Abstract
Human Papillomavirus (HPV) is the main cause of cervical cancer, which is the second most severe cancer of women worldwide, particularly in developing countries. Although vaccines against HPV infection are commercially available, they are neither affordable nor accessible to women in low income countries e.g. Africa. Thus, alternative cost-effective vaccine production approaches need to be developed. This study uses tobacco plants to express pentameric capsomeres of HPV that have been reported to generate elevated immune responses against HPV. A modified HPV-16 L1 (L1_2xCysM) protein has been expressed as a fusion protein with glutathione-S-transferase (GST) in tobacco chloroplasts following biolistic transformation. In total 7 transplastomic lines with healthy phenotypes were generated. Site specific integration of the GST-L1_2xCysM and aadA genes was confirmed by PCR. Southern blot analysis verified homogenous transformation of all transplastomic lines. Antigen capture ELISA with the conformation-specific antibody Ritti01, showed protein expression as well as the retention of immunogenic epitopes of L1 protein. In their morphology, GST-L1 expressing tobacco plants were identical to wild type plants and yielded fertile flowers. Taken together, these data enrich knowledge for future development of cost-effective plant-made vaccines against HPV.
Keywords: capsomeres, cervical cancer, GST, HPV-16 L1, Plant-made vaccines, plastid transformation
Abbreviations
- HPV
human papillomavirus
- GST
glutathione-s-transferase
- ELISA
enzyme-linked immunosorbent assay
- aadA
aminoglycoside 3'-adenylyltransferase
- L1_2xCysM
modified L1 gene
- PCR
polymerase chain reaction
- VLPs
virus-like particles
Introduction
Human Papillomavirus (HPV) is the major causative agent of cervical cancer associated with approximately 95% of cervical cancer cases, worldwide.1 It is the third most common cancer in women after breast and colorectal cancer and the second most frequent cause of cancer related death.2 Each year approximately 500,000 new cases of cervical cancer are reported throughout the world and majority of these are from developing countries.3 HPV infections are associated with about 10% of the worldwide cancer burden.4 Development and production of HPV vaccines at low cost, making them affordable and accessible in poor countries is therefore a necessity.
In meta-analyses of HPV type distribution it was revealed that HPV16 and HPV 18 are the predominant causes of invasive cervical cancer worldwide contributing to 70% of all cases.5 L1 is the major capsid protein of HPV which is capable of self-assembly into more complex molecular structures; such as virus like particles (VLPs) and capsomeres.5
The HPV virion is approximately 55nm in diameter and consists of an icosahedral capsid made up of 72 capsomeres containing a closed circular double stranded DNA genome.6 Its protein coding sequences are located on one strand of the double-stranded DNA and are divided into 2 main groups early E and late L, based on their location in the genome.7 The proteins: E1, E2, E4, E5, E6, E7 are nonstructural proteins, which are mainly involved in replication, transcription, transformation and facilitation of viral escape. The remaining 2, L1 and L2 are structural proteins that compose the capsid.8 Interestingly, it is found that L1 capsomeres alone can form virus-like particles (VLPs) that are morphologically identical to the real viral capsids9 but lack viral DNA or RNA. Therefore, the VLPs are completely noninfectious and non-oncogenic and are currently used successfully as vaccine candidates against HPV infections.10
VLP-based HPV vaccines are very immunogenic and have shown to prevent HPV infection in animals.11,12 Prophylactic human papillomavirus (HPV) L1 virus like particle (VLP) vaccines have been shown, in many clinical trials, to stimulate high titer anti-HPV serum antibody levels, to be well-tolerated and highly successful against this venereal disease caused by the different types of HPV.13 However, at present these vaccines are very expensive; they need delivery by intramuscular injection and require a continuous cold chain. Moreover, production costs related to fermenter-based production systems are considerably high. As a result these vaccines will be largely unavailable to people in resource poor countries, where more than 85% of cervical cancer cases occur.14 Therefore, there is a growing demand to develop cost-effective second generation vaccines that are easy to administer and provide long term protection.15
Plants as production platform have gained strong attention in the last decade.16 Plants offer the unique opportunity to be engineered as biofactories for the production of foreign proteins and they are discovered as an alternative and attractive source for vaccine production.17,18 The ability of plants to produce complex foreign proteins and secondary metabolites in an efficient, safe and economical way is the main reason why biopharming is gaining an increasing importance in plant biotechnology.16,19,20 Especially chloroplast transformation has emerged as a very precise alternative technique for biopharming.20-22 Plastid genetic engineering is considered particularly valuable due to numerous advantages which are mainly associated with biosecurity and subsequently with the regulatory issues of plant-made vaccines.23
Transplastomic plants are ideal for harvesting transgene products which are expressed in leaves before any of plant reproductive organs grow.24 Site specific integration in plastids is a great advantage compared to nuclear transformation25-27 evading possible epigenetic effects. Transplastomic plants address the risk of transgene spread by pollen as chloroplasts are maternally inherited in most plants28,29, although some recent studies show that there is small degree of paternal transmission of plastids in tobacco.30,31 Plastid encoded transgenes confer high levels of transgene expression and foreign protein accumulation which is due to the polyploid nature of the plastid genome.27,32
Recently, 2 licensed commercial vaccines have been introduced to the market for prevention of HPV infections, both of which are based on HPV L1 virus-like particles.14 However, these vaccines are associated with substantial manufacturing costs due to their expression and purification from eukaryotic cells i.e. insect cells or yeast. Therefore for resource-poor countries these vaccines remain unaffordable where more than 80% of all cervical cancer cases occur.33 Keeping the disease burden in developing countries in view, there is an urgent need for second-generation vaccines against cervical cancer that are cost-effective in production and affordable.
In the present study, we report the feasibility of producing a plant-based vaccine by expressing a modified HPV-16 L1 gene (L1_2xCysM) fused with glutathione-S-transferase (GST), in tobacco chloroplasts. The conformational assembly and immunogenicity of L1 capsomeres was successfully confirmed in vitro by antigen capture ELISA using a conformation specific monoclonal antibody.
Results
Plastid transformation vector
The vector pPNG1014_MCS120 served as a precursor to construct a transplastomic vector pPNGST-L1-T (Fig. 1), containing L1_2xCysM gene fused N-terminally to the sequence encoding for glutathione S-transferase (GST). Using the restriction sites NheI, AflII and NcoI, NheI the GST and L1_2xCysM were cloned into the precursor vector. The fused GST-L1_2xCysM gene was expressed by a cassette containing both, the promoters for the nuclear and plastid encoded RNA polymerases, the PrrnPEP and Prrn-62NEP respectively. For selection of transformants the aminoglycoside 3′-adenylyltransferase (aadA) gene was co-expressed under the same promoter. For homologous recombination within the tobacco chloroplast genome, the trnR served as right insertion site and trnN as left insertion site.
Figure 1.
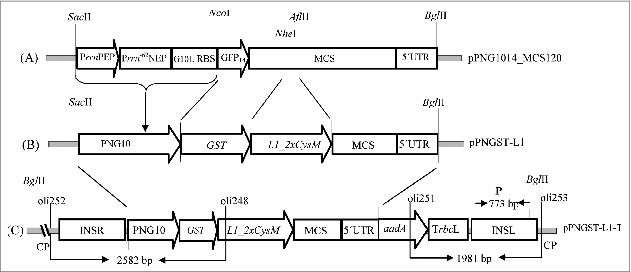
Schematic diagram of transformation vector construction (map not drawn to scale). (A) Precursor vector pPNG1014_MCS120 was used to clone transgenes. (B) Plasmid pPNGST-L1 obtained after the insertion of transgenes GST and L1_2xCysM in the precursor vector. (C) Final transformation vector pPNGST-L1-T for the transformation of plants, showing transgenes along with plastome flanks inserted within the tobacco plastid genome. PrrnPEP, plastid encoded polymerase promoter from rrn16 gene; Prrn-62NEP, nuclear encoded polymerase promoter; G10L RBS, ribosomal binding site from gene 10 leader sequence; GFP, first 14 amino acids of the green fluorescent protein; MCS, multiple cloning site; 5´ UTR, 5´ untranslated region; aadA, aminoglycoside 3'-adenylyltransferase; PNG10, cassette containing PrrnPEP, Prrn-62NEP and G10L RBS; CP, chloroplast DNA; GST, glutathione S-transferase gene; L1_2xCysM, modified L1 gene; TrbcL, terminator from large subunit of ribulose-bisphosphate carboxylase gene; INSR, right insertion site (trnR); INSL, left insertion site (trnN).
Chloroplast transformation, regeneration and morphology
The vector DNA was introduced by biolistic bombardment of 3 weeks old tobacco leaves (Petit Havana). After bombardment, leaves were cut and transferred to RMOP medium for selection on spectinomycin. After 3 weeks of incubation leaves from control plants and all untransformed explants on selection medium were bleached. Successfully transformed tissue produced green micro calli and showed rapid growth on selection medium. The leaflet pieces started to develop calli on its edges and after 2 more weeks small shoots were induced from calli. These transformed shoots were further cultured under the same selection and regeneration conditions for 6 weeks to achieve homoplasmy. All putatively transformed shoots were transferred to B5 rooting medium and after 4 weeks to the green house. Plants transformed with GST-L1_2xCysM gene showed normal morphology like wild type plants (Fig. 3). Transplastomic plants showed normal growth with green leaves, compared to phenotypes observed in earlier experiments.43 All the 7 generated transplastomic lines carrying GST-L1_2xCysM gene produced normal flowers. They set seeds by self-pollination which showed that flowers were completely fertile. From seeds obtained from T0 transplastomic plants, T1 progeny was obtained by germinating these seeds on spectinomycin containing medium. Similarly, T2 plants were raised from seeds of T1 generation. All seeds germinated uniformly on antibiotic containing medium which confirmed the fertility of flowers and viability of seeds obtained via self-pollination. All T1 and T2 transplastomic lines were also normal in morphology like WT plants.
Figure 3.

Morphology of transplastomic tobacco plants carrying transgene GST-L1_2xCysM showing healthy phenotype with fertile flowers.
Confirmation of the transgene integration
Site specific integration of GST-L1_2xCysM and aadA genes was confirmed by PCR. For GST-L1_2xCysM, an amplicon of 2582 bp was obtained (Fig. 2A). This PCR fragment was amplified by reverse primer within L1_2xCysM while the second primer (forward) located within the plastome outside the flanking sequence (INSR), resulted in a fragment that could only be obtained from the inserted cassette at INSR. For confirmation of aadA insertion on INSL, similar strategy was used using forward and reverse primers located within aadA gene and plastome outside the vector flank, respectively. Generation of 1981 bp fragment confirmed correct insertion of aadA gene within the plastid genome (Fig. 2B). Similar results were obtained for T1 and T2 progeny (data not shown). Total of 7 PCR positive lines were selected for further analysis.
Figure 2.
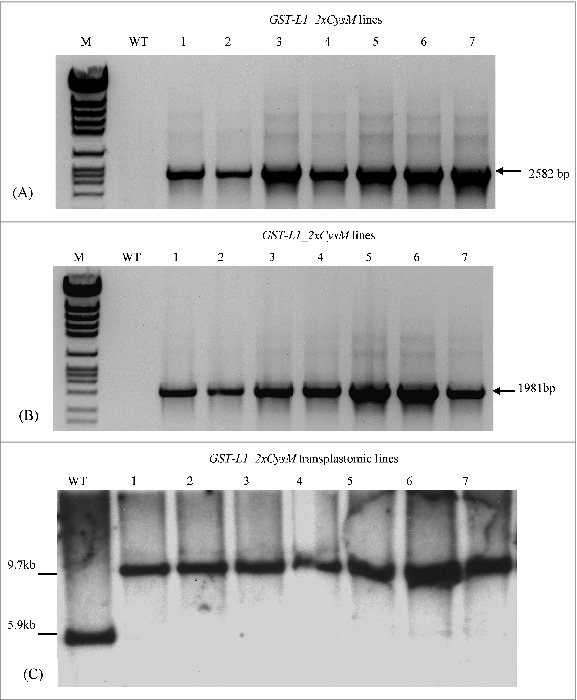
PCR and Southern analysis of the transgenic lines. (A) PCR analysis of the transgenes correctly inserted within the targeting site of the plastid genome, Amplification of GST-L1_2xCysM gene (2582 bp) with the primers oli248 in the L1_2xCysM gene and oli252 located within the plastome, (B); Amplification of the aadA gene (1981 bp) with primers oli251 in the aadA gene and oli253 present within the plastid genome. Lanes 1-7: Seven independently generated transplastomic lines (C) Southern blot analysis of GST-L1_2xCysM transplastomic plants. Total plant DNA was digested with BglII. The DNA sequence P (773 bp) located within left insertion site (INSL) of plastid genome was amplified by PCR and served for probing in the Southern analysis. Lanes 1-7: Seven independently generated transplastomic lines analyzed for the transgene integration, M: marker, WT: wild type.
Confirmation of homoplasmy
Southern blot analysis was performed to confirm homogenous transformation of all plastids (homoplasmy) of the selected PCR positive plants. Plant DNA was digested with the enzyme BglII and a site specific probe served to confirm the transgene integration. Transformed plants containing GST-L1_2xCysM were confirmed by 9.7 kb DNA fragment (Fig. 2C) while in case of wild type tobacco 5.9 kb fragment was generated as expected (Fig. 2C). The homoplasmic status of the transplastomic lines was confirmed by absence of any wild type specific 5.9 kb band in all 7 transplastomic lines. Homoplasmy was also tested and verified in transformed plants from T1 and T2 generations (data not shown).
Protein expression and analysis of immunogenic epitopes
Western blot analysis was carried out to verify the expression of GST-L1 protein. Monoclonal antibody MD2H11 was used for the detection of L1 protein. In spite of many attempts, protein concentration was under the detection limit of Western blot analysis (data not shown). To analyze the protein by a more sensitive method and for the detection of immunogenic epitopes of expressed protein, we carried out antigen capture ELISA. L1 protein was detected by conformation-specific antibody Ritti01 in all 7 transplastomic lines (Fig. 4). Binding of recombinant protein with the conformation-specific antibody confirmed that the expressed protein retained the immunogenic epitopes that are necessary for immunogenicity.
Figure 4.
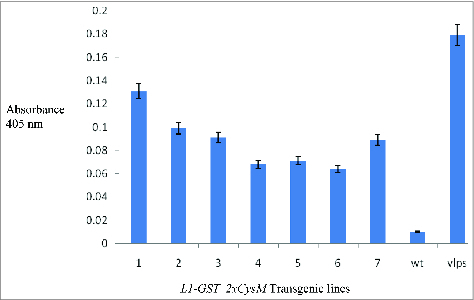
Antigen capture Enzyme-linked immunosorbent assay (ELISA) of the 7 transplastomic lines showing the L1 protein accumulation in the leaf extracts. Baculovirus-derived VLPs served as positive control for the assay. Detection of conformational epitopes was done by monoclonal antibody Ritti01. WT: Wild type, as negative control.
Discussion
To develop an alternative vaccine production platform against HPV infection, we have investigated the expression of a modified HPV-16 L1 gene fused with GST. As expression platform for the GST-L1 protein we chose tobacco plastids. Modified L1 gene (L1_2xCysM) alone leads to the assembly of L1 protein to pentameric capsomeres.32,55 The GST gene is a widely-used affinity tag gene which encodes evolutionarily conserved detoxification enzymes.44 These enzymes have the ability to conjugate a broad range of potentially harmful xenobiotics,45 hence rendering plants to detoxify directly the products of oxidative stress.46
Stable integration of the complete GST-L1_2xCysM expression cassette into the chloroplast genome and homoplasmy was verified by PCR and Southern blot analysis, respectively. The recombinant protein was under detection level in Western blot analysis, however, in antigen capture ELISA the GST-L1 fusion protein bound to conformation specific antibody, proving expression of the L1 protein and furthermore its correct folding.
Currently available vaccines against HPV are based on VLPs and are considerably expensive, but due to high disease burden of cervical cancer in developing countries, there is an urgent need for cost-effective alternatives. Pentameric capsomeres offer one such alternative due to certain advantages as reviewed by Stanley et al.47 Capsomeres have been produced in fermenter-based systems and have been shown to be highly immunogenic.48-55 In the present study, we opted for a modified L1 gene (L1_2xCysM): in this modified form the replacement of 2 cysteines by serines leads to the assembly of L1 protein into capsomeres instead of VLPs.53 In our previous study, we have shown that expression of L1_2xCysM gene without any fusion partner in tobacco plastids yielded properly folded capsomeres.32
The plastid expressed GST-L1 fusion protein was expected to assemble into pentameric capsomeres with the retention of conformational epitopes. To confirm this, an antigen capture ELISA was carried out with the conformation specific monoclonal antibody Ritti01. Conformation specific antibodies only bind to protein with accurately presented epitopes.16,53 In antigen capture ELISA, Ritti01 detected L1 capsomeres with very high specificity and all of the 7 transplastomic lines generated significant signals relative to the positive control in ELISA. This confirmed that the chloroplast derived L1 in the fusion protein retained the conformational epitopes necessary for immunogenicity. Capsomeres of HPV reported to bind with conformation-specific antibodies have also induced neutralizing antibodies in rabbits and dogs,51 which indicates that capsomeres of GST-L1 present conformational immunogenic epitopes.54,57
To verify the size of the fusion protein, Western blot analysis was performed, but unexpectedly, the protein concentration was under the detection level of this method.One possible reason for low traceability could be a shorter half-life of chloroplast-derived GST-L1. A plastidial protein with a half life less than 1h has previously been reported by Whitney and Andrews.58 Previously, Lenzi et al. have reported a VLP forming L1 protein with a plastid expression level up to 1.5% when tagged to HisXa, but when tagged to GST the expression level dropped down: 0% when expressed under rbcL 5´UTR, also 0% under psbA 5´UTR, only under atpB 5´UTR and when fused to an additional atpB peptide it reached 0.1%.59 Another possible reason for low traceability could be proteolytic degradation, which alone can account for decreased protein amounts.60 Lenzi et al. also observed the degradation of GST-L1 protein. Lenzi et al. inserted the GST-L1 genes in the trnV-rps12/7 region while in the present study we targeted the trnN and trnA region and the gene was under control of a different promoter (PrrnPEP, Prrn-62NEP) and G10L 5´UTR (Fig. 2). Another difference is that Lenzi et al. used L1 protein that folded into VLPs while in our study L1 folding was retained to capsomeres. However, it seems that both these differences did not have any effect on the expression of GST fused L1 protein. In the present study, levels of GST-L1 were very low for Western blot analysis. However, L1 protein was detected in antigen capture ELISA using conformation-specific antibody. This shows that L1 protein was expressed in tobacco plastids, albeit to minor levels. Detection of L1 protein in antigen capture ELISA is due to the higher sensitivity of this method compared to immune-blotting as it allows qualitative detection of proteins even in lowest concentrations.
In our recent reports of L1 capsomere expression as a fusion protein, we observed a series of pleiotropic effects on tobacco plants.43 Transplastomic tobacco expressing L1 protein fused with Escherichia coli heat-labile enterotoxin subunit B (LTB) exhibited stunted growth, male sterility and pale leaves. In contrast, the transformed plants expressing GST-L1 protein in the current study showed normal morphological characters like wild type tobacco plants. They developed green leaves and formed normal flowers producing viable seeds that germinated uniformly on selection medium.
Although the current study demonstrates successful transformation and integration of GST-L1_2xCysm genes in tobacco plastids, expression of the fusion protein was not observed. It would have been worthwhile to adopt a systematic approach to verify if different N-termini result in better accumulation of the GST-L1 2xCysM. However, such systematic approach will be an interesting possible future direction.
In conclusion, the current study on plant-based expression of capsomeres supports the development of cost-effective thermostable HPV vaccines, which is highly desirable for resource poor countries.
Materials and Methods
Vector construction
For the construction of transformation vector, first a precursor vector pPNG1014_MCS120 was designed, also used by Waheed et al.34 This precursor vector consisted of following components: the plastid encoded polymerase (PEP) promoter from rrn16 gene (Prrn) as reported by Svab and Maliga,35 nuclear encoded polymerase (NEP) promoter (Prrn-62NEP), the ribosomal binding site (RBS) from the leader sequence of gene 10 (G10L RBS) of the λ-phage T7,36 multiple cloning site (MCS) and 5´-untranslated region (5′ UTR) consisting of synthetic ribosomal binding site. Restriction enzymes NcoI, NheI and NheI, AflII were used to clone GST and modified L1 (L1_2xCysM) genes in the precursor vector, respectively to obtain pPNGST-L1. To get the final transformation vector pPNGST-L1-T, plasmid pT7PHB-N37 was used which already contained aadA gene which confers resistance against spectinomycin and streptomycin, 3´UTR from large subunit of ribulose-bisphosphate carboxylase gene (TrbcL) and the flanking regions INSL and INSR, homologous to the respective loci trnN and trnR in the inverted repeats (IR) of the plastid genome. This plasmid was cut with restriction enzymes SacII and BamHI and the transformation cassette was inserted by SacII and BglII. All cloning techniques were performed using the standard protocols as explained by Sambrook.38 Diagrammatic representation of the vector construction is given in Figure 1.
Transformation and regeneration of transplastomic plants
Three to 4 weeks old Nicotiana tabacum, cv. Petit Havana (Surrow Seeds, Sakskøbing, Denmark) leaflets were biolistically transformed using BIORAD PDS-1000/He Particle Delivery System, CA, USA.39 The vector DNA was coated on 0.6 μm gold particles and after bombardment leaves were cut into 5mm pieces and transferred to RMOP medium containing 500 mg/l spectinomycin. They were regenerated at 26°C in a growth chamber under standard light/dark conditions.39 After 3 weeks, leaves from control plants and all untransformed explants on selection medium bleached out. Regenerated shoots were further subjected to 3 cycles of selection on RMOP medium to obtain homoplasmic transplastomic lines. Thereafter, transformed shoots were transferred to the B540 rooting medium. Soon after root development the plants were transferred to the green house in pots. Seeds obtained via self-pollination of transplastomic plants were germinated on agar solidified B5 medium supplied with 500 mg/l spectinomycin to obtain T1 and afterwards T2 generation from the seeds of T1 plants.
Polymerase Chain Reaction analysis and Southern blot analysis
PCR analysis was used to confirm insertion of transgenes within the chloroplast genome. DNA was extracted from ∼100 mg of leaves (T0 transplastomic plants) by cetyltrimethylammonium bromide (CTAB) procedure as described by Murray and Thompson.41 For the confirmation of GST-L1_2xCysM, forward (oli252) and reverse (oli248, binding site located within L1_2xCysm gene) primers were used. The binding site of oli252 was located within the plastome (Fig. 1C). The sequences of oli252 and oli248 were: 5′-AGACAGCGACGGGTTCTCTG-3′ and 5′-GTACTTGGGGATCCTTTGCC-3′, respectively. For amplification of aadA gene, oli251 and oli253 were used as forward and reverse primers, with binding sites in aadA gene and plastome, respectively (Fig. 1C). The sequences of oli251 and 253 were: 5′-CCAGTATCAGCCCGTCATAC-3′ and 5′-GAAATTCTATGGCTCGGATC-3′, respectively. T1 and T2 transplastomic plants were also analyzed in similar way.
DNA was extracted from leaves in a similar way as described above. 3μg DNA was digested with BglII, resolved on 0.8% agarose gel and transferred onto nylon membrane (Carl Roth, Karlsruhe, Germany). Probe with a size of 773 bp was amplified by PCR (Fig 1), using forward (5′-TACCCGGGAATTGTGACCTC-3′) and reverse primers (5′-AGAGTCCGACCACAACGACC-3′). Probe labeling, hybridization and detection were performed using the DIG High Prime DNA Labeling and Detection Starter kit II, as instructed by the manufacturer (Roche, Mannheim, Germany).
Western Blot analysis
Approximately 100 mg leaves from in vitro grown transplastomic plants were ground using liquid nitrogen and powdered leaves were homogenized in SDS-buffer containing 32% glycerol, 13% β-mercaptoethanol, 185 mM Tris-HCl (with 6.8 pH), 6.5% SDS, 0.02% bromophenol blue. Soluble fraction was collected as supernatant after centrifugation of homogenized samples at 24000 g for 10 minutes at 4°C. Samples were heated for 5 minutes at 95ºC. Proteins were separated by SDS-PAGE, which were then transferred onto nitrocellulose membrane, Hybond C (GE Healthcare) and blocked with 5% of skimmed milk for 1/2h. The membrane was incubated with HPV-16 L1 specific monoclonal antibody MD2H11(DKFZ Heidelberg, Germany) diluted 1:4000 in skimmed milk for 1 hr. Membrane was washed 3 times with PBS containing Tween-20 and was incubated for 1h with peroxidase conjugated goat anti mouse IgG (Sigma) as secondary antibody diluted 1:3000 in skimmed milk. Proteins were spotted by chemiluminescence and bands were visualized on X-Ray film. Seven independently generated transplastomic lines were subjected to analysis. VLPs (DKFZ, Heidelberg, Germany) were used as positive control.
Antigen capture enzyme-linked immunosorbent assay (ELISA)
Young tobacco leaves (100mg) were obtained from the transplastomic plants grown under sterile conditions. To extract the soluble proteins, leaves were ground into fine powder using liquid nitrogen and homogenized in the extraction buffer which contained 5 mM MgCl2, 5mM CaCl2, 1M Sodium chloride, 0.01% Triton X-100, 20 mM Hepes (with 7.4 pH) and 1 mM PMSF. The homogenized mixture was centrifuged at 18000 g for 5 min and the supernatant was collected in a separate clean tube. The 96-well microtiter plate (Costar Corning, Corning, NY, USA) was coated with 50 μl of HPV-16 L1 conformation specific mouse monoclonal antibody Ritti0142 with a dilution of 1:300 in PBS and left for overnight at 4°C. Plates were rinsed with PBS-T and blocked with PBSTM for 1 h at 37°C. Plant extracts from 7 transplastomic lines were added to microtiter plate and incubated for 1h at 37°C. 50 μL of polyclonal rabbit antiserum (1:3000 in PBSTM) raised against HPV-16 L1 was added in each well and plates were again incubated for 1 h at 37°C. Plates were washed 3 times and incubated for 1 h at 37°C after the addition of 50 μL of goat anti rabbit peroxidase conjugate supplied by Sigma (1: 3000 in PBSTM) in each well. Plates were washed thoroughly before adding 100 μL of staining solution in each well. After 15 minutes the measurements at 405 nm were carried out. Baculovirus-derived purified VLPs with 10ng, 20ng, 30ng dilutions and soluble fraction of protein from Wild Type (WT) plants served as positive and negative controls, respectively.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are thankful to Prof. Hans Peter Kaul, Johanna Gottschamel, Mona N. Razavi, Evelyn Holub and Michelle Victoria Spreitz for their support during the work.
Funding
This research work was supported by Higher Education Commission (HEC), Pakistan, in co-operation with ÖAD, Austria, as well as by GoF Foundation, Germany. Also, The Research Council of Norway's grant (GLOBVAC NFR 192510) to Dr Jihong Liu Clarke.
References
- 1.Munoz N, Bosch FX, Sanjose S, Herrero R, Castellsague X, Shah KV. Epidemiologic classification of human papillomavirus types associated with cervical cancer. New Engl J Med 2003; 348: 518-527; PMID:12571259; http://dx.doi.org/ 10.1056/NEJMoa021641 [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute Cancer advances in focus: Cervical Cancer 2010; http://www.cancer.gov/cancertopics/factsheet/cancer-advances-in-focus/cervical. [Google Scholar]
- 3.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Brit J Cancer 2003; 88(1): 63-73; PMID:12556961; http://dx.doi.org/ 10.1038/sj.bjc.6600688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch FX. Human papillomavirus: science and technologies for the elimination of cervical cancer. Expert Opin Pharmaco 2011; 12(14): 2189-204; http://dx.doi.org/ 10.1517/14656566.2011.596527 [DOI] [PubMed] [Google Scholar]
- 5.Smith JS, Hoots B, Lindsay L, Keys J, Winer R, Clifford GM, Franceschi S. Human papilloma-virus type distribution in invasive cervical cancer & high grade cervical lesions. Int J Cancer 2007; 121: 621-32; PMID:17405118; http://dx.doi.org/ 10.1002/ijc.22527 [DOI] [PubMed] [Google Scholar]
- 6.Williams MG, Howatson AF, Almeida JD. Morphological characterization of the virus of the human common wart (verruca vulgaris). Nature 1961; 189: 895-7; PMID:13785470; http://dx.doi.org/ 10.1038/189895a0 [DOI] [PubMed] [Google Scholar]
- 7.Howley PM, Lowy D R. Virology. 2nd ed Philadelphia: Lippincott Williams & Wilkins; 2001; 2197-229.; . [Google Scholar]
- 8.Fehrmann F, Laimins LA. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene 2003; 22(33): 5201-7; PMID:12910257; http://dx.doi.org/ 10.1038/sj.onc.1206554 [DOI] [PubMed] [Google Scholar]
- 9.Braspenning J, Gissmanna L. Chimeric papillomavirus like particles. Virology 1997; 234: 93-111; PMID:9234950; http://dx.doi.org/ 10.1006/viro.1997.8591 [DOI] [PubMed] [Google Scholar]
- 10.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, Olsson SE, Hoye J, Steinwall M, Riis JG, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Brit J Cancer 2006; 95:1459-66; PMID:17117182; http://dx.doi.org/ 10.1038/sj.bjc.6603469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Desmarquet TD, Orth G, Schiller JT, Lowy DR. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol 1995; 69(6): 3959-63; PMID:7745754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzich JA, Ghim SJ, Palmer FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A 1995; 92(25): 11553-7; PMID:8524802; http://dx.doi.org/ 10.1073/pnas.92.25.11553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli CM, Jenkins D, Schuind A, Costa SA, Dubin G. Sustained efficacy up to 4.5 years of a bivalent L1 VLP vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet 2006; 367(9518):1247-55; PMID:16631880; http://dx.doi.org/ 10.1016/S0140-6736(06)68439-0 [DOI] [PubMed] [Google Scholar]
- 14.Waheed MT, Gottschamel J, Hassan SW, Lössl AG. Plant-derived vaccines: An approach for affordable vaccines against cervical cancer. Hum Vaccin Immunother 2012; 8(3):1-4; PMID:22251991; http://dx.doi.org/ 10.4161/hv.18568 [DOI] [PubMed] [Google Scholar]
- 15.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008. Int J Cancer 2010; 127: 2893-917; PMID:21351269; http://dx.doi.org/ 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 16.Rybicki EP. Plant made vaccines for humans and animals. Plant Biotechnol J 2010; 8 (5): 620-37; PMID:20233333; http://dx.doi.org/ 10.1111/j.1467-7652.2010.00507.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassan SW, Mehmood Z, Waheed MT, Lossl AG. Towards generation of transplastomic tobacco, expressing a 35 kda protein as an antigen: a step towards affordable plant made vaccine against Mycobacterium. Clon Transgen 2013; (3): doi: 10.4172/2168-9849.1000117; [DOI] [Google Scholar]
- 18.Stöger E, Schillberg S, Twyman RM, Fischer R, Christou P. Antibody Production in Transgenic Plants. Methods Mol Biol 2004; 248:301-18; PMID:14970504; . [DOI] [PubMed] [Google Scholar]
- 19.Hassan SW, Waheed MT, Lössl AG. New areas of plant-made pharmaceuticals. Expert Rev Vaccines 2011; 10(2):151-3; PMID:21332263; http://dx.doi.org/ 10.1586/erv.10.166 [DOI] [PubMed] [Google Scholar]
- 20.Bock R. Transgenic plastids in basic research and plant biotechnology. J Mol Biol 2001; 312: 425-38; PMID:11563907; http://dx.doi.org/ 10.1006/jmbi.2001.4960 [DOI] [PubMed] [Google Scholar]
- 21.Maliga P. Plastid transformation in higher plants. Annu Rev Plant Biol 2004; 55: 289-314; PMID:15377222; http://dx.doi.org/ 10.1146/annurev.arplant.55.031903.141633 [DOI] [PubMed] [Google Scholar]
- 22.Daniell H. Production of biopharmaceuticals and vaccines in plants via the chloroplast genome. Biotechnol J 2006; 1(10): 1071-9; PMID:17004305; http://dx.doi.org/ 10.1002/biot.200600145 [DOI] [PubMed] [Google Scholar]
- 23.Lössl AG, Waheed MT. Chloroplast derived vaccines against human diseases: achievements, challenges and scopes. Plant Biotechnol J 2011; 9(5): 527-39; PMID:21447052; http://dx.doi.org/ 10.1111/j.1467-7652.2011.00615.x [DOI] [PubMed] [Google Scholar]
- 24.Clarke JL, Daniell H. Plastid biotechnology for crop production: present status and future perspectives. Plant Mol Biol 2011; 76: 211-20; PMID:21437683; http://dx.doi.org/ 10.1007/s11103-011-9767-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streatfield SJ. Mucosal immunization using recombinant plant-based oral vaccines. Methods 2006; 38(2):150-7; PMID:16431131; http://dx.doi.org/ 10.1016/j.ymeth.2005.09.013 [DOI] [PubMed] [Google Scholar]
- 26.Bock R, Warzecha H. Solar powered factories for new vaccines and antibiotics. Trends Biotechnol 2010; 28: 246-52; PMID:20207435; http://dx.doi.org/ 10.1016/j.tibtech.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 27.Cardi T, Lenzi P, Maliga P. Chloroplasts as expression platforms. Expert Rev Vaccines 2010; 9(8): 893-911(19); PMID:20673012; http://dx.doi.org/ 10.1586/erv.10.78 [DOI] [PubMed] [Google Scholar]
- 28.Corriveau JL, Coleman AW. Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperms. Am J Bot 1988; 75: 1443-58; PMID:3272361; http://dx.doi.org/ 10.2307/24446953272361 [DOI] [Google Scholar]
- 29.Oey M, Kreikemeyer B, Lohse M, Bock R. Exhaustion of the chloroplast made protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J 2009; 57: 436-45; PMID:18939966; http://dx.doi.org/ 10.1111/j.1365-313X.2008.03702.x [DOI] [PubMed] [Google Scholar]
- 30.Ruf S, Karcher D, Bock R. Determining the transgene containment level provided by chloroplast transformation. Proc Natl Acad Sci U S A 2007; 104:6998-7002; PMID:17420459; http://dx.doi.org/ 10.1073/pnas.0700008104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svab Z, Maliga P. Exceptional transmission of plastids and mitochondria from the transplastomic pollen parent and its impact on transgene containment. Proc Natl Acad Sci. U S A 2007; 104:7003-8; PMID:17420457; http://dx.doi.org/ 10.1073/pnas.0700063104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruhlman T, Samson N, Verma D, Daniell H. The role of heterogonous chloroplast sequence elements in transgene integration and expression. Plant Physiol 2010; 152: 2088-104; PMID:20130101; http://dx.doi.org/ 10.1104/pp.109.152017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agosti JM, Goldie SJ. Introducing HPV vaccine in developing countries key challenges and issues. New Engl J Med 2007; 356(19):1908-10; PMID:17494923; http://dx.doi.org/ 10.1056/NEJMp078053 [DOI] [PubMed] [Google Scholar]
- 34.Waheed MT, Thönes N, Müller M, Hassan SW, Razavi NM, Lössl E, Kaul HP, Lössl AG. Transplastomic expression of a modified human papillomavirus L1 protein leading to the assembly of capsomeres in tobacco: a step towards cost-effective second-generation vaccines. Transgenic Res 2011; 20(2):271-82; PMID:20563641; http://dx.doi.org/ 10.1007/s11248-010-9415-4 [DOI] [PubMed] [Google Scholar]
- 35.Svab Z, Maliga P. High frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci U S A 1993; 90: 913-7; PMID:8381537; http://dx.doi.org/ 10.1073/pnas.90.3.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Studier FW, Rosenberg AH, Dunn J, Dubendorf JW. Use of T7 RNA polymerase to direct expression of cloned genes. Method Enzymol 1990; 185: 60-89; http://dx.doi.org/ 10.1016/0076-6879(90)85008-C [DOI] [PubMed] [Google Scholar]
- 37.Lössl AG, Bohmert K, Eibl C, Harloff H, Mühlbauer S, Koop HU. Inducible trans activation of plastid transgenes: expression of the phb operon in transplastomic tobacco. Plant Cell Physiol 2005; 46: 1462-71; PMID:15964903; http://dx.doi.org/ 10.1093/pcp/pci157 [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. 2nd ed. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Svab Z, Hajdukiewicz P, Maliga P. Stable transformation of plastids in higher plants. Proc Natl Acad Sci U S A 1990; 87: 8526-530; PMID:11607112; http://dx.doi.org/ 10.1073/pnas.87.21.8526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 1968;50:151-8; PMID:5650857; http://dx.doi.org/ 10.1016/0014-4827(68)90403-5 [DOI] [PubMed] [Google Scholar]
- 41.Murray SL, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucl Acid Res 1980; 8:4321-5; http://dx.doi.org/ 10.1093/nar/8.19.4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thönes N, Herreiner A, Schädlich L, Piuko K, Müller M. A direct comparison of HPV type 16 L1 particles reveals a lower immunogenicity of capsomeres than VLP with respect to the induced antibody response. J Virol 2008; 82: 5472-85; PMID:18385253; http://dx.doi.org/ 10.1128/JVI.02482-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waheed MT, Thönes N, Müller M, Hassan SW, Gottschamel J, Lössl E, Kaul HP, Lössl AG. Plastid expression of a double-pentameric vaccine candidate containing human papillomavirus-16 L1 antigen fused with LTB as adjuvant: transplastomic plants show pleiotropic phenotypes. Plant Biotechnol J 2011; 9(6):651-60; PMID:21447051; http://dx.doi.org/ 10.1111/j.1467-7652.2011.00612.x [DOI] [PubMed] [Google Scholar]
- 44.Kanai T, Takahashi K, Inoue H. Three distinct-type glutathione S-transferases from Escherichia coli important for defense against oxidative stress. J. Biochem 2006; 140: 703-11; PMID:17018556; http://dx.doi.org/ 10.1093/jb/mvj199 [DOI] [PubMed] [Google Scholar]
- 45.Burns C, Geraghty R, Neville C, Murphy A, Kavanagh K, Doyle S. Identification, cloning, and functional expression of three glutathione transferase genes from Aspergillus fumigatus. Fungal Genet Biol 2005; 42: 319-27; PMID:15749051; http://dx.doi.org/ 10.1016/j.fgb.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 46.Martret BL, Poage M, Shiel K, Nugent GD, Dix PJ. Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotechnol J 2011; 9:661-73; PMID:21450042; http://dx.doi.org/ 10.1111/j.1467-7652.2011.00611.x [DOI] [PubMed] [Google Scholar]
- 47.Stanley M, Gissmann L, Nardelli-Haefliger D. Immunobiology of human papilloma virus infection and vaccination implications for second generation vaccines. Vaccine 2008; 26 (10): 62-7; http://dx.doi.org/ 10.1016/j.vaccine.2008.05.066 [DOI] [PubMed] [Google Scholar]
- 48.Rose RC, White WI, Li M, Suzich JA, Lane C, Garcea RL. Human papillomavirus type 11 recombinant L1 capsomeres induce virus neutralizing antibodies. J Virol 1998; 72:6151-4; PMID:9621080; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell 2000; 5: 557-67; PMID:10882140; http://dx.doi.org/ 10.1016/S1097-2765(00)80449-9 [DOI] [PubMed] [Google Scholar]
- 50.Fligge C, Giroglou T, Streeck RE, Sapp M. Induction of type-specific neutralizing antibodies by capsomeres of human papillomavirus type 33. Virology 2001; 283:353-7; PMID:11336560; http://dx.doi.org/ 10.1006/viro.2000.0875 [DOI] [PubMed] [Google Scholar]
- 51.Yuan H, Estes PA, Newsome J, Chen Y, Olcese VA, Schlegel R, Garcea RL. Immunization with a pentamer L1 fusion protein protects against PV infection. J Virol 2001; 75: 7848-53; PMID:11483728; http://dx.doi.org/ 10.1128/JVI.75.17.7848-7853.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Öhlschläger P, Osen W, Dell K, Faath S, Garcea RL, Jochmus I, Müller M, Pawlita M, Schäfer K, et al. . Human papillomavirus type 16 L1 capsomeres induce L1-specific cytotoxic T lymphocytes and tumor regression in C57BL/6 mice. J Virol 2003;77:4635-45; http://dx.doi.org/ 10.1128/JVI.77.8.4635-4645.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dell K, Koesters R, Linnebacher M, Klein C, Gissmann L. Intranasal immunization with human papillomavirus type 16 capsomeres in the presence of non-toxic cholera toxin-based adjuvants elicits increased vaginal immunoglobulin levels. Vaccine 2006; 24:2238-47; PMID:16455165; http://dx.doi.org/ 10.1016/j.vaccine.2005.11.060 [DOI] [PubMed] [Google Scholar]
- 54.Schädlich L, Senger T, Carsten J. Kirschning Müller M, Gissmann L. Refining HPV 16 L1 purification from E. coli: Reducing endotoxin contaminations and their impact on immunogenicity. Vaccine 2009; 27:1511-22; http://dx.doi.org/ 10.1016/j.vaccine.2009.01.014 [DOI] [PubMed] [Google Scholar]
- 55.Schädlich L, Gerlach B, Senger T, Mücke N, Bravo IG, Müller M, Klein C, Gissmann L. Analysis of modified human papilloma-virus type 16 L1 capsomeres, the ability to assemble into larger particles correlates with higher immunogenicity. J Virol 2009; 83:7690-705; http://dx.doi.org/ 10.1128/JVI.02588-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rizk RZ, Christensen ND, Michael KM, Müller M, Sehr P, Waterboer T, Pawlita M. Reactivity pattern of 92 monoclonal antibodies with 15 human papillomavirus types. J Gen Virol 2008; 89: 117-29; PMID:18089735; http://dx.doi.org/ 10.1099/vir.0.83145-0 [DOI] [PubMed] [Google Scholar]
- 57.Thönes N, Müller M. Oral immunization with different assembly forms of the HPV 16 major capsid protein L1 induces neutralizing antibodies and cytotoxic T-lymphocytes. Virology 2007; 369: 375-88; PMID:17822733; http://dx.doi.org/ 10.1016/j.virol.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 58.Whitney SM, Andrews TJ. Plastome-encoded bacterial ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) supports photosynthesis and growth in tobacco. Proc Natl Acad Sci U S A 2001; 98 (25): 14738-43; PMID:11724961; http://dx.doi.org/ 10.1073/pnas.261417298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lenzi P, Scotti N, Alagna F, Tornesello ML, Pompa A, Vitale A, De Stradis A, Monti L, Grillo S, Buonaguro , et al. Translational fusion of chloroplast-expressed human papillomavirus type 16 L1 capsid protein enhances antigen accumulation in transplastomic tobacco. Transgenic Res 2008; 17: 1091-102; PMID:18491213; http://dx.doi.org/ 10.1007/s11248-008-9186-3 [DOI] [PubMed] [Google Scholar]
- 60.Adam Z. Protein stability and degradation in chloroplasts. Plant Mol Biol 1996; 32: 773-83; PMID:8980530; http://dx.doi.org/ 10.1007/BF00020476 [DOI] [PubMed] [Google Scholar]


