repressor. Down-regulation of REST increases neuronal genes expression (Ballas et al., 2005). Like REST, SCP1 is recruited to RE1-containing genes by REST and exerts an anti-neural role in nonneuronal tissues (Yeo et al., 2005). In our results, during the process of neural differentiation, ginsenoside Rg1 facilitated neural markers expression through miRNA-124-mediated SCP1 degradation. However, our results do not rule out the possibility that ginsenoside Rg1 may exert its role through other miRNAs or proteins.
Footnotes
Project supported by the Zhejiang Provincial Natural Science Foundation of China (No. LQ14C180001) and the Fundamental Research Funds for the Central Universities (No. 2014QNA6026), China
Compliance with ethics guidelines: Juan DONG, Guo ZHU, Tian-cheng WANG, and Fu-shan SHI declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58(11):1685–1693. doi: 10.1016/S0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 2.Ballas N, Grunseich C, Lu DD, et al. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121(4):645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Krichevsky AM, Sonntag KC, Isacson O, et al. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006;24(4):857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lagos-Quintana M, Rauhut R, Yalcin A, et al. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12(9):735–739. doi: 10.1016/S0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 5.Lu MC, Lai TY, Hwang JM, et al. Proliferation-and migration-enhancing effects of ginseng and ginsenoside Rg1 through IGF-I-and FGF-2-signaling pathways on RSC96 Schwann cells. Cell Biochem Funct. 2009;27(4):186–192. doi: 10.1002/cbf.1554. [DOI] [PubMed] [Google Scholar]
- 6.Makeyev EV, Zhang J, Carrasco MA, et al. The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27(3):435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ning H, Lin G, Lue TF, et al. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 2006;74(9-10):510–518. doi: 10.1111/j.1432-0436.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 8.Ning H, Lin G, Fandel T, et al. Insulin growth factor signaling mediates neuron-like differentiation of adipose tissue-derived stem cells. Differentiation. 2008;76(5):488–494. doi: 10.1111/j.1432-0436.2007.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi F, Yang Y, Wang T, et al. Cellular prion protein promotes neuronal differentiation of adipose-derived stem cells by upregulating miRNA-124. J Mol Neurosci. 2016;59(1):48–55. doi: 10.1007/s12031-016-0733-8. [DOI] [PubMed] [Google Scholar]
- 10.Wang P, Wei X, Zhang F, et al. Ginsenoside Rg1 of Panax ginseng stimulates the proliferation, odontogenic/osteogenic differentiation and gene expression profiles of human dental pulp stem cells. Phytomedicine. 2014;21(2):177–183. doi: 10.1016/j.phymed.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, Pan Z, Cheng M, et al. Ginsenoside Rg1 facilitates neural differentiation of mouse embryonic stem cells via GR-dependent signaling pathway. Neurochem Int. 2013;62(1):92–102. doi: 10.1016/j.neuint.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Yeo M, Lee SK, Lee B, et al. Small CTD phosphatases function in silencing neuronal gene expression. Science. 2005;307(5709):596–600. doi: 10.1126/science.1100801. [DOI] [PubMed] [Google Scholar]