Abstract
Background:
Evaluating the hemodynamic status and predicting fluid responsiveness are important in critical ultrasound assessment of shock patients. Transthoracic echocardiography with noninvasive diagnostic parameters allows the assessment of volume responsiveness. This study aimed to assess the hemodynamic changes in the liver and systemic hemodynamic changes during fluid challenge and during passive leg raising (PLR) by measuring hepatic venous flow (HVF) velocity.
Methods:
This is an open-label study in a tertiary teaching hospital. Shock patients with hypoperfusion who required fluid challenge were selected for the study. Patients <18 years old and those with contraindications to PLR were excluded from the study. Baseline values were measured, PLR tests were performed, and 500 ml of saline was infused over 30 min. Parameters associated with cardiac output (CO) in the left ventricular outflow tract were measured using the Doppler method. In addition, HVF velocity and right ventricular function parameters were determined.
Results:
Middle hepatic venous (MHV) S-wave velocity was positively correlated in all patients with CO at baseline (r = 0.706, P < 0.01) and after volume expansion (r = 0.524, P = 0.003). CO was also significantly correlated with MHV S-wave velocity in responders (r = 0.608, P < 0.01). During PLR, however, hepatic venous S-wave velocity did not correlate with CO. For the parameter ΔMHV D (increase in change in MHV D-wave velocity after volume expansion), defined as (MHV DafterVE − MHV DBaseline)/MHV DBaseline × 100%, >21% indicated no fluid responsiveness, with a sensitivity of 100%, a specificity of 71.2%, and an area under the receiver operating characteristic curve of 0.918.
Conclusions:
During fluid expansion, hepatic venous S-wave velocity can be used to monitor CO, whether or not it is increasing. ΔMHV D ≥21% indicated a lack of fluid responsiveness, thus helping to decide when to stop infusions.
Keywords: Fluid Challenge, Hepatic Venous Flow, Venous Return
Introduction
Evaluation of hemodynamic status and prediction of fluid responsiveness are important in critical ultrasound assessment of shock patients. The gold standard for testing fluid responsiveness is a fluid challenge, in which a volume of fluid sufficient to increase the preload is infused over a short period of time and the response of the ventricle is tested. Although patients in shock are initially managed with basic resuscitation measures, bedside ultrasound should be performed if hemodynamic instability persists. A number of minimally invasive and noninvasive diagnostic parameters allow the assessment of volume responsiveness using transesophageal and transthoracic echocardiography.
Respiratory variation in inferior vena cava (IVC) diameter is a guide to fluid therapy.[1] Ultrasound evaluation of the IVC is classically performed using a subcostal view. Since the IVC has an angular appearance, IVC blood flow velocity cannot be used to measure the systemic venous return flow velocities. Since the distal portion of the IVC is intrahepatic and the liver is large, imaging through the liver from any lower right intercostal position provides an acoustic window to the IVC. This allows accurate measurement of the IVC diameter as well as hepatic venous flow (HVF) velocity from the same view. Anatomically, the hepatic vein joins the systemic venous blood flow. Thus, monitoring HVF can theoretically detect systemic venous return. As cardiac output (CO) is dependent on the return of venous blood,[2] it is speculated that HVF is related to both CO and systemic venous return.
Doppler ultrasound is used to measure the velocity of blood in the aorta, which can be converted into a volume, provided the diameter of the aorta is known. This technique makes the assumption that the angle of the ultrasound beam to the blood flow direction is the same as that of the transducer and probe. If the angle of ultrasound probe changes, the stroke volume (SV)/CO will not be accurately reflected. Hence, we tried to explore the Doppler waveform of the hepatic vein to reflect the fluid responsiveness.
The Doppler waveform of the hepatic vein shows a triphasic pattern in healthy controls, consisting of two anterograde flow peaks toward the heart and one retrograde flow peak toward the liver. HVF depends on right atrial pressure, thoracoabdominal pressure, and hepatic parenchymal compliance.[3] Abnormal waveforms are present in patients with severe tricuspid regurgitation, constrictive pericarditis, right ventricular hypokinesia, cirrhosis, atrial fibrillation, and thrombosis of IVC or hepatic veins.[4] This study hypothesized that HVF velocity is associated with system venous return. Thus, monitoring HVF may reveal its relationship with CO and help identify patients with fluid responsiveness. Pulsed wave Doppler ultrasound of the hepatic vein was used to assess HVF to identify systemic hemodynamic changes during fluid challenge and passive leg raising (PLR).
Methods
Ethical approval
This was an observational study undertaken in patients admitted to the Department of Critical Care Medicine, Peking Union Medical College Hospital (PUMCH). The study protocol was approved by the Ethics Committee of PUMCH (No. S-167). Informed consent was obtained from all patients and their family members before data were included in the study.
Patients
All patients with shock admitted to the 30-bed general Intensive Care Unit of the PUMCH between March 2015 and July 2015 were consecutively included in this study. Patients were included if they (1) had a systolic arterial pressure ≤90 mmHg (or a ≥40 mmHg reduction in systolic arterial pressure in known hypertensive patients) and at least one of the following signs: urinary flow ≤0.5 ml·kg−1·min−1 for ≥2 h, tachycardia ≥100 beats/min, or skin mottling; and (2) required fluid challenge, as determined by the attending physician.
Patients were excluded from the study if they were younger than 18 years old, or if they had a condition contraindicating PLR, including head trauma, the wearing of venous compression stockings, amputations of one or two lower limbs, or intra-abdominal hypertension (intra-abdominal pressure ≥16 mmHg).[5]
Study design
At baseline, the patients were placed in the semirecumbent position, and CO, hepatic vein flow velocities, and parameters related to right ventricular function were measured in the left ventricular outflow tract (LVOT) by Doppler ultrasonography.
To perform PLR tests, CO and HVF were measured in a semirecumbent position and 1–3 min after raising lower limbs.[6]
The patients then returned to the semirecumbent position, and after a 5-min rest, the PLR test was repeated and HVF velocities were measured.
After the PLR tests, 500 ml of saline was infused over 30 min. At the end of fluid infusion, CO was measured in the LVOT using the Doppler method, as were HVF velocities and parameters associated with right ventricular function [Figure 1].
Figure 1.
The test flow chart of this study. PLR: Passive leg raising; VE: Volume expansion; CO: Cardiac output; HVF: Hepatic venous flow; RV function: Right ventricular function.
Transthoracic echocardiography
TTE was performed using a Philips Envisor Ultrasound System (Philips Medical System, Suresnes, France) by a physician experienced in echocardiography and blinded to the study design and outcomes, who recorded the echocardiography data.
LVOT area was derived from the LVOT diameter, which was measured in parasternal long axis, at a mid-systolic frame, at 1 cm of aortic valve of the patient. LVOT area was calculated as SLVOT (cm2) = π × DLVOT2/4. Aortic velocity-time integral (VTILVOT) was measured using pulsed Doppler on a 5-chamber apical view, and VTILVOT was measured in 3- or 5-chamber view, with the sample volume placed approximately at 1 cm of the aortic valve. SV was calculated as VTILVOT × SLVOT. CO was calculated using the following formula: CO (ml/min) = SV × heart rate, and cardiac index (ml∙min−1∙m−2) as CO/body surface area.[7]
Tricuspid annular systolic velocity at the lateral wall (Sa), early diastolic velocity at the lateral wall (Ea), and late diastolic velocity at the lateral wall (Aa) were measured by the tissue-Doppler pulse wave.
M-mode annular systolic excursion plane (tricuspid annular plane systolic excursion) was measured by M-mode sample volume at the level of the basal right ventricular free wall.
IVC diameter was measured on a subcostal view. Mean echocardiographic parameters were the average of five measurements, regardless of the respiratory cycle. Fluid responsiveness was defined as a ≥15% increase in CO after fluid challenge when compared with baseline CO.
Scanning parameters for HVF were as follows: depth of the display 16.0 cm; one focal zone at the target vessel, dynamic range, 75 dB; and frame rate 31 Hz. Moreover, the sample volume was placed within the hepatic vein. Ultrasonograms of the middle hepatic vein (MHV) and IVC were obtained through a subcostal view. Pulse Doppler ultrasonography was used to measure the vascular flow of the MHV. MHV waveforms were classified as triphasic, biphasic without a reverse flow, and monophasic. Using a concurrent electrocardiography (ECG) tracing, the waves in the hepatic vein spectrum can be reliably correlated with the cardiac cycle. Atrial depolarization, which causes the P-wave on the ECG, corresponds to the beginning of the spectral Doppler A-wave. The Doppler S-waves occur between the ECG QRS complex and T-wave, and Doppler D-waves are seen following the ECG T-wave during cardiac diastole. Maximum flow velocities were recorded. Increase in change in MHV D-wave velocity after volume expansion (ΔMHV D) was calculated as (MHV DafterVE − MHV DBaseline)/MHV DBaseline× 100%.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD). Hemodynamic variables and Doppler parameters were compared between responders and nonresponders using independent-samples t-test. Hemodynamic variables and Doppler parameters were compared between baseline and after volume expansion, baseline, and during PLR using paired Student's t-test. The simple linear regression analysis was used to assess the correlation between MHV S-wave and CO. Univariate analyses were used to assess the relationship between middle hepatic vein flow and right ventricular fillings. The sensitivity, specificity, and positive and negative predictive values are expressed as mean and 95% confidence interval (CI). Receiver operating characteristic (ROC) curves were constructed to test the ability of PPV. A P < 0.05 was considered statistically significant. All statistical analyses were undertaken using the SPSS software package (version 16.0, SPSS, Chicago, IL, USA).
Results
Patient characteristics
Among the 44 patients enrolled in the present study, two patients with their echocardiographic parameters were unmeasurable because of technical difficulties. The study included 42 patients finally. The characteristics of the patients at baseline are summarized in Table 1. Of the 42 patients, 19 (45%) responded to fluid challenge, whereas 23 (55%) did not.
Table 1.
Characteristics of patients with shock at baseline (n = 42)
| Characteristics | Values |
|---|---|
| Age (years), mean ± SD | 59 ± 14 |
| Sex (male/female) | 22/20 |
| APACHE II score, mean ± SD | 18 ± 6 |
| Source of infection, n (%) | |
| Pneumonia | 12 (29) |
| Bacteremia | 20 (48) |
| Urinary | 2 (5) |
| Abdominal | 7 (17) |
| Unknown | 1 (2) |
| Vasopressors | |
| Norepinephrine, n (%) | 42 (100) |
| Norepinephrine dosage (µg·kg−1·min−1), mean ± SD | 0.34 ± 0.11 |
| Dobutamine, n (%) | 10 (24) |
| LVEF (%), mean ± SD | 53.0 ± 10.4 |
| Acute respiratory distress syndrome, n (%) | 16 (33) |
| Atrial fibrillation, n (%) | 13 (31) |
| Spontaneous breathing activity, n (%) | 30 (71) |
LVEF: Left ventricular ejection fraction; APACHE II: Acute Physiology and Chronic Health Evaluation II scores; SD: Standard deviation.
Effects of volume expansion and passive leg raising on middle hepatic vein flow and hemodynamics
Table 2 shows hemodynamic variables of the patients between baseline and during PLR of the 42 patients, and Table 3 shows hemodynamic variables of the patients between baseline and after volume expansion. At baseline, RV end-diastolic diameter was significantly lower in responders than in nonresponders (2.7 ± 0.3 cm vs. 3.2 ± 0.4 cm, P = 0.014). No other parameter differed in these two groups at baseline, and no parameter differed in responders and nonresponders after volume expansion.
Table 2.
Hemodynamic variables of patients with shock between baseline and during PLR
| Variables | Baseline | During PLR | t | P |
|---|---|---|---|---|
| Heart rate | ||||
| Responders (n = 19) | 87.5 ± 18.1 | 86.1 ± 19.2 | −0.132 | 0.893 |
| Nonresponders (n = 23) | 81.5 ± 16.4 | 80.5 ± 16.5 | 0.432 | 0.674 |
| Systolic arterial pressure (mmHg) | ||||
| Responders (n = 19) | 120.3 ± 17.2 | 127.3 ± 14.4 | −1.597 | 0.133 |
| Nonresponders (n = 23) | 118.7 ± 17.1 | 125.3 ± 15.2 | −1.357 | 0.202 |
| Diastolic artery pressure (mmHg) | ||||
| Responders (n = 19) | 60.4 ± 9.1 | 63.2 ± 9.5 | −1.317 | 0.212 |
| Nonresponders (n = 23) | 62.3 ± 8.4 | 65.2 ± 9.2 | −0.927 | 0.375 |
| Mean arterial pressure (mmHg) | ||||
| Responders (n = 19) | 89.1 ± 11.7 | 92.4 ± 9.4 | −1.529 | 0.150 |
| Nonresponders (n = 23) | 85.1 ± 10.1 | 91.2 ± 10.3 | −1.414 | 0.183 |
| VTIAo (cm) | ||||
| Responders (n = 19) | 18.3 ± 5.5 | 22.3 ± 6.3 | −7.335 | 0.000 |
| Nonresponders (n = 23) | 19.1 ± 4.8 | 21.7 ± 5.4 | −0.857 | 0.402 |
| Stroke volume (ml) | ||||
| Responders (n = 19) | 57.2 ± 22.8 | 69.2 ± 26.3 | −6.631 | 0.000 |
| Nonresponders (n = 23) | 61.3 ± 14.1 | 68.5 ± 15.4 | −1.122 | 0.284 |
| Cardiac output (L/min) | ||||
| Responders (n = 19) | 4.7 ± 1.9 | 5.5 ± 1.8 | −5.035 | 0.000 |
| Nonresponders (n = 23) | 5.0 ± 1.3 | 5.2 ± 1.5 | −1.091 | 0.244 |
| Middle hepatic vein S-wave (cm/s) | ||||
| Responders (n = 19) | 30.1 ± 10.2 | 31.2 ± 12.4 | −0.745 | 0.473 |
| Nonresponders (n = 23) | 33.9 ± 12.2 | 37.5 ± 15.3 | −1.802 | 0.090 |
| Middle hepatic vein D-wave | ||||
| Responders (n = 19) | 23.3 ± 6.1 | 26.2 ± 6.3 | −2.295 | 0.115 |
| Nonresponders (n = 23) | 24.6 ± 9.8 | 26.0 ± 10.7 | −1.345 | 0.302 |
| Middle hepatic vein A-wave (cm/s) | ||||
| Responders (n = 19) | 29.3 ± 11.1 | 22.7 ± 6.7 | −0.140 | 0.891 |
| Nonresponders (n = 23) | 25.5 ± 5.3 | 24.4 ± 7.2 | −0.695 | 0.529 |
| Middle hepatic vein S/D | ||||
| Responders (n = 19) | 1.3 ± 0.3 | 1.1 ± 0.1 | 1.241 | 0.313 |
| Nonresponders (n = 23) | 1.3 ± 0.3 | 1.1 ± 0.4 | 1.921 | 0.129 |
All results reported as mean ± SD. VTIAo: Aortic velocity-time integral; SD: Standard deviation; PLR: Passive leg raising.
Table 3.
Hemodynamic variables of patients with shock between baseline and after volume expansion
| Variables | Baseline | After volume expansion | t | P |
|---|---|---|---|---|
| Heart rate (beats/min) | ||||
| Responders (n = 19) | 87.5 ± 18.1 | 84.7 ± 18.2 | 2.473 | 0.029 |
| Nonresponders (n = 23) | 81.5 ± 16.4 | 79.8 ± 17.1 | 0.531 | 0.608 |
| Systolic arterial pressure (mmHg) | ||||
| Responders (n = 19) | 120.3 ± 17.2 | 126.2 ± 19.8 | −1.943 | 0.076 |
| Nonresponders (n = 23) | 118.7 ± 17.1 | 123.2 ± 14.4 | −0.765 | 0.462 |
| Diastolic artery pressure (mmHg) | ||||
| Responders (n = 19) | 60.4 ± 9.1 | 61.1 ± 9.3 | −0.361 | 0.721 |
| Nonresponders (n = 23) | 62.3 ± 8.4 | 62.3 ± 9.2 | 0.338 | 0.752 |
| Mean arterial pressure (mmHg) | ||||
| Responders (n = 19) | 89.1 ± 11.7 | 91.0 ± 12.4 | −1.145 | 0.275 |
| Nonresponders (n = 23) | 85.1 ± 10.1 | 86.3 ± 10.2 | −0.095 | 0.924 |
| VTIAo (cm) | ||||
| Responders (n = 19) | 18.3 ± 5.5 | 22.8 ± 5.3 | −7.781 | 0.000 |
| Nonresponders (n = 23) | 19.1 ± 4.8 | 22.1 ± 5.3 | −1.039 | 0.124 |
| Stroke volume (ml) | ||||
| Responders (n = 19) | 57.2 ± 22.8 | 70.1 ± 24.2 | −6.859 | 0.000 |
| Nonresponders (n = 23) | 61.3 ± 14.1 | 63.7 ± 15.2 | −0.194 | 0.236 |
| Cardiac output (L/min) | ||||
| Responders (n = 19) | 4.7 ± 1.9 | 5.9 ± 2.1 | −8.788 | 0.000 |
| Nonresponders (n = 23) | 5.0 ± 1.3 | 5.4 ± 1.4 | −1.025 | 0.244 |
| Middle hepatic vein S wave (cm/s) | ||||
| Responders (n = 19) | 30.1 ± 10.2 | 37.1 ± 12.5 | −3.717 | 0.003 |
| Nonresponders (n = 23) | 33.9 ± 12.2 | 34.8 ± 15.7 | −0.415 | 0.475 |
| Middle hepatic vein D wave (cm/s) | ||||
| Responders (n = 19) | 23.3 ± 6.1 | 25.4 ± 7.1 | −0.984 | 0.355 |
| Nonresponders (n = 23) | 24.6 ± 9.8 | 36.8 ± 15.1† | −6.429 | 0.000 |
| Middle hepatic vein A wave (cm/s) | ||||
| Responders (n = 19) | 29.3 ± 11.1 | 27.5 ± 4.2 | 0.085 | 0.933 |
| Nonresponders (n = 23) | 25.5 ± 5.3 | 25.5 ± 5.5 | 0.009 | 0.994 |
| Middle hepatic vein S/D | ||||
| Responders (n = 19) | 1.3 ± 0.3 | 1.5 ± 0.2 | −1.392 | 0.169 |
| Nonresponders (n = 23) | 1.3 ± 0.3 | 1.4 ± 0.3 | 1.401 | 0.181 |
| IVC diameter (cm) | ||||
| Responders (n = 19) | 1.5 ± 0.4 | 1.7 ± 0.4 | −3.578 | 0.004 |
| Nonresponders (n = 23) | 1.7 ± 0.6 | 1.8 ± 0.6 | 0.298 | 0.771 |
| TAPSE (cm) | ||||
| Responders (n = 19) | 1.8 ± 0.4 | 1.8 ± 0.4 | 0.589 | 0.547 |
| Nonresponders (n = 23) | 2.1 ± 0.3 | 2.1 ± 0.3 | 0.321 | 0.715 |
| RV end-diastolic diameter (cm) | ||||
| Responders (n = 19) | 2.7 ± 0.3 | 2.8 ± 0.7 | −0.586 | 0.579 |
| Nonresponders (n = 23) | 3.2 ± 0.4* | 3.2 ± 0.5 | 0.296 | 0.772 |
| Tricuspid valve | ||||
| E peak velocity (cm/s) | ||||
| Responders (n = 19) | 45.1 ± 14.5 | 53.1 ± 20.6 | 1.950 | 0.077 |
| Nonresponders (n = 23) | 51.7 ± 14.9 | 53.2 ± 15.1 | −0.782 | 0.451 |
| A peak velocity (cm/s) | ||||
| Responders (n = 19) | 51.5 ± 9.7 | 53.7 ± 12.2 | −0.782 | 0.448 |
| Nonresponders (n = 23) | 50.6 ± 10.2 | 49.7 ± 14.3 | −0.724 | 0.486 |
| Sa (cm/s) | ||||
| Responders (n = 19) | 11.6 ± 3.3 | 12.2 ± 4.3 | −0.973 | 0.352 |
| Nonresponders (n = 23) | 12.7 ± 3.2 | 12.0 ± 3.1 | 1.651 | 0.133 |
| Ea (cm/s) | ||||
| Responders (n = 19) | 8.4 ± 4.4 | 9.2 ± 4.2 | −0.696 | 0.501 |
| Nonresponders (n = 23) | 10.9 ± 5.2 | 11.2 ± 5.3 | −0.538 | 0.604 |
| Aa (cm/s) | ||||
| Responders (n = 19) | 13.9 ± 4.5 | 14.5 ± 4.5 | −0.432 | 0.674 |
| Nonresponders (n = 23) | 16.2 ± 5.7 | 16.9 ± 8.5 | −0.390 | 0.714 |
| E/Ea | ||||
| Responders (n = 19) | 6.1 ± 2.3 | 6.5 ± 2.7 | −0.292 | 0.771 |
| Nonresponders (n = 23) | 5.7 ± 3.2 | 5.4 ± 2.8 | 0.380 | 0.714 |
All results reported as mean ± SD. *P<0.05 comparing responders with nonresponders in baseline; †P<0.05 comparing responders with nonresponders after volume expansion. VTIAo: Aortic velocity-time integral; IVC: Inferior vena cava; TAPSE: Tricuspid annular plane systolic excursion; RV: Right ventricular; Sa: Tricuspid annular systolic velocity at the lateral wall measured by tissue-Doppler pulse wave; Ea: Early diastolic velocity at the lateral wall measured by tissue-Doppler pulse wave; Aa: Late diastolic velocity at the lateral wall measured by tissue-Doppler pulse wave; SD: Standard deviation.
In addition, variables in the two groups were compared at baseline and after volume expansion. In responders, MHV S-wave velocity was 30.1 ± 10.2 cm/s at baseline, increasing to 37.1 ± 12.5 cm/s after volume expansion (P = 0.003). In nonresponders, MHV S-wave velocity was similar at baseline and after volume expansion. Thus, in all patients, CO significantly correlated with MHV S-wave velocity at baseline (r = 0.706, P < 0.01) and after volume expansion (r = 0.524, P = 0.003). CO also correlated with MHV S-wave velocity at baseline and after volume expansion in responders (r = 0.608, P < 0.01).
Baseline MHV D-wave velocity was similar in responders and nonresponders (23.3 ± 6.1 vs. 24.6 ± 9.8, P = 0.793), but was significantly lower in responders than in nonresponders after volume expansion (25.4 ± 7.1 vs. 36.8 ± 15.1, P = 0.019). In nonresponders, MHV D-wave velocity differed significantly at baseline and after volume expansion (P < 0.01). ΔMHV D was significantly lower in responders than in nonresponders (8.6 ± 21.6 vs. 50.2 ± 24.9, P < 0.01). ΔMHV D was able to accurately detect <15% increases in CO on ROC curve analysis. Area under the curves (AUCs) for ΔMHV D and MHV DafterVE are shown in Figure 2. ΔMHV D >21% was associated with no increase in CO during volume expansion, with a sensitivity of 100% (95% CI: 77–100%), a specificity of 71% (95% CI: 49–90%), and an AUC of 0.918 ± 0.046. MHV DafterVE >31.4 cm/s was associated with no increase in CO during volume expansion, with a sensitivity of 73% (95% CI: 43–91%), a specificity of 84% (95% CI: 55–93%), and an AUC of 0.772 ± 0.090.
Figure 2.
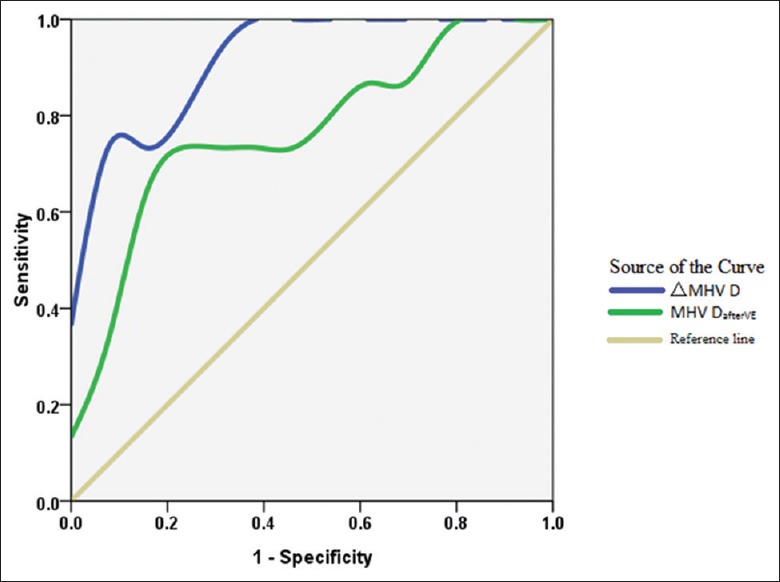
ROC curve analysis showing the relationship between CO and ΔMHV D. ΔMHV D was able to accurately detect <15% increase in CO on ROC curve analysis. ΔMHV D >21% was associated with no increase in CO during volume expansion, with a sensitivity of 100%, a specificity of 71%, and an AUC of 0.918. MHV DafterVE >31.4 cm/s was associated with no increase in CO during volume expansion, with a sensitivity of 73%, a specificity of 84%, and an AUC of 0.772. CO: Cardiac output; AUC: Area under the curve; ROC: Receiver operating characteristic; MHV: Middle hepatic venous.
The effects of PLR on CO and MHV S wave velocity were compared in responders and nonresponders. Responders to volume expansion showed a statistically significant difference between COPLR and COBaseline (5.5 ± 1.8 L/min vs. 4.7 ± 1.9 L/min, P < 0.01), but not between MHV SPLR and MHV SBaseline (31.2 ± 12.4 cm/s vs. 30.1 ± 10.2 cm/s, P = 0.473). In nonresponders, there were no statistically significant differences between COPLR and COBaseline or between MHV SPLR and MHV SBaseline.
Univariate analysis of the correlations between MHV wave velocity and right ventricular function variables showed significant negative correlations between MHV S-wave velocity and IVC diameter (P = 0.030) and between MHV D-wave velocity and IVC diameter (P = 0.035) at baseline [Table 4]. After volume expansion, there was a significant positive correlation between MHV A-wave velocity and tricuspid valve blood flow A-wave velocity (P = 0.009).
Table 4.
Univariate analyses of middle hepatic vein flow in relation to right ventricular filling
| Ranked variables | IVC diameter | TAPSE | RVEDD | Tricuspid valve | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E | A | E/A | Sa | Ea | Aa | E/Ea | ||||
| Baseline | ||||||||||
| MHV S | ↑* | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| MHV D | ↑† | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| MHV A | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| MHV S/D | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| Postventricular | ||||||||||
| MHV S | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| MHV D | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| MHV A | ↓ | ↓ | ↓ | ↓ | ↑‡ | ↓ | ↓ | ↓ | ↓ | ↓ |
| MHV S/D | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
↓: Nonsignificant; ↑: Significant; *↑: P = 0.030; †↑: P = 0.035; ‡↑: P = 0.009. MHV: Middle hepatic venous; IVC: Inferior vena cava; TAPSE: Tricuspid annular plane systolic excursion; RVEDD: Right ventricular end-diastolic diameter; Sa: Tricuspid annular systolic velocity at the lateral wall measured by tissue-Doppler pulse wave; Ea: Early diastolic velocity at the lateral wall measured by tissue-Doppler pulse wave; Aa: Late diastolic velocity at the lateral wall measured by tissue-Doppler pulse wave.
The hepatic vein waveform obtained in baseline and after volume expansion was shown in Figure 3.
Figure 3.
The hepatic vein waveform obtained in baseline and after volume expansion. (a) The patient who has fluid responsiveness: The S-wave increased with volume expansion. (b) The patient who lack fluid responsiveness: The D-wave increased with volume expansion, and the ΔMHV D, calculated as (38.4 − 17.4)/17.4 × 100% = 121%, far more than 21% as this study shows. MHV: Middle hepatic venous.
Discussion
This study showed that during volume expansion, MHV S-wave velocity was positively correlated with CO, and that ΔMHV D >21% indicated a complete lack of fluid responsiveness. That is, an increase in MHV S-wave velocity during volume expansion indicated that CO was also increasing, whereas a >21% increase from baseline in MHV D-wave velocity suggested not giving fluid bolus anymore.
Positive correlation between hepatic venous S-wave velocity and cardiac output
The normal hepatic vein wave form has three components: an antegrade S-wave, an antegrade D-wave, and a retrograde A-wave. During ventricular systole, the initial downward-sloping portion is generated by a decrease in right atrial pressure caused by the sucking effect created by the downward motion of the atrioventricular septum, which descends toward the cardiac apex during early systole. The lowest point, which occurs in systole, is the point at which negative pressure is minimally opposed and antegrade velocity is maximal.[8] The tricuspid valve remains closed, as according to Guyton's law, the systemic venous return is equal to CO such that the maximal antegrade velocity reflects the CO at this point.
Using the LiDCO™ plus and the Navigator™ technique, CO was found to correlate with the pressure gradient of venous return (dVR).[9] According to the law of Guyton, (venous return) Q = dVR/(venous return resistance) Rv; Q is venous return, which is equal to CO; dVR is the pressure gradient of venous return, which is equal to mean systemic filling pressure minus CVP; Rv is venous return resistance, but which cannot be directly measured. As blood flow velocity represents the pressure gradient, the hepatic venous S-wave velocity represents the gradient of pressure for venous return in the liver. Volume expansion may also immediately reduce RV because of improvements in red blood cell rheology/fluid viscosity resulting from hemodilution, because hemoglobin level is the primary determinant of blood viscosity.[10] This study found that hepatic venous S-wave velocity was positively correlated with CO, both at baseline and after fluid expansion. Similarly, studies of pulmonary vein waveforms have shown that CO was linearly correlated with pulmonary vein S-wave velocity.[11,12]
ΔMHV D ≥21% is indicative of lack of fluid responsiveness
Monitoring of ΔMHV D may be useful for confirming a lack of fluid responsiveness, allowing infusion during volume expansion to be halted as soon as possible. To our knowledge, there are few other clinical parameters those are associated with halting of fluid challenge. A CO increase <15% was found to be indicative of a lack of fluid responsiveness, indicating that less fluid should be used to reduce the risk of volume overload, i.e. mini-fluid challenge.[13] Although clinically the appearance of lung edema after fluid challenges and the aggravation of B-lines in lung ultrasound are clinical parameters that preclude further fluid challenges, we need more early warning signals. A cutoff of <10–15% change in CO after a fluid challenge defines a lack of fluid responsiveness and indicates that an individual patient's CO does not change significantly with a proper fluid bolus, and that also lung and systemic edema may occur or aggravate. ΔMHV D ≥21% can unify the concepts of fluid challenge and fluid therapy, indicating when infusion should be halted in shock patients undergoing fluid resuscitation.
The right atrium is thought to act as a conduit in diastole. Thus, hepatic venous diastolic flow should largely reflect tricuspid flow-velocity pattern. However, we did not observe a correlation between hepatic venous diastolic flow and tricuspid E velocity, perhaps because of the effect of right atrial compliance. When the right atrium is fully filled, its compliance should be reduced, with even a small amount of initial atrial emptying in early diastole leading to a steep drop in right atrial pressure. This drop in right atrial pressure should facilitate atrial filling from the IVC, increasing hepatic venous diastolic flow. Alterations in right atrial compliance due to infused fluid should also alter MHV D velocity. In pulmonary venous flow, the peak early diastolic velocity correlated strongly with mean left atrial pressure.[14] Furthermore, peak diastolic pulmonary venous forward flow velocity was found to be higher in patients with higher rather than lower mean pulmonary capillary wedge pressure. Although these studies showed the relationship between left atrial and pulmonary venous flow, the relationship between right atrial and HVF is similar.
During passive leg raising, hepatic venous S-wave velocity does not correlate with cardiac output
During PLR, we found that hepatic venous S-wave velocity did not correlate with CO as did volume expansion. The PLR technique can mobilize venous blood in the splanchnic reservoir as well as in the legs, significantly enhancing the sensitivity of the PLR test and reducing the number of false-negative results.[6] Under physiologic conditions, the volume of blood contained in capacitance veins in the legs and recruited during PLR is estimated to be close to 300 ml.[15] PLR induces an increase in splanchnic reservoir venous return, not the main part of systemic venous return.[16] HVF only represents the change in the splanchnic reservoir, which can influence the results of PLR tests. In addition, PLR instantly increases intra-abdominal pressure, which in turn reduces hepatic perfusion[17] and hepatic venous wave velocity, counteracting the PLR-induced increase on hepatic venous return.
Acquiring Doppler waveforms of the hepatic veins requires a bridge from the systemic venous return to the liver. Therefore, many echocardiographic parameters representative of right heart function were analyzed. Hepatic venous A-wave flow velocity was found to be related to tricuspid A-wave velocity. Right ventricular function has been analyzed by evaluating HVF.[18] This study also found that HVF was associated with IVC diameter and right ventricular end-diastolic diameter. Studies are needed to determine how to better apply HVF parameters to guide hemodynamic treatment in shock patients.
Study limitations
First, some patients were in atrial fibrillation. SV measurements were averaged over 5 readings, which was the limitation for CO calculations. Second, although this study found that hepatic venous S-wave flow rate was significantly correlated with CO, in the physics of meaning, S-wave velocity is the velocity (cm/s), different from the flow rate (L/min). Velocity is different from volumetric blood flow rate.[19] Several studies have analyzed the systolic velocity-time integrals (VTIs) of HVF for hepatic vein systolic filling fraction,[20] but the VTI of HVF was not analyzed in this study. During volume expansion, fluid responsiveness can be best determined by fast, simple, direct, and reliable parameters. Hepatic venous wave velocity can be measured directly, reducing the impact of technology and the measurer's subjectivity. Third, there are technical difficulties to obtain Doppler signals of MHV because that respiratory or abdominal movement probably changes velocities. Fourth, there are other limitations such as single expert operator and small sample size.
In conclusions, during fluid expansion, hepatic venous S-wave velocity can be used to monitor CO, whether or not it is increasing. ΔMHV D ≥21% indicated a lack of fluid responsiveness, thus helping to decide when to stop infusions. Although the hepatic venous S-wave could not be a substitute for CO during the fluid challenge test, the change of hepatic venous D-wave can tell us when to stop.
Financial support and sponsorship
This work is partially supported by a grant from the National Natural Science Foundations of China (No. 81501639).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
References
- 1.Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: Systematic review and meta-analysis. Ultrasound Med Biol. 2014;40:845–53. doi: 10.1016/j.ultrasmedbio.2013.12.010. doi: 10.1016/j.ultrasmedbio.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev. 1955;35:123–9. doi: 10.1152/physrev.1955.35.1.123. [DOI] [PubMed] [Google Scholar]
- 3.Bang DH, Son Y, Lee YH, Yoon KH. Doppler ultrasonography measurement of hepatic hemodynamics during Valsalva maneuver: Healthy volunteer study. Ultrasonography. 2015;34:32–8. doi: 10.14366/usg.14029. doi: 10.14366/usg.14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheinfeld MH, Bilali A, Koenigsberg M. Understanding the spectral Doppler waveform of the hepatic veins in health and disease. Radiographics. 2009;29:2081–98. doi: 10.1148/rg.297095715. doi: 10.1148/rg.297095715. [DOI] [PubMed] [Google Scholar]
- 5.Mahjoub Y, Touzeau J, Airapetian N, Lorne E, Hijazi M, Zogheib E, et al. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med. 2010;38:1824–9. doi: 10.1097/CCM.0b013e3181eb3c21. doi: 10.1097/CCM.0b013e3181eb3c21. [DOI] [PubMed] [Google Scholar]
- 6.Jabot J, Teboul JL, Richard C, Monnet X. Passive leg raising for predicting fluid responsiveness: Importance of the postural change. Intensive Care Med. 2009;35:85–90. doi: 10.1007/s00134-008-1293-3. doi: 10.1007/s00134-008-1293-3. [DOI] [PubMed] [Google Scholar]
- 7.Du W, Wang XT, Long Y, Liu DW. Efficacy and safety of esmolol in treatment of patients with septic shock. Chin Med J. 2016;129:1658–65. doi: 10.4103/0366-6999.185856. doi: 10.4103/0366-6999.185856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNaughton DA, Abu-Yousef MM. Doppler US of the liver made simple. Radiographics. 2011;31:161–88. doi: 10.1148/rg.311105093. doi: 10.1148/rg.311105093. [DOI] [PubMed] [Google Scholar]
- 9.Cecconi M, Aya HD, Geisen M, Ebm C, Fletcher N, Grounds RM, et al. Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med. 2013;39:1299–305. doi: 10.1007/s00134-013-2928-6. doi: 10.1007/s00134-013-2928-6. [DOI] [PubMed] [Google Scholar]
- 10.Funk DJ, Jacobsohn E, Kumar A. Role of the venous return in critical illness and shock: Part II-shock and mechanical ventilation. Crit Care Med. 2013;41:573–9. doi: 10.1097/CCM.0b013e31827bfc25. doi: 10.1097/CCM.0b013e31827bfc25. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura RA, Abel MD, Hatle LK, Tajik AJ. Relation of pulmonary vein to mitral flow velocities by transesophageal Doppler echocardiography. Effect of different loading conditions. Circulation. 1990;81:1488–97. doi: 10.1161/01.cir.81.5.1488. [DOI] [PubMed] [Google Scholar]
- 12.Masuyama T, Lee JM, Nagano R, Nariyama K, Yamamoto K, Naito J, et al. Doppler echocardiographic pulmonary venous flow-velocity pattern for assessment of the hemodynamic profile in acute congestive heart failure. Am Heart J. 1995;129:107–13. doi: 10.1016/0002-8703(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 13.Muller L, Toumi M, Bousquet PJ, Riu-Poulenc B, Louart G, Candela D, et al. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: The mini-fluid challenge study. Anesthesiology. 2011;115:541–7. doi: 10.1097/ALN.0b013e318229a500. doi: 10.1097/ALN.0b013e318229a500. [DOI] [PubMed] [Google Scholar]
- 14.Kuecherer HF, Muhiudeen IA, Kusumoto FM, Lee E, Moulinier LE, Cahalan MK, et al. Estimation of mean left atrial pressure from transesophageal pulsed Doppler echocardiography of pulmonary venous flow. Circulation. 1990;82:1127–39. doi: 10.1161/01.cir.82.4.1127. [DOI] [PubMed] [Google Scholar]
- 15.Rutlen DL, Wackers FJ, Zaret BL. Radionuclide assessment of peripheral intravascular capacity: A technique to measure intravascular volume changes in the capacitance circulation in man. Circulation. 1981;64:146–52. doi: 10.1161/01.cir.64.1.146. [DOI] [PubMed] [Google Scholar]
- 16.Xu Q, Yan J, Cai G, Chen J, Li L, Hu C. Effect of two volume responsiveness evaluation methods on fluid resuscitation and prognosis in septic shock patients. Chin Med J. 2014;127:483–7. [PubMed] [Google Scholar]
- 17.Diebel LN, Wilson RF, Dulchavsky SA, Saxe J. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma. 1992;33:279–82. doi: 10.1097/00005373-199208000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Ghio S, Recusani F, Sebastiani R, Klersy C, Raineri C, Campana C, et al. Doppler velocimetry in superior vena cava provides useful information on the right circulatory function in patients with congestive heart failure. Echocardiography. 2001;18:469–77. doi: 10.1046/j.1540-8175.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 19.Blanco P. Volumetric blood flow measurement using Doppler ultrasound: Concerns about the technique. J Ultrasound. 2015;18:201–4. doi: 10.1007/s40477-015-0164-3. doi: 10.1007/s40477-015-0164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagueh SF, Kopelen HA, Zoghbi WA. Relation of mean right atrial pressure to echocardiographic and Doppler parameters of right atrial and right ventricular function. Circulation. 1996;93:1160–9. doi: 10.1161/01.cir.93.6.1160. [DOI] [PubMed] [Google Scholar]




