Abstract
Background:
Propofol is increasingly used during partial support mechanical ventilation such as pressure support ventilation (PSV) in postoperative patients. However, breathing pattern, respiratory drive, and patient-ventilator synchrony are affected by the sedative used and the sedation depth. The present study aimed to evaluate the physiologic effects of varying depths of propofol sedation on respiratory drive and patient-ventilator synchrony during PSV in postoperative patients.
Methods:
Eight postoperative patients receiving PSV for <24 h were enrolled. Propofol was administered to achieve and maintain a Ramsay score of 4, and the inspiratory pressure support was titrated to obtain a tidal volume (VT) of 6–8 ml/kg. Then, the propofol dose was reduced to achieve and maintain a Ramsay score of 3 and then 2. At each Ramsay level, the patient underwent 30-min trials of PSV. We measured the electrical activity of the diaphragm, flow, airway pressure, neuro-ventilatory efficiency (NVE), and patient-ventilator synchrony.
Results:
Increasing the depth of sedation reduced the peak and mean electrical activity of the diaphragm, which suggested a decrease in respiratory drive, while VT remained unchanged. The NVE increased with an increase in the depth of sedation. Minute ventilation and inspiratory duty cycle decreased with an increase in the depth of sedation, but this only achieved statistical significance between Ramsay 2 and both Ramsay 4 and 3 (P < 0.05). The ineffective triggering index increased with increasing sedation depth (9.5 ± 4.0%, 6.7 ± 2.0%, and 4.2 ± 2.1% for Ramsay 4, 3, and 2, respectively) and achieved statistical significance between each pair of depth of sedation (P < 0.05). The depth of sedation did not affect gas exchange.
Conclusions:
Propofol inhibits respiratory drive and deteriorates patient-ventilator synchrony to the extent that varies with the depth of sedation. Propofol has less effect on breathing pattern and has no effect on VT and gas exchange in postoperative patients with PSV.
Keywords: Electrical Activity of Diaphragm, Patient-ventilator Synchrony, Propofol, Respiratory Drive
Introduction
Patient-ventilator interaction is influenced by the machine's features, such as ventilatory mode and settings[1,2,3] and the patient's own breathing pattern, respiratory center drive and effort.[4,5] Patient breathing pattern, drive, and effort are affected by sedatives, whose effects vary depending on the drug used and the sedation depth.[6,7] To enhance tolerance to an endotracheal tube, decrease the stress response, and minimize patient discomfort and pain, sedation is often necessary even with modes of partial support such as pressure support ventilation (PSV) in postoperative patients. An observational study recently reported a 4- to 5-fold increase in ineffective triggering with sedation, as opposed to wakefulness.[8] However, in this study, the sedation regimen and depth were not standardized and included various drugs with different effects on breathing pattern and respiratory drive.
As a short-acting sedative-hypnotic agent, propofol is increasingly used for sedation during mechanical ventilation, especially for postoperative patients.[9] Propofol is at least as effective as midazolam, though with a more rapid and predictable time of emergence[10,11] and a shorter interval to extubation.[12,13,14] However, propofol was previously shown to affect the ventilatory response to CO2 and the breathing pattern in healthy controls during spontaneous unassisted breathing[15] and to influence the outcome of the spontaneous breathing trial by altering the rapid shallow breathing index.[16] To the best of our knowledge, few studies evaluated the effects of propofol in postoperative patients receiving PSV. The aim of this physiologic study was to evaluate the effects of varying depths of propofol sedation on breathing pattern, gas exchange, respiratory drive, and patient-ventilator synchrony during PSV.
Methods
Ethical approval
The trial was conducted in a 20-bed general Intensive Care Unit (ICU) of Zhongda Hospital, which is affiliated with Southeast University. The protocol was approved by the Institutional Ethics Committee of Zhongda Hospital (Approval Number: 2010ZDLL018.0), and written informed consent was obtained from the patients or next of kin.
Patients
All postoperative patients who were admitted to the ICU were screened from October 1, 2010, to November 1, 2010. Patients were eligible for inclusion based on the following criteria: (1) PSV for ≤24 h; (2) central venous and arterial indwelling catheters; and (3) administration of only short-acting sedative agents (i.e., propofol and/or remifentanil). Exclusion criteria were (1) younger than 18 years or older than 85 years; (2) contraindications for an electrical activity of the diaphragm (EAdi) catheter placement, i.e., esophageal varices, upper gastroesophageal bleeding in the previous 30 days, gastroesophageal surgery in the previous 12 months, and coagulation disorders (international normalized ratio >1.5 and activated partial thromboplastin time >44 s); (3) history of an acute, central or peripheral nervous system disorder or a severe neuromuscular disease; (4) hemodynamic instability (i.e., need for epinephrine or vasopressin infusion or need for dopamine or dobutamine >5 μg∙kg−1∙min−1, or norepinephrine >5 μg/min to maintain mean arterial blood pressure >65 mmHg); (5) hypertension (arterial systolic pressure >180 mmHg) and tachycardia (>130 beats/min) or unbearable patient discomfort; (5) pregnancy; or (6) history of allergy to propofol components. Criteria for protocol discontinuation were as follows: (1) agitation; (2) respiratory rate >40 or <8 breaths/min or SPO2 <90%; (3) hemodynamic instability, as defined in exclusion criteria; or (4) malignant arrhythmias.
Study protocol
The enrolled patients were switched to an SERVO-i ventilator (Maquet, Solna, Stockholm, Sweden). A 16-F nasogastric feeding tube (Maquet, Solna, Stockholm, Sweden) with electrodes measuring the EAdi was inserted through the nose and secured after confirming the positioning according to the guidelines for NAVA catheter positioning (Maquet, Solna, Stockholm, Sweden). After positioning the EAdi catheter, propofol (2%) was administered into a central vein. We adjusted the dose of propofol every 5 min to achieve and maintain a Ramsay score of 4 and titrated the inspiratory pressure support to obtain a tidal volume (VT) of 6–8 ml/kg of the predicted body weight. SERVO-i default inspiratory (level 5, corresponding to 50% of the 2 L/min bias flow) and expiratory (30% of peak inspiratory flow) trigger settings were used for PSV throughout the study period. The positive end-expiratory pressure (PEEP) and FiO2 were maintained at the values in use before patient enrolment, with consistent, constant maintenance throughout the study period. Then, we reduced the dose of propofol to achieve and maintain a Ramsay score of 3 and then 2. The patient was evaluated at three levels of sedation (Ramsay score 4, 3, and 2), and each patient underwent 30-min trials of PSV at each level of sedation. During the study, morphine was given as a continuous intravenous infusion at a dose of 1 mg/h.
Measurements
The depth of sedation was continuously assessed by the bispectral index (BIS) (BIS Monitor, Aspect, USA), which provides a numeric output indicating the patient's level of consciousness in a range between 0 (the absence of brain electrical activity) and 100 (wide awake). The depth of sedation was evaluated using the Ramsay score, which was assessed by the investigators at the beginning and at the end of each trial. Airway occlusion pressure (P0.1) was measured at the end of each trial. The last 10 min of each trial were recorded and stored on a dedicated personal computer for further analysis of breathing pattern and patient-ventilatory synchrony. Arterial blood gases were taken at the end of each trial.
Data analysis
EAdi, airway pressure (Paw), and flow were acquired from the ventilator through an RS232 interface at a sampling rate of 100 Hz, recorded by means of dedicated software (NAVA Tracker V. 2.0, Maquet Critical Care, Sölna, Sweden), and analyzed using customized software (Maquet) by one of the researchers.[17] With the analysis software, the researcher placed cursors on the EAdi and flow waveform to confirm the beginning and 70% of peak of EAdi (which represents the beginning and end of neural inspiration) and the beginning and end of the inspiratory flow (which represents the beginning and end of mechanical inspiration). Then, the inspiratory trigger (Delayinsp) and expiratory trigger (Delayexp) delays were calculated from the time gap between the beginning and the end of neural and mechanical inspiration by the software. Ineffectively triggered, auto-triggered, and double-triggered breaths were determined by comparing the EAdi and flow waveform as previously described[2,8] for 1 min in all trials in each patient. The ineffective triggering index was computed by dividing ineffectively triggered breaths by the total breaths.[8] VT, minute ventilation (VE), respiratory rate (RR), and inspiratory duty cycle (Ti/TT) were also determined.[2] The EAdi was measured to assess neural drive and diaphragm function and was estimated by neuromechanical efficiency (NME) and neuro-ventilatory efficiency (NVE).[17] The NME was calculated as (Paw-PEEP) divided by EAdi during inspiratory occlusion. NVE was calculated as the ratio of VT and EAdi during inspiration. All parameters and indices (except NME) were calculated as the mean value of five inspirations at each time point. NVE was calculated from one inspiratory occlusion at each trial.
Statistical analysis
Statistical analysis was performed with SPSS 16.0 (IBM, New York, USA). Data are reported as the mean ± standard deviation (SD), and statistically significant difference was defined as P < 0.05. One-way repeated-measures analysis of variance with the Student–Newman–Keuls post hoc comparison test was used to compare variables between different depths of sedation.
Results
We enrolled 8 consecutive patients (4 males and 4 females, 61.1 ± 8.6 years old, predicted body weight of 64.5 ± 6.2 kg), and all of them completed the study protocol. Anthropometric and clinical characteristics of the 8 patients who concluded the study protocol are provided in Table 1. PEEP and FiO2 were 5.0 ± 0.5 cmH2O and 0.4 ± 0.0, respectively. The levels of inspiratory assistance were 10.8 ± 1.8 cmH2O. No patient had a comorbidity of the respiratory system or died in hospital. Table 2 lists the dose of propofol and BIS value at different depths of sedation. The dose of propofol increased and the BIS value decreased with increasing depth of sedation. The BIS values were 69.0 ± 10.3, 84.8 ± 1.2, and 91.9 ± 1.6 at a Ramsay score of 4, 3, and 2, respectively (P < 0.05).
Table 1.
Characteristics of postoperative patients receiving PSV for <24 h at enrolment
| Patient number | Gender | Age (years) | Predicted body weight (kg) | Comorbidity | Diagnosis | Operation | PEEP (cmH2O) | Inspiratory pressure support above PEEP (cmH2O) | FiO2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 73 | 69 | Hypertension and diabetes | Coronary heart disease | Coronary artery bypass grafting | 5 | 12 | 0.4 |
| 2 | Female | 56 | 61 | – | Hysteromyoma | Abdominal hysterectomy | 5 | 10 | 0.4 |
| 3 | Female | 68 | 65 | Chronic cardiac dysfunction | Rheumatic heart disease, tricuspid regurgitation | Tricuspid valve replacement | 5 | 12 | 0.4 |
| 4 | Female | 57 | 55 | Chronic cardiac dysfunction | Rheumatic heart disease, mitral stenosis | Mitral valve replacement | 5 | 10 | 0.4 |
| 5 | Male | 56 | 67 | Chronic cardiac dysfunction | Senile calcific valve disease, mitral stenosis and aortic valve insufficiency | Mitral valve and aortic valve replacement | 5 | 10 | 0.4 |
| 6 | Male | 57 | 72 | Chronic cardiac dysfunction and Hypertension | Senile calcific valve disease, mitral stenosis | Mitral valve replacement | 5 | 10 | 0.4 |
| 7 | Male | 72 | 70 | Diabetes | Coronary heart disease | Coronary artery bypass grafting | 6 | 14 | 0.5 |
| 8 | Female | 50 | 57 | Hypertension | Left atrial myxoma | Excision of left trial myxoma | 4 | 8 | 0.4 |
PSV: Pressure support ventilation; PEEP: Positive end-expiratory pressure.
Table 2.
Dose of propofol and BIS value at different depth of sedation
| Patient number | Ramsay 4 | Ramsay 3 | Ramsay 2 | |||
|---|---|---|---|---|---|---|
| Propofol dose (mg/h) | BIS value | Propofol dose (mg/h) | BIS value | Propofol dose (mg/h) | BIS value | |
| 1 | 60 | 73 | 40 | 85 | 20 | 93 |
| 2 | 40 | 78 | 20 | 83 | 10 | 92 |
| 3 | 50 | 74 | 30 | 86 | 10 | 90 |
| 4 | 40 | 57 | 15 | 85 | 5 | 94 |
| 5 | 50 | 78 | 30 | 86 | 20 | 90 |
| 6 | 50 | 77 | 30 | 86 | 10 | 93 |
| 7 | 60 | 63 | 25 | 85 | 10 | 90 |
| 8 | 30 | 52 | 15 | 83 | 5 | 93 |
| Mean ± SD | 47.5 ± 10.4 | 69.0 ± 10.3 | 25.6 ± 8.6* | 84.9 ± 1.2* | 11.3 ± 5.8*,† | 91.9 ± 1.6*,† |
*P<0.05 compared with Ramsay 4; †P<0.05 compared with Ramsay 3. BIS: Bispectral index; SD: Standard deviation.
As shown in Table 3, increasing the depth of sedation reduced the peak EAdi [Figure 1] and mean EAdi (P < 0.05), which suggested a decrease in respiratory drive, while P0.1 and VT/Ti remained unchanged. NVE increased with increasing depth of sedation unexpectedly, and NME had an increasing trend with increasing depth of sedation but did not achieve statistical significance. The depth of sedation did not significantly affect the Ppeak, RR, VT, and Ti (P > 0.05). Increasing the depth of sedation decreased minute ventilation and Ti/TT, but the only differences that achieved statistical significance were those between Ramsay 2 and both Ramsay 4 and 3 (P < 0.05).
Table 3.
Respiratory drive, breath pattern, and patient-ventilatory synchrony at different depths of sedation
| Parameters | Ramsay 4 (n = 8) | Ramsay 3 (n = 8) | Ramsay 2 (n = 8) | F | P |
|---|---|---|---|---|---|
| EAdi peak (µV) | 2.9 ± 1.3 | 4.1 ± 1.8* | 7.4 ± 4.6*,† | 5.991 | 0.037 |
| EAdi mean (µV) | 1.7 ± 0.7 | 2.3 ± 0.8* | 4.2 ± 2.5*,† | 7.067 | 0.026 |
| P0.1 (cmH2O) | 0.9 ± 0.5 | 1.0 ± 0.4 | 1.0 ± 0.6 | 0.255 | 0.783 |
| NVE (ml/µV) | 180.4 ± 78.2 | 129.2 ± 41.2* | 85.9 ± 35.5*,† | 7.850 | 0.021 |
| NME (cmH2O/µV) | 4.8 ± 4.0 | 3.4 ± 1.7 | 1.6 ± 1.3 | 4.323 | 0.069 |
| Ppeak (cmH2O) | 15.6 ± 2.3 | 15.8 ± 1.8 | 15.4 ± 2.4 | 0.255 | 0.783 |
| RR (breaths/min) | 15.1 ± 6.0 | 14.3 ± 7.8 | 17.6 ± 8.6 | 3.097 | 0.119 |
| VT (ml/kg of PBW) | 6.5 ± 1.5 | 7.1 ± 2.7 | 7.4 ± 2.3 | 0.985 | 0.427 |
| Minute ventilation (L/min) | 5.9 ± 1.7 | 5.9 ± 0.9 | 7.8 ± 2.0*,† | 3.842 | 0.038 |
| Neural Ti (s) | 1.2 ± 0.2 | 1.4 ± 0.4 | 1.3 ± 0.4 | 0.779 | 0.472 |
| Neural Ti/TT (%) | 0.26 ± 0.05 | 0.28 ± 0.05 | 0.34 ± 0.07*,† | 7.255 | 0.025 |
| Ineffective triggering index (%) | 9.5 ± 4.0 | 6.7 ± 2.0* | 4.2 ± 2.1*,† | 6.718 | 0.006 |
| Inspiratory trigger delay (ms) | 231.8 ± 61.7 | 199.4 ± 62.8 | 144.7 ± 30.2 | 1.503 | 0.246 |
| Expiratory trigger delay (ms) | 189.5 ± 83.6 | 107.3 ± 60.2 | 102.1 ± 50.4 | 0.660 | 0.527 |
Data were shown as mean ± SD. *P<0.05 compared with Ramsay 4; †P<0.05 compared with Ramsay 3. VT: Tidal volume; EAdi: Electrical activity of the diaphragm; RR: Respiratory rate; NVE: Neuro-ventilatory efficiency; NME: Neuro-mechanical efficiency; PBW: Predicted body weight; P0.1: Airway occlusion pressure; Ti: Neural inspiratory time; TT: Neural total respiratory time; SD: Standard deviation.
Figure 1.
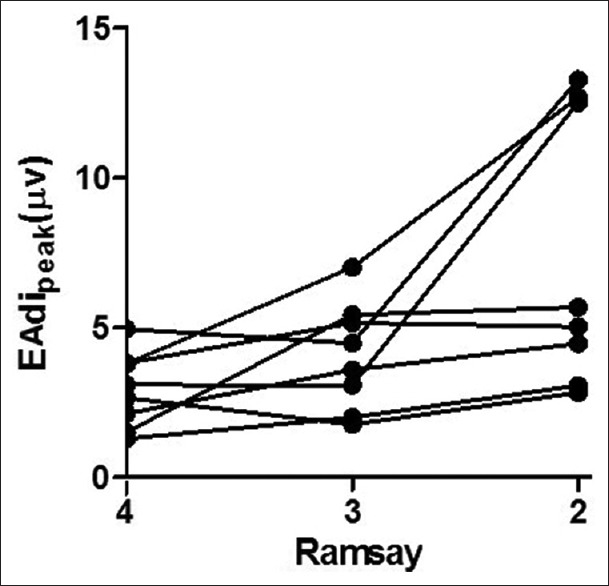
Individual change in peak electrical activity of the diaphragm at different depths of sedation.
The ineffective triggering index was 9.5 ± 4.0% in Ramsay 4, which was significantly higher than that for Ramsay 3 (6.7 ± 2.0%, P < 0.05) and 2 (4.2 ± 2.1%, P < 0.05). It is noteworthy that because auto triggering and double triggering were absent, the ineffective triggering index actually corresponded to the asynchrony index used in the previous studies.[18] In our postoperative patients, 3 patients at Ramsay 4 and 1 patient at Ramsay 3 had an ineffective triggering index greater than 10% [Figure 2]. The inspiratory trigger and expiratory trigger delay tend to increase with the increasing depth of sedation; however, this did not achieve statistical significance (P > 0.05) [Table 3].
Figure 2.
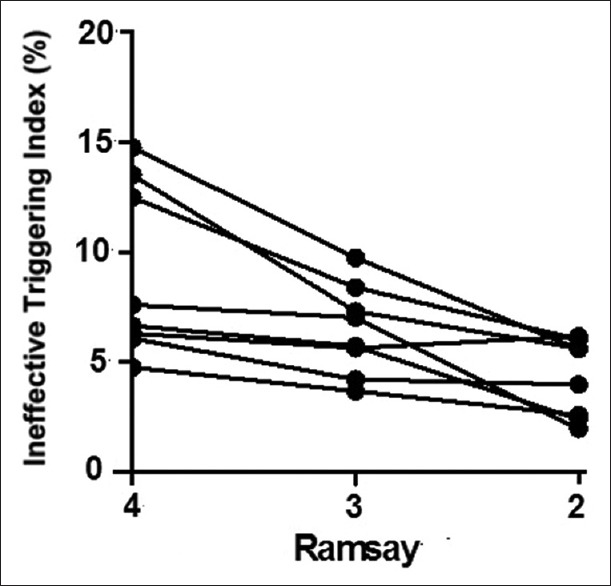
Individual change in ineffective triggering at different depths of sedation.
As depicted in Table 4, the pH, PaO2, PaCO2, heart rate, and mean arterial pressure were unaffected by the depth of sedation (P > 0.05).
Table 4.
Blood gas and hemodynamics at different depths of sedation
| Parameter | Ramsay 4 (n = 8) | Ramsay 3 (n = 8) | Ramsay 2 (n = 8) | F | P |
|---|---|---|---|---|---|
| pH | 7.42 ± 0.03 | 7.44 ± 0.02 | 7.45 ± 0.03 | 4.435 | 0.066 |
| PaCO2 (mmHg) | 35.1 ± 2.8 | 33.4 ± 2.2 | 33.4 ± 4.2 | 2.193 | 0.193 |
| PaO2 (mmHg) | 123.7 ± 35.0 | 130.9 ± 33.4 | 136.4 ± 38.4 | 2.375 | 0.174 |
| SaO2 (%) | 98.7 ± 1.3 | 99.0 ± 1.0 | 99.0 ± 1.5 | 0.719 | 0.525 |
| HR (breaths/min) | 84.6 ± 11.9 | 84.1 ± 12.6 | 84.9 ± 11.5 | 0.162 | 0.854 |
| MAP (mmHg) | 78.6 ± 11.7 | 78.5 ± 10.0 | 79.4 ± 9.4 | 1.068 | 0.935 |
Data were shown as mean ± SD. HR: Heart rate; MAP: Mean arterial pressure; SD: Standard deviation.
Discussion
This study showed that during PSV, propofol significantly inhibited respiratory drive and decreased patient-ventilator synchrony, especially at high doses, resulting in deep sedation. Propofol reduced diaphragmatic effort with increasing depth of sedation, while it did not significantly affect VT and gas exchange.
Sedatives influence the output of the respiratory centers by affecting either the respiratory drive or timing. In past studies, respiratory drive and timing have been inferred from the flow-time curve as the mean inspiratory flow (VT/Ti) and inspiratory duty cycle (Ti/TT) in spontaneously breathing healthy animals and human volunteers, respectively.[19,20] Through this approach, previous studies showed the different effects of various sedatives on the respiratory output in healthy controls.[21,22] However, in most ICU patients requiring mechanical ventilation, VT/Ti ceases to be a valid surrogate estimate of respiratory drive because of the alternative respiratory system impedance.[23] EAdi has been recently introduced in the clinical setting, and offers the unique possibility to directly assess respiratory drive and timing from the closest respiratory center signal and thus achieve better understanding of patient-ventilator interaction.[24]
The respiratory drive and patient's own (neural) timing were assessed through EAdi in the present study. We found that propofol had little and insignificant effects on neural inspiratory time, whereas it significantly decreased the respiratory drive. Consistent with the previous study,[25] the present study showed that EAdi decreased gradually from Ramsay 2 to 4. Notably, consequent to diverse mechanisms of action, other sedatives, particularly opioids,[26] may produce different effects on drive and timing. Administration of morphine at a dose of 1 mg/h in the present study might be a reason for the low absolute value of EAdi peak (only 2.9 ± 1.3 μV and 4.1 ± 1.8 μV at Ramsay 4 and 3, respectively) because of the synergistic effect between propofol and morphine.
We found that with increasing depth of sedation, ineffective triggering increased due to a decrease in respiratory drive. The previous study showed that deep sedation (Ramsay 6 and BIS 40) but not light sedation (Ramsay 3.9 ± 1.3) with propofol increased ineffective triggering when compared with wakefulness.[25] However, in the present study, ineffective triggering gradually increased with an increase in the depth of sedation from Ramsay 2 to 4 (which was considered as light sedation in the previous study). Different patient enrolment methods in the two studies (patients with respiratory failure and postoperative patients with healthy lungs) might be a reasonable explanation. Our data indicate that an increased depth of sedation with propofol mainly affected the diaphragmatic effort; however, the extent of other inspiratory muscles, except the diaphragm, was less affected. Unexpectedly, NVE, which is the VT/EAdi ratio, expresses the ability to generate volume normalized to neural drive, increased with increasing depths of sedation. Further analysis of the two factors related to NVE showed that EAdi decreased with increasing depth of sedation; however, VT remained unchanged. EAdi allows the quantification of the neural respiratory drive to the diaphragm.[27,28] Another useful parameter to indicate respiratory drive, P0.1, remained unchanged with increasing depth of sedation; however, it is a comprehensive parameter that reflects neural respiratory drive to all the inspiratory muscles. The most likely explanation of the decreased EAdi and consistent VT and P0.1 might be that in spite of decreasing diaphragmatic effort with higher propofol doses, other inspiratory muscle effort compensates for the decline in diaphragm effort. A previous study showed that sedation with midazolam reduced the electrical activity of diaphragm but increased the electrical activity of the intercostal and abdominal muscles.[29] These results suggested that during sedation, the role of diaphragm decreased in respiratory activity.
There are several limitations of this study. First, the number of patients enrolled is relatively small. However, based on the pilot study by de Wit et al.,[8] 8 patients might be sufficient to identify an ineffective triggering increase from wakefulness to deep sedation. Second, subjective assessment of the Ramsay score to monitor the depth of sedation is questionable because of the subjectivity and individual variation between physicians. In the present study, the same researcher performed the assessment, and the BIS was monitored to objectively quantify the depth of sedation. Third, esophageal pressure was not available in the present study, and auto-PEEP, which was one of the main reasons for ineffective triggering, cannot be measured in patients with PSV. Finally, since 7 of the 8 enrolled patients were cardiac surgery patients, it was arbitrary to extend our conclusion to all postoperative patients.
In conclusion, propofol affects respiratory drive, patient-ventilator synchrony, and diaphragm effort to the extent that varies with the depth of sedation. Propofol has less of an effect on breathing pattern and does not affect VT and gas exchange in postoperative patients during PSV.
Financial support and sponsorship
This study was supported by grants from Clinical Science and Technology Specific Projects of Jiangsu Province (No. BL2013030) and National Natural Science Foundation of China (No. 81670074).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
References
- 1.Younes M, Brochard L, Grasso S, Kun J, Mancebo J, Ranieri M, et al. A method for monitoring and improving patient: Ventilator interaction. Intensive Care Med. 2007;33:1337–46. doi: 10.1007/s00134-007-0681-4. doi: 10.1007/s00134-007-0681-4. [DOI] [PubMed] [Google Scholar]
- 2.Colombo D, Cammarota G, Bergamaschi V, De Lucia M, Corte FD, Navalesi P. Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med. 2008;34:2010–8. doi: 10.1007/s00134-008-1208-3. doi: 10.1007/s00134-008-1208-3. [DOI] [PubMed] [Google Scholar]
- 3.Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006;32:1515–22. doi: 10.1007/s00134-006-0301-8. doi: 10.1007/s00134-006-0301-8. [DOI] [PubMed] [Google Scholar]
- 4.Tobin MJ, Jubran A, Laghi F. Patient-ventilator interaction. Am J Respir Crit Care Med. 2001;163:1059–63. doi: 10.1164/ajrccm.163.5.2005125. doi: 10.1164/ajrccm.163.5.2005125. [DOI] [PubMed] [Google Scholar]
- 5.Appendini L, Purro A, Patessio A, Zanaboni S, Carone M, Spada E, et al. Partitioning of inspiratory muscle workload and pressure assistance in ventilator-dependent COPD patients. Am J Respir Crit Care Med. 1996;154:1301–9. doi: 10.1164/ajrccm.154.5.8912740. doi: 10.1164/ajrccm.154.5.8912740. [DOI] [PubMed] [Google Scholar]
- 6.Cavaliere F, Antonelli M, Arcangeli A, Conti G, Costa R, Pennisi MA, et al. A low-dose remifentanil infusion is well tolerated for sedation in mechanically ventilated, critically-ill patients. Can J Anaesth. 2002;49:1088–94. doi: 10.1007/BF03017909. doi: 10.1007/BF03017909. [DOI] [PubMed] [Google Scholar]
- 7.Conti G, Arcangeli A, Antonelli M, Conti G, Costa R, Pennisi MA, et al. Sedation with sufentanil in patients receiving pressure support ventilation has no effects on respiration: A pilot study. Can J Anaesth. 2004;51:494–9. doi: 10.1007/BF03018315. doi: 10.1007/BF03018315. [DOI] [PubMed] [Google Scholar]
- 8.de Wit M, Pedram S, Best AM, Epstein SK. Observational study of patient-ventilator asynchrony and relationship to sedation level. J Crit Care. 2009;24:74–80. doi: 10.1016/j.jcrc.2008.08.011. doi: 10.1016/j.jcrc.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the Intensive Care Unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. doi: 10.1097/CCM.0b013e3182a16ff0. [DOI] [PubMed] [Google Scholar]
- 10.Barr J, Egan TD, Sandoval NF, Zomorodi K, Cohane C, Gambus PL, et al. Propofol dosing regimens for ICU sedation based upon an integrated pharmacokinetic-pharmacodynamic model. Anesthesiology. 2001;95:324–33. doi: 10.1097/00000542-200108000-00011. doi.org/10.1097/00000542-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 11.McKeage K, Perry CM. Propofol: A review of its use in intensive care sedation of adults. CNS Drugs. 2003;17:235–72. doi: 10.2165/00023210-200317040-00003. doi. org/10.2165/00023210-200317040-00003. [DOI] [PubMed] [Google Scholar]
- 12.Anis AH, Wang XH, Leon H, Hall R Propofol Study Group. Economic evaluation of propofol for sedation of patients admitted to Intensive Care Units. Anesthesiology. 2002;96:196–201. doi: 10.1097/00000542-200201000-00034. doi. org/10.1097/00000542-200201000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Ostermann ME, Keenan SP, Seiferling RA, Sibbald WJ. Sedation in the Intensive Care Unit: A systematic review. JAMA. 2000;283:1451–9. doi: 10.1001/jama.283.11.1451. doi.org/10.1001/jama.283.11.1451. [DOI] [PubMed] [Google Scholar]
- 14.Hughes CG, McGrane S, Pandharipande PP. Sedation in the intensive care setting. Clin Pharmacol. 2012;4:53–63. doi: 10.2147/CPAA.S26582. doi: 10.2147/CPAA.S26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman NW, Black AM, Carter JA. Some ventilatory effects of propofol as sole anaesthetic agent. Br J Anaesth. 1987;59:1497–503. doi: 10.1093/bja/59.12.1497. doi.org/10.1093/bja/59.12.1497. [DOI] [PubMed] [Google Scholar]
- 16.Khamiees M, Amoateng-Adjepong Y, Manthous CA. Propofol infusion is associated with a higher rapid shallow breathing index in patients preparing to wean from mechanical ventilation. Respir Care. 2002;47:150–3. [PubMed] [Google Scholar]
- 17.Liu L, Liu H, Yang Y, Huang Y, Liu S, Beck J, et al. Neuroventilatory efficiency and extubation readiness in critically ill patients. Crit Care. 2012;16:R143. doi: 10.1186/cc11451. doi: 10.1186/cc11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cammarota G, Olivieri C, Costa R, Vaschetto R, Colombo D, Turucz E, et al. Noninvasive ventilation through a helmet in postextubation hypoxemic patients: Physiologic comparison between neurally adjusted ventilatory assist and pressure support ventilation. Intensive Care Med. 2011;37:1943–50. doi: 10.1007/s00134-011-2382-2. doi: 10.1007/s00134-011-2382-2. [DOI] [PubMed] [Google Scholar]
- 19.Grunstein MM, Derenne JP, Milic-Emili J. Control of depth and frequency of breathing during baroreceptor stimulation in cats. J Appl Physiol. 1975;39:395–404. doi: 10.1152/jappl.1975.39.3.395. [DOI] [PubMed] [Google Scholar]
- 20.Davis JN, Stagg D. Interrelationships of the volume and time components of individual breaths in resting man. J Physiol. 1975;245:481–98. doi: 10.1113/jphysiol.1975.sp010857. doi.org/10.1113/jphysiol.1975.sp010857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morel DR, Forster A, Bachmann M, Suter PM. Effect of intravenous midazolam on breathing pattern and chest wall mechanics in human. J Appl Physiol Respir Environ Exerc Physiol. 1984;57:1104–10. doi: 10.1152/jappl.1984.57.4.1104. [DOI] [PubMed] [Google Scholar]
- 22.Berggren L, Eriksson I, Mollenholt P. Changes in breathing pattern and chest wall mechanics after benzodiazepines in combination with meperidine. Acta Anaesthesiol Scand. 1987;31:381–6. doi: 10.1111/j.1399-6576.1987.tb02588.x. doi. org/10.1111/j.1399-6576.1987.tb02588.x. [DOI] [PubMed] [Google Scholar]
- 23.Milic-Emili J, Grunstein MM. Drive and timing components of ventilation. Chest. 1976;70(1 Suppl):131–3. doi: 10.1378/chest.70.1_supplement.131. doi.org/10.1378/chest.70.1_Supplement.131. [DOI] [PubMed] [Google Scholar]
- 24.Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, et al. Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999;5:1433–6. doi: 10.1038/71012. doi: 10.1038/71012. [DOI] [PubMed] [Google Scholar]
- 25.Vaschetto R, Cammarota G, Colombo D, Longhini F, Grossi F, Giovanniello A, et al. Effects of propofol on patient-ventilator synchrony and interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. 2014;42:74–82. doi: 10.1097/CCM.0b013e31829e53dc. doi: 10.1097/CCM.0b013e31829e53dc. [DOI] [PubMed] [Google Scholar]
- 26.Costa R, Cammarota G, Spinazzola G, Longhini F, Grossi F, Giovanniello A, et al. Effects of remifentanil on respiratory drive and timing during PSV and NAVA. Intensive Care Med. 2010;36:s346. [Google Scholar]
- 27.Beck J, Sinderby C, Lindström L, Grassino A. Crural diaphragm activation during dynamic contractions at various inspiratory flow rates. J Appl Physiol. 1998;85:451–8. doi: 10.1152/jappl.1998.85.2.451. [DOI] [PubMed] [Google Scholar]
- 28.Beck J, Gottfried SB, Navalesi P, Skrobik Y, Comtois N, Rossini M, et al. Electrical activity of the diaphragm during pressure support ventilation in acute respiratory failure. Am J Respir Crit Care Med. 2001;164:419–24. doi: 10.1164/ajrccm.164.3.2009018. doi: 10.1164/ajrccm.164.3.2009018. [DOI] [PubMed] [Google Scholar]
- 29.Fujii Y, Uemura A, Toyooka H. The dose-range effects of propofol on the contractility of fatigued diaphragm in dogs. Anesth Analg. 2001;93:1194–8. doi: 10.1097/00000539-200111000-00029. doi: 10.1213/ANE.0b013e31828ac45c. [DOI] [PubMed] [Google Scholar]


