Abstract
Background:
The antibiotic meropenem is commonly administered in patients with severe sepsis and septic shock. We compared the pharmacokinetic, clinical, and bacteriological efficacies of continuous infusion of meropenem versus intermittent administration in such patients.
Methods:
Patients admitted to the Intensive Care Unit (ICU) with severe sepsis or septic shock who received meropenem were randomly assigned to either the continuous (n = 25) or intermittent groups (n = 25). The continuous group received a loading dose of 0.5 g of meropenem followed by a continuous infusion of 3 g/day; the intermittent group received an initial dose of 1.5 g followed by 1 g for every 8 h. Clinical success, microbiological eradication, superinfection, ICU mortality, length of ICU stay, and duration of meropenem treatment were assessed. Serial plasma meropenem concentrations for the first and third dosing periods (steady state) were also measured.
Results:
Clinical success was similar in both the continuous (64%) and intermittent (56%) groups (P = 0.564); the rates of microbiological eradication and superinfection (81.8% vs. 66.7% [P = 0.255] and 4% vs. 16% [P = 0.157], respectively) showed improvement in the continuous group. The duration of meropenem treatment was significantly shorter in the continuous group (7.6 vs. 9.4 days; P = 0.035), where a better steady-state concentration was also achieved. Peak and trough concentrations were significantly different between the continuous and intermittent groups both in the first (Cmax: 19.8 mg/L vs. 51.8 mg/L, P = 0.000; Cmin: 11.2 mg/L vs. 0.5 mg/L, P = 0.000) and third dosing periods (Cmax: 12.5 mg/L vs. 46.4 mg/L, P = 0.000; Cmin: 11.4 mg/L vs. 0.6 mg/L, P = 0.000). For medium-susceptibility pathogens, continuous infusion concentrations above the minimal inhibitory concentration were 100%, which was better than that in the intermittent group.
Conclusions:
Continuous infusion of meropenem provides significantly shorter treatment duration and a tendency for superior bacteriological efficacy than intermittent administration. Continuous infusion may be more optimal against intermediate-susceptibility pathogens.
Keywords: Continuous Infusion, Intermittent Infusion, Meropenem, Pharmacodynamic, Pharmacokinetic
Introduction
Severe sepsis and septic shock are common problems in the Intensive Care Units (ICUs) and produce high morbidity and mortality.[1,2] Early initiation of effective antimicrobial treatment is an important component of therapy against severe sepsis and septic shock.[3,4] Increasing antibiotic resistance is a major health-care problem in the ICU, but the discovery and development of suitable antibacterial drugs are slow paced and have become more costly.[5,6] It is critical to use antibiotics at optimal doses and appropriate routes to improve their efficacy and decrease the chances of developing drug resistance in bacteria.[7]
Meropenem is a common choice for the treatment of severe sepsis and septic shock. Similar to other beta-lactam antibiotics, it is time dependent and its antibacterial activity is related to the duration of the maintenance of its free concentration above the minimal inhibitory concentration (MIC) during each dosing interval (referred to as % of T>MIC). The T>MIC required for carbapenems has been reported to be at least 40%,[8] and a T>MIC of 100% displayed significantly greater clinical and bacteriological outcomes in patients with serious bacterial infections.[9] Extended infusions, especially continuous infusion of beta-lactam antibiotics, can prolong the T>MIC and improve antibacterial activity. Furthermore, the pathophysiological changes associated with severe sepsis and septic shock often affect the volume of distribution (Vd), drug clearance, and pharmacokinetic parameters.[10,11] The use of continuous administration of meropenem was studied in some trials and indicated greater pharmacokinetic efficacy,[10] bacteriological eradication,[12] and clinical cure rates.[13] However, these trials have not been conducted among patients with severe sepsis and septic shock, and combination pharmacokinetic/pharmacodynamic studies are scarce.
The aim of this study was to compare the pharmacokinetic, bacteriological, and clinical efficacy of continuous infusion of meropenem versus traditional intermittent administration (as recommended by the manufacturer[14]) in patients with severe sepsis and septic shock.
Methods
Ethical approval
This was a single-center, prospective, randomized, comparative study; the trial was approved by the local research Ethics Committee of our institution (2012[19]) and conformed to the tenets of the Declaration of Helsinki. Written informed consent was obtained from patients’ next of kin or legal representatives.
Inclusion criteria
All the patients who were diagnosed with severe sepsis or septic shock and were admitted to the ICU, received meropenem therapy, and provided informed consent were included in the study. Meropenem administration was indicated as empirical therapy for severe infection without a proven pathogen, or as a second-line antibiotic based on microbiological findings. Concomitant antimicrobial therapy was permitted. The diagnosis of severe sepsis and septic shock was made in accordance with the International Guidelines for Management of Severe Sepsis and Septic Shock.[15]
Exclusion criteria
Exclusion criteria included age <18 years, pregnancy, acute or chronic renal failure with a glomerular filtration rate (GFR; calculated with the Cockcroft formula) <50 ml/min, immunodeficiency or taking immunosuppressant medication, allergy to meropenem, and previous application of meropenem in the past 2 weeks. According to the manufacturer's product information, meropenem dose reduction is required when the GFR is <50 ml/min.[14]
Patient randomization and treatment protocol
Patients meeting the inclusion criteria with no presence of exclusion criteria were randomized into equally numbered groups using sealed opaque envelopes without stratification. They were assigned to receive either continuous infusion (continuous group, n = 25) or intermittent intravenous (i.v.) application (intermittent group, n = 25). The patients in the continuous group received a loading dose of 0.5 g of meropenem in 100 ml of normal saline i.v. infused over 30 min followed immediately by continuous infusion of 3 g of meropenem over 24 h. Regarding meropenem stability, 0.5 g of meropenem was continuously infused over 4 h in 50 ml of normal saline.[16,17] The patients in the intermittent group received the first dose of 1.5 g of meropenem in 100 ml of normal saline infused over 30 min, and then 1 g in 100 ml of normal saline infused over 30 min for every 8 h. The dose for both groups on day 1 was 3.5 g and 3 g/day thereafter. Patients in both groups were treated during their ICU stays by the regular team of ICU physicians and received standard intensive care (the researchers were not involved in the clinical strategy). Meropenem administration was stopped under the following conditions: further bacterial cultures and MIC testing indicated resistance to meropenem, bacterial cultures and MIC testing indicated increased sensitivity to other narrow-spectrum antibiotics, which could better permeate the infection region (de-escalation of antimicrobial therapy), and significant resolution of clinical symptoms and negative bacterial cultures.
Clinical end points
The primary end points were clinical and microbiological results of meropenem therapy. Clinical success was defined as complete or partial resolution of temperature, clinical signs and symptoms of infection, and leukocytosis. Clinical failure was defined as the appearance of any of the following: persistent or progressing signs and symptoms of infection, or death because of infection. Microbiological outcomes included microbiological eradication and superinfection (which was defined as requiring other antibiotics to target a new Gram-negative bacterial infection). Appropriate routine bacterial cultures (including two sets of blood cultures) were obtained before commencing antimicrobial therapy and were repeated daily if clinical manifestations did not resolve or were exacerbated. Secondary end points included ICU mortality, length of ICU stay (LOS), and duration of meropenem treatment.
The following clinical data were collected: sex, age, weight, diagnosis, site and etiology of infection treated by meropenem, pathogens, MICs of identified pathogens, the Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores at the start of meropenem therapy, white blood cell (WBC) counts at 1 and 5 days of meropenem therapy, procalcitonin (PCT) at 1 and 5 days of meropenem therapy, daily body temperature, serum creatinine at therapy commencement, and the total fluid infusion in the first 24 h.
Microbiologic methods
Identification of antimicrobial susceptibility and MIC testing were performed in the clinical microbiology laboratory using the VITEK 2 automated system (bioMérieux, Marcy l’Etoile, France).
Blood sampling
Two milliliters of blood were collected using an indwelling arterial catheter for each blood sample to determine plasma meropenem concentrations. In the first dosing period (the first 8 h), samples were collected at 0, 30, 60, 150, 200, 360, and 480 min. In the third dosing period (the first 8 h; steady state), blood samples were acquired in line with an intermittent infusion dose or change of continuous infusion bag at 0, 30, 60, 150, 200, and 480 min. The 200-min time point corresponded to nearly 40% of the dosing interval and was regarded as T40%. Specimens were centrifuged at 3000 rpm for 10 min and then frozen at −20°C for subsequent analysis. All samples were assayed individually within 7 days of collection.
Drug assay
Plasma meropenem concentrations were measured using an ultra-high-performance liquid chromatography (UPLC)-diode array detector-column switching method with the Shimadzu LC-20A Prominence System. The chromatographic column was the ACQUITY UPLC® BEH C18 column using the gradient elution method (mobile Phase A and extracting mobile Phase C: methanol-0.05 mol/L K2HPO4 5:95, adjusted to a pH of 7; mobile Phase B: methanol). The absorbance wavelength was 299 nm.
Pharmacokinetic analysis
The pharmacokinetic profile of meropenem in the intermittent group was individually assessed using the WinNonlin Professional version 5.0.1 software (Pharsight Corporation, Mountain View, CA, USA). A one-compartment model with the first-order elimination was selected to fit the data. Investigated pharmacokinetic parameters included the Vd and total clearance (CL).
Statistical analysis
Kolmogorov–Smirnov test was used to check for the normal distribution of data. Continuous normally distributed data were tested with unpaired t-tests; nonnormally distributed data were analyzed using Mann–Whitney U-test. Categorical data were evaluated using Chi-square test. Unless stated otherwise, normally distributed data are presented as mean ± standard deviation, and as median (interquartile ranges) where nonnormally distributed. A two-sided P < 0.05 was considered statistically significant for all tests. Statistical analyses were performed using the SPSS statistical package version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient demographics
Fifty patients who fulfilled the participation criteria were enrolled between June 2012 and December 2014; 25 patients were randomized to each of the intermittent group and the continuous group. The main infection site was intra-abdominal (54%); other sites included the lung (38%), bloodstream (16%), urinary tract (6%), wound (2%), central nervous system (2%), and multiple sites (18%). Bacterial cultures of 43 patients (86%) were positive for Escherichia coli (26%), Pseudomonas aeruginosa (24%), Klebsiella pneumonia (16%), Acinetobacter baumannii (12%), Enterobacter cloacae (2%), Enterobacter aerogenes (2%), Providencia stuartii (2%), and Burkholderia cepacia (2%).
Comparison of clinical characteristics between the two groups
There were no significant differences in age, weight, severity of illness (APACHE II and SOFA scores), site of infection, or rate of isolated bacteria and other evaluated parameters [Table 1]. In addition, the rates of clinical success (the primary endpoint) were similar at 16 (64%) in the continuous group and 14 (56%) in the intermittent group (P = 0.564). The duration of meropenem treatment was significantly shorter in the continuous group (P = 0.035); however, there were no significant differences in other secondary end points including ICU mortality and LOS. Moreover, the rate of superinfection among participants in the continuous group (4%) was less than that in the intermittent group (16%), although the difference was not statistically significant (P = 0.157).
Table 1.
Comparison of clinical characteristics between the continuous and intermittent groups
| Clinical characteristics | Continuous group (n = 25) | Intermittent group (n = 25) | Statistical value | P |
|---|---|---|---|---|
| Male sex, n (%) | 10 (40.0) | 11 (44.0) | 0.082* | 0.774 |
| Age (years) | 68.0 ± 15.4 | 67.0 ± 12.2 | 0.275‡ | 0.309 |
| Weight (kg) | 60.5 ± 10.2 | 63.8 ± 11.8 | −1.067‡ | 0.445 |
| APACHE II score | 19.4 ± 5.0 | 19.7 ± 5.9 | −0.181‡ | 0.523 |
| SOFA score | 8.0 ± 2.8 | 8.5 ± 2.4 | −0.657‡ | 0.577 |
| GFR (ml/min) | 97.5 ± 43.4 | 91.1 ± 34.0 | 0.578‡ | 0.295 |
| WBC1 (×109/L) | 11.5 ± 4.0 | 11.9 ± 5.0 | −0.268‡ | 0.410 |
| WBC5 (×109/L) | 9.2 ± 3.9 | 10.2 ± 4.3 | −0.822‡ | 0.325 |
| PCT1 (µg/L), value (range) | 1.3 (0.3–4.0) | 1.2 (0.3–23.8) | 292.500† | 0.696 |
| PCT5 (µg/L), value (range) | 0.2 (0.1–0.6) | 0.3 (0.1–1.3) | 80.000† | 0.610 |
| Fluid infusion (ml) | 4267.8 ± 1074.7 | 4225.7 ± 858.3 | 0.153‡ | 0.477 |
| Site of infection, n (%) | ||||
| Lung | 9 (36.0) | 10 (40.0) | 0.085* | 0.771 |
| Intra-abdominal | 14 (56.0) | 13 (52.0) | 0.081* | 0.777 |
| Bloodstream | 5 (20.0) | 3 (12.0) | 0.595* | 0.440 |
| Urinary tract | 1 (4.0) | 2 (8.0) | 0.355* | 0.552 |
| Wound | 1 (4.0) | 0 | 1.020* | 0.312 |
| Central nervous system | 0 | 1 (4.0) | 1.020* | 0.312 |
| Multiple sites | 5 (20.0) | 4 (16.0) | 0.136* | 0.713 |
| Isolated bacteria, n (%) | 22 (88.0) | 21 (84.0) | 1.296* | 0.684 |
| Length of ICU stay (days) | 10.0 (7.0–26.5) | 10.0 (8.0–27.0) | 263.000† | 0.336 |
| Duration of meropenem treatment (days) | 7.6 ± 2.3 | 9.4 ± 5.5 | −2.258‡ | 0.035 |
| ICU mortality, n (%) | 7 (28.0) | 8 (32.0) | 0.095* | 0.758 |
| Clinical success, n (%) | 16 (64.0) | 14 (56.0) | 0.333* | 0.564 |
| Superinfection, n (%) | 1 (4.0) | 4 (16.0) | 2.000* | 0.157 |
*χ2 value; †U value; ‡t value. APACHE II: Acute Physiology and Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment; GFR: Glomerular filtration rate (calculated with Cockcroft formula); WBC1 and WBC5: White blood cell count at the 1st and the 5th days of meropenem therapy; PCT1 and PCT5: Procalcitonin at the 1st and the 5th days of meropenem therapy; ICU: Intensive Care Unit.
Comparison of microbiological characteristics between the two groups
The main bacterial MIC in both groups was ≤0.25 (68.2% of the continuous group and 61.9% of the intermittent group); the difference was not significant. The rate of microbiological eradication between participants was slightly, but not significantly, higher in the continuous group (81.8%) than in the intermittent group (66.7%; P = 0.255) [Table 2].
Table 2.
Comparison of microbiological characteristics between the continuous and intermittent groups
| Microbiological characteristics | Continuous group (n = 22) | Intermittent group (n = 21) | χ2 | P |
|---|---|---|---|---|
| Bacterial MIC, n (%) | ||||
| ≤0.25 | 15 (68.2) | 13 (61.9) | 4.322 | 0.364 |
| 1 | 0 | 2 (9.5) | ||
| 2 | 1 (4.5) | 3 (14.2) | ||
| 4 | 3 (13.6) | 1 (4.8) | ||
| ≥16 | 3 (13.6) | 2 (9.5) | ||
| Microbiological eradication, n (%) | 18 (81.8) | 14 (66.7) | 1.296 | 0.255 |
MIC: Minimal inhibitory concentration.
Pharmacokinetic data
The observed concentration-time profiles of meropenem administered to the intermittent and continuous groups are shown in Figure 1a (the first dosing period) and Figure 1b (the third dosing period). The comparative Cmax, Cmin, and concentrations of T40% (CT40%) in both dosing periods are shown in Table 3. The Vd was 27.3 ± 4.8 L while the CL was 0.23 ± 0.1 L/min.
Figure 1.
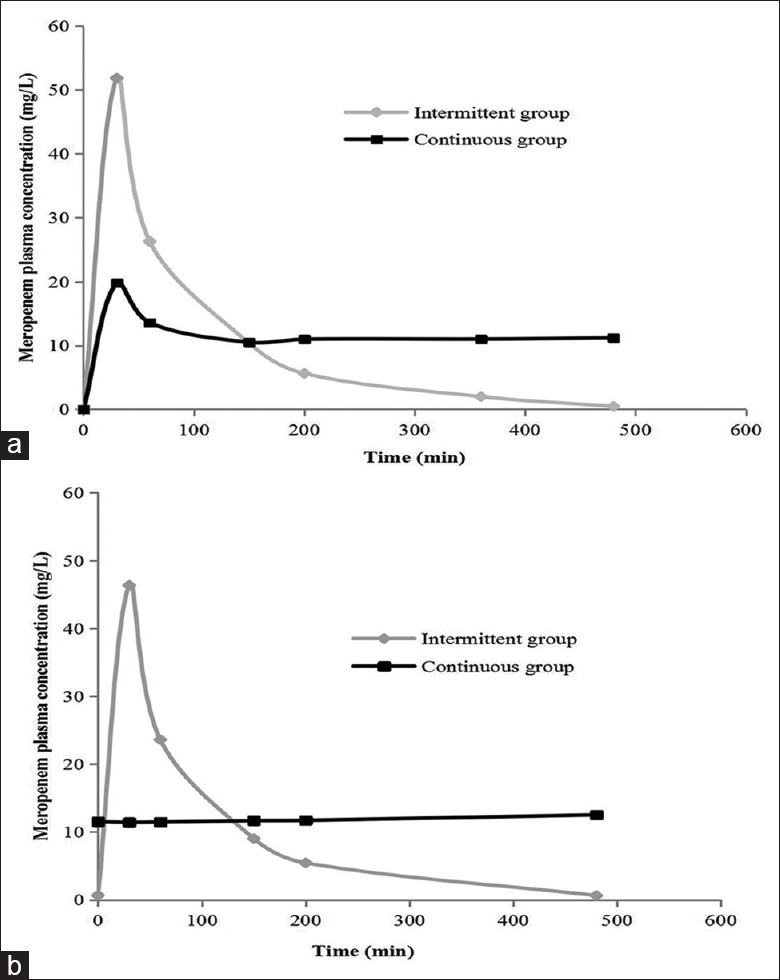
Plasma concentrations of meropenem administered to patients with severe sepsis or septic shock by intermittent infusion and continuous infusion for (a) the first dosing period and (b) third dosing period.
Table 3.
Comparison of pharmacokinetic data between the continuous and intermittent groups
| Pharmacokinetic data (mg/L) | Continuous group (n = 22) | Intermittent group (n = 21) | U | P |
|---|---|---|---|---|
| The first dosing period Cmax | 19.8 (16.0–21.4) | 51.8 (44.3–54.25) | 0.000 | 0.000 |
| The first dosing period Cmin | 11.2 (7.6–11.7) | 0.5 (0–1.0) | 0.000 | 0.000 |
| The first dosing period CT40% | 10.98 (8.18–12.38) | 5.62 (4.22–6.83) | 65.000 | 0.000 |
| The third dosing period Cmax | 12.5 (8.7–12.9) | 46.4 (40.8–49.0) | 0.000 | 0.000 |
| The third dosing period Cmin | 11.4 (7.9–12.5) | 0.6 (0–1.1) | 0.000 | 0.000 |
| The third dosing period CT40% | 11.7 (8.4–12.6) | 5.4 (4.1–6.7) | 41.000 | 0.000 |
Cmax: Peak concentrations; Cmin: Trough concentrations; CT40%: Concentrations of T40% (the time point of 200 min was nearly 40% of a dosing interval and was marked as T40%).
Discussion
In this prospective randomized pilot study, we found that continuous infusion of 3 g of meropenem per day preserved a similar rate of clinical cure as intermittent infusion, but showed a tendency for higher microbiological efficacy and required a significantly shorter duration of treatment. We also found that continuous infusion of meropenem achieved a better steady concentration. Both intermittent and continuous infusion regimens for meropenem were effective against most Gram-negative pathogens that remain highly susceptible (MIC ≤0.25). Against intermediate-susceptibility bacteria (MIC = 4 for nonfermentative bacteria and MIC = 2 for enterobacteriaceae), better pharmacokinetic results are likely to be achieved by continuous infusion than by intermittent infusion of the same daily dose. However, administering 3 g of meropenem using either infusion regimen was pharmacokinetically ineffective against resistant bacteria (i.e., MIC ≥8 and particularly MIC ≥16) in our study.
There were no significant differences in the primary clinical outcomes (cure rates) between the continuous and intermittent groups. Our results are most comparable to those of Chytra et al. who found a nonsignificant difference in cure rates in a trial of 240 critically ill patients randomized to receive meropenem by continuous infusion versus intermittent (bolus) administration (83% vs. 75%, respectively).[12] Recently, Dulhunty et al.[18] conducted a randomized controlled trial in 25 ICUs to evaluate the efficacy of continuous versus intermittent infusion of beta-lactam antibiotics in patients with severe sepsis and found no difference between treatment groups in clinical cure rates (52.4% vs. 49.5%). In contrast, a retrospective study of meropenem[13] and that of other beta-lactam agents found that continuous infusion resulted in better results than intermittent bolus administration did.[19,20,21]
Several factors could explain our results. First, ours was a pilot study, and our sample size was small. Second, the theoretical advantage of continuous infusion is crucially dependent on the MIC. In our study, 86% of participants had an identified pathogen, and the MICs in a majority of our isolated pathogens (65.1%) were ≤0.25 (MICs were measured by semi-quantitative determination). However, according to our pharmacokinetic data, both the continuous and intermittent groups achieved T>MIC values close to 100% for the highly susceptible pathogens [Table 3 and Figure 1]. For intermediate-susceptibility bacteria (MIC = 4 for nonfermentative bacteria and MIC = 2 for enterobacteriaceae), continuous infusion may achieve a better pharmacokinetic profile. However, only 18.6% of the pathogens were in this category; 11.6% of the isolated pathogens were resistant bacteria (MIC ≥16) against which adequate pharmacokinetic efficacy was not achieved in either group with 3 g of meropenem per day (the CT40% of the intermittent group was 5.4 mg/L, while the Cmin of the continuous group was 11.7 mg/L). It is possible that increasing the dosage to 6 g/day in renal function normal patients can achieve better efficacy against resistant bacteria in the continuous group than in the intermittent group. Jamal et al.[22] observed the pharmacokinetics of meropenem in critically ill patients receiving continuous venovenous hemofiltration and found that administration of 3 g/day with continuous infusion resulted in a higher steady-state concentration of 21.91 mg/L. Third, physiological changes associated with severe sepsis and septic shock can increase Vd and drug clearance[10,23] leading to lower plasma[11,24] and tissue concentrations[25] than is commonly observed in noncritically ill patients. In our study of patients with severe sepsis and septic shock, Vd (27.3 L) and CL (0.23 L/min) were higher, and plasma concentrations of meropenem were lower than that in other critically ill patients.[11,26,27] Taccone et al.[28] conducted a prospective, multicenter study to determine whether the first dose of beta-lactam antibiotics would result in inadequate serum drug concentrations in patients with severe sepsis and septic shock and found that the target pharmacokinetic profiles were achieved in only 75% of patients for meropenem.
Although there was no significant difference in the rate of superinfection and microbiological eradication between participants in the continuous and intermittent groups, the former tended to show more favorable results. Other studies had similar outcomes that showed an improved bacteriological efficacy associated with the continuous application of meropenem[12] and beta-lactams.[20] A possible explanation for the better microbial clearance in the continuous group may be the higher tissue concentrations of meropenem in patients of this group than in those of the intermittent group;[10,25] patients with pneumonia in particular experience poor penetration of most beta-lactams into lung tissue.[29]
We observed a significantly shorter duration of meropenem treatment in the continuous group. Similar results were observed in the study of Chytra et al.[12] However, we did not find differences in ICU mortality rates, LOS, and values of WBC and PCT. Our results are concurrent with those of Dulhunty et al.,[18] although previous studies had conflicting results.[30] Meta-analyses by Roberts et al.[31] and Shiu et al.[32] found no significant differences in cumulative mortality between the two groups. In contrast, Falagas et al. found a significant mortality difference between continuous and intermittent infusion in their meta-analysis of observational and randomized controlled trials comparing infusion methods of carbapenems and piperacillin-tazobactam.[33] Such studies have not been conducted in patients with severe sepsis and septic shock; their mortality rates were much lower than that in our patients.
Our study has the following limitations. This was a pilot investigation, and our results may have been influenced by our small sample size (only fifty patients). Furthermore, the study was not blinded and was conducted at a single center; therefore, bias in clinical evaluations may exist. We did not record the time between the onset of sepsis and administration of antibiotic therapy, but all the patients were treated according to the Surviving Sepsis Campaign guideline. We set the third dosing period as the steady state despite it being longer than 5 half-lives; the sixth dosing period may be more stable.
In conclusion, we found that continuous infusion of meropenem in lieu of intermittent dosing provides equal clinical efficacy and mortality, but also produces a significantly shorter duration of meropenem treatment plus a tendency toward superior bacteriological efficacy in patients with severe sepsis and septic shock. Optimal administration modalities and dosing should be chosen carefully according to the MICs of the pathogens. Both continuous and intermittent infusion of meropenem can achieve good pharmacokinetics against high-susceptibility pathogens; however, continuous infusion is better for intermediate susceptibility. Neither method is effective against drug-resistant bacteria in regular doses, although a continuous regimen may be better with incrementally increasing doses. Therapeutic drug monitoring based on pathogen MICs and doses must be the strategic focus of the future studies.
Financial support and sponsorship
This study was supported by grants from the Clinical Research Special Foundation of Chinese Medical Association (No. 11030140258).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
References
- 1.Kadri SS, Rhee C, Strich JR, Morales MK, Hohmann S, Menchaca J, et al. Estimating ten-year trends in septic shock incidence and mortality in United States academic medical centers using clinical data. Chest. 2017;151:278–85. doi: 10.1016/j.chest.2016.07.010. doi: 10.1016/j.chest.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y, Xie JF, Yu KJ, Yao C, Li JG, Guan XD, et al. Epidemiological study of sepsis in China: Protocol of a cross-sectional survey. Chin Med J (Engl) 2016 Dec;129:2967–73. doi: 10.4103/0366-6999.195474. doi: 10.4103/0366-6999.195474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellinger RP. The surviving sepsis campaign: 2013 and beyond. Chin Med J (Engl) 2013;126:1803–5. doi: 10.3760/cma.j.issn.0366-6999. [PubMed] [Google Scholar]
- 5.Livermore DM British Society for Antimicrobial Chemotherapy Working Party on the Urgent Need: Regenerating Antibacterial Drug Discovery and Development. Discovery research: The scientific challenge of finding new antibiotics. J Antimicrob Chemother. 2011;66:1941–4. doi: 10.1093/jac/dkr262. doi: 10.1093/jac/dkr262. [DOI] [PubMed] [Google Scholar]
- 6.Wei WJ, Yang HF, Ye Y, Li JB. New Delhi Metallo-ß-lactamase-mediated carbapenem resistance: Origin, diagnosis, treatment and public health concern. Chin Med J (Engl) 2015;128:1969–76. doi: 10.4103/0366-6999.160566. doi: 10.4103/0366-6999.160566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chastre J, Luyt CE. Continuous ß-lactam infusion to optimize antibiotic use for severe sepsis. A knife cutting water? Am J Respir Crit Care Med. 2015;192:1266–8. doi: 10.1164/rccm.201507-1487ED. doi: 10.1164/rccm.201507-1487ED. [DOI] [PubMed] [Google Scholar]
- 8.Drusano GL. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat Rev Microbiol. 2004;2:289–300. doi: 10.1038/nrmicro862. doi: 10.1038/nrmicro862. [DOI] [PubMed] [Google Scholar]
- 9.McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31:345–51. doi: 10.1016/j.ijantimicag.2007.12.009. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: Intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother. 2009;64:142–50. doi: 10.1093/jac/dkp139. doi: 10.1093/jac/dkp139. [DOI] [PubMed] [Google Scholar]
- 11.Novelli A, Adembri C, Livi P, Fallani S, Mazzei T, De Gaudio AR. Pharmacokinetic evaluation of meropenem and imipenem in critically ill patients with sepsis. Clin Pharmacokinet. 2005;44:539–49. doi: 10.2165/00003088-200544050-00007. doi: 10.2165/00003088-200544050-00007. [DOI] [PubMed] [Google Scholar]
- 12.Chytra I, Stepan M, Benes J, Pelnar P, Zidkova A, Bergerova T, et al. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: A randomized open-label controlled trial. Crit Care. 2012;16:R113. doi: 10.1186/cc11405. doi: 10.1186/cc11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorente L, Lorenzo L, Martín MM, Jiménez A, Mora ML. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann Pharmacother. 2006;40:219–23. doi: 10.1345/aph.1G467. doi: 10.1345/aph.1G467. [DOI] [PubMed] [Google Scholar]
- 14.Product Information: Mepem®, Meropenem for Injection (Revised February 2010) Oita, Japan: Sumitomo Dainippon Pharma Co., Ltd; 2010. [Google Scholar]
- 15.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 16.Kuti JL, Nightingale CH, Knauft RF, Nicolau DP. Pharmacokinetic properties and stability of continuous-infusion meropenem in adults with cystic fibrosis. Clin Ther. 2004;26:493–501. doi: 10.1016/s0149-2918(04)90051-3. doi: 10.1016/0149-2918(04)90051-3. [DOI] [PubMed] [Google Scholar]
- 17.Franceschi L, Cojutti P, Baraldo M, Pea F. Stability of generic meropenem solutions for administration by continuous infusion at normal and elevated temperatures. Ther Drug Monit. 2014;36:674–6. doi: 10.1097/FTD.0000000000000054. doi: 10.1097/FTD.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 18.Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, et al. A multicenter randomized trial of continuous versus intermittent ß-lactam infusion in severe sepsis. Am J Respir Crit Care Med. 2015;192:1298–305. doi: 10.1164/rccm.201505-0857OC. doi: 10.1164/rccm.201505-0857OC. [DOI] [PubMed] [Google Scholar]
- 19.Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: A multicenter double-blind, randomized controlled trial. Clin Infect Dis. 2013;56:236–44. doi: 10.1093/cid/cis856. doi: 10.1093/cid/cis856. [DOI] [PubMed] [Google Scholar]
- 20.Roberts JA, Boots R, Rickard CM, Thomas P, Quinn J, Roberts DM, et al. Is continuous infusion ceftriaxone better than once-a-day dosing in intensive care? A randomized controlled pilot study. J Antimicrob Chemother. 2007;59:285–91. doi: 10.1093/jac/dkl478. doi: 10.1093/jac/dkl478. [DOI] [PubMed] [Google Scholar]
- 21.Lorente L, Jiménez A, Martín MM, Iribarren JL, Jiménez JJ, Mora ML. Clinical cure of ventilator-associated pneumonia treated with piperacillin/tazobactam administered by continuous or intermittent infusion. Int J Antimicrob Agents. 2009;33:464–8. doi: 10.1016/j.ijantimicag.2008.10.025. doi: 10.1016/j.ijantimicag.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 22.Jamal JA, Mat-Nor MB, Mohamad-Nor FS, Udy AA, Wallis SC, Lipman J, et al. Pharmacokinetics of meropenem in critically ill patients receiving continuous venovenous haemofiltration: A randomised controlled trial of continuous infusion versus intermittent bolus administration. Int J Antimicrob Agents. 2015;45:41–5. doi: 10.1016/j.ijantimicag.2014.09.009. doi: 10.1016/j.ijantimicag.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Gonçalves-Pereira J, Póvoa P. Antibiotics in critically ill patients: A systematic review of the pharmacokinetics of ß-lactams. Crit Care. 2011;15:R206. doi: 10.1186/cc10441. doi: 10.1186/cc10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeurissen A, Rutsaert R. ß-lactam antibiotics in continuous infusion in critically ill patients. Crit Care. 2010;14:446. doi: 10.1186/cc9288. doi: 10.1186/cc9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts JA, Roberts MS, Robertson TA, Dalley AJ, Lipman J. Piperacillin penetration into tissue of critically ill patients with sepsis – Bolus versus continuous administration? Crit Care Med. 2009;37:926–33. doi: 10.1097/CCM.0b013e3181968e44. doi: 10.1097/CCM.0b013e3181968e44. [DOI] [PubMed] [Google Scholar]
- 26.Kitzes-Cohen R, Farin D, Piva G, De Myttenaere-Bursztein SA. Pharmacokinetics and pharmacodynamics of meropenem in critically ill patients. Int J Antimicrob Agents. 2002;19:105–10. doi: 10.1016/s0924-8579(01)00474-5. doi: 10.1016/S0924-8579(01)00474-5. [DOI] [PubMed] [Google Scholar]
- 27.Thalhammer F, Traunmüller F, El Menyawi I, Frass M, Hollenstein UM, Locker GJ, et al. Continuous infusion versus intermittent administration of meropenem in critically ill patients. J Antimicrob Chemother. 1999;43:523–7. doi: 10.1093/jac/43.4.523. doi: 10.1093/jac/43.4.523. [DOI] [PubMed] [Google Scholar]
- 28.Taccone FS, Laterre PF, Dugernier T, Spapen H, Delattre I, Wittebole X, et al. Insufficient ß-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care. 2010;14:R126. doi: 10.1186/cc9091. doi: 10.1186/cc9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodvold KA, Yoo L, George JM. Penetration of anti-infective agents into pulmonary epithelial lining fluid: Focus on antifungal, antitubercular and miscellaneous anti-infective agents. Clin Pharmacokinet. 2011;50:689–704. doi: 10.2165/11592900-000000000-00000. doi: 10.2555/11592900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Cotta MO, Dulhunty JM, Roberts JA, Myburgh J, Lipman J. Should ß-lactam antibiotics be administered by continuous infusion in critically ill patients? A survey of Australia and New Zealand intensive care unit doctors and pharmacists. Int J Antimicrob Agents. 2016;47:436–8. doi: 10.1016/j.ijantimicag.2016.02.017. doi: 10.1016/j.ijantimicag.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Roberts JA, Webb S, Paterson D, Ho KM, Lipman J. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit Care Med. 2009;37:2071–8. doi: 10.1097/CCM.0b013e3181a0054d. doi: 10.1097/CCM.0b013e3181a0054d. [DOI] [PubMed] [Google Scholar]
- 32.Shiu J, Wang E, Tejani AM, Wasdell M. Continuous versus intermittent infusions of antibiotics for the treatment of severe acute infections. Cochrane Database Syst Rev. 2013;28:CD008481. doi: 10.1002/14651858.CD008481.pub2. doi: 10.1002/14651858.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: A systematic review and meta-analysis. Clin Infect Dis. 2013;56:272–82. doi: 10.1093/cid/cis857. doi: 10.1093/cid/cis857. [DOI] [PubMed] [Google Scholar]


