Abstract
Background:
Aerosolized amikacin (AA) is a current option for the management of ventilator-associated pneumonia (VAP) caused by multidrug-resistant Gram-negative bacteria (MDR-GNB), as it is reported that AA could increase the alveolar level of the drug without increasing systemic toxicity. This study aimed to evaluate the efficacy and safety of AA as an adjunctive therapy for VAP caused by MDR-GNB.
Methods:
In this single-center, double-blind study conducted in a 36-bed general Intensive Care Unit (ICU) in a tertiary hospital from June 2014 to June 2016, 52 ICU patients with confirmed MDR-GNB VAP were randomized to two groups (AA group, n = 27 and placebo group, n = 25). Amikacin (400 mg, q8h) or saline placebo (4 ml, q8h) was aerosolized for 7 days. The attending physician determined the administration of systemic antibiotics for VAP. Patients were followed up for 28 days. Bacteriological eradication, clinical pulmonary infection score (CPIS), and serum creatinine were assessed on day 7 of therapy. New resistance to amikacin, cure rate of VAP, weaning rate, and mortality were assessed on day 28.
Results:
The baseline characteristics of patients in both groups were similar. At the end of the treatment, 13 of the 32 initially detected bacterial isolates were eradicated in AA group, compared to 4 of 28 in placebo group (41% vs. 14%, P = 0.024). As for patients, 11 of 27 patients treated with AA and 4 of 25 patients treated with placebo have eradication (41% vs. 16%, P = 0.049). The adjunction of AA reduced CPIS (4.2 ± 1.6 vs. 5.8 ± 2.1, P = 0.007). New drug resistance to amikacin and the change in serum creatinine were not detected in AA group. No significant differences in the clinical cure rate in survivors (48% vs. 35%, P = 0.444), weaning rate (48% vs. 32%, P = 0.236), and mortality (22% vs. 32%, P = 0.427) were detected between the two groups on day 28.
Conclusions:
As an adjunctive therapy of MDR-GNB VAP, AA successfully eradicated existing MDR organisms without inducing new resistance to amikacin or change in serum creatinine. However, the improvement of mortality was not found.
Keywords: Aerosol Drug Therapy, Amikacin, Gram-negative Bacteria, Multidrug Resistance, Pneumonia, Ventilator-associated
Introduction
Multidrug-resistant (MDR) Gram-negative bacteria (GNB) are becoming a growing threat to the ventilator-associated pneumonia (VAP) patients in Intensive Care Unit (ICU).[1] The increasing incidence of the MDR-GNB and the paucity of new effective antibiotics have contributed to the renewed enthusiasm for employing amikacin. However, the application of amikacin is limited by adverse effects and the poor penetration into infected lung tissues with intravenous (IV) administration.[2,3]
Aerosolized amikacin (AA) is an effective method to deliver drug to lung tissues. This method can achieve a drug concentration in the lung tissues which is multiple times more than the minimal inhibitory concentration (MIC) of evenly resistant pathogens, and hence inhibiting and eradicating pathogens. Moreover, the amikacin concentration in blood is extremely low due to local administration, so the dose-dependent systemic side effects could be bypassed.[4,5] Pharmacokinetics and effect of AA have been revealed in animal models and clinical studies.[6,7,8] However, due to the great heterogeneity and conflicting conclusions of existing publications, the true contribution of AA needs further exploration in clinical studies. The objective of this study was to test whether AA as an adjunctive therapy of MDR-GNB VAP was effective and safe in a prospective, randomized, controlled trial.
Methods
Ethical approval
This double-blind, randomized, placebo-controlled, single-center study was conducted from June 2014 to June 2016 in a 36-bed general ICU in Zhongnan Hospital of Wuhan University, China. The study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (No. 2015042). Consent was obtained from either the patient or his/her health-care proxy.
Patients and randomization
Patients with mechanical ventilation (MV) were chosen as the research candidates, especially those with high-risk infection of MDR bacteria. The known risk factors include (1) hospitalization of greater than 5 days, (2) prior use of systemic antibiotics (SA) in the past 90 days, and (3) high frequency of resistance in the patient's unit. Vital signs, characters of airway secretions, and oxygenation index were recorded daily. Once fever, purulence of sputum, or hypoxemia was detected, complete blood counts (CBCs) with differential, chest X-ray or computed tomography, airway secretions cultivation would be conducted.
The inclusion criteria included (1) age >18 years old, (2) invasive MV >48 h, (3) new onset and/or progressive pulmonary infiltrates on chest radiography, (4) at least two of three clinical features such as (a) fever (≥38°C), (b) leukocytosis (≥10,000/mm3) or leucopenia (<4000/mm3), and (c) purulent tracheal secretions, and (5) MDR-GNB on bacterial culture of airway secretion and drug sensitive test, namely, GNB drug resistant to three or more than three kinds of antibiotics on the drug susceptibility test.
The exclusion criteria included (1) pregnancy, perinatal period, and feeding period; (2) history of allergy or adverse effect to amikacin or aerosolized therapy; (3) acute or chronic renal insufficiency; (4) existence of airway obstructive factors or limitation; (5) immunosuppression; and (6) requirement of small tidal volume MV (<6 ml/kg).
The final definite diagnosis of each patient and the final enrollment were made by an expert team following the above criteria. The expert group consisted of 4 professors of critical care medicine from Zhongnan Hospital. The final definite diagnosis and enrollment of each patient needed to be agreed by at least three professors; otherwise, the patient would be excluded from the study.
Block randomization was performed by a computer-generated randomization algorithm; the allocation sequence was kept by an independent statistician and was reported to the investigator. Investigator and clinical staff were blinded to the treatment arms.
Interventions
The patients in AA group were treated with amikacin (Qilu Pharma, China) 400 mg for 20 min every 8 h, while those in placebo group were treated with normal saline (Fuxing Bio-Pharma, China) 4 ml at the same frequency. Aerosol treatment was conducted for 7 days. During the treatment, there were several possible outcomes and the corresponding actions as follows: (1) weaning, use T-tube for nebulization, (2) extubation, use oronasal nebulizer mask, (3) death, end study, and (4) change of bacteriology result, unsuitable for AA, then end study. The use of SA during the treatment was determined by the attending physician based on clinical criteria and available culture results.
The trachea secretions were cleared with a suction catheter before the onset of nebulization. Amikacin or placebo was nebulized through a jet nebulizer (BD Medical Technology, USA). The following ventilator settings were followed during nebulization: volume control mode, tidal volume of 8 ml/kg, constant inspiratory flow rate of 40 L/min, nebulization set during inspiration, with heat and moisture exchangers remove or heated humidifiers off, inspiratory to expiratory (I:E) ratio ≤ 50%, and an end-inspiratory pause of 20% of the duty cycle. To optimize the synchronization of the patients and the nebulizer, the patients were sedated with propofol (Corden Pharma, Italy) and fentanyl (Humanwell Pharma, China) during treatment.
Definitions and outcomes
At the time of randomization, demographic data as well as Acute Physiology and Chronic Health Evaluation II (APACHE II) score, simplified clinical pulmonary infection score (CPIS), cause of MV, length of MV, and ICU length of stay were collected. Vital signs, oxygenation index, airway secretion quantity and character, SA, and adverse events were tracked on a daily basis. The highest temperature and the minimum oxygenation index of each day were noted. CBCs with differential, serum creatinine, and lung imaging were performed at the time of randomization and by end of the treatment. Bacteriological samples were performed on tracheal aspirate before the first dose on day 1 and every other day within the 1st week, and then weekly between day 7 and day 28. A culture result of ≥105 CFU/ml was defined as positive.
All patients were followed up for 28 days. The primary endpoints were bacteriological eradication and emergency of new drug resistant to amikacin. The eradication of an organism is defined as no growth in culture and no visible organisms seen on Gram staining identified at randomization. Secondary endpoints were CPIS, serum creatinine assessed at day 7, and cure of VAP, weaning rate, and mortality assessed at day 28. The cure of VAP was defined as the resolution of clinical and biological signs of infection.
Statistical analysis
We estimated that a minimum sample size of 42 patients (21 in each group) would be required to provide the trial with 90% power to detect a between-group difference of 20% in the rate of bacteriological eradication at day 7, under the assumption of a one-sided alpha level of 0.05 and a mean standard deviation (SD) of 22%. The parametric continuous variables were described as mean ± SD and compared using unpaired t-test. Wilcoxon's rank-sum test was used to test the difference between nonparametric continuous variables reported as median (interquartile range). Pearson Chi-square was used to test differences between percentages in categorical variables. The significance level was fixed at a two-sided alpha level of P < 0.05. All statistical analyses were performed using SPSS version 13.0 (IBM Corp, Armonk, NY, USA).
Results
Patients
Of 200 candidates, 60 had met our eligibility criteria, while three patients in AA group and five in placebo group withdrew the study shortly after enrollment. Finally, 52 patients were retained for analysis [Figure 1]. The demographic and clinical data were similar between the two groups at the time of randomization [Table 1]. Table 2 demonstrates the MDR-GNB pathogens isolated from tracheal aspirates and the sensitivity to amikacin at the time of randomization (41% vs. 54%, χ2 = 1.006, P = 0.316) in the two groups. During the treatment period, the choices of sensitive SA were based on the culture results, 19 of 27 patients in AA group and 15 of 25 patients in placebo group accepted combination therapy against MDR-GNB (70% vs. 60%, χ2 = 0.617, P = 0.432). The use of SA demonstrated no significant difference between the two groups [Table 3].
Figure 1.
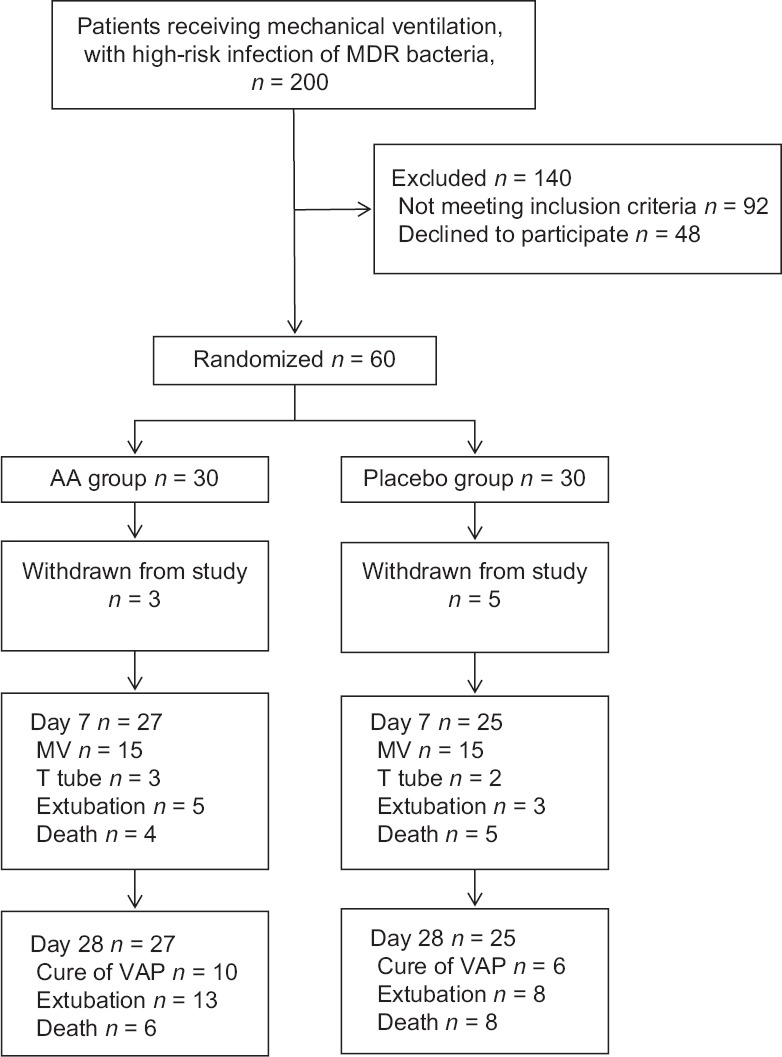
Flowchart for patient recruitment, enrollment, and analysis. MDR: Multidrug resistant; MV: Mechanical ventilation; VAP: Ventilator-associated pneumonia.
Table 1.
Characteristics of the enrolled patients with ventilator-associated pneumonia caused by MDR-GNB
| Characteristics | Amikacin group (n = 27) | Placebo group (n = 25) | Statistics | P |
|---|---|---|---|---|
| Cause of mechanical ventilation, n (%) | ||||
| Respiratory disease | 13 (48) | 12 (48) | – | |
| Cardiac disease | 4 (15) | 2 (8) | – | |
| Neurological disease | 4 (15) | 5 (20) | – | |
| Surgical intervention | 1 (4) | 0 | – | |
| Multiple organ failure | 2 (7) | 3 (12) | – | |
| Sepsis | 3 (11) | 3 (12) | – | |
| Gender, n (%) | ||||
| Male | 16 (59) | 16 (64) | 0.123* | 0.726 |
| Female | 11 (41) | 9 (36) | ||
| Mean age (years) | 68.1 ± 16.7 | 64.7 ± 10.6 | 0.869† | 0.389 |
| APACHE II scores | 21.8 ± 3.8 | 19.3 ± 5.3 | 1.966† | 0.055 |
| Vent days before randomization (days) | 16.7 ± 7.2 | 18.3 ± 6.9 | 0.817† | 0.418 |
| ICU length of stay before randomization (days) | 16.0 ± 6.3 | 14.4 ± 5.2 | 0.994† | 0.325 |
Data are reported as mean ± SD for quantitative value and n (%) for qualitative value. *Pearson χ2 value; †t values. –: Not applicable; APACHE II: Acute Physiology and Chronic Health Evaluation II; ICU: Intensive Care Unit; SD: Standard deviation; MDR-GNB: Multidrug-resistant Gram-negative bacteria.
Table 2.
Bacterial isolates from tracheal aspirates and sensitivity to amikacin at randomization in two groups
| MDR-GNB | Amikacin group | Placebo group | ||
|---|---|---|---|---|
| Isolates (n = 32) | Sensitive to amikacin (n = 13) | Isolates (n = 28) | Sensitive to amikacin (n = 15) | |
| Acinetobacter baumannii | 9 | 0 | 7 | 0 |
| Pseudomonas aeruginosa | 8 | 4 | 7 | 3 |
| Klebsiella pneumoniae | 6 | 5 | 5 | 5 |
| Stenotrophomonas maltophilia | 4 | 0 | 2 | 0 |
| Escherichia coli | 2 | 2 | 3 | 3 |
| Enterobacter sp. | 2 | 2 | 4 | 4 |
| Burkholderia cepacia | 1 | 0 | 0 | 0 |
MDR-GNB: Multidrug-resistant Gram-negative bacteria.
Table 3.
The systemic use of antibiotics against MDR-GNB in two groups (n (%))
| Groups | Carbapenem* | β-lactam/β-lactamase inhibitor† | Fluoroquinolone‡ | Cephalosporin§ | Total (N) | P|| |
|---|---|---|---|---|---|---|
| Amikacin group | 17 (37) | 9 (20) | 14 (30) | 6 (13) | 46 | 0.6202 |
| Placebo group | 14 (35) | 5 (13) | 12 (30) | 9 (23) | 40 |
*Meropenem or imipenem; †Piperacillin/tazobactam or cefoperazone/sulbactam; ‡Moxifloxacin, ciprofloxacin, or levofloxacin; §Ceftazidime, ceftriaxone, or cefoselis; ||Pearson Chi-square test, χ2 = 1.776. MDR-GNB: Multidrug-resistant Gram-negative bacteria.
Bacteriological effects
In AA group, 13 of the 32 initially detected bacterial isolates were eradicated at the end of treatment, but only 4 of 28 in placebo group (41% vs. 14%, χ2 = 5.102, P = 0.024). As for patients, 11 of 27 patients treated with AA and 4 of 25 patients treated with placebo have eradication (41% vs.16%, χ2 = 3.871, P = 0.049). On 28-day follow-up, new resistance to amikacin was not detected.
Clinical effects
The CPIS of the two groups at the time of randomization reached the diagnostic standard of lung infection (8.1 ± 2.0 vs.8.5 ± 2.5, P = 0.526). By the end of treatment, the CPIS of the two groups had a tendency to decline. Compared to placebo, AA reduced CPIS significantly (4.2 ± 1.6 vs. 5.8 ± 2.1, P = 0.007). Other parameters of CPIS, i.e., temperature (37.0°C ± 1.3°C vs. 38.0°C ± 0.9°C, P = 0.002), oxygenation index (321 ± 120 mmHg vs. 261 ± 75 mmHg, P = 0.043), and the WBC (8.4 ± 6.1 × 103/mm3 vs. 12.1 ± 4.7 × 103/mm3, P = 0.031), were significantly reduced in AA group.
Nephrotoxicity was monitored by serum creatinine assessment. No significant difference of serum creatinine values between AA and placebo group was detected at the time of randomization (66.7 [37.0–81.6] μmol/L vs. 56.9 [35.7–74.8] μmol/L, P = 0.857) and day 7 (72.9 [32.4–93.3] μmol/L vs. 61.4 [34.8–77.4] μmol/L, P = 0.614). As to the other adverse effects, three and one episodes of bronchospasm were reported in AA and placebo group, respectively, during the treatment, which were relieved after treated with bronchodilator.
Followed up to 28 days, the clinical cure rate of VAP in survivors was similar between two groups (48% vs. 35%, χ2 = 0.585, P = 0.444); 13 of 27 patients were weaned from ventilation in AA group while 8 of 25 in placebo group (48% vs. 32%, χ2 = 1.406, P = 0.236). AA group demonstrated a trend toward lower 28-day mortality, but no difference was detected (22% vs. 32%, χ2 = 0.631, P = 0.427).
Discussion
This study revealed that as an adjunctive therapy of the VAP caused by MDR-GNB, AA effectively eradicated existing MDR organisms and improved CPIS without inducing new drug resistance or change in serum creatinine. However, improvement of morality was not found.
VAP is a challenging complication for critical illness. The global spread of MDR pathogens and particularly the MDR-GNB further complicates VAP therapy.[1,9,10] Pharmacokinetic studies showed that in MV patients, AA achieved very high local drug concentrations in the lung reaching 30–200-fold greater concentrations in the respiratory secretions compared to the relevant systemic levels.[2,3,5,11] Therefore, there is an increasing interest in the use of AA for MDR-GNB VAP patients to (a) overcome pharmacokinetic issues in the lung compartment with traditional systemic use, (b) avoid dose-dependent systemic side effects, and (c) prevent the emergence of MDR pathogens.
To our knowledge, few clinical trials studied the effects of AA in the treatment of VAP. In a retrospective observational study, Mohr et al.[6] evaluated the effect of adjunctive inhaled aminoglycoside (either aerosolized tobramycin or amikacin) in 22 surgical ICU patients with GNB VAP, reporting a cure rate of 59%. The cure rate was not impressive, and most of the treatment failures were in patients with previous episodes of VAP or MDR organisms. However, in another observational study, Czosnowski et al.[7] conducted a study that enrolled 53 ICU patients who were treated with aerosolized tobramycin or amikacin added to IV therapy, and the clinical cure rate was 73% despite a high rate of patients with previous treatment failure with SA alone or the presence of MDR organisms. Similarly, in a retrospective case-matched study in cancer patients with GNB VAP, Ghannam et al.[8] compared 16 patients treated with aerosolized aminoglycosides or colistin with 16 patients who received the same agents only IV. The overall clinical response rate was 100% compared to 55% in a matched group of patients who did not receive aerosolized therapy. However, the outcomes for colistin and aminoglycosides were not reported separately.
As discussed above, the results of previous trials were observational and controversial. In our study, the main endpoints were microbiologic. We believed that our results truly reflected the effect of AA on MDR-GNB. First, AA leads deposition of amikacin in respiratory tract secretions, culturing the sputum samples rich in amikacin may lead to false negative results due to the in vitro effect of inhibiting bacteria growth by high concentration of amikacin in the sputum. However, we do not hold the view that the eradication of MDR-GNB in our study was an in vitro effect of amikacin, as Gram staining was added to routine culture of sputum sample in this study. Only no growth in culture and no visible organisms seen on Gram staining could be defined as “eradication.” The combination of these two methods would minimize the errors in this study.[11] Second, the choice of SA during the treatment was based on culture results and susceptibility test. Similar coverage and combination of SA were prescribed in two groups. Therefore, we do not ascribe the eradication of MDR-GNB to the effect of SA.
The second endpoints were clinical effects. Microbial eradication in AA group was associated with an improvement in clinical manifestation. The severity of respiratory infection can be quantified by CPIS. By the end of treatment, compared to placebo group, CPIS of AA group decreased significantly. The parameters in CPIS such as WBC, temperature, and oxygenation index also showed obvious improvements, demonstrating that the addition of AA facilitated the infection control of VAP. However, the improvement in microbial eradication and infection control did not translate into a statistically significant improvement in cure rate, weaning rate, and mortality.[11,12] This might result from two major factors: (1) this study enrolled critically ill patients. The severity of illness was confirmed by high levels of patients’ mean age and APACHE II scores, and also by long time of ICU stay and MV before enrollment. Several factors including severity of infections, cause of MV, and primary and underlying diseases determined the prognosis of critically ill VAP patients. Even though AA effectively decreased the bacterial burden of pulmonary and alleviated the clinical manifestation of VAP, it might fail to turn around the primary and underlying diseases and eliminate the cause of MV. Thus, the prognosis of patients could not improve significantly; (2) the small sample size and the short follow-up time of this study may be one of the reasons why we found no positive results. In addition, the leave of eight patients (withdrew or transferred to another facility) might cause bias.
During the study, new resistance to amikacin or change in serum creatinine was not detected. These findings were consistent with previous studies and often interpreted by the pharmacokinetic characteristics of AA, mainly the high pulmonary concentrations and extremely low blood concentrations of amikacin due to the local drug delivery. The high pulmonary concentrations not only exceed the MIC but also surpass the relevant mutant-prevention concentration of the pathogens. As a result, the emergence of new resistance could be prevented.[4,13] However, two aspects need to be taken into account in the interpretation of this and previous study results: (1) the follow-up time may be not enough to detect new resistance and nephrotoxicity; (2) serum creatinine was a common and almost the only biomarker employed to evaluate the injury of kidney in the type of studies; this may be insufficient to detect the amikacin-related nephrotoxicity early, comprehensively, and accurately.[6,7,8,11]
This study contributes to the existing literature in the following aspects. First, strict inclusion criteria and procedure were set for the study to ensure that the included patients were truly MDR-GNB infected patients, rather than colonization or contamination.[14,15] Second, before enrollment, the expert team carefully examined the clinical and bacteriological data of each patient, to ensure that they were definitely diagnosed with VAP. CPIS at randomization of two groups all reached 8, implying that it was likely to be infection of deep lung parenchyma, rather than bacteria colonization or ventilator-associated tracheobronchitis.[16] Third, to optimize the drug delivery and to improve the deposition of amikacin particles in the deep lung tissue, a nebulization procedure protocol including operational specification, nebulizer type, drug formulation, and ventilator settings were optimized and standardized before the beginning of this study and carefully followed up during the study.[17,18,19,20,21] We believed that these three improvements make our results to be a better evaluation of the therapeutic effects of AA on VAP caused by MDR-GNB.
This study has several limitations, within which two need to be taken into account particularly. The first one is the study design of single center, small sample size, short follow-up time, and a 13.33% dropout rate as mentioned above. The second one is the amikacin formulations, the currently available formulations of amikacin are not intended for aerosolized use in China, and the same puzzle troubled doctors and researchers worldwide. The IV formulation of amikacin has been adopted off-label for the aerosolized use in clinical trial and treatment of VAP patients in previous publications.[4,6,7,8,11,12] Given the paucity of specialized formulation, after obtaining consent from the ethics committee and every enrolled patient or his/her proxy, the same approach was employed by our study team. The freshly prepared amikacin solutions were used in this study, and adverse events had been monitored. Three episodes of mild bronchospasm were reported in AA group during the study, the probability that it was related to the IV formulation of amikacin cannot be ruled out.
In conclusion, as an adjunctive therapy of MDR-GNB VAP, AA successfully eradicated existing MDR organisms and provided improvement of CPIS, without inducing new resistance to amikacin or change in serum creatinine. However, an improvement of mortality was not found. Large-scale clinical trials are needed to confirm these findings.
Financial support and sponsorship
This work was funded by grants of the Natural Science Foundation of Hubei Province (No. 2015CFB695) and the Natural Science Foundation of Wuhan University (No. 2042015kf0152).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
References
- 1.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–111. doi: 10.1093/cid/ciw353. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein I, Wallet F, Nicolas-Robin A, Ferrari F, Marquette CH, Rouby JJ. Lung deposition and efficiency of nebulized amikacin during Escherichia coli pneumonia in ventilated piglets. Am J Respir Crit Care Med. 2002;166:1375–81. doi: 10.1164/rccm.200204-363OC. doi: 10.1164/rccm.200204-363OC. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein I, Wallet F, Robert J, Becquemin MH, Marquette CH, Rouby JJ. Lung tissue concentrations of nebulized amikacin during mechanical ventilation in piglets with healthy lungs. Am J Respir Crit Care Med. 2002;165:171–5. doi: 10.1164/ajrccm.165.2.2107025. doi: 10.1164/ajrccm.165.2.2107025. [DOI] [PubMed] [Google Scholar]
- 4.Poulakou G, Siakallis G, Tsiodras S, Arfaras-Melainis A, Dimopoulos G. Nebulized antibiotics in mechanically ventilated patients: Roadmap and challenges. Expert Rev Anti Infect Ther. 2017;2:1–19. doi: 10.1080/14787210.2017.1268052. doi: 10.1080/14787210.2017.1268052. [DOI] [PubMed] [Google Scholar]
- 5.Petitcollin A, Dequin PF, Darrouzain F, Vecellio L, Boulain T, Garot D, et al. Pharmacokinetics of high-dose nebulized amikacin in ventilated critically ill patients. J Antimicrob Chemother. 2016;71:3482–6. doi: 10.1093/jac/dkw313. doi: 10.1093/jac/dkw313. [DOI] [PubMed] [Google Scholar]
- 6.Mohr AM, Sifri ZC, Horng HS, Sadek R, Savetamal A, Hauser CJ, et al. Use of aerosolized aminoglycosides in the treatment of Gram-negative ventilator-associated pneumonia. Surg Infect (Larchmt) 2007;8:349–57. doi: 10.1089/sur.2006.041. doi: 10.1089/sur.2006.041. [DOI] [PubMed] [Google Scholar]
- 7.Czosnowski QA, Wood GC, Magnotti LJ, Croce MA, Swanson JM, Boucher BA, et al. Adjunctive aerosolized antibiotics for treatment of ventilator-associated pneumonia. Pharmacotherapy. 2009;29:1054–60. doi: 10.1592/phco.29.9.1054. doi: 10.1592/phco.29.9.1054. [DOI] [PubMed] [Google Scholar]
- 8.Ghannam DE, Rodriguez GH, Raad II, Safdar A. Inhaled aminoglycosides in cancer patients with ventilator-associated Gram-negative bacterial pneumonia: Safety and feasibility in the era of escalating drug resistance. Eur J Clin Microbiol Infect Dis. 2009;28:253–9. doi: 10.1007/s10096-008-0620-5. doi: 10.1007/s10096-008-0620-5. [DOI] [PubMed] [Google Scholar]
- 9.Qi F, Zhang GX, She DY, Liang ZX, Wang RT, Yang Z, et al. Healthcare-associated pneumonia: Clinical features and retrospective analysis over 10 years. Chin Med J. 2015;128:2707–13. doi: 10.4103/0366-6999.167294. doi: 10.4103/0366-6999.167294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei WJ, Yang HF, Ye Y, Li JB. New Delhi metallo-ß-lactamase-mediated carbapenem resistance: Origin, diagnosis, treatment and public health concern. Chin Med J. 2015;128:1969–76. doi: 10.4103/0366-6999.160566. doi: 10.4103/0366-6999.160566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer LB, Smaldone GC. Reduction of bacterial resistance with inhaled antibiotics in the Intensive Care Unit. Am J Respir Crit Care Med. 2014;189:1225–33. doi: 10.1164/rccm.201312-2161OC. doi: 10.1164/rccm.201312-2161OC. [DOI] [PubMed] [Google Scholar]
- 12.Kollef MH, Ricard JD, Roux D, Francois B, Ischaki E, Rozgonyi Z, et al. A randomized trial of the amikacin fosfomycin inhalation system for the adjunctive therapy of Gram-negative ventilator-associated pneumonia: IASIS Trial. Chest. 2016 doi: 10.1016/j.chest.2016.11.026. pii: S0012-369262463-7. doi: 10.1016/j.chest.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Palmer LB. Ventilator-associated infection: The role for inhaled antibiotics. Curr Opin Pulm Med. 2015;21:239–49. doi: 10.1097/MCP.0000000000000160. doi: 10.1097/MCP.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 14.Cook DJ, Johnstone J, Marshall JC, Lauzier F, Thabane L, Mehta S, et al. Probiotics: Prevention of severe pneumonia and endotracheal colonization trial-prospect: A pilot trial. Trials. 2016;17:377–86. doi: 10.1186/s13063-016-1495-x. doi: 10.1186/s13063-016-1495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russotto V, Cortegiani A, Raineri SM, Giarratano A. Bacterial contamination of inanimate surfaces and equipment in the Intensive Care Unit. J Intensive Care. 2015;3:54. doi: 10.1186/s40560-015-0120-5. doi: 10.1186/s40560-015-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craven DE, Hudcova J, Rashid J. Antibiotic therapy for ventilator-associated tracheobronchitis: A standard of care to reduce pneumonia, morbidity and costs? Curr Opin Pulm Med. 2015;21:250–9. doi: 10.1097/MCP.0000000000000158. doi: 10.1097/MCP.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery AB, Vallance S, Abuan T, Tservistas M, Davies A. A randomized double-blind placebo-controlled dose-escalation phase 1 study of aerosolized amikacin and fosfomycin delivered via the PARI investigational eFlow® inline nebulizer system in mechanically ventilated patients. J Aerosol Med Pulm Drug Deliv. 2014;27:441–8. doi: 10.1089/jamp.2013.1100. doi: 10.1089/jamp.2013.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhanani J, Fraser JF, Chan HK, Rello J, Cohen J, Roberts JA, et al. Fundamentals of aerosol therapy in critical care. Crit Care. 2016;20:269–84. doi: 10.1186/s13054-016-1448-5. doi: 10.1186/s13054-016-1448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dugernier J, Reychler G, Wittebole X, Roeseler J, Depoortere V, Sottiaux T, et al. Aerosol delivery with two ventilation modes during mechanical ventilation: A randomized study. Ann Intensive Care. 2016;6:73–82. doi: 10.1186/s13613-016-0169-x. doi: 10.1186/s13613-016-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassetti M, Luyt CE, Nicolau DP, Pugin J. Characteristics of an ideal nebulized antibiotic for the treatment of pneumonia in the intubated patient. Ann Intensive Care. 2016;6:35–43. doi: 10.1186/s13613-016-0140-x. doi: 10.1186/s13613-016-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubosky MN, Chen YF, Henriksen ME, Vines DL. Vibrating mesh nebulizer compared with metered-dose inhaler in mechanically ventilated subjects. Respir Care. 2017;62:391–5. doi: 10.4187/respcare.04823. doi: 10.4187/respcare.04823. [DOI] [PubMed] [Google Scholar]


