Abstract
Background:
The normal range of red cell distribution width (RDW) level is <15%. Several studies have indicated that a high RDW level was associated with mortality in critically ill patients, and the patients with a high RDW level need increased focus in clinical practice. In view of the difficulty in defining the specific value of high RDW level, the key is to focus on the patient with the level beyond the normal upper limit. This study aimed to determine whether dynamic change of RDW levels, rather than the level itself, is predictive of death in elderly patients with septic shock when RDW level is beyond 15%.
Methods:
Between September 2013 and September 2015, the elderly septic shock patients with RDW level beyond 15% were enrolled in this study. The RDW levels were measured at enrollment (day 1), and days 4 and 7 after enrollment. Sequential Organ Failure Assessment (SOFA) scores were recorded simultaneously.
Results:
A total of 45 patients, including 32 males and 13 females, were included in the final analysis. Based on their hospital outcomes, these patients were divided into the survivor group (n = 26) and the nonsurvivor group (n = 19). There were no significant differences in age, gender, body mass index, initial level of RDW, Acute Physiology and Chronic Health Evaluation II scores, and SOFA scores between survivors and nonsurvivors. At days 4 and 7 measurement, both RDW level (median [interquartile range]: day 4: 15.8 [2.0]% vs. 16.7 [2.0]%, P = 0.011; and day 7: 15.6 [1.8]% vs. 17.7 [2.5]%, P = 0.001) and SOFA scores (day 4: 7.0 [4.0] vs. 16.0 [5.0], P < 0.001, day 7: 5.5 [4.0] vs. 17.0 [5.0], P < 0.001) were significantly lower in survivors than those in nonsurvivors. Dynamic changes of RDW and SOFA scores in survivor group were significantly different from those in nonsurvivor group (all P < 0.05). Continuous increase in RDW level was observed in 10 of the 13 nonsurvivors, but only in 3 of the 26 survivors. The level of RDW7 and dynamic changes significantly correlated with their counterparts of SOFA scores (all P < 0.05), whereas the levels of RDW1 and RDW4 had no significant correlation with their counterparts of SOFA scores (all P > 0.05).
Conclusions:
Continuous increase in RDW level, rather than the level of RDW itself, was more useful in predicting hospital death in elderly patients with septic shock when the level of RDW was >15%. The dynamic changes of RDW were highly correlated with the SOFA score in the patients.
Keywords: Prognosis, Red Cell Distribution Width, Septic Shock, Sequential Organ Failure Assessment Score
Introduction
Red blood cell distribution width (RDW) is a measure of red blood cell size heterogeneity. RDW has been widely accepted as a useful biomarker associated with mortality in patients with various cardiovascular and cerebrovascular diseases, such as heart failure,[1] acute pulmonary embolism,[2] acute coronary syndrome,[3,4] stroke,[5,6] and peripheral artery disease.[7,8] Inflammation and oxidative state play important roles in the pathophysiologic mechanisms underlying the association between RDW and mortality.[9,10] Furthermore, several studies have indicated that a high RDW level was associated with mortality in patients with community-acquired pneumonia,[11,12] severe sepsis or septic shock,[13,14,15] and even in unselected critically ill patients.[16] The studies also suggested that high RDW level had a very close relationship with poor prognosis, indicating that patients with high RDW level need increased focus in clinical practice. However, it is difficult to define the accurate value of high RDW, the key is to focus on the patient with the level beyond the upper limit of normal value. When the value is beyond 15%, it is considered as abnormality. We should pay more attention to these patients.
RDW value is influenced by a lot of interindividual variances. In fact, it is invalid using RDW value to predict the discrimination between survivors and nonsurvivors. Ku et al.[17] pointed out that the dynamic change of RDW levels was more likely to be predictive of outcome in a patient with Gram-negative bacteremia. It demonstrated that the significant increase of RDW levels over a 3-day period was associated with hospital mortality. In addition, Kim et al.[15] have found that a combination of baseline RDW value and an increase in RDW can be a promising independent prognostic marker in patients with severe sepsis or septic shock. Lorente et al.'s research[14] indicated that nonsurviving septic patients showed persistently higher RDW during the 1st week. Therefore, the clinical observation of dynamic RDW seems more valuable.
However, it is unclear whether the dynamic change of RDW level is of prognostic value in septic shock patients with extremely high RDW levels such as beyond 15%. Furthermore, as the population is aging in China, more and more septic shock patients are old people. Sequential Organ Failure Assessment (SOFA) score, a simple but effective index, has been well established to describe the severity of critical ill patients.[18,19] It is unclear whether a relationship exists between RDW and SOFA in septic shock patients. Therefore, in this observational study, we aimed to investigate whether dynamic change of RDW level and the level itself over a 6-day management still have a relationship with patients’ outcome when RDW level is beyond 15% in Chinese elderly septic shock patients. We also aimed to further evaluate the correlation between the dynamic change of RDW level and the dynamic change of SOFA score when RDW level is >15%.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by Medical Ethics Review Board of Shanghai Jiading District Center Hospital (No. 2013-QN-02). Since this was an observational study and all the observed laboratory indices were commonly measured for all patients in Intensive Care Unit (ICU), the need for written informed consent was waived by the Medical Ethics Review Board.
Patients and ethical approval
From September 2013 to September 2015, a total of 332 patients received RDW measurements in ICU of Shanghai Jiading District Central Hospital. RDW measurements were performed for the suspicion of sepsis, severe sepsis, and septic shock on daily rounds.
Patients who met the following criteria were enrolled in this study: (1) age of patients ≥65 years; (2) patients those who met the consensus criteria of septic shock;[20] and (3) the RDW level >15% (normal range <15%) no matter whether the RDW measurement was performed at ICU admission or during ICU stay. Patients who met the following criteria were excluded from the study: (1) patients had prior anemia, chronic heart failure, neoplasm, history of renal diseases or thyroid diseases; and (2) patients with autoimmune disease.
All patients received empirical and culture-guided antibiotic treatment and hemodynamic support therapy. All the patients were managed by their responsible physicians. Mortality was defined as all-cause mortality.
Blood samples’ measurement
Serum C-reactive protein and whole blood cell tests were measured using the Blood cell analyzer (ABX Pentra 60, HORIBA ABX SAS, France). Procalcitonin concentration was measured using the Elecsys electrochemiluminescence assay (Cobas e411 Analyzer; Roche Diagnostics; Mannheim, Germany). Serum creatinine, albumin, sodium, potassium, cholesterol, and total bilirubin levels were measured using the automatic biochemical analyzer (Roche cobas® 8000 modular analyzer series c 702, Germany).
The RDW levels were measured at enrollment (day 1), and days 4 and 7 after enrollment. The normal range of RDW level was <15%. Dynamic change of RDW was shown as dRDW and was expressed as dRDW14, dRDW47, or dRDW17 at the time node.
Sequential Organ Failure Assessment score and Acute Physiology and Chronic Health Evaluation II score
The SOFA scores were recorded simultaneously with the RDW measurement. Dynamic change of SOFA score was expressed as dSOFA. They were defined as the differences between the values of subsequent and initial measurements and were expressed as dSOFA14, dSOFA47, and dSOFA17 at the time node. Acute Physiology and Chronic Health Evaluation II Score (APACHE II score) has been widely used in clinical practice. It has an important clinical value for predicting the prognosis of the critically ill patients.[21,22]
Statistical analysis
All statistical analyses were performed using IBM SPSS 19.0 (IBM, Armonk, New York, USA). Continuous variables are presented as mean ± standard deviation (SD) or median (interquartile range), and categorical variables are expressed as percentages. Baseline characteristics between the two groups were compared with unpaired Student's t-test or Mann-Whitney U-test for continuous variables and Chi-square test or Fisher's exact test for categorical variables. Spearman rank correlation was used for the analysis of correlation between RDW levels and SOFA scores and dRDW and dSOFA. The diagnostic accuracy of dRDW and dSOFA between survivors and nonsurvivors was analyzed by the area under the corresponding receiver operating characteristic curve. Odds ratio (OR) was calculated to evaluate the relative risk. When one element of the equation was zero, Woolf's formula with Haldane's modification (OR, [2a + 1] [2d + 1]/[2b + 1] [2c + 1]) was adopted to assess the relative risk.[22] A P < 0.05 was considered statistically significant.
Results
Finally, a total of 45 patients, including 32 males and 13 females, were included in the final analysis. The mean age was 75.6 ± 7.1 years. Six patients died within 4 days and other 13 patients died after management 6 days later. The overall mortality of these patients was 42.2% (19/45). Based on their hospital outcomes, these patients were divided into the survivor group (n = 26) and the nonsurvivor group (n = 19). The baseline characteristics and laboratory parameters between the two groups are shown in Table 1. There were no significant differences in age, gender, BMI, APACHE II scores, primary site of infections, and comorbidity between the two groups (all P > 0.05).
Table 1.
Baseline characteristics and laboratory parameters between two groups in this study
| Characteristics | Survivor group (n = 26) | Nonsurvivor group (n = 19) | Statistical values | P |
|---|---|---|---|---|
| Age (years), mean ± SD | 75.1 ± 6.1 | 78.6 ± 7.9 | −1.678* | 0.101 |
| Male, n (%) | 16 (61.5) | 11 (57.9) | 0.061† | 0.805 |
| BMI (kg/m2), mean ± SD | 23.2 ± 4.4 | 22.6 ± 4.8 | 0.451* | 0.654 |
| APACHE II scores, mean ± SD | 20.3 ± 3.9 | 22.0 ± 3.5 | −1.498* | 0.141 |
| Primary site of infection, n (%) | ||||
| Respiratory | 20 (76.9) | 12 (63.2) | 1.013† | 0.314 |
| Urinary | 1 (3.9) | 0 (0.0) | ||
| Hepatobiliary | 1 (3.9) | 2 (10.5) | ||
| Soft tissue | 1 (3.9) | 0 (0.0) | ||
| Miscellaneous | 3 (11.5) | 5 (26.3) | ||
| Comorbidity, n (%) | ||||
| Hypertension | 14 (53.8) | 5 (26.3) | 3.411† | 0.065 |
| Diabetes mellitus | 10 (38.5) | 6 (31.6) | 0.227† | 0.634 |
| COPD | 9 (34.6) | 3 (15.8) | 1.990† | 0.158 |
| Laboratory parameters | ||||
| WBC (×109/L), mean ± SD | 11.2 ± 4.8 | 15.3 ± 6.2 | −2.527* | 0.015 |
| HGB (×109/L), mean ± SD | 125.2 ± 23.3 | 109.4 ± 33.1 | 1.881* | 0.067 |
| PLT (×109/L), median (IQR) | 208.5 (120.0) | 168.0 (147.0) | 1.184‡ | 0.237 |
| Serum sodium (mmol/L), mean ± SD | 137.2 ± 5.7 | 135.5 ± 7.0 | 0.932* | 0.356 |
| Serum potassium (mmol/L), mean ± SD | 3.4 ± 0.6 | 3.8 ± 0.8 | −1.836* | 0.073 |
| Serum creatinine (µmol/L), median (IQR) | 75.3 (52.1) | 190.2 (150.1) | −2.781‡ | 0.005 |
| Serum cholesterol (mmol/L), mean ± SD | 3.93 (1.2) | 3.44 (1.6) | 1.804‡ | 0.071 |
| Serum albumin (g/L), mean ± SD | 32.0 ± 5.9 | 24.9 ± 5.8 | 3.995* | <0.001 |
| TBIL (µmol/L), median (IQR) | 11.5 (17.0) | 7.9 (8.9) | 0.896‡ | 0.370 |
| CRP (mg/L), median (IQR) | 38.4 (79.1) | 104.0 (124.0) | −1.517‡ | 0.129 |
| PCT (ng/ml), median (IQR) | 0.4 (4.0) | 1.6 (4.9) | −1.384‡ | 0.166 |
*t value; †Chi-square value; ‡Z value. BMI: Body mass index; APACHE II: Acute Physiology and Chronic Health Evaluation II; COPD: Chronic obstructive pulmonary diseases; WBC: White blood cell; HGB: Hemoglobin; PLT: Platelet; TBIL: Total bilirubin; CRP: C-reactive protein; PCT: Procalcitonin; IQR: Interquartile range; SD: Standard deviation. *t value, †: χ2 value, ‡: Z value.
There were no significant differences in initial RDW levels and SOFA scores between survivor and nonsurvivor groups (all P > 0.05). However, both RDW levels and SOFA scores were significantly lower at days 4 and 7 in survivor group, compared with nonsurvivor group (P < 0.05; Figure 1).
Figure 1.
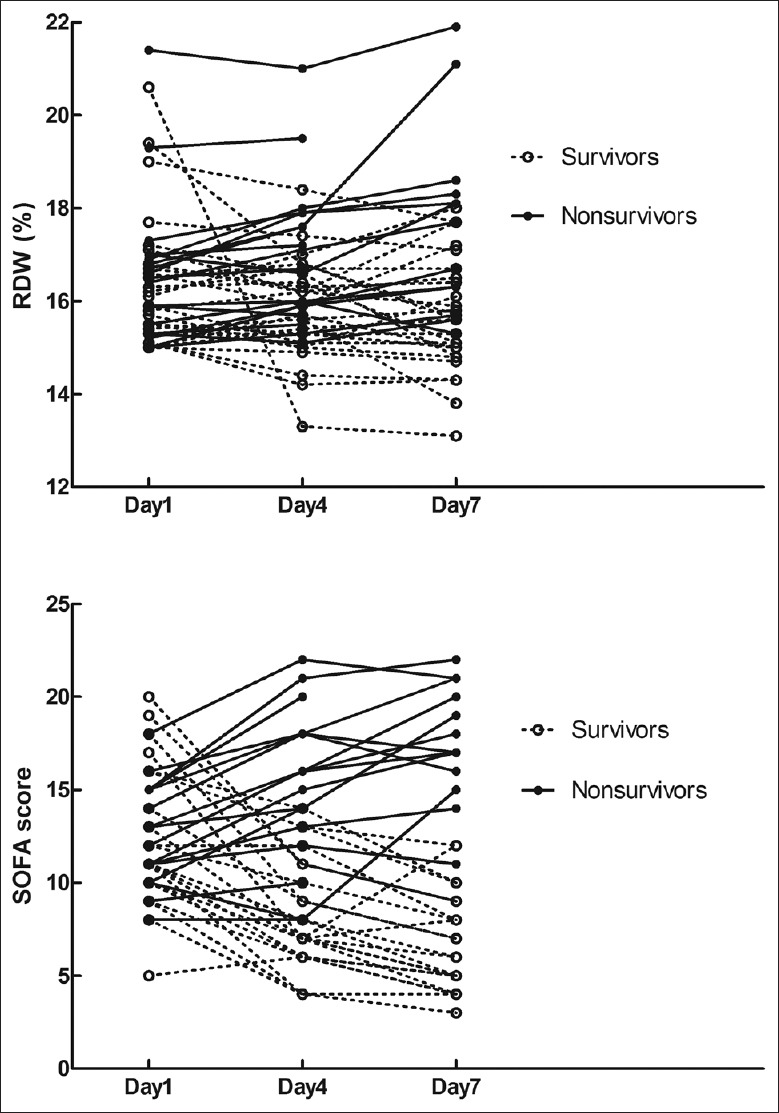
All individual measurements of RDW and SOFA scores at days 1, 4, and 7. Black solid dots represent the nonsurvivors; open circles represent the survivors. RDW: Red cell distribution width; SOFA: Sequential Organ Failure Assessment.
The survivor group had declining tendency in RDW levels with median decreases of 0.4% (day 1–day 4), 0.2% (day 4–day 7), and 0.5% (day 1–day 7). However, the nonsurvivor group had increasing tendency in RDW levels with median increases of 0.3% (day 1–day 4), 0.5% (day 4–day 7), and 1.1% (day 1–day 7). Dynamic changes of RDW levels and SOFA scores in all patients are shown in Table 2. Dynamic changes of RDW levels in survivor group were significantly different from that of nonsurvivor group (all P < 0.05, Table 2).
Table 2.
RDW levels, SOFA scores, and their dynamic changes between two groups in this study
| Parameters | Survivor group (n = 26) | Nonsurvivor group (n = 19) | Z values | P |
|---|---|---|---|---|
| RDW1 (%) | 16.0 (1.5) | 16.5 (1.7) | −0.598 | 0.550 |
| RDW4 (%) | 15.8 (2.0) | 16.7 (2.0) | −2.530 | 0.011 |
| RDW7 (%) | 15.6 (1.8) | 17.7 (2.5) | −3.176 | 0.001 |
| dRDW14 (%) | −0.4 (0.9) | 0.3 (0.7) | −3.351 | 0.001 |
| dRDW47 (%) | −0.2 (0.9) | 0.5 (0.5) | −3.163 | 0.002 |
| dRDW17 (%) | −0.5 (1.4) | 1.1 (0.9) | −3.951 | <0.001 |
| SOFA1 | 11.0 (5.0) | 13.0 (4.0) | −0.928 | 0.353 |
| SOFA4 | 7.0 (4.0) | 16.0 (5.0) | −4.938 | <0.001 |
| SOFA7 | 5.5 (4.0) | 17.0 (5.0) | −4.993 | <0.001 |
| dSOFA14 | −4.0 (3.0) | 3.0 (3.0) | −5.414 | <0.001 |
| dSOFA47 | −2.0 (1.0) | 1.0 (5.0) | −3.883 | <0.001 |
| dSOFA17 | −6.0 (3.0) | 5.0 (4.0) | −4.758 | <0.001 |
The data are shown as median (IQR). RDW: Red cell distribution width; RDW1: The level of RDW on day 1; RDW4: The level of RDW on day 4; RDW7: The level of RDW on day 7; SOFA: Sequential Organ Failure Assessment; SOFA1: The score of SOFA on day 1; SOFA4: The score of SOFA on day 4; SOFA7: The score of SOFA on day 7; dRDW14: The level of RDW on day 4 – the level of RDW on day 1; dSOFA14: The score of SOFA on day 4 – the score of SOFA on day 1; dRDW17: The level of RDW on day 7 – the level of RDW on day 1; dSOFA17: The score of SOFA on day 7 – the score of SOFA on day 1; dRDW47: The level of RDW on day 7 – the level of RDW on day 4; dSOFA47: The score of SOFA on day 7 – the score of SOFA on day 4. IQR: Interquartile range.
At day 4 measurement, an increase in RDW level was observed in 9 out of 26 survivors, whereas 15 out of 19 nonsurvivors had an increase in RDW level. Sensitivity and specificity of RDW increase for predicting death in elderly patients with septic shock were 81% and 62.5%, respectively. Similarly, an increase in SOFA scores was observed in 2 out of 26 survivors, whereas 17 of the 19 nonsurvivors had an increase in SOFA scores at day 4. Sensitivity and specificity of increase in SOFA scores for predicting death were 83.9% and 100%, respectively.
At day 7 measurement, increase in RDW level was observed in 8 of the 26 survivors, whereas 12 of the 13 nonsurvivors had an increase in RDW level. Sensitivity and specificity of RDW increase for predicting death in elderly patients with septic shock were 94.7% and 60%, respectively. Moreover, increase in SOFA scores was observed in 2 of the 26 survivors, whereas 12 of the 13 nonsurvivors had an increase in SOFA scores. Sensitivity and specificity of increase in SOFA score for predicting death in elderly patients with septic shock were 96.0% and 85.7%, respectively.
About 3 of 26 survivors had continuous increases in RDW level and SOFA score, and 10 and 9 out of the 13 nonsurvivors had continuous increases in RDW level and SOFA score, respectively [Table 3]. Sensitivity and specificity of continuous increases in RDW level and SOFA score for predicting death in elderly patients with septic shock were 88.5%, 76.9% and 92.3%, 89.5%, respectively. After Haldane's modification, the ORs for death in patients with continuous increases of RDW level and SOFA score were 25.6 (95% confidence interval: 4.38–149.15) and 81.9 (95% confidence interval: 9.84–681.55), respectively.
Table 3.
Changes of RDW level and SOFA score at days 4 and 7 between two groups
| Parameters | Survivor group | Nonsurvivor group | Chi-square value | P |
|---|---|---|---|---|
| RDW14 increase | 9/26 (34.6) | 15/19 (78.9) | 8.668 | 0.003 |
| RDW17 increase | 8/26 (30.8) | 12/13 (92.3) | 13.137 | <0.001 |
| RDW147 continuous increase | 3/26 (11.5) | 10/13 (76.9) | 16.673 | <0.001 |
| SOFA14 increase | 2/26 (7.7) | 17/19 (89.5) | 30.097 | <0.001 |
| SOFA17 increase | 2/26 (7.7) | 12/13 (92.3) | 29.966 | <0.001 |
| SOFA147 continuous increase | 3/26 (11.5) | 9/13 (69.2) | 10.969 | 0.001 |
The data are shown as n/N (%). RDW: Red cell distribution width; SOFA: Sequential Organ Failure Assessment.
Among the patients, there was no correlation between RDW levels and their counterparts of SOFA scores on days 1 and 4 (all P > 0.05). RDW level on day 7 and its dynamic changes significantly correlated with their counterparts of SOFA scores (P = 0.001, 0.001, 0.002, and 0.002, Table 4). Relationship between dRDW and dSOFA is shown in Figure 2.
Table 4.
Spearman’s correlation of RDW level and SOFA score in septic shock patients
| Parameters | Correlation efficient | P |
|---|---|---|
| RDW1 with SOFA1 | −0.207 | 0.172 |
| RDW4 with SOFA4 | 0.241 | 0.112 |
| RDW7 with SOFA7 | 0.495 | 0.001 |
| dRDW14 with dSOFA14 | 0.469 | 0.001 |
| dRDW17 with dSOFA17 | 0.481 | 0.002 |
| dRDW147 with dSOFA147 | 0.487 | 0.002 |
RDW: Red cell distribution width; SOFA: Sequential Organ Failure Assessment.
Figure 2.
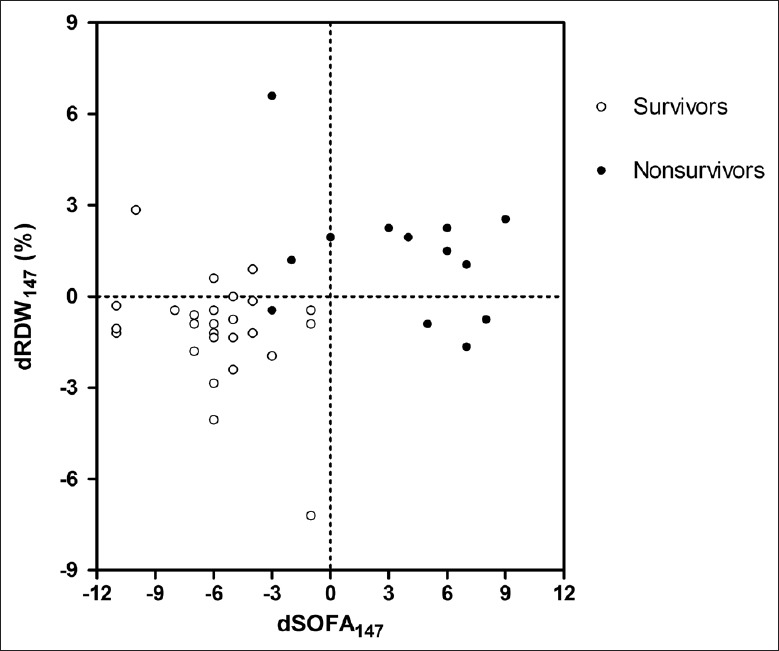
Scatterplot of dRDW and dSOFA. dRDW: Dynamic change of red cell distribution width; dSOFA: Dynamic change of Sequential Organ Failure Assessment.
Discussion
The major novel finding of the present study was the significantly continuous increase in RDW levels, but not the level itself, might be a useful indicator for predicting poor outcome in Chinese elderly patients with septic shock, when the RDW level was beyond 15%. Dynamic changes of RDW level closely correlated with dynamic changes of SOFA score.
Previous studies have demonstrated a potential prognostic value of RDW level on day 1 in the infective patients, including patients with septic shock.[12,17,23,24] Most of the studies showed that high RDW level was associated with the likelihood of poor outcome. However, in clinical practice, we are unsure of the definition of high RDW level since many patients whose RDW levels were higher than the maximum limit still survived. Kim et al.[15] found that an increase in RDW level from baseline during the first 72 h after hospitalization was significantly associated with adverse clinical outcomes. Another study[14] further demonstrated that RDW levels on days 1, 4, and 8 were associated with prognosis in septic patients. This meant that dynamic observation of RDW levels seemed more valuable in clinical practice. Since we could not define what a high value of RDW level is, a better criterion would be an RDW level beyond 15%. At least, this value pointed out that the patients were in the status of high RDW level. We also wanted to know the changing tendency of RDW levels among the patients with septic shock.
This study showed that the initial level of RDW was not associated with poor outcome in elderly septic shock patients when the RDW level was beyond 15%. The reasons could be: (1) the severities of patients’ diseases were similar between the two groups based on APACHE II scores. A previous study confirmed that RDW levels and APACHE II scores had significant positive correlation in shock patients; (2) All the patients who were enrolled in this study had RDW levels beyond 15% which were different from the previous study; (3) Renal dysfunction, which is rather common in septic shock patients, may influence the diagnostic accuracy of RDW level.[25,26]
This study showed that, when the RDW level was beyond 15%, the survivors had declining tendency in RDW levels, but the nonsurvivors had increasing tendency in RDW levels. Therefore, dynamic observation of RDW levels is necessary. Inflammatory cytokines can inhibit erythrocyte maturation, which leads to release of immature RBCs into the circulation and hence elevated RDW level.[9,27] The inflammatory cytokines in the process of disease development, especially in patients with sepsis, showed a “waterfall effect”.[28] This could cause further increase of inflammation factors and could affect further change of RDW levels. Oxidative stress accompanied the development of the disease process. The tendency of the disease's development was closely related to the level of oxidative stress. High oxidative stress could also lead to elevated RDW level by reducing RBC survival and by increasing the release of large premature RBCs into the peripheral circulation.[10] This would definitely worsen the disease process and RDW levels will increase significantly. Inflammation could also inhibit erythropoietin production, induce resistance to erythropoietin, decrease iron bioavailability, directly suppress erythroid precursors in the bone marrow, and activate red cell apoptosis and peripheral phagocytosis.[9,25,29,30,31]
RDW level has been shown to correlate with SOFA score and it is an index of multiple organ dysfunction in sepsis.[23] The data of this study were in accordance with previous studies and further showed that, even in septic shock patients with high RDW levels beyond 15%, the RDW level still highly correlated with the SOFA score.
There were several limitations in our study. This was a single-center study with small sample size, and the patients involved in this study were of specific population (septic shock, RDW >15%, and elderly), we did not know whether our result was also applicable to younger ages, so caution should be taken in any application of this study. Since the enrollment of this study was based on the criteria of RDW >15% and clinical diagnosis of septic shock, the patients might be either in the beginning of treatment or during the treatment, we could not exclude the possibility that some mixing of variables that would affect the outcome may have been missed. Since Ku et al.[17] suggested that 72-h RDW after the onset of bacteremia could be a predictor of all-cause mortality in patients with Gram-negative bacteremia. In addition, the patients may have either a community-acquired infection or nosocomial infection. This study did not investigate erythropoietin levels, iron levels, or Vitamin B12 levels. The reticulocyte count could also affect RDW values, so this might have limited the interpretation of the study results. In the future, a larger multicenter study with repeated RDW measurements is necessary for further investigating the role of changes in RDW.
In conclusion, continuous increase in RDW level, rather than the level of RDW itself, is more useful for predicting hospital death in elderly patients with septic shock when the level of RDW is beyond 15%. It is better to monitor the dynamic changes of RDW levels. The dynamic changes of RDW were highly correlated with the SOFA score in the patients.
Financial support and sponsorship
The study was supported by grants from Key Subjects of Jiading District (No. ZD01) and Foundation of the Public Health Bureau of Jiading (No. 2013-QN-02).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
References
- 1.Muhlestein JB, Lappe DL, Anderson JL, Muhlestein JB, Budge D, May HT, et al. Both initial red cell distribution width (RDW) and change in RDW during heart failure hospitalization are associated with length of hospital stay and 30-day outcomes. Int J Lab Hematol. 2016;38:328–37. doi: 10.1111/ijlh.12490. doi: 10.1111/ijlh.12490. [DOI] [PubMed] [Google Scholar]
- 2.Zorlu A, Bektasoglu G, Guven FM, Dogan OT, Gucuk E, Ege MR, et al. Usefulness of admission red cell distribution width as a predictor of early mortality in patients with acute pulmonary embolism. Am J Cardiol. 2012;109:128–34. doi: 10.1016/j.amjcard.2011.08.015. doi: 10.1016/j.amjcard.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Turcato G, Serafini V, Dilda A, Bovo C, Caruso B, Ricci G, et al. Red blood cell distribution width independently predicts medium-term mortality and major adverse cardiac events after an acute coronary syndrome. Ann Transl Med. 2016;4:254. doi: 10.21037/atm.2016.06.35. doi: 10.21037/atm.2016.06.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekler A, Tenekecioglu E, Erbag G, Temiz A, Altun B, Barutçu A, et al. Relationship between red cell distribution width and long-term mortality in patients with non-ST elevation acute coronary syndrome. Anatol J Cardiol. 2015;15:634–9. doi: 10.5152/akd.2014.5645. doi: 10.5152/akd.2014.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demir R, Saritemur M, Atis O, Ozel L, Kocaturk I, Emet M, et al. Can we distinguish stroke and stroke mimics via red cell distribution width in young patients? Arch Med Sci. 2015;11:958–63. doi: 10.5114/aoms.2014.40995. doi: 10.5114/aoms.2014.40995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Kim YD, Song TJ, Park JH, Lee HS, Nam CM, et al. Red blood cell distribution width is associated with poor clinical outcome in acute cerebral infarction. Thromb Haemost. 2012;108:349–56. doi: 10.1160/TH12-03-0165. doi: 10.1160/TH12-03-0165. [DOI] [PubMed] [Google Scholar]
- 7.Ye Z, Smith C, Kullo IJ. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol. 2011;107:1241–5. doi: 10.1016/j.amjcard.2010.12.023. doi: 10.1016/j.amjcard.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zalawadiya SK, Veeranna V, Panaich SS, Afonso L. Red cell distribution width and risk of peripheral artery disease: Analysis of National Health and Nutrition Examination Survey 1999-2004. Vasc Med. 2012;17:155–63. doi: 10.1177/1358863X12442443. doi: 10.1177/1358863X12442443. [DOI] [PubMed] [Google Scholar]
- 9.Scharte M, Fink MP. Red blood cell physiology in critical illness. Crit Care Med. 2003;31(12 Suppl):S651–7. doi: 10.1097/01.CCM.0000098036.90796.ED. doi: 10.1097/01.CCM.0000098036.90796.ED. [DOI] [PubMed] [Google Scholar]
- 10.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10:1923–40. doi: 10.1089/ars.2008.2142. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bello S, Fandos S, Lasierra AB, Mincholé E, Panadero C, Simon AL, et al. Red blood cell distribution width [RDW] and long-term mortality after community-acquired pneumonia. A comparison with proadrenomedullin. Respir Med. 2015;109:1193–206. doi: 10.1016/j.rmed.2015.07.003. doi: 10.1016/j.rmed.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Chung HJ, Kim K, Jo YH, Rhee JE, Kim YJ, et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am J Emerg Med. 2013;31:72–9. doi: 10.1016/j.ajem.2012.06.004. doi: 10.1016/j.ajem.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Özdogan HK, Karateke F, Özyazici S, Özdogan M, Özaltun P, Kuvvetli A, et al. The predictive value of red cell distribution width levels on mortality in intensive care patients with community-acquired intra-abdominal sepsis. Ulus Travma Acil Cerrahi Derg. 2015;21:352–7. doi: 10.5505/tjtes.2015.26737. doi: 10.5505/tjtes.2015.26737. [DOI] [PubMed] [Google Scholar]
- 14.Lorente L, Martín MM, Abreu-González P, Solé-Violán J, Ferreres J, Labarta L, et al. Red blood cell distribution width during the first week is associated with severity and mortality in septic patients. PLoS One. 2014;9:e105436. doi: 10.1371/journal.pone.0105436. doi: 10.1371/journal.pone.0105436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim CH, Park JT, Kim EJ, Han JH, Han JS, Choi JY, et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit Care. 2013;17:R282. doi: 10.1186/cc13145. doi: 10.1186/cc13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Xu X, Ni H, Deng H. Red cell distribution width is associated with hospital mortality in unselected critically ill patients. J Thorac Dis. 2013;5:730–6. doi: 10.3978/j.issn.2072-1439.2013.11.14. doi: 10.3978/j.issn.2072-1439.2013.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ku NS, Kim HW, Oh HJ, Kim YC, Kim MH, Song JE, et al. Red blood cell distribution width is an independent predictor of mortality in patients with gram-negative bacteremia. Shock. 2012;38:123–7. doi: 10.1097/SHK.0b013e31825e2a85. doi: 10.1097/SHK.0b013e31825e2a85. [DOI] [PubMed] [Google Scholar]
- 18.Safari S, Shojaee M, Rahmati F, Barartloo A, Hahshemi B, Forouzanfar MM, et al. Accuracy of SOFA score in prediction of 30-day outcome of critically ill patients. Turk J Emerg Med. 2016;16:146–50. doi: 10.1016/j.tjem.2016.09.005. doi: 10.1016/j.tjem.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair R, Bhandary NM, D'souza AD. Initial Sequential Organ Failure Assessment score versus simplified acute physiology score to analyze multiple organ dysfunction in infectious diseases in Intensive Care Unit. Indian J Crit Care Med. 2016;20:210–5. doi: 10.4103/0972-5229.180041. doi: 10.4103/0972-5229.180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui Y, Wang T, Bao J, Tian Z, Lin Z, Chen D. Comparison of Charlson's weighted index of comorbidities with the chronic health score for the prediction of mortality in septic patients. Chin Med J. 2014;127:2623–7. doi: 10.3760/cma.j.issn.0366-6999.20132684. [PubMed] [Google Scholar]
- 22.Claeys R, Vinken S, Spapen H, ver Elst K, Decochez K, Huyghens L, et al. Plasma procalcitonin and C-reactive protein in acute septic shock: clinical and biological correlates. Crit Care Med. 2002;30:757–62. doi: 10.1097/00003246-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Braun E, Domany E, Kenig Y, Mazor Y, Makhoul BF, Azzam ZS. Elevated red cell distribution width predicts poor outcome in young patients with community acquired pneumonia. Crit Care. 2011;15:R194. doi: 10.1186/cc10355. doi: 10.1186/cc10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadaka F, O’Brien J, Prakash S. Red cell distribution width and outcome in patients with septic shock. J Intensive Care Med. 2013;28:307–13. doi: 10.1177/0885066612452838. doi: 10.1177/0885066612452838. [DOI] [PubMed] [Google Scholar]
- 25.Akin F, Celik O, Altun I, Ayca B, Ozturk D, Satilmis S, et al. Relation of red cell distribution width to contrast-induced acute kidney injury in patients undergoing a primary percutaneous coronary intervention. Coron Artery Dis. 2015;26:289–95. doi: 10.1097/MCA.0000000000000223. doi: 10.1097/MCA.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 26.Qin JP, Yu XY, Qian CY, Li SS, Qin TH, Chen EZ, et al. Value of kidney disease improving global outcomes urine output criteria in critically ill patients: A secondary analysis of a multicenter prospective cohort study. Chin Med J. 2016;129:2050–7. doi: 10.4103/0366-6999.189059. doi: 10.4103/0366-6999.189059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan R, Wang B, Lu W, Maeda Y, Dowling P. A distinct region in erythropoietin that induces immuno/inflammatory modulation and tissue protection. Neurotherapeutics. 2015;12:850–61. doi: 10.1007/s13311-015-0379-1. doi: 10.1007/s13311-015-0379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–74. doi: 10.1001/jama.2016.0288. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: Prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158:659–66. doi: 10.1016/j.ahj.2009.07.024. doi: 10.1016/j.ahj.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Laftah AH, Sharma N, Brookes MJ, McKie AT, Simpson RJ, Iqbal TH, et al. Tumour necrosis factor alpha causes hypoferraemia and reduced intestinal iron absorption in mice. Biochem J. 2006;397:61–7. doi: 10.1042/BJ20060215. doi: 10.1042/BJ20060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86–105. doi: 10.3109/10408363.2014.992064. doi: 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]


