Abstract
Background:
Triggering receptor expressed on myeloid cell-1 (TREM-1) may play a vital role in mammalian target of rapamycin (mTOR) modulation of CD8+ T-cell differentiation through the transcription factors T-box expressed in T-cells and eomesodermin during the immune response to invasive pulmonary aspergillosis (IPA). This study aimed to investigate whether the mTOR signaling pathway modulates the proliferation and differentiation of CD8+ T-cells during the immune response to IPA and the role TREM-1 plays in this process.
Methods:
Cyclophosphamide (CTX) was injected intraperitoneally, and Aspergillus fumigatus spore suspension was inoculated intranasally to establish the immunosuppressed IPA mouse model. After inoculation, rapamycin (2 mg·kg−1·d−1) or interleukin (IL)-12 (5 μg/kg every other day) was given for 7 days. The number of CD8+ effector memory T-cells (Tem), expression of interferon (IFN)-γ, mTOR, and ribosomal protein S6 kinase (S6K), and the levels of IL-6, IL-10, galactomannan (GM), and soluble TREM-1 (sTREM-1) were measured.
Results:
Viable A. fumigatus was cultured from the lung tissue of the inoculated mice. Histological examination indicated greater inflammation, hemorrhage, and lung tissue injury in both IPA and CTX + IPA mice groups. The expression of mTOR and S6K was significantly increased in the CTX + IPA + IL-12 group compared with the control, IPA (P = 0.01; P = 0.001), and CTX + IPA (P = 0.034; P = 0.032) groups, but significantly decreased in the CTX + IPA + RAPA group (P < 0.001). Compared with the CTX + IPA group, the proportion of Tem, expression of IFN-γ, and the level of sTREM-1 were significantly higher after IL-12 treatment (P = 0.024, P = 0.032, and P = 0.017, respectively), and the opposite results were observed when the mTOR pathway was blocked by rapamycin (P < 0.001). Compared with the CTX + IPA and CTX + IPA + RAPA groups, IL-12 treatment increased IL-6 and downregulated IL-10 as well as GM, which strengthened the immune response to the IPA infection.
Conclusions:
mTOR modulates CD8+ T-cell differentiation during the immune response to IPA. TREM-1 may play a vital role in signal transduction between mTOR and the downstream immune response.
Keywords: CD8+ T Effector Memory Cells, Immunosuppression, Invasive Pulmonary Aspergillosis, Mammalian Target of Rapamycin, Triggering Receptor Expressed on Myeloid Cell-1
Introduction
Invasive pulmonary aspergillosis (IPA) has become a leading cause of severe fungal infections in critically ill patients and has a high mortality rate, especially in patients with immune dysfunction such as those undergoing immunosuppressive or high-dose glucocorticoid therapy, and solid organ or hematopoietic stem cell transplantation patients.[1] The acquired immune response is mediated by T-lymphocytes, which determine whether the host can effectively clear the pathogen. CD8+ T-cells are a vital component of the acquired immune system.[2,3] Effector-phenotype CD8+ memory T-cells (Tem) can trigger a rapid, potent, and effective immune response to specific antigens in the early stages of infection. Investigating the mechanism of differentiation of CD8+ T cells in the early stages of infection could provide new insights into effectively controlling infection in IPA patients with immune dysfunction.[4,5]
The mammalian target of rapamycin (mTOR) signaling pathway regulates cell metabolism, survival, growth, and proliferation. The mTOR downstream signaling pathway contains two key substrates, the ribosomal S6 kinase (S6K) and the eukaryotic translational initiation factor 4E binding protein 1 (4E-BP1). When activated, mTOR phosphorylates S6K and 4E-BP1 to promote protein translation and is sensitive to rapamycin inhibition.[6] Recent studies have shown that mTOR may regulate T-cell differentiation and adaptive immunity following the binding of the T-cell receptor to antigen.[7] In preliminary experiments, we found that mTOR modulates lymphocyte differentiation in immunosuppressed animals with fungal infections by regulating the transcription factors T-box expressed in T-cells (T-bet) and eomesodermin.[8] T-bet is a key transcription factor regulating T-cell differentiation and belongs to the T-box transcription factor family.[9] Eomesodermin plays a synergistic role in T-bet function and regulates the differentiation of CD8+ T-cells into effector and memory T-cells. A recent study suggests that interleukin (IL)-12 can affect memory/effector CD8+ T-cell phenotype differentiation by regulating T-bet and eomesodermin.[10] IL-12 can also enhance the activity of mTOR kinase in naïve CD8+ T-cells.[11] In contrast, rapamycin inhibits the activity of mTOR kinase in CD8+ T-cells, thereby blocking the expression of T-bet and promoting the expression of eomesodermin. Increased expression of eomesodermin in CD8+ T-cells may promote the generation of memory T-cells, while high expression of T-bet could promote effector CD8+ T-cell proliferation.[12]
Triggering receptors expressed on myeloid cells (TREMs) were identified as a new family of receptors that regulate both innate and adaptive immune responses to infection.[13,14] TREM-1 is mainly expressed on neutrophils, monocytes, and macrophages. High TREM-1 levels are indicative of acute or chronic conditions caused by fungi or bacteria.[14,15] The TREM-1 extracellular domain can also be found as soluble TREM-1 (sTREM-1). It is proposed that sTREM-1 may arise directly from a splice variant or following the shedding of membrane-bound TREM-1 by metalloproteinase-mediated proteolytic cleavage.[16] sTREM-1 has proven to be a valuable diagnostic and prognostic marker since it is easily detected with immunochemical assays in biological fluid samples. Previous studies have found that sTREM-1 levels are elevated in plasma and bronchoalveolar lavage fluid from patients with bacterial or fungal pneumonia.[17,18] In preliminary studies, we found that plasma sTREM-1 is closely correlated with T-bet and eomesodermin levels in immunocompromised Aspergillus-infected rats, suggesting that TREM-1 may be involved in lymphocyte regulation and differentiation during fungal infection.[19]
Based on the theories and experimental results described above, we hypothesized that TREM-1 may play a vital role in mTOR modulation of CD8+ T-cell differentiation through the transcription factors T-bet and eomesodermin during the immune response to IPA.
Methods
Pathogen preparation
The strain of Aspergillus fumigatus, provided by the Department of Clinical Laboratory, Peking Union Medical College Hospital (PUMCH), was obtained from a case of pulmonary aspergillosis. Viable conidia (>95%) were obtained by growth on Sabouraud dextrose agar for 5 days at 35°C. Conidia were harvested in 10 ml 0.1% Tween-80/PBS and filtered through five layers of gauze. The concentration of conidia was adjusted to 1 × 108 CFU/ml by turbidity adjustment method.
Animal model preparation
Healthy 4-week-old female BALB/c mice, weighing 20 ± 5 g, were obtained from the Animal Facility Center, PUMCH. All animals were housed in a pathogen-free facility and treated according to protocols approved by the Institutional Animal Care and Use Committee of PUMCH. Thirty mice were randomly divided into the following groups (six mice per group): (1) control group: nontreated mice. (2) IPA group: animals were inoculated with 0.1 ml A. fumigatus conidia solution intranasally. (3) Immunosuppression plus aspergillosis group (cyclophosphamide [CTX] + IPA): CTX (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) was injected intraperitoneally at a dose of 200 mg·kg−1·d−1 for 5 consecutive days. Animals were then infected with A. fumigatus as mentioned above. (4) Immunosuppression plus aspergillosis plus rapamycin treatment group (CTX + IPA + RAPA): mice were given 2 mg·kg−1·d−1 rapamycin for the 7 consecutive days after intraperitoneal injection of CTX and A. fumigatus infection. (5) Immunosuppression plus IPA plus IL-12 treatment group (CTX + IPA + IL-12): mice were given 5 μg/kg IL-12 every other day for the 7 days following intraperitoneal injection of CTX and A. fumigatus infection. Blood samples were obtained by retro-orbital bleeding. Part of the lung tissue was minced and used for A. fumigatus culture, the rest was fixed with 4% formaldehyde, and paraffin-embedded tissue sections were stained with hematoxylin and eosin (H and E), Masson trichrome, and periodic acid-silver methenamine.
CD8+ effector memory T-cells counts and interferon-γ, mammalian target of rapamycin, and S6 kinase expression
Peripheral blood mononuclear cells were isolated from blood samples and counted by flow cytometry. Cells were then labeled with the following fluorescence-conjugated monoclonal antibodies: anti-rat CD45 PE (12-0451-81, eBioscience, San Diego, CA, USA), anti-rat CD8a APC (17-0081-81, eBioscience), anti-rat CD44 PE (25-0441-81, eBioscience), and anti-rat CD62L (104432, Biolegend). CD8+ Tem were sorted by flow cytometry (EPICS-XL, Beckman-Coulter, Indianapolis, IN, USA) and then stained for interferon (IFN)-γ (11-7311-81, eBioscience), mTOR (ab87540, Abcam, Cambridge, MA, USA), and S6K (ab32529, Abcam) expression.
Cytokine and soluble triggering receptors expressed on myeloid cell-1 quantification
Cytokines and sTREM-1 were quantified using the following ELISA kits as per the manufacturer's instructions; IL-6 (cat#: ab168538, Abcam), IL-10 (cat#: ab176665, Abcam), GM (cat#: 85-86051, Affymetrix Bioscience, San Diego, CA, USA), and sTREM-1 (cat#: SEA213Mu, USCN, Wuhan, China).
Statistical analysis
Data were analyzed using SPSS version 18.0 software (SPSS Inc., Armonk, NY, USA). All the data for the continuous variables in this study were proven to have normal distributions and are given as mean ± standard deviations (SD). Results for continuous variables that were not normally distributed are given as medians (interquartile ranges) and were compared using nonparametric tests. Student's t-test or analysis of variance followed by Bonferroni's test were used to determine the statistical significance (P) of differences. Pearson's correlation coefficient was used to analyze the correlation of two parameters. A value of P < 0.05 was considered statistically significant.
Results
Tissue culture and histology
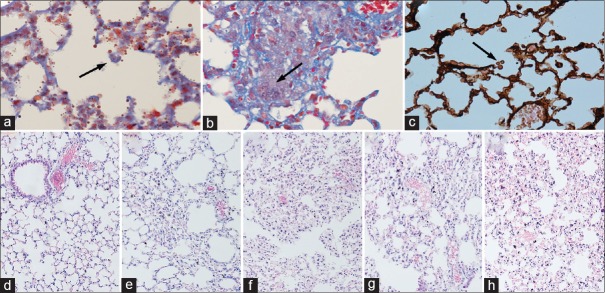
Viable A. fumigatus was cultured from lung tissue of both IPA and CTX + IPA mice treated with rapamycin or IL-12 or without treatment [Figure 1a–1c], while control mice were negative. Histological examination indicated that the lung tissue structure was intact in the normal control [Figure 1d]. In contrast, infiltration of inflammatory cells, hemorrhage, and interstitial lung tissue injury were found in the lungs of the IPA, CTX + IPA, CTX + IPA + IL-12, and CTX + IPA + RAPA groups [Figure 1e–1h]. Compared with the IPA group, Figure 1 suggests that CTX + IPA mice treated with rapamycin or IL-12 or without treatment had greater inflammation and hemorrhage in the interstitial lung tissue.
Figure 1.
Histology of lung tissue stained with H and E, Masson trichrome, and PASM. (a–c) The fungal spores of Aspergillus fumigatus. (d) Control animal. (e) Animal infected with IPA. (f) Immunosuppression plus aspergillosis group (CTX + IPA). (g) Immunosuppression plus IPA plus IL-12 treatment group (CTX + IPA + IL-12). (h) Immunosuppression plus aspergillosis plus rapamycin treatment group (CTX + IPA + RAPA). Original magnification: (a) Masson trichrome staining, original magnification ×200; (b) Masson trichrome staining, original magnification ×400; (c) PASM staining, original magnification ×600; (d–h) H and E, original magnification ×100. CTX: Cyclophosphamide; IPA: Invasive pulmonary aspergillosis; PASM: Periodic acid silver methenamine; IL: Interleukin.
Regulation of the mammalian target of rapamycin pathway
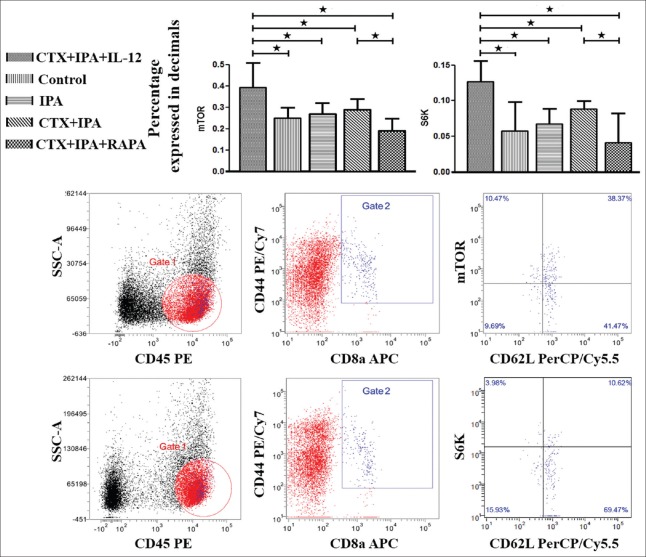
As shown in Figure 2, compared with the control group (0.25 ± 0.04, P = 0.004), IPA group (0.27 ± 0.04, P = 0.01), and CTX + IPA group (0.29 ± 0.04, P = 0.034), mTOR activity was significantly increased in the CTX + IPA + IL-12 group (0.39 ± 0.12). To verify that the induction of mTOR phosphorylation also led to its kinase activity, we monitored the kinetics of S6K, a direct target of mTOR kinase activity. As anticipated, IL-12 stimulation significantly enhanced the expression of S6K in CD8+ Tem (0.13 ± 0.03) compared with the control (0.06 ± 0.04, P < 0.001), IPA (0.07 ± 0.02, P = 0.001), and CTX + IPA (0.09 ± 0.01, P = 0.032) groups.
Figure 2.
IL-12 increases the expression of mammalian target of rapamycin and S6 kinase, which was blocked by rapamycin. The peripheral blood mononuclear cells obtained from IPA mice, CTX + IPA mice, CTX + IPA mice treated with IL-12 or rapamycin, and control animals were detected using flow cytometry 7 days after intranasal inoculation of Aspergillus fumigatus. Side scatter and CD8a were used to gate on CD8+ T-lymphocytes, and CD44+ CD45+ CD62+/− cells represent CD8+ effector memory T-cells. The data are presented as the mean ± standard deviation. *P < 0.05. CTX: Cyclophosphamide; IPA: Invasive pulmonary aspergillosis; IL: Interleukin.
Similarly, when we blocked mTOR activity by adding rapamycin during IPA infection, we found that the expression of mTOR was significantly lower in the CTX + IPA + RAPA group (0.19 ± 0.05) compared with the CTX + IPA (0.29 ± 0.04, P = 0.034) and CTX + IPA + IL-12 (0.39 ± 0.12, P < 0.001) groups. The expression of S6K was also significantly lower in the CTX + IPA + RAPA (0.04 ± 0.04) group than in the CTX + IPA (0.09 ± 0.01, P = 0.01) and CTX + IPA + IL-12 (0.13 ± 0.03, P < 0.001) groups.
Mammalian target of rapamycin modulates CD8+ T-cell differentiation and expression of triggering receptor expressed on myeloid cell-1
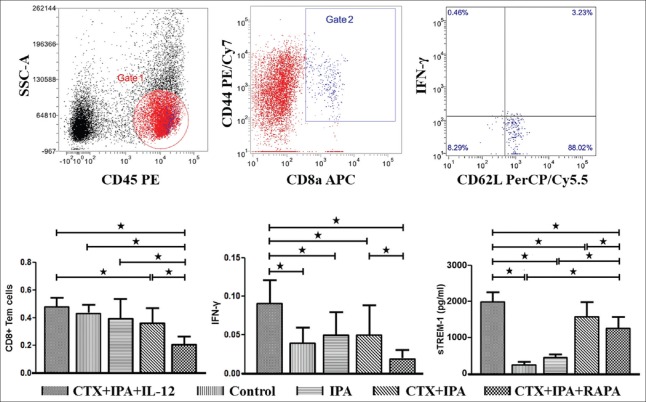
As shown in Figure 3, the proportion of CD8+ Tem, IFN-γ production, and serum sTREM-1 expression were significantly increased in the CTX + IPA + IL-12 group (0.47 ± 0.06, 0.09 ± 0.03, and 1876.91 ± 247.80, respectively) compared with the CTX + IPA group (0.35 ± 0.10, P = 0.024; 0.05 ± 0.04, P = 0.032; and 1537.64 ± 359.52, P = 0.017, respectively). The result indicates that the addition of IL-12 could improve CD8+ Tem differentiation, IFN-γ production, and resulted in robust serum sTREM-1 expression during IPA infection by activating the mTOR pathway.
Figure 3.
The proportion of CD8+ effector memory T-cells, expression of interferon-γ, and sTREM-1 levels were significantly increased by IL-12 stimulation but significantly decreased by rapamycin treatment. The peripheral blood mononuclear cells obtained from IPA mice, CTX + IPA mice, CTX + IPA mice treated with IL-12 or rapamycin, and control animals were detected using flow cytometry 7 days after Aspergillus fumigatus intranasal inoculation. Side scatter and CD8a were used to gate on CD8+ T-lymphocytes, and CD44+ CD45+ CD62+/- cells represented CD8+ effector memory T-cells. The data are presented as the mean ± standard deviation. *P < 0.05. CTX: Cyclophosphamide; IPA: Invasive pulmonary aspergillosis; IL: Interleukin; sTREM-1: Soluble triggering receptors expressed on myeloid cell-1.
More importantly, we also found that adding rapamycin could significantly decrease the proportion of CD8+ Tem (0.22 ± 0.05), IFN-γ production (0.02 ± 0.01), and serum TREM-1 expression (1214.45 ± 293.24) compared with the CTX + IPA (0.35 ± 0.10, P = 0.017; 0.05 ± 0.04, P = 0.039; and 1537.64 ± 359.52, P = 0.022, respectively) and CTX + IPA + IL-12 (0.47 ± 0.06, P < 0.001; 0.09 ± 0.03, P < 0.001; and 1876.91 ± 247.80, P < 0.001, respectively) groups. These results indicate that sustained mTOR activity is essential for CD8+ Tem differentiation, Type I effector functions, and serum sTREM-1 expression.
Alteration of inflammatory responses and severity of fungal infection
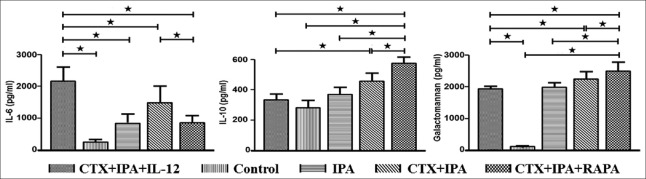
IL-6 levels reflect the inflammatory response, and IL-10 levels reflect anti-inflammatory responses. Figure 4 shows that the IL-12-treated group had the highest IL-6 levels (2053.91 ± 402.37 pg/ml), followed by the CTX + IPA (1426.57 ± 488.03 pg/ml, P = 0.036), CTX + IPA + RAPA (822.74 ± 211.30 pg/ml, P < 0.001), IPA (811.58 ± 247.03 pg/ml, P < 0.001), and control (245.65 ± 85.78 pg/ml, P < 0.001) groups. In contrast, the concentration of IL-10 showed the reverse trend. The IL-10 level of the IL-12-treated group (314.94 ± 36.97 pg/ml), as well as the control (267.60 ± 46.10 pg/ml) and IPA (350.93 ± 43.11 pg/ml) groups, were significantly lower than the CTX + IPA group (437.95 ± 53.41 pg/ml, P < 0.001) and especially the CTX + IPA + RAPA group (542.44 ± 37.32 pg/ml, P < 0.001).
Figure 4.
IL-12 significantly increased the IL-6 level, which was significantly decreased by rapamycin treatment. In contrast, the concentration of IL-10 and galactomannan showed the reverse trend. Plasma samples obtained from IPA mice, CTX + IPA mice, CTX + IPA mice treated with IL-12 or rapamycin, and control animals were detected by ELISA 7 days after Aspergillus fumigatus intranasal inoculation. The data are presented as the mean ± standard deviation. *P < 0.05. CTX: Cyclophosphamide; IPA: Invasive pulmonary aspergillosis; IL: Interleukin.
Galactomannan (GM) is used to demonstrate the severity of fungal infection in clinical practice. In this study, we found that, after A. fumigates inoculation, the level of GM was significantly increased in the IPA (1985.98 ± 152.79 pg/ml, P < 0.001), CTX + IPA (2251.06 ± 230.50 pg/ml, P < 0.001), CTX + IPA + IL-12 (1920.15 ± 86.16 pg/ml, P < 0.001), and CTX + IPA + RAPA (2496.87 ± 278.05 pg/ml, P < 0.001) groups compared with the control group (126.82 ± 10.31 pg/ml). However, as shown in Figure 4, treatment with IL-12 significantly decreased the level of GM compared with the CTX + IPA (P = 0.002) and CTX + IPA + RAPA (P < 0.001) groups.
Discussion
In the current study, we demonstrated that IL-12 increased the number and effector response (IFN-γ release) of CD8+ Tem and the level of sTREM-1 during IPA infection in CTX-induced immunocompromised animals, through increased expression and activity of the mTOR signal transduction pathway. Furthermore, inflammatory cytokines (IL-6) were increased and anti-inflammatory cytokines (IL-10) were decreased significantly, which markedly affect the fungal burden of the host. These effects could be blocked by the mTOR inhibitor rapamycin.
CD8+ T-cells play a vital role in the adaptive immune system. After infection, some of the CD8+ T-cells will display a memory phenotype and can survive long term. CD8+ effector memory T-cells (CD8+ Tem), memory T-cells with effector cell phenotype, will establish a rapid immune response when the host comes into contact with the same pathogen, which is crucial for patients who are at a high risk of repeated infections such as immunosuppressed patients.[5,20] The mTOR signaling pathway has the ability to sense cellular metabolic state, extracellular nutrient availability, presence of growth factors/cytokines, and control key cellular processes, including apoptosis/autophagy, proliferation, and cell growth that govern cell fate. Studies indicate that mTOR contributes to the proliferation and differentiation of T-cells in response to antigen stimulation.[7,21] mTOR inhibition by rapamycin induces CD4+ T-cell anergy and/or differentiation into FoxP3+ regulatory T-cells and attenuates mTOR kinase signaling in regulating CD8+ T-cell trafficking.[22,23] Consistent with these results, the current study demonstrated that the presence of IL-12 augments mTOR activity and influences CD8+ Tem proliferation and Type I effector maturation following immunosuppression by CTX and A. fumigatus infection. Effector functions induced by IL-12 treatment were significantly attenuated by adding the mTOR inhibitor rapamycin, confirming that sustained mTOR kinase activity is required for IL-12-programed proliferation and Type I effector function in CD8+ Tem in response to a fungal infection.
TREM-1 is largely known as an activating receptor on neutrophils and monocytes, playing an important role in amplification of the inflammatory response. Studies demonstrated that activation of TREM-1 on monocytes drove robust production of pro-inflammatory chemokines such as monocyte chemoattractant protein 1 and IL-8. Engagement of TREM-1 in combination with microbial ligands that activate toll-like receptors also synergistically increased the production of the pro-inflammatory cytokines, TNF-α and IL-1β.[24,25] Moreover, in primary human monocytes, TREM-1 activation was found to be upregulated in the context of host defense directed against bacterial and fungal infections. These cells had an improved ability to elicit T-cell proliferation and production of IFN-γ. The balance between the transcription factors T-bet and eomesodermin has been shown to determine effector and memory cell differentiation in CD8+ T-cells. Although in our previous studies, we found that both sTREM-1 and mTOR activity was significantly correlated with T-bet and eomesodermin levels in immunocompromised animals with IPA, little is known regarding its specific involvement in mTOR modulation of CD8+ T-cell differentiation and Type I effector function.[8,19] Consistent with previous studies, the findings of the current study demonstrated that, similar to the number and effector cell response of CD8+ Tem, the level of serum sTREM-1 expression was also significantly increased after IL-12 treatment and significantly decreased after adding rapamycin. These findings suggest that the mTOR signaling pathway is the central regulator of transcriptional programs which determine effector and/or memory cell differentiation in CD8+ T-cells through the transcription factors, T-bet and eomesodermin. Thus, sTREM-1 might be the molecular link between mTOR modulation and immune cell differentiation in the immunocompromised mice with a fungal infection. However, elucidation of a clear mechanism requires further study.
The current study also elucidated the final immune effects of the mTOR signal pathway. We found that IL-12 could increase plasma pro-inflammatory cytokine (IL-6) level, decrease the anti-inflammatory cytokine (IL-10), and reduce the fungal load (GM) significantly. The mTOR inhibitor rapamycin completely caused the opposite effects. These results indicated that the mTOR signaling pathway plays a vital role in CD8+ T-cell differentiation and function, resulting in changes of the immune response and fungal load in infected hosts with immunosuppression.
To summarize, mTOR could modulate CD8+ T-cell differentiation during immune responses to IPA. sTREM-1 may play a vital role in signal transduction between mTOR and the immune response. Whether this mechanism could be used as a target of antifungal immunotherapy in the future requires further study.
Financial support and sponsorship
The work was supported by the Beijing Municipal Natural Science Foundation (No. 7152119) and Chinese National Natural Science Foundation for Young Scholars (No. 81601657).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
References
- 1.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–50. doi: 10.1093/cid/civ933. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander-Miller MA. High-avidity CD8+ T cells: Optimal soldiers in the war against viruses and tumors. Immunol Res. 2005;31:13–24. doi: 10.1385/IR:31:1:13. doi: 10.1385/IR: 31:1:13. [DOI] [PubMed] [Google Scholar]
- 3.Zhang N, Bevan MJ. CD8(+) T cells: Foot soldiers of the immune system. Immunity. 2011;35:161–8. doi: 10.1016/j.immuni.2011.07.010. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams MA. Instant recall: A key role for effector-phenotype CD8+ memory T cells in immune protection. Immunity. 2013;38:1090–1. doi: 10.1016/j.immuni.2013.06.007. doi: 10.1016/j.immuni.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Plumlee CR, Sheridan BS, Cicek BB, Lefrançois L. Environmental cues dictate the fate of individual CD8+ T cells responding to infection. Immunity. 2013;39:347–56. doi: 10.1016/j.immuni.2013.07.014. doi: 10.1016/j.immuni.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobbold SP. The mTOR pathway and integrating immune regulation. Immunology. 2013;140:391–8. doi: 10.1111/imm.12162. doi: 10.1111/imm.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–91. doi: 10.1126/science.1139393. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 8.Cui N, Su LX, Wang H, Xiao M, Yang F, Zheng M, et al. mTOR modulates lymphocyte differentiation through T-bet and eomesodermin in response to invasive pulmonary aspergillosis in rats. Chin Med J. 2016;129:1704–10. doi: 10.4103/0366-6999.185858. doi: 10.4103/0366-6999.185858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–44. doi: 10.1038/ni1268. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 10.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–9. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 12.Keppler SJ, Rosenits K, Koegl T, Vucikuja S, Aichele P. Signal 3 cytokines as modulators of primary immune responses during infections: The interplay of type I IFN and IL-12 in CD8 T cell responses. PLoS One. 2012;7:e40865. doi: 10.1371/journal.pone.0040865. doi: 10.1371/journal.pone.0040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu M, Peng A, Sun M, Deng Q, Hazlett LD, Yuan J, et al. TREM-1 amplifies corneal inflammation after Pseudomonas aeruginosa infection by modulating toll-like receptor signaling and Th1/Th2-type immune responses. Infect Immun. 2011;79:2709–16. doi: 10.1128/IAI.00144-11. doi: 10.1128/IAI.00144-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–7. doi: 10.1038/35074114. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 15.Molad Y, Pokroy-Shapira E, Carmon V. CpG-oligodeoxynucleotide-induced TLR9 activation regulates macrophage TREM-1 expression and shedding. Innate Immun. 2013;19:623–30. doi: 10.1177/1753425913476970. doi: 10.1177/1753425913476970. [DOI] [PubMed] [Google Scholar]
- 16.Gómez-Piña V, Soares-Schanoski A, Rodríguez-Rojas A, Del Fresno C, García F, Vallejo-Cremades MT, et al. Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J Immunol. 2007;179:4065–73. doi: 10.4049/jimmunol.179.6.4065. doi: 10.4049/jimmunol.179.6.4065. [DOI] [PubMed] [Google Scholar]
- 17.Dimopoulou I, Pelekanou A, Mavrou I, Savva A, Tzanela M, Kotsaki A, et al. Early serum levels of soluble triggering receptor expressed on myeloid cells-1 in septic patients: Correlation with monocyte gene expression. J Crit Care. 2012;27:294–300. doi: 10.1016/j.jcrc.2011.06.013. doi: 10.1016/j.jcrc.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451–8. doi: 10.1056/NEJMoa031544. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 19.Cui N, Su L, Xiao M, Yang F, Liu D. The expression of triggering receptor expresses on myeloid cells receptor-1, T cell-specific transcription factor, and eomesodermin in Aspergillus infected immunosuppressed rats (in Chinese) Chin J Intern Med. 2016;55:40–4. doi: 10.3760/cma.j.issn.0578-1426.2016.01.010. doi: 10.3760/cma.j.issn.0578-1426.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Cui N, Wang H, Long Y, Liu D. CD8+ T-cell counts: An early predictor of risk and mortality in critically ill immunocompromised patients with invasive pulmonary aspergillosis. Crit Care. 2013;17:R157. doi: 10.1186/cc12836. doi: 10.1186/cc12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–77. doi: 10.1038/ni.1743. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–44. doi: 10.1016/j.immuni.2009.04.014. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–21. doi: 10.1038/ni.1603. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulevitch RJ, Tobias PS. Recognition of Gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 1999;11:19–22. doi: 10.1016/s0952-7915(99)80004-1. doi: 10.1016/S0952-7915(99)80004-1. [DOI] [PubMed] [Google Scholar]
- 25.Downey GP, Fukushima T, Fialkow L, Waddell TK. Intracellular signaling in neutrophil priming and activation. Semin Cell Biol. 1995;6:345–56. doi: 10.1016/s1043-4682(05)80005-4. doi: 10.1016/S1043-4682(05)80005-4. [DOI] [PubMed] [Google Scholar]