Abstract
Preterm infants ≤ 35 weeks' gestational age (GA), and children ≤ 24 months of age with bronchopulmonary dysplasia (BPD) or hemodynamically significant congenital heart disease (hsCHD) are at high risk for developing severe respiratory syncytial virus (RSV) disease. In 3 previous randomized, placebo-controlled trials, palivizumab efficacy varied significantly based on these underlying conditions, and trial enrollment was not proportional to condition prevalence. This analysis provides the first estimate of the population-weighted efficacy of palivizumab in high-risk children, adjusting for condition prevalence. Palivizumab efficacy by high-risk condition was obtained from the clinical trials. The annual number of US children with each condition was obtained from the 2010 Centers for Disease Control and Prevention (CDC) natality statistics and the medical literature. Data from specialty pharmacies in the US palivizumab distribution network were used to estimate the population for each condition receiving at least 1 dose in the outpatient setting in 2012–2013. The weighted efficacy estimate was derived by summing the products of the condition-specific relative risk reductions and the relative frequency of each condition among those receiving palivizumab. The US population-weighted efficacy estimate for those receiving palivizumab was 68%. Due to the low prevalence of BPD and hsCHD and the higher efficacy observed in preterm infants without BPD or CHD, the population-weighted estimate of palivizumab efficacy is higher than the overall 45–55% efficacy observed in initial clinical trials. Consistent with 2012 American Academy of Pediatrics RSV prophylaxis recommendations, a low proportion of preterm infants 32–35 weeks' gestational age receive palivizumab.
Keywords: palivizumab, efficacy, RSV-related hospitalization, population-weighted efficacy, condition-adjusted efficacy, respiratory syncytial virus, severe RSV disease
Abbreviations
- BPD
bronchopulmonary dysplasia
- CDC
Centers for Disease Control and Prevention
- CHD
congenital heart disease
- FDA
US Food and Drug Administration
- GA
gestational age
- hsCHD
hemodynamically significant congenital heart disease
- RSV
respiratory syncytial virus
- RSVH
respiratory syncytial virus-related hospitalization
Introduction
Respiratory syncytial virus (RSV), an important pathogen of infants and young children, is the most common cause of bronchiolitis and pneumonia worldwide.1 Severe RSV disease causes significant morbidity and is the leading cause of hospitalization among infants in the United States (US).2-4 Preterm infants ≤ 35 wk gestational age (GA), and children ≤24 mo of age with bronchopulmonary dysplasia (BPD)5 or hemodynamically significant congenital heart disease (hsCHD) are at high risk for developing severe RSV disease.6
Palivizumab is a humanized monoclonal antibody indicated for the prevention of serious lower respiratory tract disease in these high-risk populations.7 Common side effects of palivizumab include fever and rash, and children should not receive palivizumab if they have ever had a severe allergic reaction to palivizumab.7 In 3 previous randomized, placebo-controlled trials,5,6,8 palivizumab efficacy varied significantly based on the presence of the aforementioned underlying high-risk conditions. Additionally, trial enrollment was not proportional to condition prevalence; for example, a trial of preterm infants with or without BPD overrepresented those with BPD. Palivizumab was shown to reduce RSV-related hospitalizations (RSVH) in children ≤ 24 mo of age at RSV season start with BPD or hsCHD by 39% (95% CI: 20, 58) and 45% (95% CI: 23, 67), respectively.5,6 Among preterm infants ≤ 35 wk GA without BPD, palivizumab reduced RSVH by 72% (95% CI: 17.4, 90.8) among <32-wk GA infants and by 82% (95% CI: 45.4, 94.2) among 32- to 35-wk GA infants.5 A recent randomized clinical trial also demonstrated that the efficacy of palivizumab in reducing RSVH among 32- to 35-wk GA infants without BPD was 82% (95% CI: 18, 157).8 Lastly, utilization of palivizumab also varies significantly by condition based on recommendations for use.
This analysis provides the first estimate of the population-weighted efficacy of palivizumab in US children at high risk for severe RSV disease, adjusting for condition prevalence.
Materials and Methods
Palivizumab efficacy by specific high-risk condition (ie, preterm infants ≤35 wk GA and children ≤24 mo of age with BPD or hsCHD) was obtained from the results of the following 3 clinical trials.5,6,8 The IMpact-RSV study was a multicenter, randomized, double-blind, placebo-controlled trial that enrolled preterm infants born at ≤35 wk GA who were ≤6 mo of age and children ≤24 mo of age with BPD.5 The multicenter, randomized, double-blind, placebo-controlled study conducted by Feltes et al.6 enrolled children ≤ 24 mo of age with hsCHD. Blanken et al.8 conducted a double-blind, placebo-controlled study that included preterm infants 32–35 wk GA ≤ 6 mo of age who were otherwise healthy. Study procedures were conducted in accordance with the principles of the Declaration of Helsinki, the US Code of Federal Regulations for protection of human subjects, and Institutional Review Boards.5,6,8
The annual number of US infants with prematurity by GA subgroup was obtained from the 2010 Centers for Disease Control and Prevention (CDC) natality statistics.9 Estimates of the number of children with BPD or moderate/severe CHD were obtained from the medical literature.10-12 The estimated prevalence of BPD by GA subgroup was 25.4% of infants <32 wk GA12; 1.5% of infants 32–33 wk GA10; and 0.34% of infants 34–35 wk GA.10 The estimated number of children with moderate or severe CHD was 0.6%.11 Estimates of the number of preterm infants without BPD or moderate/severe CHD were obtained by subtracting the estimates for moderate/severe CHD and the GA-specific estimates of BPD from the number of births reported in the CDC natality statistics. For estimates of the populations eligible to receive palivizumab, no data were available regarding the percentage of children with BPD requiring oxygen, steroids, diuretics, or bronchodilators in the 6 mo before the start of RSV season; the percentage of children with moderate or severe CHD who have hsCHD; or the percentage of infants 32–35 wk GA with American Academy of Pediatrics (AAP) 2012 risk factors (ie, attends child care or has ≥1 older child <5 y of age living in the same household; excluding multiple-birth siblings < 1 y of age).13
Data from specialty pharmacies in the US palivizumab distribution network were used to estimate the population for each condition receiving ≥ 1 dose of palivizumab in the outpatient setting in 2012–2013. The weighted efficacy estimate was derived by summing the products of the condition-specific relative risk reductions from the clinical trials (Table 1) and the relative frequency of each condition among those receiving palivizumab.
Table 1.
Population-Weighted Efficacy of Palivizumab in Children at High Risk for Severe RSV Disease
| Underlying Condition | Estimated Population Size | Estimated Annual Population Receiving Palivizumab (% of Population) | Condition-Specific Relative Risk Reduction in RSV-Related Hospitalization, % (P Value) |
|---|---|---|---|
| Children ≤24 mo with BPD | 43,000* | 10,900 (25) | 39 (0.038) |
| Children ≤24 mo with moderate/severe CHD | 19,300† | 6600 (34) | 45 (0.003) |
| Preterm infants <32 wk GA and age ≤6 mo without BPD or CHD | ,44,800‡ | 36,500 (81) | 72 (0.027) |
| Preterm infants 32–35 wk GA and age ≤6 mo without BPD or CHD | 159,000‡ | 23,600 (15) | 82§ (0.001) |
AAP, American Academy of Pediatrics; BPD, bronchopulmonary dysplasia; CHD, congenital heart disease; wk GA, weeks' gestational age; RSV, respiratory syncytial virus; *Data source10,12; †Data source11; ‡Data source9; §Among preterm infants 32–35 wk GA with 2012 AAP risk factors (attends child care or has ≥1 older child <5 y of age living in the same household; excluding multiple-birth siblings <1 y of age), efficacy was consistent at 93% (RSV-related hospitalization: 12.5% for placebo vs 0.9% for palivizumab; P = 0.004).
Results
The estimated total US population eligible to receive palivizumab and the estimated population receiving at least one dose of palivizumab in the outpatient setting by underlying condition is shown in Table 1 and Figure 1. Overall, among the total annual US population of 250,500 high-risk children potentially eligible to receive palivizumab based on the US Food and Drug Administration (FDA)-approved indication, an estimated 31% receive at least one dose of palivizumab in the outpatient setting. For each respective condition, the proportion of children receiving palivizumab is substantially lower than the estimated population potentially eligible to receive it (Table 1 and Figure 1). Depending on the condition, an estimated 15%‒67% of the eligible population receives palivizumab. Consistent with 2012 AAP recommendations for RSV prophylaxis, the lowest estimated proportion of palivizumab receipt (15%) occurs among infants 32–35 wk GA; this is 4-fold lower compared with infants <32 wk GA who have an estimated receipt proportion of 67%.
Figure 1.
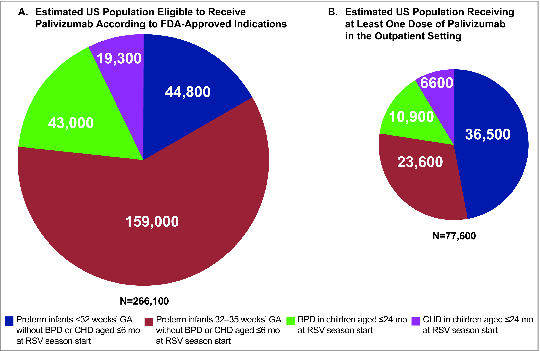
Estimated US population (A) eligible to receive palivizumab according to FDA-approved indication and (B) receiving at least one dose of palivizumab in the outpatient setting. BPD, bronchopulmonary dysplasia; CHD, congenital heart disease; FDA, US Food and Drug Administration; GA, gestational age.
Compared with placebo, the relative risk reduction in RSVH with palivizumab was greater in preterm infants without BPD or CHD than in children with BPD or moderate/severe CHD (Table 1). The population-weighted efficacy estimates were 71% for those potentially eligible to receive palivizumab and 68% for those receiving palivizumab.
Discussion
Because of the low prevalence of BPD and hsCHD and the higher efficacy observed in preterm infants without BPD or CHD, the population-weighted estimate of palivizumab efficacy is approximately 70%, which is higher than the overall 45–55% efficacy observed in the initial clinical trials. Previous studies have demonstrated the efficacy of palivizumab in helping to prevent hospitalizations due to severe RSV disease.5,6,8 This is the first estimate of the population-weighted efficacy of palivizumab and may provide a more accurate estimate of the cumulative efficacy among the US populations receiving palivizumab.
Based on the 2012 AAP guidelines, RSV prophylaxis is recommended throughout the RSV season for all infants born at <32 wk GA.9 In preterm infants 32–34 wk GA, palivizumab is recommended only through 90 d of age and only if the infant has at least 1 of the following 2 risk factors: attends child care or has ≥1 older sibling <5 y of age or other children <5 y of age who live permanently in the same household.9 Consistent with these recommendations, the present analysis demonstrates a substantially lower proportion of preterm infants 32–35 wk GA receive palivizumab compared with those who are < 32 wk GA.
A strength of this analysis is that it included data from 3 randomized, double-blind, placebo-controlled trials. Limitations include assumptions inherent in modeled projections and the fact that no distribution data were available for hospital utilization of palivizumab. However, hospital utilization represents a small fraction of palivizumab use as eligible infants would only receive their initial dose before discharge from the birth hospitalization during the RSV season.13 Additionally, as noted above, in estimating the populations potentially eligible to receive palivizumab, no data were available regarding the percentage of children with BPD requiring oxygen, steroids, diuretics, or bronchodilators in the 6 mo before the start of RSV season; the percentage of children with moderate or severe CHD who have hsCHD; or the percentage of infants 32–35 wk GA with 2012 AAP risk factors.
In conclusion, this analysis estimates the population-weighted efficacy of palivizumab in reducing RSVH at approximately 70%, which is higher than the overall 45–55% efficacy observed in initial clinical trials. The proportion of children receiving palivizumab on an annual basis is substantially lower relative to the total population that is eligible to receive it.
Disclosure of Potential Conflicts of Interest
Christopher Ambrose, Xin Chen, and Veena Kumar are employees of AstraZeneca, the parent company of MedImmune, and may hold stock or stock options.
Acknowledgments
Medical writing assistance was provided by John E. Fincke, PhD, and Anny Wu, PharmD, of Complete Healthcare Communications, Inc. (Chadds Ford, PA), and funded by MedImmune.
Funding
This study was sponsored by MedImmune. Medical writing and editorial assistance was provided by Complete Healthcare Communications, Inc., (Chadds Ford, PA, USA) and supported by MedImmune.
References
- 1.Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997-2006. Pediatr Infect Dis J 2012; 31:5-9; PMID:21817948; http://dx.doi.org/ 10.1097/INF.0b013e31822e68e6 [DOI] [PubMed] [Google Scholar]
- 2.Boyce TG, Mellen BG, Mitchel EF Jr., Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr 2000; 137:865-70; PMID:11113845; http://dx.doi.org/ 10.1067/mpd.2000.110531 [DOI] [PubMed] [Google Scholar]
- 3.Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J 2002; 21:629-32; PMID:12237593; http://dx.doi.org/ 10.1097/00006454-200207000-00005 [DOI] [PubMed] [Google Scholar]
- 4.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999; 282:1440-6; PMID:10535434; http://dx.doi.org/ 10.1001/jama.282.15.1440 [DOI] [PubMed] [Google Scholar]
- 5.The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102:531-7; PMID:9738173; http://dx.doi.org/ 10.1542/peds.102.3.531 [DOI] [PubMed] [Google Scholar]
- 6.Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH Jr., Connor EM, Sondheimer HM; Cardiac Synagis Study Group. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003; 143:532-40; PMID:14571236; http://dx.doi.org/ 10.1067/S0022-3476(03)00454-2 [DOI] [PubMed] [Google Scholar]
- 7.Palivizumab Full Prescribing Information, MedImmune, Gaithersburg, MD, 2014. [Google Scholar]
- 8.Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, Bont L; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791-9; PMID:23656644; http://dx.doi.org/ 10.1056/NEJMoa1211917 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Natality public-use data 2007-2011, on CDC WONDER Online Database, November 2013. Available at: http://wonder.cdc.gov/natality-current.html. Accessed April 4, 2014. [Google Scholar]
- 10.Escobar GJ, Masaquel AS, Li SX, Walsh EM, Kipnis P. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr 2013; 13:97; PMID:23782528; http://dx.doi.org/ 10.1186/1471-2431-13-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39:1890-900; PMID:12084585; http://dx.doi.org/ 10.1016/S0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 12.Yoder BA, Harrison M, Clark RH. Time-related changes in steroid use and bronchopulmonary dysplasia in preterm infants. Pediatrics 2009; 124:673-9; PMID:19620192; http://dx.doi.org/ 10.1542/peds.2008-2793 [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics Respiratory syncytial virus. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, eds. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed Elk Grove Village, IL: American Academy of Pediatrics; 2012:609-617. [Google Scholar]


