Abstract
Background:
Little is known about the long-term outcomes of severe acute respiratory distress syndrome (ARDS) patients requiring extracorporeal membrane oxygenation (ECMO). This study aimed to investigate the 1-year outcomes of these patients or patients receiving mechanical ventilation (MV) and compare their health-related quality of life (HRQoL) to the general population.
Methods:
Severe ARDS survivors admitted to two ICUs in China between January 2012 and January 2014 were enrolled. Of the severe ARDS survivors enrolled, 1-year postdischarge, HRQoL assessment using the Short-Form 36 (SF-36) and EuroQol questionnaire dimensions, 6-min walking distance, chest computed tomography scan, pulmonary function, and arterial blood gas analysis were compared for ARDS patients with or without ECMO.
Results:
ARDS patients receiving ECMO had a significantly higher Acute Physiology and Chronic Health Evaluation II score (30.3 ± 6.7 vs. 26.5 ± 7.3, P = 0.036), lung injury score (3.3 ± 0.4 vs. 2.8 ± 0.5, P = 0.000), Sequential Organ Failure Assessment score (10.8 ± 3.5 vs. 7.9 ± 3.1, P = 0.000), lower PaO2/FiO2 ratio ([mmHg, 1 mmHg = 0.133 kPa], 68.3 ± 16.1 vs. 84.8 ± 16.5, P = 0.000), and increased extrapulmonary organ failure (2 [1, 3] vs. 1 [1, 1], P = 0.025) compared with patients not receiving ECMO. ECMO and non-ECMO survivors showed similar pulmonary function, morphological abnormalities, resting arterial blood gas values, and 6-min walking distance. Mild pulmonary dysfunction and abnormal morphology were observed in a few survivors. In addition, ECMO and non-ECMO survivors showed a similar quality of life. ECMO survivors showed lower SF-36 physical functioning and role-physical domain scores (minimum clinically significant difference at least 5 points), and non-ECMO survivors had similar outcome.
Conclusions:
One-year posthospital discharge, severe ARDS survivors receiving ECMO or MV demonstrated comparable outcomes. Compared with the general population, ARDS survivors showed reduced HRQoL. Pulmonary function and lung morphology revealed sufficient recovery with minor lung impairment.
Keywords: Acute Respiratory Distress Syndrome, Extracorporeal Membrane Oxygenation, Mechanical Ventilation, Outcome
Introduction
Acute respiratory distress syndrome (ARDS) is a condition of acute respiratory failure with various causes that is often treated by mechanical ventilation (MV) to provide respiratory support.[1] Promising results from a multicenter randomized controlled trial, and benefits described during the influenza-A (H1N1) pandemic, made extracorporeal membrane oxygenation (ECMO) a therapeutic option for severe ARDS patients who are unresponsive to conventional intensive care and ventilation.[2] Due to substantial costs, potential complications, and particularly poor recognition of long-term outcomes of survivors, this approach remains a challenge.[3] Previous studies have reported considerable health issues for ARDS survivors receiving MV, including reduced exercise capacity, cognitive dysfunction, significant depression, and posttraumatic stress disorder.[4,5,6,7] It is thought that ARDS patients requiring ECMO are at a higher risk compared to patients receiving MV, but whether these patients also have worse long-term outcomes remains to be elucidated.
The main goal of this study was to evaluate the 1-year outcomes of severe ARDS patients receiving ECMO treatment by comparing quality of life, 6-min walking distance, chest computed tomography (CT) scan, and pulmonary function with ARDS patients who received conventional MV. In addition, we compared the health-related quality of life (HRQoL) of ARDS survivors to that of the general population.
Methods
Ethical approval
This study was approved by the Ethics Committees of Tianjin Third Centre Hospital and all participants provided written informed consent prior to enrollment.
Patient selection
This study was performed at two 30-bed multidisciplinary adult Intensive Care Units (ICUs) of Tianjin Third Centre Hospital and Shandong Provincial Hospital in China. Patients were admitted between January 2012 and January 2014, and severe ARDS diagnosis was as per the Berlin ARDS definition.[8] Patients were included in the study if they survived hospital discharge and did not meet one of the exclusion criteria [Figure 1]. Exclusion criteria included chronic lung diseases, such as chronic obstructive pulmonary disease, bronchial asthma, and interstitial lung disease; heart disease with a cardiac function level of II–IV; history of cerebral accident sequelae, or severe neurological diseases; chronic kidney impairment; chronic liver failure; tumor; neuromuscular disease; or history of immune suppression.
Figure 1.
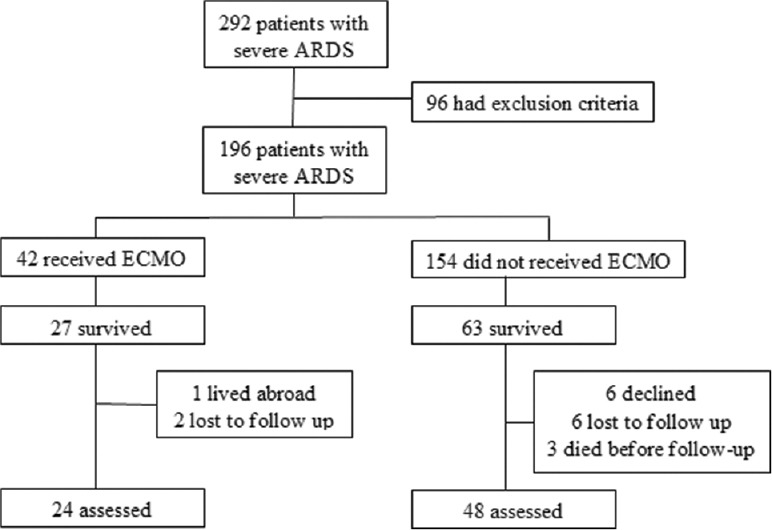
Flowchart of patients into the study. ARDS: Acute respiratory distress syndrome; ECMO: Extracorporeal membrane oxygenation.
Patient management
All patients received standard combined therapy that was based on the guidelines for the management of ARDS. In the participating ICUs, indications for ECMO were used as follows:[9] severe respiratory failure (PaO2/FiO2< 100 mmHg on FiO2 of 1, a Murray lung injury score [LIS] of 3 or higher, decompensated hypercapnia with a pH of <7.2) despite optimal conventional treatment, age <65 years, and less invasive MV time (<7 days) prior to ECMO. ECMO patients underwent cannulation through the jugular and femoral vein for veno-venous-ECMO. After initiation of ECMO support, ECMO patients were ventilated in pressure control mode with a peak inspiratory pressure of <20–25 cmH2O (1 cmH2O = 0.098 kPa), positive end expiratory pressure (PEEP) of 10–15 cmH2O, and FiO2 of 0.3–0.5. Non-ECMO patients were given a lung protective ventilation strategy.[10]
Data collection
From all patients, the following data were collected in the ICU: age, sex, height, weight, origin of ARDS, admission Acute Physiology and Chronic Health Evaluation II (APACHE II) score, mortality risk, Sequential Organ Failure Assessment (SOFA) score, Charlson comorbidity score, LIS, PaO2/FiO2 ratio, smoking history, number of organ failure, corticosteroids, muscle relaxants, sedatives, vasopressors, prone position ventilation, continuous renal replacement therapy (CRRT), tracheotomy, time on ECMO, ventilator days, length of ICU stay, hospitalization time and costs, condition at discharge, and location after discharge.
Follow-up protocol
ARDS survivors were telephoned and invited to participate in the follow-up, 1 year after discharge. Survivors were interviewed in the participating institutions. The follow-up evaluation included a pulmonary function test, chest CT scanning, 6-min walking test, arterial blood gas analysis, return to work, and evaluation of HRQoL as evaluated by the Short-Form 36 (SF-36) and the EuroQol-5 dimensions (EQ-5D) health survey questionnaire. The predicted 6-min walking distance was calculated as described by Enright and Sherrill.[11] For pulmonary function and the 6-min walking test, data were presented as a percentage of the predicted value. The obstructive ventilation dysfunction is defined as forced expiratory volume 1 s/forced vital capacity (FEV1/FVC) <70%, whereas the restrictive ventilation dysfunction is defined as the absence of airflow obstruction (FEV1/FVC ≥70%) with a reduced FVC <80% predicted. Arterial blood samples were measured for resting patients who were not assisted in breathing. The SF-36 health survey questionnaire is the most common evaluation of HRQoL and covers eight dimensions of physical and mental health, including physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health.[12] The SF-36 questionnaire was scored using a conversion score that was based on the scores for each dimension. A lower score indicated a poorer quality of life, a score of five points was considered the minimal clinically meaningful magnitude of decrement.[13] The Chinese normal values for SF-36 were adapted from Zhu et al.[14] The EQ-5D questionnaire measures five aspects of health: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The questionnaire consists of two parts: (1) the self-classifier and (2) the visual analog scale, a self-rated health status ranging from 0 (worst) to 100 (best health state). Each dimension covers three levels of severity: none, some/moderate, and severe/extreme.[15]
Chest CT scanning was performed from the lung apices to the bases. To obtain thin-section CT images with a thickness of 1.25 mm at 1.25 mm intervals, images were reconstructed. CT scans were reviewed by two radiologists who were blinded to the patients’ characteristics. Abnormal morphologic alterations were established by consensus. For each scan, observers assessed the presence, pattern, and distribution of the following abnormal criteria: groundglass opacity, consolidation, reticulation, and decreased attenuation as per standard morphologic descriptors based on the Fleischner Society Nomenclature Committee recommendations.[16]
Statistical analysis
Data were analyzed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). Data were analyzed for normal distribution and homoscedasticity using Kolmogorov–Smirnov test and Levene's test. Normally distributed data were expressed as the mean ± standard deviation and compared by Student's t-test. Data that did not follow a normal distribution were described using median and interquartile range and were compared using Mann–Whitney U-tests. Chi-square tests were used for categorical data and were expressed as numbers and percentages. Mann–Whitney U-test was adopted to evaluate the quality of life assessed using EQ-5D between ECMO and non-ECMO ARDS survivors. A P < 0.05 was considered statistically significant.
Results
During the study, 292 patients diagnosed with severe ARDS were admitted, of which 96 patients (33%) were excluded by the exclusion criteria. Of the 42 patients who received ECMO, 27 patients (64%) survived; one patient lived abroad, two patients declined to participate, and 24 of 27 patients (89%) participated to follow-up. Among the 154 non-ECMO patients, 63 patients (41%) survived to discharge and 60 of these patients were alive at follow-up (two died of severe sepsis, one died unexpectedly). Six patients could not be contacted, and another six patients declined to participate. Therefore, 48 of 60 patients (80%) were included in the follow-up study [Figure 1]. The mean follow-up time for ECMO and non-ECMO patients after discharge was 12.7 and 14.8 months, respectively.
Clinical characteristics at admission to Intensive Care Unit
Patients’ clinical characteristics at the time of ICU admission are shown in Table 1. ECMO patients had significantly higher mean APACHE II death risk, LIS and SOFA scores, lower PaO2/FiO2 ratios, and greater median number of extrapulmonary organ failure when compared to patients who did not receive ECMO treatment.
Table 1.
Characteristics of severe ARDS survivors at admission to the Intensive Care Unit according to ECMO status
| Parameters | ECMO survivors (n = 24) | Non-ECMO survivors (n = 48) | Statistics | P |
|---|---|---|---|---|
| Age (years) | 38.0 ± 15.1 | 44.3 ± 15.6 | −1.632* | 0.107 |
| Male, n (%) | 18 (75) | 33 (69) | 0.303† | 0.582 |
| BMI (kg/m2) | 26.5 ± 6.1 | 26.9 ± 6.2 | −0.259* | 0.796 |
| Origin of ARDS, n (%) | ||||
| Pneumonia | 18 (75) | 35 (73) | 0.036† | 0.850 |
| Trauma | 1 (4) | 3 (6) | 0.000† | 1.000 |
| Sepsis | 4 (17) | 6 (13) | 0.015† | 0.904 |
| Pancreatitis | 1 (4) | 4 (8) | 0.027† | 0.870 |
| APACHE II score | 30.3 ± 6.7 | 26.5 ± 7.3 | −2.138* | 0.036 |
| APACHE II death risk (%) | 78.2 ± 14.1 | 60.0 ± 8.6 | 6.789* | 0.000 |
| Lung injury score | 3.3 ± 0.4 | 2.8 ± 0.5 | 4.260* | 0.000 |
| SOFA score | 10.8 ± 3.5 | 7.9 ± 3.1 | 3.584* | 0.000 |
| PaO2/FiO2 (mmHg) | 68.3 ± 16.1 | 84.8 ± 16.5 | −4.032* | 0.000 |
| Smoker, n (%) | 12 (50) | 19 (40) | 0.708† | 0.400 |
| Charlson score | 0.00 (0.00, 0.75) | 0.00 (0.00, 0.75) | 0.000‡ | 1.000 |
| Number of extrapulmonary organs failure§ | 2 (1, 3) | 1 (1, 1) | −2.238‡ | 0.025 |
Data are expressed as n (%), median (interquartile range), or mean ± SD. *t-test; †Chi-square test; ‡MannWhitney U-test; §Renal dysfunction was defined as a serum creatinine concentration of >1.2 mg/dl, liver impairment as a serum bilirubin >1.2 mg/dl, and cardiovascular dysfunction when mean arterial pressure <75 mmHg in the absence of vasopressor support. 1 mmHg = 0.133 kPa. ECMO: Extracorporeal membrane oxygenation; BMI: Body mass index; ARDS: Acute respiratory distress syndrome; APACHE II: Acute Physiology and Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment; PaO2/FiO2: Arterial oxygen partial pressure/inspired oxygen fraction; SD: Standard deviation.
Treatments and outcomes
As shown in Table 2, ECMO and non-ECMO patients demonstrated a similar use of corticosteroids, muscle relaxants, sedatives, prone position ventilation, and tracheostomy. However, when compared to non-ECMO patients, more ECMO patients received CRRT and vasopressors prior to ECMO treatment (P < 0.05). After the start of ECMO treatment, ECMO patients showed no significant differences in PEEP. However, significantly lower levels of plateau pressure, vital volume, and compliance were found in ECMO patients when compared to non-ECMO patients (P < 0.05). In addition, compared with non-ECMO patients, ECMO patients demonstrated similar durations of MV, ICU stay, and hospital stay. Moreover, total hospital costs tended to be higher for ECMO patients compared to non-ECMO patients (P > 0.05). The proportion of ARDS survivors who returned to work was high when compared to non-ECMO patients. In addition, ECMO survivors returned to work earlier compared to non-ECMO survivors, however this difference was not statistically significant (P = 0.245).
Table 2.
Treatment characteristics and Intensive Care Unit, hospital and posthospital outcomes of severe ARDS survivors according to ECMO status
| Parameters | ECMO survivors (n = 24) | Non-ECMO survivors (n = 48) | Statistics | P |
|---|---|---|---|---|
| Treatment, n (%) | ||||
| Corticosteroids | 10 (42) | 29 (60) | 2.266† | 0.132 |
| Muscle relaxants | 16 (67) | 29 (60) | 0.267† | 0.606 |
| Sedatives | 24 (100) | 30 (100) | – | – |
| Vasopressors | 22 (92) | 26 (54) | 10.125† | 0.001 |
| Prone position ventilation | 14 (58) | 22 (46) | 1.000† | 0.317 |
| CRRT | 16 (67) | 15 (31) | 8.186† | 0.004 |
| Ventilation parameters* | ||||
| Plateau pressure (cmH2O) | 23 (20, 24) | 28 (26, 32) | −4.790‡ | 0.000 |
| Tidal volume (ml/kg) | 3.4 (3.1, 4.2) | 6.3 (5.8, 6.9) | −0.445‡ | 0.000 |
| PEEP (cmH2O) | 13 (12, 14) | 12 (10, 14) | −5.370‡ | 0.656 |
| Compliance (ml/cmH2O) | 21 (18, 24) | 24 (22, 26) | −2.349‡ | 0.019 |
| ICU and hospital outcomes | ||||
| ECMO duration (days) | 6.0 ± 2.3 | – | – | – |
| Tracheostomy, n (%) | 4 (17) | 10 (21) | 0.011† | 0.916 |
| MV duration (days) | 10.0 (6.0, 16.3) | 9.0 (6.0, 13.0) | 0.054‡ | 0.658 |
| ICU stay (days) | 13.0 (9.8, 22.3) | 11.0 (8.0, 18.0) | 0.449‡ | 0.430 |
| Hospital stay (days) | 25.5 (16.5, 31.3) | 26.0 (15.0, 56.3) | −0.488‡ | 0.711 |
| Bedridden at hospital discharge, n (%) | 2 (8) | 7 (15) | 0.143† | 0.705 |
| Hospital costs (RMB) | 230,727.0 ± 1.1 | 202,095.7 ± 1.9 | 0.682§ | 0.497 |
| Posthospital outcomes | ||||
| Discharge destination, n (%) | ||||
| Home | 22 (92) | 43 (90) | 0.000† | 1.000 |
| Other hospital | 2 (8) | 5 (10) | 0.000† | 1.000 |
| Returned to work, n (%) | 16 (67) | 24 (50) | 1.800† | 0.180 |
| Time from hospital discharge to return to work (months) | 6.0 ± 3.4 | 6.9 ± 2.9 | −1.171§ | 0.245 |
| Time from hospital discharge to follow-up evaluation (months) | 12.7 ± 5.8 | 14.8 ± 6.5 | −1.338§ | 0.185 |
Data are expressed as n (%), median (interquartile range), or mean ± SD. *Ventilation parameters are shown after ECMO in ECMO group; †Chi-square test; ‡MannWhitney U-test; §t-test. 1 cmH2O = 0.098 kPa. ECMO: Extracorporeal membrane oxygenation; CRRT: Continuous renal replacement therapy; MV: Mechanical ventilation; SD: Standard deviation; ICU: Intensive Care Unit; PEEP: Positive end-expiratory pressure; ARDS: Acute respiratory distress syndrome; –: No data available.
Pulmonary function, arterial blood gas analysis, and 6-min walking distance 1-year posthospital discharge
At follow-up, ECMO survivors demonstrated normal resting arterial blood gas values, and pulmonary function tests were not significantly different between ECMO and non-ECMO survivors. For patients in both groups, spirometry tests were in the lower normal range, and carbon monoxide diffusion capacity was low and similar [Table 3]. None of the ECMO patients and two non-ECMO survivors had obstructive ventilation dysfunction. Restrictive ventilation dysfunction was observed in 5 of 24 ECMO patients (21%) and 8 of 48 non-ECMO patients (17%, P = 0.912). Ten out of 24 ECMO patients (42%) and 24 out of 48 non-ECMO patients (50%) had a diffusion barrier (P = 0.504). The 6-min walking distance was close to 80% of the predicted value, with no statistically significant difference for ECMO treatment.
Table 3.
Pulmonary function test, arterial blood gas analysis, and 6-min walk distance of severe ARDS survivors according to ECMO status, 1 year after hospital discharge
| Parameters | ECMO survivors (n = 24) | Non-ECMO survivors (n = 48) | t values | P |
|---|---|---|---|---|
| Pulmonary function test (% predicted) | ||||
| FEV1 | 85.6 ± 8.6 | 83.0 ± 19.1 | 0.634 | 0.528 |
| FVC | 82.4 ± 8.9 | 80.5 ± 18.0 | 0.487 | 0.628 |
| FEV1/FVC | 108.6 ± 9.4 | 104.8 ± 13.1 | 1.266 | 0.210 |
| PEFR | 79.2 ± 17.7 | 82.2 ± 24.7 | −0.530 | 0.598 |
| MVV | 88.5 ± 16.7 | 79.1 ± 21.3 | 1.889 | 0.063 |
| DLco | 72.3 ± 6.3 | 72.4 ± 6.0 | −0.066 | 0.948 |
| Arterial blood-gas values | ||||
| pH | 7.42 ± 0.04 | 7.41 ± 0.04 | 1.000 | 0.321 |
| PaO2 (mmHg) | 99.6 ± 13.4 | 97.4 ± 14.3 | 0.628 | 0.532 |
| PaCO2 (mmHg) | 39.8 ± 4.3 | 39.6 ± 5.5 | 0.156 | 0.877 |
| P(A-a)O2 (mmHg) | 10.8 ± 8.2 | 12.7 ± 7.6 | −0.974 | 0.333 |
| Distance walked in 6 min (m) | 530.6 ± 83.6 | 495.0 ± 90.1 | 1.618 | 0.110 |
| % predicted | 81.3 ± 12.9 | 78.3 ± 13.2 | 0.916 | 0.363 |
Data are expressed as mean ± SD. 1 mmHg = 0.133 kPa. ECMO: Extracorporeal membrane oxygenation; FEV1: Forced expiratory volume in 1 s; FVC: Forced vital capacity; PEFR: Peak expiratory flow rate; MVV: Maximal minute ventilation; DLco: Carbon monoxide diffusion capacity; P(A-a)O2: Alveolar arterial oxygen partial pressure difference; ARDS: Acute respiratory distress syndrome; SD: Standard deviation.
Lung morphology by chest computed tomography scan
In 10 out of 24 ECMO patients (42%) and 22 out of 48 non-ECMO patients (46%), abnormal morphological alterations were observed. The most common alterations were groundglass opacity and reticulation, which were found in 4/24 ECMO patients (17%) and in 9/48 non-ECMO patients (19%), followed by decreased attenuation and consolidation. The abnormal changes were mild and minor, with no significant differences in ventro-dorsal distribution of CT changes.
Quality of life 1 year after hospital discharge
SF-36 scores are depicted in Table 4. ECMO and non-ECMO survivors demonstrated a similar score for SF-36 domains. Given the average age of survivors in the study, a general control population was chosen that was aged between 35 and 44 and published by Yan-Bo et al.[14] When compared to the general population, ECMO and non-ECMO survivors showed decreased SF-36 scores for physical functioning and role-physical domain. As shown in Table 5, no statistically significant differences were found in EQ-5D scores between ECMO and non-ECMO survivors. However, several survivors complained about issues with mobility and usual activities. There were 13% of ECMO survivors and 15% of non-ECMO survivors who reported fatigue and decreased endurance. In addition, 42% of ECMO and 27% of non-ECMO survivors reported symptoms of anxiety or depression.
Table 4.
Comparison of SF-36 scores between severe ARDS survivors who received ECMO, survivors who did not receive ECMO, and normative values for the general Chinese population
| Parameters | General population | ECMO survivors (n = 24) | Non-ECMO survivors (n = 48) | t values* | P* |
|---|---|---|---|---|---|
| Physical function | 91.5 | 81.0 ± 16.6 | 80.6 ± 19.6 | 0.086 | 0.932 |
| Physical role | 86.7 | 58.5 ± 44.7 | 50.0 ± 39.5 | 0.824 | 0.413 |
| Bodily pain | 82.1 | 83.4 ± 16.4 | 77.1 ± 30.3 | 0.949 | 0.346 |
| General health | 67.4 | 64.9 ± 21.0 | 64.3 ± 28.6 | 0.091 | 0.928 |
| Vitality | 71.7 | 78.0 ± 16.2 | 79.1 ± 22.7 | −0.212 | 0.833 |
| Social functioning | 85.8 | 81.3 ± 25.2 | 82.4 ± 22.6 | −0.187 | 0.852 |
| Emotional role | 81.8 | 80.0 ± 42.2 | 74.5 ± 40.0 | 0.540 | 0.591 |
| Mental health | 76.0 | 77.1 ± 20.8 | 78.1 ± 18.9 | −0.205 | 0.838 |
| Physical component score | 81.9 | 72.0 ± 20.6 | 68.0 ± 22.6 | 0.729 | 0.469 |
| Mental component score | 78.8 | 79.3 ± 15.7 | 78.5 ± 22.5 | 0.156 | 0.877 |
Data are expressed as mean ± SD. *: ECMO survivors vs. Non-ECMO survivors. ECMO: Extracorporeal membrane oxygenation; SF-36: Short-Form 36 questionnaire; ARDS: Acute respiratory distress syndrome; SD: Standard deviation.
Table 5.
Quality of life assessed using EQ-5D of severe ARDS survivors who received ECMO or did not receive ECMO
| Parameters | ECMO survivors (n = 24) | Non-ECMO survivors (n = 48) | Statistics | P |
|---|---|---|---|---|
| Problems with mobility, n (%) | ||||
| None | 21 (86) | 41 (85) | −0.239* | 0.811 |
| Some | 3 (13) | 7 (15) | ||
| Confined to bed | 0 | 0 | ||
| Problems with self-care, n (%) | ||||
| None | 24 (100) | 43 (90) | −1.628* | 0.104 |
| Some problems washing or dressing | 0 | 5 (11) | ||
| Unable to wash or dress | 0 | 0 | ||
| Problems with usual activities, n (%) | ||||
| None | 21 (88) | 43 (90) | −0.263* | 0.792 |
| Some | 3 (13) | 5 (11) | ||
| Unable | 0 | 0 | ||
| Pain or discomfort, n (%) | ||||
| None | 17 (71) | 38 (79) | −0.721* | 0.471 |
| Moderate | 7 (29) | 9 (19) | ||
| Extreme | 0 | 1 (2) | ||
| Anxiety or depression, n (%) | ||||
| None | 14 (58) | 35 (73) | −1.256* | 0.209 |
| Moderate | 5 (21) | 7 (15) | ||
| Extreme | 5 (21) | 6 (13) | ||
| Overall health status (VAS, 0–100) | 81.5 ± 12.0 | 79.1 ± 15.0 | 0.682† | 0.673 |
Data are expressed as n (%), or mean ± SD. *MannWhitney U-test; †t-test. ECMO: Extracorporeal membrane oxygenation; VAS: Visual analogue scale; ARDS: Acute respiratory distress syndrome; SD: Standard deviation; EQ-5D: EuroQol-5 dimensions.
Discussion
This prospective study, including patients with severe ARDS, demonstrated that although ECMO patients presented with more severe ARDS, patients receiving ECMO or treated with conventional MV showed comparable outcomes 1 year posthospital discharge. This included HRQoL, pulmonary function, abnormal morphologic alterations, arterial blood gas analysis, and 6-min walking distance. In both groups, and compared to the general Chinese population, physical HRQoL was significantly worse for severe ARDS survivors. Pulmonary function tests revealed sufficient recovery; however, some patients showed residual lung sequelae with impaired pulmonary ventilation and diffusion. In addition, in several patients, minor morphologic abnormalities were observed, which were limited on CT scans. At follow-up, some patients demonstrated physical limitations and reported symptoms of anxiety or depression.
In recent years, it has been shown that advances in respiratory support, including ECMO, decreased ARDS-related mortality.[17] However, survivors of ARDS, who received conventional MV, may experience issues for up to 5 years after ICU discharge, which include muscle wasting and weakness, abnormal pulmonary function, psychological and cognitive impairments, and decreased quality of life.[4,18] Compared with conventional MV, ECMO treatment reduced the risk of ventilator-induced lung injury, indicating its promising approach for improving the long-term outcomes of ARDS survivors. However, in patients requiring ECMO, ARDS may be more severe, and long-term dysfunction may be more challenging to treat. Currently, little information is available that focuses on the differences in the long-term outcome between ECMO and non-ECMO ARDS survivors. In this study, we attempted to highlight the differences in long-term outcomes between ARDS patients treated with or without ECMO.
Respectable outcomes combined with persistent pulmonary dysfunction and lower quality of life were reported for 21 long-term survivors of severe ARDS, who received ECMO.[19] In a study by Peek et al.,[20] no significant differences in HRQoL were observed in patients with severe ARDS receiving ECMO or conventional MV at 6 months after treatment. In a relatively small study of severe H1N1-associated ARDS survivors in France, it was demonstrated that, for patients with or without ECMO, HRQoL was similar.[21] Likewise, at 1 year postdischarge, no significant differences were found in SF-36 scores between ECMO and non-ECMO survivors in this study. ARDS survivors showed a decent quality of life and more than 50% of survivors returned to work. Compared with the general population, for both ECMO and non-ECMO survivors, the SF-36 physical component scores were significantly lower. In addition, SF-36 mental component scores were comparable, which was consistent with previous findings of ECMO[21,22,23] and non-ECMO survivors.[4,5,24,25,26]
ARDS survivors exhibit varying degrees of pulmonary damage, including restrictive or obstructive ventilation dysfunction and decreased diffusion capacity.[5,26,27,28] Luyt et al.[21] demonstrated that pulmonary function test results were similar and near normal in patients with and without extracorporeal lung assistance. In a previous study, it was found that, in ARDS survivors who received ECMO, pulmonary ventilation function was roughly normal; however, 65% of patients demonstrated decreased diffusion capacity.[19] In this study, we also found that, in most ECMO and non-ECMO survivors, pulmonary function recovery was comparable and relatively complete, however 20% of ARDS survivors showed pulmonary ventilation lesions and 50% of survivors had diffusion barrier. Compared to previous studies, we reported significantly less lung morphological abnormalities on CT scans.[28,29] This difference may be due to MV strategy and differences related to follow-up.
In our study, we found that, in patients who were treated with ECMO, illness was more severe; however, long-term outcome of patients receiving ECMO and patients treated with conventional MV was similar. One possible explanation for this difference may be the difference in respiratory support means. Thus, the ventilatory strategies used in patients receiving ECMO may offer better protection against lung injury resulting in improved long-term outcomes.
In this study, near-complete recovery of respiratory function was observed, however 14% of the survivors reported varying degrees of weakness and fatigue. In another study, weakness and substantial impairments in physical function and HRQoL were reported at 24 months.[30] Weakness and fatigue may be related to ICU-acquired weakness (ICUAW), which occurs in up to 60% of patients in the ICU.[31] Currently, early mobilization/rehabilitation (including passive movement) in both general ICU patients and ECMO patients is considered the best treatment regimen to counteract ICUAW.[32] Early mobilization is associated with improved rates of returning to independent functioning, shorter durations of MV, and reduced length of ICU and hospital stay.[33] Despite this improvement, many survivors reported symptoms of anxiety and depression. However, assessment of survivor's mental status using a standard scale was beyond the scope of the current study.
This study has several limitations. First, the sample size was relatively small. Therefore, a larger sample size may have revealed additional significant differences between ECMO and non-ECMO survivors. Second, the ECMO support in ECMO patients was relatively short. Further studies would be needed to evaluate the long-term outcomes of patients receiving long-term ECMO support. A previous report indicated that, in patients who received ECMO support for over 3 weeks, long-term outcomes were favorable;[34] however, we were limited in test aspect. Third, our patients were healthy individuals without a diagnosis of serious underlying diseases; therefore, the results cannot be generalized to all ARDS patients.
In conclusion, although symptoms in patients receiving ECMO were more severe compared to patients receiving conventional MV, their quality of life, pulmonary function, activity ability, and mental health were comparable at 1 year posthospital discharge. Compared to the general Chinese population, ARDS survivors demonstrated a reduction in HRQoL, particularly in SF-36 domains of physical functioning and the role-physical domain. Pulmonary function and morphological abnormalities could still be detected; however, the impairments found were mild. Future studies should focus on large-scale and long-term follow-up to evaluate the long-term outcomes of severe ARDS patients. The establishment of outpatient services and the development of specific guidelines for follow-up will help in achieving this goal.
Financial support and sponsorship
This study was supported by a grant from Tianjin Municipal Bureau of Health (No. 12KG106).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
References
- 1.Cardenas VJ, Lynch JE. Mechanical ventilation and acute respiratory distress syndrome. Semin Thorac Cardiovasc Surg. 2006;18:8–12. doi: 10.1053/j.semtcvs.2006.01.002. doi: 10.1053/j.semtcvs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Rozencwajg S, Pilcher D, Combes A, Schmidt M. Outcomes and survival prediction models for severe adult acute respiratory distress syndrome treated with extracorporeal membrane oxygenation. Crit Care. 2016;20:392. doi: 10.1186/s13054-016-1568-y. doi: 10.1186/s13054-016-1568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang L, Li T, Xu L, Hu XM, Duan DW, Li ZB, et al. Performance of multiple risk assessment tools to predict mortality for adult respiratory distress syndrome with extracorporeal membrane oxygenation therapy: An external validation study based on chinese single-center data. Chin Med J. 2016;129:1688–95. doi: 10.4103/0366-6999.185871. doi: 10.4103/0366-6999.185871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–93. doi: 10.1056/NEJMoa022450. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 5.Orme J, Jr, Romney JS, Hopkins RO, Pope D, Chan KJ, Thomsen G, et al. Pulmonary function and health-related quality of life in survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2003;167:690–4. doi: 10.1164/rccm.200206-542OC. doi: 10.1164/rccm.200206-542OC. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–7. doi: 10.1164/rccm.200406-763OC. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 7.Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matté A, Barr A, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:538–44. doi: 10.1164/rccm.200505-693OC. doi: 10.1164/rccm.200505-693OC. [DOI] [PubMed] [Google Scholar]
- 8.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 9.Schuerer DJ, Kolovos NS, Boyd KV, Coopersmith CM. Extracorporeal membrane oxygenation: Current clinical practice, coding, and reimbursement. Chest. 2008;134:179–84. doi: 10.1378/chest.07-2512. doi: 10.1378/chest.07-2512. [DOI] [PubMed] [Google Scholar]
- 10.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 11.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384–7. doi: 10.1164/ajrccm.158.5.9710086. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 12.Chiumello D, Taccone P, Berto V, Marino A, Migliara G, Lazzerini M, et al. Long-term outcomes in survivors of acute respiratory distress syndrome ventilated in supine or prone position. Intensive Care Med. 2012;38:221–9. doi: 10.1007/s00134-011-2445-4. doi: 10.1007/s00134-011-2445-4. [DOI] [PubMed] [Google Scholar]
- 13.Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, et al. Quality of life after acute respiratory distress syndrome: A meta-analysis. Intensive Care Med. 2006;32:1115–24. doi: 10.1007/s00134-006-0217-3. doi: 10.1007/s00134-006-0217-3. [DOI] [PubMed] [Google Scholar]
- 14.Zhu YB, Wang Q, Chen KF, Luo XX, Tang F. Predictors of health-related quality of life in the general population (in Chinese) Chin J Behav Med Brain Sci. 2009;18:254–9. doi: 10.3760/cma.j.issn.1674-6554.2009.03.026. [Google Scholar]
- 15.EuroQol Group. EuroQol – A new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 16.Xing ZH, Sun X, Xu L, Wu Q, Li L, Wu XJ, et al. Thin-section computed tomography detects long-term pulmonary sequelae 3 years after novel influenza A virus-associated pneumonia. Chin Med J. 2015;128:902–8. doi: 10.4103/0366-6999.154285. doi: 10.4103/0366-6999.154285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blank R, Napolitano LM. Epidemiology of ARDS and ALI. Crit Care Clin. 2011;27:439–58. doi: 10.1016/j.ccc.2011.05.005. doi: 10.1016/j.ccc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304. doi: 10.1056/NEJMoa1011802. doi: 10.1056/NEJMe1101057. [DOI] [PubMed] [Google Scholar]
- 19.Lindén VB, Lidegran MK, Frisén G, Dahlgren P, Frenckner BP, Larsen F. ECMO in ARDS: A long-term follow-up study regarding pulmonary morphology and function and health-related quality of life. Acta Anaesthesiol Scand. 2009;53:489–95. doi: 10.1111/j.1399-6576.2008.01808.x. doi: 10.1111/j.1399-6576.2008.01808.x. [DOI] [PubMed] [Google Scholar]
- 20.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–63. doi: 10.1016/S0140-6736(09)61069-2. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 21.Luyt CE, Combes A, Becquemin MH, Beigelman-Aubry C, Hatem S, Brun AL, et al. Long-term outcomes of pandemic 2009 influenza A(H1N1)-associated severe ARDS. Chest. 2012;142:583–92. doi: 10.1378/chest.11-2196. doi: 10.1378/chest.11-2196. [DOI] [PubMed] [Google Scholar]
- 22.Hodgson CL, Hayes K, Everard T, Nichol A, Davies AR, Bailey MJ, et al. Long-term quality of life in patients with acute respiratory distress syndrome requiring extracorporeal membrane oxygenation for refractory hypoxaemia. Crit Care. 2012;16:R202. doi: 10.1186/cc11811. doi: 10.1186/cc11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt M, Zogheib E, Rozé H, Repesse X, Lebreton G, Luyt CE, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39:1704–13. doi: 10.1007/s00134-013-3037-2. doi: 10.1007/s00134-013-3037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999;281:354–60. doi: 10.1001/jama.281.4.354. doi: 10.1001/jama.281.4.354. [DOI] [PubMed] [Google Scholar]
- 25.Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1389–94. doi: 10.1164/ajrccm.163.6.2005123. doi: 10.1164/ajrccm.163.6.2005123. [DOI] [PubMed] [Google Scholar]
- 26.Heyland DK, Groll D, Caeser M. Survivors of acute respiratory distress syndrome: Relationship between pulmonary dysfunction and long-term health-related quality of life. Crit Care Med. 2005;33:1549–56. doi: 10.1097/01.ccm.0000168609.98847.50. doi: 10.1097/01.CCM.0000168609.98847.50. [DOI] [PubMed] [Google Scholar]
- 27.Neff TA, Stocker R, Frey HR, Stein S, Russi EW. Long-term assessment of lung function in survivors of severe ARDS. Chest. 2003;123:845–53. doi: 10.1378/chest.123.3.845. doi: 10.1378/chest.123.3.845. [DOI] [PubMed] [Google Scholar]
- 28.Masclans JR, Roca O, Muñoz X, Pallisa E, Torres F, Rello J, et al. Quality of life, pulmonary function, and tomographic scan abnormalities after ARDS. Chest. 2011;139:1340–6. doi: 10.1378/chest.10-2438. doi: 10.1378/chest.10-2438. [DOI] [PubMed] [Google Scholar]
- 29.Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology. 1999;210:29–35. doi: 10.1148/radiology.210.1.r99ja2629. doi: 10.1148/radiology.210.1.r99ja2629. [DOI] [PubMed] [Google Scholar]
- 30.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, et al. Physical complications in acute lung injury survivors: A two-year longitudinal prospective study. Crit Care Med. 2014;42:849–59. doi: 10.1097/CCM.0000000000000040. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermans G, Van Mechelen H, Clerckx B, Vanhullebusch T, Mesotten D, Wilmer A, et al. Acute outcomes and 1-year mortality of Intensive Care Unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190:410–20. doi: 10.1164/rccm.201312-2257OC. doi: 10.1164/rccm.201312-2257OC. [DOI] [PubMed] [Google Scholar]
- 32.Abrams D, Javidfar J, Farrand E, Mongero LB, Agerstrand CL, Ryan P, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: A retrospective cohort study. Crit Care. 2014;18:R38. doi: 10.1186/cc13746. doi: 10.1186/cc13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet. 2009;373:1874–82. doi: 10.1016/S0140-6736(09)60658-9. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kon ZN, Dahi S, Evans CF, Byrnes KA, Bittle GJ, Wehman B, et al. Long-term venovenous extracorporeal membrane oxygenation support for acute respiratory distress syndrome. Ann Thorac Surg. 2015;100:2059–63. doi: 10.1016/j.athoracsur.2015.05.088. doi: 10.1016/j.athoracsur.2015.05.088. [DOI] [PubMed] [Google Scholar]


