Abstract
Background:
Leakage of the intestinal mucosal barrier may cause translocation of bacteria, then leading to multiorgan failure. This study hypothesized that rhubarb monomers might protect the gut mucosal barrier in sepsis through junction proteins.
Methods:
Healthy male Sprague-Dawley rats (weighing 230–250 g) under anesthesia and sedation were subjected to cecal ligation and perforation (CLP). After surgical preparation, rats were randomly assigned to eight groups (n = 6 or 8 each group): sham group (Group A: normal saline gavage); sepsis group (Group B: normal saline gavage); Group C (intraperitoneally, dexamethasone 0.5 mg/kg) immediately after CLP surgery; and rhubarb monomer (100 mg/kg in normal saline)-treated groups (Group D: rhein; Group E: emodin; Group F: 3,8-dihydroxy-1-methyl-anthraquinone-2-carboxylic acid; Group G: 1-O-caffeoyl-2-(4-hydroxy-O-cinnamoyl)-D-glucose; and Group H: daucosterol linoleate). Animals were sacrificed after 24 h. Intestinal histology, lactulose, mannitol concentrations were measured, and zonula occludens (ZO)-1, occludin and claudin-5 transcription (polymerase chain reaction), translation (by Western blot analysis), and expression (by immunohistochemistry) were also measured.
Results:
Intestinal histology revealed injury to intestinal mucosal villi induced by sepsis in Group B, compared with Group A. Compared with Group A (0.17 ± 0.41), the pathological scores in Groups B (2.83 ± 0.41, P < 0.001), C (1.83 ± 0.41, P < 0.001), D (2.00 ± 0.63, P < 0.001), E (1.83 ± 0.41, P < 0.001), F (1.83 ± 0.75, P < 0.001), G (2.17 ± 0.41, P < 0.001), and H (1.83 ± 0.41, P < 0.001) were significantly increased. Lactulose/mannitol (L/M) ratio in Group B (0.046 ± 0.003) was significantly higher than in Group A (0.013 ± 0.001, P < 0.001) while L/M ratios in Groups C (0.028 ± 0.002, P < 0.001), D (0.029 ± 0.003, P < 0.001), E (0.026 ± 0.003, P < 0.001), F (0.027 ± 0.003, P < 0.001), G (0.030 ± 0.005, P < 0.001), and H (0.026 ± 0.002, P < 0.001) were significantly lower than that in Group B. ZO-1, occludin and claudin-5 transcription, translation, and expression in Group B were significantly lower than that in Group A (P < 0.001), but they were significantly higher in Groups C, D, E, F, G, and H than those in Group B (P < 0.05).
Conclusion:
Rhubarb monomer treatment ameliorated mucosal damage in sepsis via enhanced transcription, translation, and expression of junction proteins.
Keywords: Junction Proteins, Mucosal Barrier, Rhubarb Monomers, Sepsis
Introduction
Sepsis and septic shock are a major healthy challenge worldwide and associated with high morbidity and mortality.[1] Sepsis changes the intestinal integrity by increasing epithelial permeability and apoptosis and decreasing epithelial proliferation and mucosal integrity.[2] Injured gut mucosa enables bacterial translocation and their virulence factors, further contributing to activation of the host immune inflammatory defense mechanisms resulted in multiorgan failure.[3] Therapies for protecting gastrointestinal (GI) tract have been shown to improve patient's outcome.
The intestinal mucosa is the physical and metabolic barrier, regulated by an epithelial junctional complex referred to as the tight junction (TJ).[4] This barrier function is reflected by the intestinal permeability. TJ is a cell-to-cell adhesion structure and plays a role in the barrier function. Different molecules have been identified as the components of TJ such as transmembrane protein claudins, occludin, and peripheral membrane protein zonula occludens (ZO).[5] Abundant studies showed that claudin-5, a major determinant of the properties of the endothelial barrier, played an important role in the blood-brain barrier and blood–retina barrier. Occludin, an integral membrane protein of the epithelial TJ, is responsible for maintaining the integrity and barrier function of the TJ. ZO-1 belongs to the membrane-associated guanylate kinase family of proteins and usually acts as a scaffold and recruits various signaling molecules and the actin cytoskeleton to TJs.[6] Rhubarb, a traditional Chinese herb, has been used to treat diarrhea in traditional Chinese medicine,[7] but the pharmacological mechanism is unclear. There were 21 monomers extracted from the rhubarb, and 5 of these 21 monomers (rhein, emodin, 3,8-dihydroxy-1-methyl-anthraquinone-2-carboxylic acid, 1-O-caffeoxyl-2 (4-hydroxy-O-cinnamoyl)-β-D-glucose, and daucosterol linoleate) have shown efficiency in vitro.[8] We hypothesized that rhubarb monomers could protect GI mucosal barrier via modulation of transcription, translation, and expression of junction proteins in sepsis.
Methods
Experimental animals
Healthy adult male Sprague-Dawley rats (weighing 230–250 g) were purchased from the Animal Experiment Center of Second Military Medical University (Shanghai, China). The rats were allowed to acclimatize in the laboratory for one week before experiments. Care and handling of the animals were in accordance with the National Institute of Health Guidelines (Institute of Laboratory Animal Resources). All experimental protocols were performed according to the guidelines of the Ethical Committee for Animal Use of the Second Military Medical University. The Ethics Committee of Biomedicine Research of the Second Military Medical University had approved this study. In our preexperiment, a total of 30 rats were used for survival study. The rats were randomized into the sham group, the cecal ligation and perforation (CLP) group, and the rhubarb-treated CLP group (n = 10 in each group). In our experiment, animals were randomized to eight groups: sham group and seven other groups (details presented later). For the investigation of the intestinal histology and junction protein expression, at least 6 rats per group were used. For measuring the intestinal permeability, at least 8 rats per group were used.
Surgical preparation
The rats were anesthetized by an abdominal injection of 3% pentobarbital sodium (30 mg/kg). Surgery was performed under sterile conditions. A midline abdominal incision was performed and the cecum was exposed and the distal 1/3 was ligated with a 3–0 silk. At the end of the appendix, two holes were punctured with an 18-gauge needle. The cecum was then replaced in its original position within the abdomen, which was closed in two layers (in sham group, cecum was replaced). Surgery lasted for 20 min. Warm Ringer's lactate (50 ml/kg) with buprenorphine (0.01 mg/kg) was administered subcutaneously via abdominal wall. Immediately after the surgical operation, animals received gavage of normal saline in the sham-operated group (Group A) and sepsis (Group B); or dexamethasone intraperitoneally injected with dexamethasone (0.5 mg/kg; Group C); and rhubarb monomer (100 mg/kg diluted in 5 ml normal saline)-treated groups (Group D: rhein; Group E: emodin; Group F: 3,8-dihydroxy-1-methyl-anthraquinone-2- carboxylic acid; Group G: 1-O-caffeoyl-2-(4-hydroxy -O-cinnamoyl)-D-glucose; and Group H: daucosterol linoleate). The rhubarb dose was selected based on a previous study.[9] They were housed individually with free access to water without food. All of the rats were sacrificed at 24 h after gavage.[10,11]
Histological studies
The intestinal tract was washed with normal saline. Five-centimeter terminal ileum tissue was harvested and fixed with 4% paraformaldehyde. After dehydration, the ileum was embedded in paraffin, sliced, and then stained with hematoxylin and eosin. Under light microscopy (×100), intestinal mucosal morphology was observed [Table 1].
Table 1.
Gastrointestinal pathological score criteria
| Score | Criterion |
|---|---|
| 0 | Mucosal layer was intact with compact villi |
| 1 | Mild mucosal atrophy, necrosis and bleeding, mild neutrophil infiltration, and mucosal layer was intact |
| 2 | Moderate mucosal atrophy, necrosis and bleeding, moderate neutrophil infiltration, and mucosal layer was intact |
| 3 | Severe mucosal atrophy, necrosis and bleeding, moderate, massive neutrophil infiltration, and mucosal layer was damaged |
| 4 | Widespread mucosal necrosis and bleeding, neutrophil infiltration was complete, and mucosal layer disappeared |
Measurement of intestinal permeability
After emptying the urinary bladder, the rats were administered an intragastric test solution consisting of 100 mg lactulose and 50 mg mannitol diluted in 2 ml water. Rats were transferred into a metabolic cage immediately, and urine was collected for the next 24 h. The urine of rat was then stored in a container with a conservative. The total volume of each collection was measured and an aliquot was stored at −20°C until further use.[12]
The determination of lactulose and mannitol was based on a fully enzymatic method. Lactulose was hydrolyzed into fructose and galactose by β-galactosidase. The formed fructose was further quantitatively converted through three consecutive enzymatic reactions, in the presence of hexokinase, glucosephosphate isomerase, and glucose 6-phosphate dehydrogenase, to gluconate-6-phosphate and nicotinamide adenine dinucleotide phosphate (NADPH). The formed NADPH in the reactions was assessed by measurement of changes in absorbance at 340 nm.[13]
Mannitol assay was based on formaldehyde production after periodic acid oxidation. Periodic acid (0.5 ml) was added to the urine sample (2 ml) and allowed to stand at room temperature for 10 min. Stannous chloride (0.125 mol/L) was added, and the tube was shaken until a milky precipitate developed. The precipitate was dissolved in 5 ml of chromotropic reagent and vigorously shaken, and the tube was then placed in a boiling water bath for 30 min. After cooling, the sample was diluted to 25 ml with distilled water and allowed to stabilize at 25°C in a water bath. The absorbance was read at 570 nm.[14]
Expression of junction protein
Immunohistochemical analysis
The expression of proteins (claudin-5, ZO-1, and occludin) was analyzed by a two-step EnVision/horseradish peroxidase (HRP) technique, following the manufacturer's instructions. Polyclonal rabbit antihuman antibody (dilution 1:150) was acquired from Abcam Inc., (Cambridge, MA, USA). Paraffin sections were dewaxed in water. A pH 6.0, 0.01 mol/L citrate buffer was used to thermally induce repair, and 0.03% H2O2 was used to inhibit endogenous peroxidase at room temperature. The sections were incubated with a 20% normal goat serum at room temperature and at an appropriate dilution of primary antibody at 37°C for 2h. After additional incubation with EnVision reagent (HRP/R) and development with 3,3’-diaminobenzidine after 8–12 min, hematoxylin staining was carried out. After blow-drying, they were mounted on resin and visualized under a microscope. The nucleus stained violet blue and brownish yellow suggesting that the intramembrane structures were positive. Immunohistochemistry was carried out using medical image quantitative analysis system (Qiu Wei Inc., Shanghai, China).
Quantitative polymerase chain reaction analysis
Tissue total RNA was extracted with Trizol (Invitrogen Life Technologies, Carlsbad, CA, USA) and reversely transcribed to cDNA by BIOTAQ PCR Kit (Bioline Life Company, Boston, Massachusetts, USA) followed the manufacturer's instructions. Rat claudin-5 specific primers (NCBI Reference Sequence: NM_031701.2) used were: 5’-ATCGGTGAAGTAGCCACCAA-3’ (forward) and 5’-CTGCCCTTTCAGGTTAGCAG-3’ (reverse); Rat ZO-1-specific primers (NCBI Reference Sequence: NM_001106266.1) used were: 5’-GAAGGGGATGTTGTCCTGAA-3’ (forward) and 5’-GTAGCCCGCTCATCTCTTTG-3’ (reverse); occludin-specific primers (NCBI Reference Sequence: NM_031329.2) used were: 5’-ATCGGTGAAGTAGCCACCAA-3’ (forward) and 5-CTGCCCTTTCAGGTTAGCAG-3’ (reverse); β-actin (NCBI Reference Sequence: NM_031144.3) was used as a control to normalize messenger RNA (mRNA) expression level of the protein, and the primers were 5’-TTGCTGACAGGATGCAGAAG-3’ (forward) and 5’-CAGTGAGGCCAGGATAGAGC -3 (reverse). The polymerase chain reaction (PCR) reaction was set at 50°C (2 min), 95°C (5 min), 95°C (15 s) for a total of 45 cycles with a final extension at 60°C for 45 s. RT-PCR reactions were performed by the default PCR cycle on a sequence detection system ABI prism 7500 (Applied Biosystems Inc., USA). The results were analyzed using 2−ΔCT data analysis, ΔCT = CT value of target gene − CT value of internal reference (β-actin), mRNA relative expression = 2−ΔCT × 100%.
Western blot analysis
The ileum epithelial tissue was weighed, cut into small fragments, and transferred into tubes and then into a prerefrigerated protein extractor, homogenized with a homogenizer until complete lysis was achieved. Lysates were centrifuged in a precooled centrifuge (14,000 ×g, 15 min). The supernatant was immediately transferred into a centrifuge tube and stored for later use. Proteins were analyzed using the bicinchoninic acid method (Pierce, Rockford, IL, USA). A standard curve of protein concentration (μg/ml) was plotted by measuring the absorbance of corrected BSA standard protein at 562 nm. We used a standard curve to quantify the protein concentration of the sample. Using BioRad electrophoresis apparatus (mini protean 3 cell. BIO-RAD, Hercules, California, USA), the sample was subjected to a 20 mA constant current electrophoresis until the dye was nearly at the top of the separating gel. Next, a 60 mA constant current electrophoresis was carried out with the bromophenol blue until the dye reached the bottom of the gel. Gel electrophoresis was performed at 4°C. Gel was slightly cleaned with 1x transfer buffer. Under 30 mA constant current conditions, it was stored at 4°C overnight. The film contained the primary antibody (rabbit polyclonal anti-occludin, anti-ZO-1, and anti-claudin-5: Abcam Inc., Cambridge, MA, USA), which was added and left overnight at 4°C, to facilitate antigen-antibody binding. HRP-labeled secondary antibody (anti-rabbit IgG; Abcam Inc.) was added to bind with the primary antibody and HRP-labeled anti-biotin antibody based on molecular weight standards. The membranes were incubated at room temperature for 1 h and then were transferred to the reaction mixture. After excess solution was removed, the membrane was sandwiched between two plastic films and exposed to X-ray. The image was scanned and saved as a computer file. ImageJ analysis software (Qiu Wei Inc., Shanghai, China) was used for the digitalization of grayscales of specific bands.
Statistical analysis
Data were analyzed for normality using the Kolmogorov-Smirnov test. As no deviation from normality was detected, data were described as mean ± standard deviation (SD). Multiple comparisons were performed with one-way analysis of variance (ANOVA) with post hoc comparisons by least significant difference test, if significance was found, followed by post hoc test. The differences between two groups were detected with Student's t-test. The statistical analyses were performed using SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). A P < 0.05 was considered statistically significant.
Results
Sepsis model
All CLP-operated animals exhibited signs of sepsis in the form of tachypnea, piloerection, diarrhea, periorbital exudations, and lethargy. Sham-operated animals were active within their cages and showed no sign of disease. There was no dead rat in the sham group, while rhubarb therapy significantly increased survival rate of rhubarb-treated CLP group compared with the CLP group [Figure 1].
Figure 1.
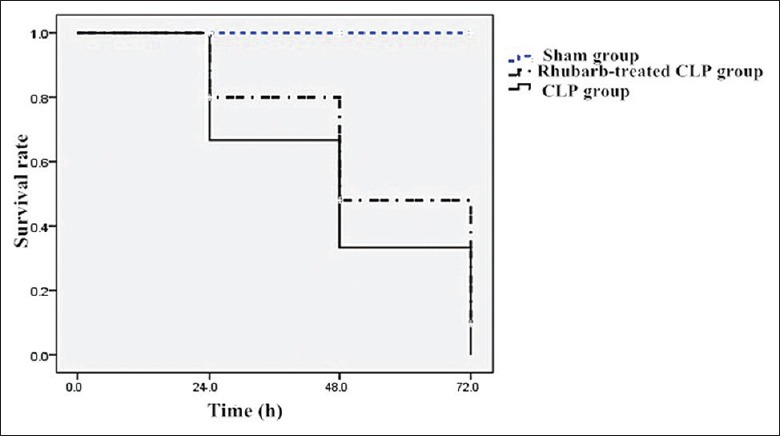
The survival rates in sham, CLP, and rhubarb-treated CLP groups. CLP: Cecal ligation and perforation.
Intestinal histology
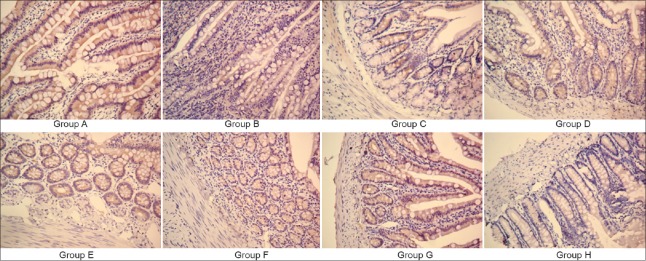
In the Group A, mucosal layer was intact with compact villi, whereas villi were severely damaged in Group B. There was edema within the intestinal villi of the subcutaneous gap, along with neutrophil infiltration. In the Group C, mucosal atrophy was significantly reduced compared with Group B. All Groups C, D, E, F, G, and H showed attenuated epithelial damage and neutrophil infiltration and partially protected villi [Figure 2a–2h]. Compared with Group A (0.17 ± 0.41), the pathological scores in Groups B (2.83 ± 0.41, t = 8.90, P < 0.001), C (1.83 ± 0.41, t = 5.23, P < 0.001), D (2.00 ± 0.63, t = 5.76, P < 0.001), E (1.83 ± 0.41, t = 5.23, P < 0.001), F (1.83 ± 0.75, t = 5.23, P < 0.001), G (2.17 ± 0.41, t = 6.28, P < 0.001), and H (1.83 ± 0.41, t = 5.23, P < 0.001) were significantly increased. Compared with Group B, the pathological scores in Groups C (t =5.23, P < 0.001), D (t = 3.14, P < 0.001), E (t = 5.23, P < 0.001), F (t = 5.23, P < 0.001), G (t =2.62, P < 0.001), and H (t = 5.23, P < 0.001) were significantly decreased [Figure 2i].
Figure 2.
Hematoxylin and eosin staining in rat intestinal mucosa (slice thickness 5 μm; original magnification ×100): (a) Group A; (b) Group B; (c) Group C; (d) Group D; (e) Group E; (f) Group F; (g) Group G; and (h) Group H. (i) Mucosal pathological scores among groups. Data are shown as mean ± SD. *P < 0.05, versus Group B; †P < 0.05, versus Group A; ‡P < 0.05, versus Group C. SD: Standard deviation.
Intestinal permeability
Lactulose/mannitol (L/M) ratio in Group B (0.046 ± 0.003) was significantly higher than in Group A (0.013 ± 0.001, t = 24.00, P < 0.001), while L/M ratios in Groups C (0.028 ± 0.002, t = 12.79, P < 0.001), D (0.029 ± 0.003, t = 12.07, P < 0.001), E (0.026 ± 0.003, t = 14.57, P < 0.001), F (0.027 ± 0.003, t = 14.56, P < 0.001), G (0.030 ± 0.005, t = 11.83, P < 0.001), and H (0.026 ± 0.002, t = 14.17, P < 0.001) were significantly lower than that in Group B. However, the Groups C (t = 11.21, P < 0.001), D (t = 11.93, P < 0.001), E (t = 9.36, P < 0.001), F (t = 9.39, P < 0.001), G (t = 12.20, P < 0.001), and H (t = 9.86, P < 0.001) showed higher permeability compared with Group A. No significant difference was found between Groups C and D (t = 0.73, P = 0.474), E (t = 1.79, P = 0.084), F (t = 1.78, P = 0.082), G (t = 0.93, P = 0.334), and H (t = 1.35, P = 0.192; Figure 3).
Figure 3.
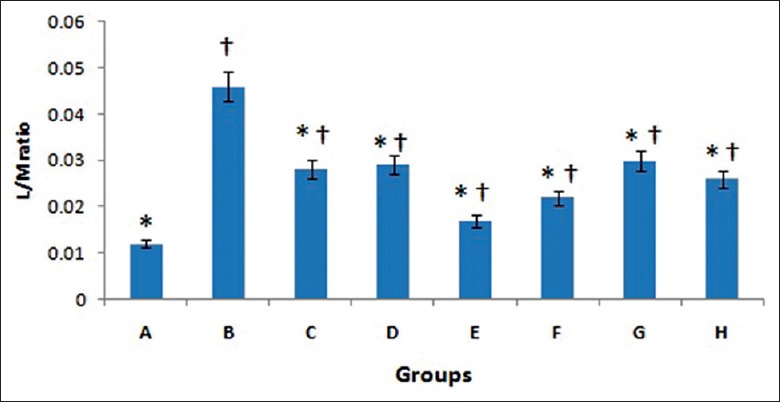
Lactulose/mannitol ratios among groups. Data are shown as mean ± SD. *P < 0.05, versus Group B; †P < 0.05, versus Group A. SD: Standard deviation.
Relative messenger RNA expression of zonula occludens-1, occludin, and claudin-5
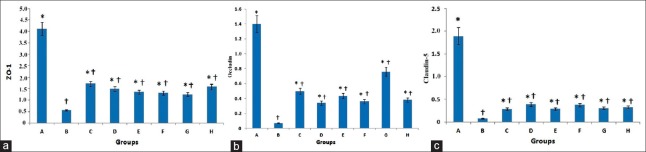
Compared with Group A, the relative mRNA expressions of ZO-1 in Group B (t = 11.73, P < 0.001), C (t = 7.94, P < 0.001), D (t = 8.72, P < 0.001), E (t = 9.12, P < 0.001), F (t = 9.27, P < 0.001), G (t = 9.47, P < 0.001), and H (t = 8.36, P < 0.001) were significantly decreased. Compared with Group B, the relative mRNA expressions of ZO-1 in Groups C (t = 3.76, P = 0.001), D (t = 3.02, P = 0.004), E (t = 2.62, P = 0.012), F (t = 2.47, P = 0.018), G (t = 2.25, P = 0.030), and H (t = 3.38, P = 0.002) were significantly increased. There was no significant difference between Groups C and D (t = 0.77, P = 0.449), E (t = 1.12, P = 0.249), F (t = 1.31, P = 0.197), G (t = 1.53, P = 0.134), and H (t = 0.41, P = 0.686; Figure 4a).
Figure 4.
Relative messenger RNA expression of junction protein ZO-1 (a), occludin (b), and claudin-5 (c). Data are shown as mean ± SD. *P < 0.05, versus Group B; †P < 0.05, versus Group A. SD: Standard deviation. ZO-1: Zonula occludens-1.
Compared with Group A, the relative mRNA expressions of occludin in Groups B (t = 10.10, P < 0.001), C (t = 6.85, P < 0.001), D (t = 8.05, P < 0.001), E (t = 7.32, P < 0.001), F (t = 7.92, P < 0.001), G (t = 4.87, P < 0.001), and H (t = 7.68, P < 0.001) were significantly decreased. Compared with Group B, the relative mRNA expressions of occludin in Groups C (t = 3.25, P = 0.002), D (t = 2.04, P = 0.047), E (t = 21, P = 0.008), F (t = 2.18, P = 0.035), G (t = 5.23, P < 0.001), and H (t = 2.41, P = 0.021) were significantly increased. There was no significant difference between Groups C and D (t = 1.20, P = 0.235), E (t = 0.47, P = 0.639), F (t = 1.07, P = 0.291), G (t = 1.97, P = 0.055), and H (t = 0.84, P = 0.491; Figure 4b).
Compared with Group A, the relative mRNA expressions of claudin-5 in Groups B (t = 20.14, P < 0.001), C (t = 17.77, P < 0.001), D (t = 16.67, P < 0.001), E (t = 17.78, P < 0.001), F (t = 16.66, P < 0.001), G (t = 17.55, P < 0.001), and H (t = 17.22, P < 0.001) were significantly decreased. Compared with Group B, the relative mRNA expressions of claudin-5 in Groups C (t = 2.29, P = 0.028), D (t = 3.48, P = 0.001), E (t = 2.38, P = 0.023), F (t = 3.43, P = 0.001), G (t = 2.57, P = 0.014), and H (t = 2.88, P = 0.007) were significantly increased. There was no difference between Groups C and D (t = 1.18, P = 0.244), E (t = 1.17, P = 0.930), F (t = 0.08, P = 0.263), G (t = 0.27, P = 0.784), and H groups (t = 0.58, P = 0.562; Figure 4c).
Protein expression of zonula occludens-1, occludin, and claudin-5
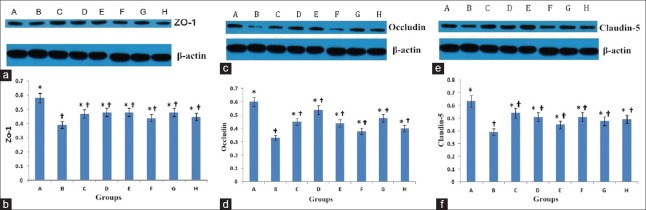
ZO-1 (194,000) was significantly decreased in Groups B (t = 10.78, P < 0.001), C (t = 5.95, P < 0.001), D (t = 5.03, P < 0.001), E (t = 5.29, P < 0.001), F (t = 7.63, P < 0.001), G (t = 5.47, P < 0.001), and H (t = 7.43, P < 0.001) as compared to the Group A. The expression of ZO-1 protein was significantly higher in Groups C (t = 4.83, P < 0.001), D (t = 5.48, P < 0.001), E (t = 5.49, P < 0.001), F (t = 3.14, P = 0.003), G (t = 5.31, P < 0.001), and H (t = 3.31, P = 0.002), compared with Group B [Figure 5a and 5b].
Figure 5.
Western blot analysis and band grayscale of ZO-1 (a and b), occludin (c and d); and claudin-5 (e and f) expressions. Data are shown as mean ± SD. *P < 0.05, versus Group B; †P < 0.05, versus Group A. SD: Standard deviation; ZO-1: Zonula occludens-1.
Occludin (59,000) was significantly decreased in Groups B (t = 17.87, P < 0.001), C (t = 9.58, P < 0.001), D (t = 3.65, P < 0.001), E (t = 10.50, P < 0.001), F (t = 14.67, P < 0.001), G (t = 7.61, P < 0.001), and H (t = 12.88, P < 0.001) as compared to the Group A. The expression of occludin protein was significantly higher in Groups C (t = 8.28, P < 0.001), D (t = 14.20, P < 0.001), E (t = 7.35, P < 0.001), F (t = 3.20, P < 0.001), G (t = 10.24, P < 0.001), and H (t = 4.98, P < 0.001), compared with Group B [Figure 5c and 5d].
Claudin-5 (24,000) was significantly decreased in Groups B (t = 16.22, P < 0.001), C (t = 6.41, P < 0.001), D (t = 8.26, P < 0.001), E (t = 12.29, P < 0.001), F (t = 8.44, P < 0.001), G (t = 10.34, P < 0.001), and H (t = 9.73, P < 0.001) as compared to the Group A. The expression of claudin-5 protein was significantly higher in Groups C (t = 9.81, P < 0.001), D (t = 7.98, P < 0.001), E (t = 3.95, P < 0.001), F (t = 7.77, P < 0.001), G (t = 5.87, P < 0.001), and H (t = 6.49, P < 0.001), compared with Group B [Figure 5e and 5f].
Immunohistochemistry
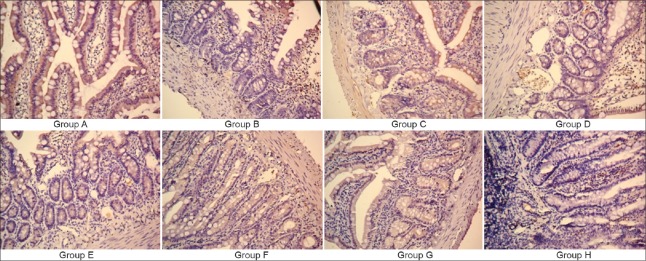
Light microscopy showed that ZO-1 protein in Group A was uniformly distributed in the intestinal epithelial cells at the apex of the connection, tightly packed and with smooth edges; in the Group B, ZO-1 was unevenly distributed, with rough edges and fading staining. In Groups C, D, E, F, G, and H, ZO-1 was less and unevenly distributed. In Groups D, E, F, and G, ZO-1 staining was darker and with continuous distribution [Figure 6].
Figure 6.
Immunohistochemical determination of the expression of junction protein ZO-1 (slice thickness 5 μm; original magnification ×250). ZO-1: Zonula occludens-1.
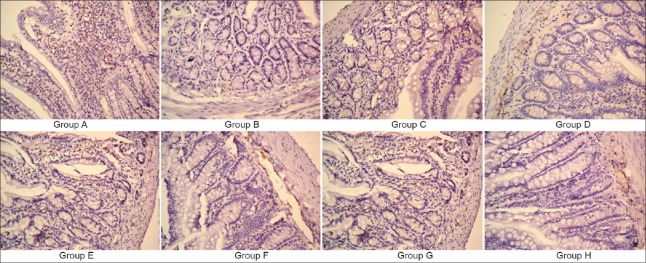
Light microscopy showed that occludin protein in Group A was distributed in a continuous uniform manner beneath the mucosal villi, expressing a relatively strong tan signal. In the Group B, only a very small amount of occludin in a scattered distribution was seen by sides of the villi, and Group B had the weakest expression. In Groups C, D, E, F, G, and H, there was a small amount of occludin aggregating in the sides of the villi in the mucous layer, the distribution was uneven and intermittent, and the expression of the occludin color was centered [Figure 7].
Figure 7.
Immunohistochemical determination of the expression of junction protein occludin (slice thickness 5 μm; original magnification ×250).
Claudin-5 protein in Group A was uniformly distributed at the junction of the apex of the intestinal epithelial cells and presented with a very strong tan color; in the Group B, the expression of claudin-5 protein faded, the distribution was uneven, and the expression gradually became less pronounced. In Groups C, D, E, F, G, and H, the expression level of claudin-5 protein signal color and expression was moderate [Figure 8].
Figure 8.
Immunohistochemical determination of the expression of junction protein claudin-5 (slice thickness 5 μm; original magnification ×250).
Discussion
This study found that rhubarb monomer treatment significantly ameliorated mucosal damage in sepsis, which was mainly due to increased junction protein transcription, translation, and expression. It has been suggested that the increased intestinal permeability demonstrated in critical patients was associated with the development of sepsis and multiple organ failure.[15] Modulation of this permeability might improve sepsis outcomes from literature review. Adherens junctions and TJs play an important role for protecting the intestinal barrier. TJs are made up of occludin, claudin, and ZO-1.[16] Although previous studies have thoroughly investigated the molecular structure of TJs,[17] studies related to sepsis-induced damage to intestinal mucosa and the ultrastructure of TJs are scarce.
ZO-1 plays an important role in junction assembly[18,19] and permeability.[20] In the absence of ZO-1 and -2, cells fail to form TJs.[21] Both ZO-1 and -2 knockout mice were embryonically lethal due to apoptosis and reduced yolk sac angiogenesis and proliferation.[22,23] As an important scaffold protein, ZO-1 regulates TJ assembly in the epithelial cells.[18] ZO protein is the only factor, which reversibly controls the effect of intercellular junction proteins on intestinal permeability.[24]
Occludin is a transmembrane protein that is capable of linking with ZO-1. It was first identified as a transmembrane protein component of TJs.[25] The main functions of occludin are regulation and sealing of TJs. Alterations in occludin expression contribute to the disruption of TJ barrier function in epithelial cell lines and animal models. Analysis of its level may reflect the degree intestinal bacteria affect TJs and cause barrier destruction.
Claudin-5 is expressed in vascular endothelia, as well as in uterine epithelium, stomach surface and glands, and throughout the intestine, without gradation along the crypt-to-villus surface axis.[26] Claudin-5 protein by forming chains of TJs creates a barrier between the epithelial cells, which, in cases of abnormal expression, can lead to epithelial cell structure damage, dysfunction, causing increased permeability of the intestinal epithelial cell gap. As a result, bacterial endotoxins and other macromolecules can penetrate the systemic circulation through TJs, leading to the development and progression of many diseases.[27]
The important role of junction protein ZO-1, occludin, and claudin-5 in the regulation of intestinal permeability has already been recognized, but its exact physiological role is still very unclear.[28] We analyzed the changes in junction protein and mRNA levels in the rat intestinal epithelium during sepsis. Our study showed that in the sham group, ZO-1, occludin, and claudin-5 were continuously and evenly distributed within the intestinal epithelial cells near the membrane. It was tightly packed, with smooth edges and strong positive expression. In the sepsis group, the connective tissue proteins showed light staining, uneven distribution, rough edges, decreased positive expression, indicating that sepsis resulted in damage to intestinal mucosal barrier. Western blot analysis showed that band grayscales of ZO-1, occludin, and claudin-5 protein were significantly decreased in the sepsis group, compared with the sham group, suggesting a decreased expression of connective tissue proteins. The PCR analysis of mRNA levels showed that in the sepsis group, the junction expression was significantly decreased, compared with the sham group. Immunohistochemistry and Western blot analyses confirmed tissue protein expression.
Our previous study showed that raw rhubarb could be used to control intestinal bacterial translocation, improve intestinal mucosa blood perfusion, and finally reduce MODS incidence in patients with severe sepsis.[7] However, raw rhubarb is a complex herb with a diverse chemical composition. Therefore, the medicinal ingredients of the raw rhubarb were extracted. The following five monomers were effective in minimizing the intensity of the increase in intestinal permeability in vitro: rhein, emodin, 3,8-dihydroxy-1-methyl-anthraquinone-2-carboxylic acid, 1-O- coffee acyl-2-(4-hydroxy-O-cinnamoyl)-β-D-glucose, and daucosterol linoleate.[8] Therefore, rhubarb monomer can significantly prevent the increase in mucosal permeability and enhance the expression of tight junctional protein, thus maintaining the functional integrity of intestinal microvascular endothelial cells, improving intestinal TJs, reducing intestinal damage, and protecting the intestinal mucosal barrier during sepsis. Further studies are necessary to test it in large animal studies and later might even in clinical trials.
Our study had some limitations related to the measurement of ZO-1, occludin, and claudin-5, and had no measurement of the related proteins such as ZO-2. In addition, the small number of animals limited the statistical power of the present study. We selected a fixed dose of each monomer while any dose-dependent effects were not investigated in this model.
In conclusion, rhubarb monomer treatment significantly ameliorated mucosal damage in sepsis primarily via enhanced transcription, translation, and expression of junction proteins.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundations of China (No. 81173402, No. 81571943, No. 81272137, No.30472270), the Shanghai Health System Advanced Suitable Technology Popularization Project (No. 2013SY070), the Traditional Chinese Medicine Research Fund of Shanghai Municipal Health and Family Planning Commission (No. 2010L051A).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
References
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Zheng S, Wang X, Shi Q, Wang X, Yuan S, et al. Carbon monoxide-releasing molecule-2 reduces intestinal epithelial tight-junction damage and mortality in septic rats. PLoS One. 2015;10:e0145988. doi: 10.1371/journal.pone.0145988. doi: 10.1371/journal.pone.0145988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20:214–23. doi: 10.1016/j.molmed.2013.08.004. doi: 10.1016/j.molmed.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B, Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr. 2009;102:687–93. doi: 10.1017/S0007114509289033. doi: 10.1017/S0007114509289033. [DOI] [PubMed] [Google Scholar]
- 5.Hamada K, Kakigawa N, Sekine S, Shitara Y, Horie T. Disruption of ZO-1/claudin-4 interaction in relation to inflammatory responses in methotrexate-induced intestinal mucositis. Cancer Chemother Pharmacol. 2013;72:757–65. doi: 10.1007/s00280-013-2238-2. doi: 10.1007/s00280-013-2238-2. [DOI] [PubMed] [Google Scholar]
- 6.Shen ZY, Zhang J, Song HL, Zheng WP. Bone-marrow mesenchymal stem cells reduce rat intestinal ischemia-reperfusion injury, ZO-1 downregulation and tight junction disruption via a TNF-a-regulated mechanism. World J Gastroenterol. 2013;19:3583–95. doi: 10.3748/wjg.v19.i23.3583. doi: 10.3748/wjg.v19.i23.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen DC, Wang L. Mechanisms of therapeutic effects of rhubarb on gut origin sepsis. Chin J Traumatol. 2009;12:365–9. doi: 10.3760/cma.j.issn.1008-1275.2009.06.008. [PubMed] [Google Scholar]
- 8.Cui YL, Zhang S, Tian ZT, Lin ZF, Chen DC. Rhubarb antagonizes matrix metalloproteinase-9-induced vascular endothelial permeability. Chin Med J. 2016;129:1737–43. doi: 10.4103/0366-6999.185859. doi: 10.4103/0366-6999.185859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan L, Cheng Y, Dang S, Zhang Z, Chen Y, Jia X. Preventive effect of emodin and astragalus polysaccharides on experimental hepatocarcinoma in rats (in Chinese) China Pharm. 2005;16:574–6. doi: 10.3969/j.issn. 1001-0408.2005.08.005. [Google Scholar]
- 10.Cui YL, Wang L, Tian ZT, Lin ZF, Chen DC. Effect of rhubarb pre-treatment on intestinal microcirculation in septic rats. Am J Chin Med. 2014;42:1215–27. doi: 10.1142/S0192415X14500761. doi: 10.1142/S0192415x14500761. [DOI] [PubMed] [Google Scholar]
- 11.Wan J, Shan Y, Shan H, Li G, Wang T, Guan J, et al. Thymosin-alpha1 promotes the apoptosis of regulatory T cells and survival rate in septic mice. Front Biosci (Landmark Ed) 2011;16:3004–13. doi: 10.2741/3894. doi: 10.2741/3894. [DOI] [PubMed] [Google Scholar]
- 12.Li K, Ning L, Li J, Yang B, Li Y. Effect of hepatocyte growth factor on intestinal permeability and bacterial translocation after small bowel transplantation in rat. Chin J Reparative Reconstr Surg. 2007;21:532–5. doi: 10.3321/j.issn:1002-1892.2007.05.022. [PubMed] [Google Scholar]
- 13.Dastych M, Dastych M, Jr, Novotná H, Cíhalová J. Lactulose/mannitol test and specificity, sensitivity, and area under curve of intestinal permeability parameters in patients with liver cirrhosis and Crohn's disease. Dig Dis Sci. 2008;53:2789–92. doi: 10.1007/s10620-007-0184-8. doi: 10.1007/s10620-007-0184-8. [DOI] [PubMed] [Google Scholar]
- 14.Di Leo V, D’Incà R, Diaz-Granado N, Fries W, Venturi C, D’Odorico A, et al. Lactulose/mannitol test has high efficacy for excluding organic causes of chronic diarrhea. Am J Gastroenterol. 2003;98:2245–52. doi: 10.1111/j.1572-0241.2003.07697.x. doi: 10.1111/j.1572-0241.2003.07697. [DOI] [PubMed] [Google Scholar]
- 15.De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: Effect of glutamine. Crit Care Med. 2005;33:1125–35. doi: 10.1097/01.ccm.0000162680.52397.97. doi: 10.1097/01.ccm.0000162680.52397.97. [DOI] [PubMed] [Google Scholar]
- 16.Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363:689–91. doi: 10.1056/NEJMcibr1007320. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 17.Dejana E. Endothelial cell-cell junctions: Happy together. Nat Rev Mol Cell Biol. 2004;5:261–70. doi: 10.1038/nrm1357. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 18.McNeil E, Capaldo CT, Macara IG. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2006;17:1922–32. doi: 10.1091/mbc.E05-07-0650. doi: 10.1091/mbc.E05-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, et al. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem. 2004;279:44785–94. doi: 10.1074/jbc.M406563200. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez S, Chavez Munguia B, Gonzalez-Mariscal L. ZO-2 silencing in epithelial cells perturbs the gate and fence function of tight junctions and leads to an atypical monolayer architecture. Exp Cell Res. 2007;313:1533–47. doi: 10.1016/j.yexcr.2007.01.026. doi: 10.1016/j.yexcr.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–54. doi: 10.1016/j.cell.2006.06.043. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 22.Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, et al. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell. 2008;19:2465–75. doi: 10.1091/mbc.E07-12-1215. doi: 10.1091/mbc.E07-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Kausalya PJ, Phua DC, Ali SM, Hossain Z, Hunziker W. Early embryonic lethality of mice lacking ZO-2, but Not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol Cell Biol. 2008;28:1669–78. doi: 10.1128/MCB.00891-07. doi: 10.1128/MCB.00891-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klaus DA, Motal MC, Burger-Klepp U, Marschalek C, Schmidt EM, Lebherz-Eichinger D, et al. Increased plasma zonulin in patients with sepsis. Biochem Med (Zagreb) 2013;23:107–11. doi: 10.11613/BM.2013.013. doi: 10.11613/BM.2013.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong Y, Smart EJ, Weksler B, Couraud PO, Hennig B, Toborek M. Caveolin-1 regulates human immunodeficiency virus-1 Tat-induced alterations of tight junction protein expression via modulation of the Ras signaling. J Neurosci. 2008;28:7788–96. doi: 10.1523/JNEUROSCI.0061-08.2008. doi: 10.1523/JNEUROSCI.0061-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watari A, Hasegawa M, Yagi K, Kondoh M. Checkpoint kinase 1 activation enhances intestinal epithelial barrier function via regulation of claudin-5 expression. PLoS One. 2016;11:e0145631. doi: 10.1371/journal.pone.0145631. doi: 10.1371/journal.pone.0145631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu LL, Lyu YQ, Jiang HT, Shan T, Zhang JB, Li QR, et al. Effect of alemtuzumab on intestinal intraepithelial lymphocytes and intestinal barrier function in cynomolgus model. Chin Med J. 2015;128:680–6. doi: 10.4103/0366-6999.151675. doi: 10.4103/0366-6999.151675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci. 2012;1258:25–33. doi: 10.1111/j.1749-6632.2012.06538.x. doi: 10.1111/j. 1749-6632.2012.06538. [DOI] [PMC free article] [PubMed] [Google Scholar]