Abstract
Pre-existing immunity against human adenovirus (HAd) serotype 5 derived vector in the human population is widespread, thus hampering its clinical use. Various components of the immune system, including neutralizing antibodies (nAbs), Ad specific T cells and type I IFN activated NK cells, contribute to dampening the efficacy of Ad vectors in individuals with pre-existing Ad immunity. In order to circumvent pre-existing immunity to adenovirus, numerous strategies, such as developing alternative Ad serotypes, varying immunization routes and utilizing prime-boost regimens, are under pre-clinical or clinical phases of development. However, these strategies mainly focus on one arm of pre-existing immunity. Selection of alternative serotypes has been largely driven by the absence in the human population of nAbs against them with little attention paid to cross-reactive Ad specific T cells. Conversely, varying the route of immunization appears to mainly rely on avoiding Ad specific tissue-resident T cells. Finally, prime-boost regimens do not actually circumvent pre-existing immunity but instead generate immune responses of sufficient magnitude to confer protection despite pre-existing immunity. Combining the above strategies and thus taking into account all components regulating pre-existing Ad immunity will help further improve the development of Ad vectors for animal and human use.
Keywords: Adenovirus vectors, cellular responses, humoral responses, innate immunity, pre-existing immunity
Introduction
The great majority of commercially available vaccines, such as the tetanus, measles and yellow fever vaccines, are designed to induce robust humoral responses. Neutralization titers or index above 0.01, 200mIU/ml and 5 are indicative of protective immunity against tetanus,1 measles2 and yellow fever,3 respectively, in vaccine recipients. However, for protection against numerous disease causing agents such as human immunodeficiency virus (HIV), hepatitis C virus (HCV), Plasmodium parasites, Mycobacterium tuberculosis, Lassa virus, Ebolavirus and Marburg virus, induction of both humoral and cellular immune responses are desirable. As a result, considerable efforts are put into developing vectors capable of generating both cellular and antibody (Ab) responses against the target gene (transgene).
Adenovirus (Ad) derived vectors have been shown to successfully elicit strong cellular and humoral immune responses in rodents, non-human primates (NHP) as well as in humans after a single injection.4-7 In preclinical studies, among all human Ad vectors tested, those derived from human adenovirus serotype 5 (HAd5) have emerged as the gold standard for immunization due to their superior immunogenicity. As a result, earlier clinical trials were almost exclusively performed using HAd5 vectors.8 However, although HAd5 vectors were highly efficacious in pre-clinical studies, they did not perform as anticipated in clinical trials due to pre-existing HAd5 immunity in the participants from natural exposure. Adenoviruses are some of the pathogens that can cause the common cold and a significant proportion of the human population have antibodies against these viruses due to past infections naturally acquired in the community, notably during childhood.9,10 These natural exposures can be responsible for long-lasting immunity (pre-existing immunity) that can interfere with HAd-based vaccines. In a phase I trial, individuals with pre-existing HAd5 immunity mounted lower immune responses compared with participants without pre-existing immunity.5 Pre-existing immunity to HAd5 was even associated with undesirable effects beyond neutralization of the benefits intended from vaccination. The negative impact of pre-existing HAd5 immunity was illustrated in the infamous STEP trial during which a lower frequency of individuals with high HAd5 Ab titers developed a cellular response to the transgenes (HIV Gag, Pol and Nef). Furthermore, pre-existing HAd5 immunity was associated with an increase in HIV acquisition in vaccinated participants in comparison to the placebo.7 Pre-existing HAd5 immunity was not taken into account in pre-clinical studies as animals used in these studies, such as mice and NHPs, are not naturally infected by HAd5 or other human Ads. In contrast, the percentage of the African, European and American population with neutralizing antibodies (nAbs) against HAd5 has been documented to be between 65–100, 61 and 37–70%, respectively.11-14
Since realizing the negative impact of pre-existing Ad immunity in early clinical trials, better understanding and ultimately circumventing pre-existing immunity has been a major focus in the development of new Ad based vaccines. In order to reach these goals, pre-existing immunity has been artificially generated in animal models by immunization with HAd vectors either lacking a foreign gene or encoding an irrelevant one. In addition, in order to determine the contribution of Ad specific T cells and Ab in pre-existing immunity, T cells or serum from Ad immune animals can be transferred in naïve ones. This review will first summarize the contributions of the different arms of the immune system toward pre-existing immunity against Ad vectors. Then, the mechanisms of evasion of the main strategies developed to circumvent pre-existing Ad immunity will be reviewed.
Components of pre-existing Ad vector immunity
Work on pre-existing Ad immunity revealed that nAbs, Ad specific T cells, as well as Ad induced inflammatory responses, all contribute to different extents to reducing Ad vector efficacy in pre-immune subjects.
Humoral responses
The importance of nAbs in reducing Ad vector immunogenicity has been extensively demonstrated. Passive transfer of serum from Ad immune mice or purified nAbs against Ad decreases Ad transgene expression, as well as transgene specific cellular and humoral response in rodents.15,16 Depletion of Ab against the three main Ad capsid proteins (fiber, penton, hexon) by affinity chromatography in Ad immune serum, greatly reduces the inhibitory effect of these sera on vaccine vector efficacy, as demonstrated by an almost complete restoration of transgene expression level.15
Although nAbs targeting the main 3 capsid proteins have been detected in vitro,15, 17-19 they do not equally contribute to Ad vector neutralization in vivo. The relative contribution of each subset of nAbs has been teased apart by vaccinating Ad pre-immuned animals with capsid chimeric Ad vectors. From these studies, nAbs targeting the hexon protein appears to play a dominant role in vivo.18-22 Hexon specific nAbs are directed against exposed loops on the surface of the virus particle. These exposed loops are also known as hypervariable regions (HVR). Replacing the entire HAd5 hexon sequence22 or simply the exposed epitopes of HAd5 hexon21 with hexon or HVR from a different serotype, was sufficient to overcome pre-existing HAd5 immunity in rodents. In addition, a three amino acid substitution mutation in one of the HVR was able to greatly reduce in vitro neutralization by a NHP polyclonal serum raised against chimpanzee Ad serotype 68 (ChAd68) derived vector.19 Conversely, addition of the hexon domain of simian Ad serotype 23 (SAd23/Pan6) into a SAd24/Pan7 vector rendered the chimeric vector susceptible to pre-existing SAd23/Pan6 immunity, as illustrated by reduce transgene expression in rodents.18
In contrast, nAbs against the Ad fiber only play a limited role in in vivo neutralization. NAbs generated after a single HAd7 injection can only weakly neutralize chimeric HAd5 vector expressing HAd7 fiber, indicating that most nAbs were generated against other capsid proteins. Conversely, nAbs generated after a single HAd5 injection can readily neutralize chimeric HAd5 vector expressing HAd7 fiber.23 In vivo, pre-existing ChAd6 immunity inhibited expression of the transgene from chimeric SAd24/Pan7 vectors that possess the ChAd6 hexon protein but not those that possess the ChAd6 fiber protein.18 Passive transfer of mouse serum containing Abs against a chimeric HAd35 expressing HAd5 fiber, did not impact cellular and humoral immune responses against the transgene generated after HAd5 injection.20 One caveat of the above studies is that in vivo, nAbs targeting the fiber are less common after a single injection23 but are more readily detectable after two or more immunizations with the same adenovirus.15,17,20 However, in most animal studies, pre-existing immunity is generated by a single Ad injection. The induced Ab response is probably of limited breath and neutralizing titer compared with humoral responses generated in individuals after repeated natural infections with replication competent Ad. As a result, these studies may underestimate the role played by nAbs targeting Ad fiber; especially as nAbs against Ad fiber and penton can act synergistically.17
Although nAbs against Ad strongly reduce Ad vector immunogenicity, they are serotype specific with limited to no cross-neutralization of different Ad serotypes (Fig. 1A). The lack of cross-neutralization is due to high sequence heterogeneity of targeted epitopes such as hexon HVR or fiber knob.19,21,23,24 However, non-neutralizing but cross-reactive Ab are also generated, especially after repeated Ad injections.25 These non- neutralizing Abs can also reduce transgene expression, presumably using various Fc receptor dependent (such as antibody dependent cellular cytotoxicity, complement mediated lysis or opsonisation) and independent mechanisms. The impact of non-neutralizing Abs was elegantly illustrated by Pichla-Gollon and colleagues who demonstrated that passive transfer of an Ab able to bind, but not neutralize, could reduce transgene expression as well as immunogenicity of a modified Chad68 vector.26 The inhibitory effect of cross-reactive but non neutralizing Ab is of particular concern, as these Abs seem to be readily inducible.25
Figure 1.
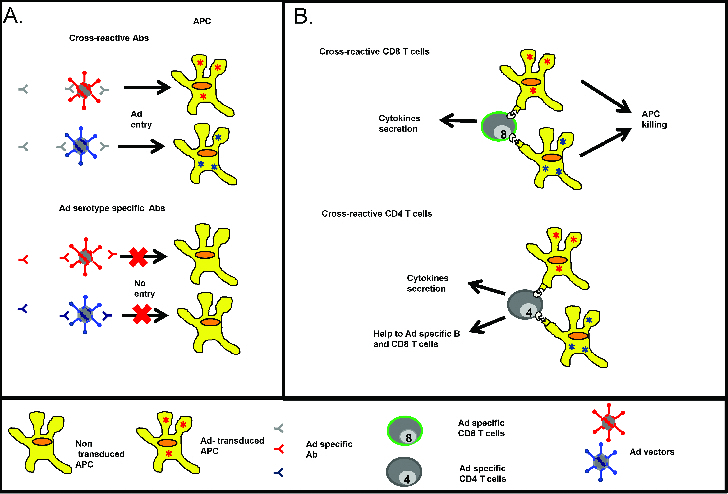
Humoral and cellular recognition of Ad vector. (A) Cross-reactive Ad specific Ab (gray) can bind to conserved regions (depicted in gray) within various serotypes but they lack neutralizing ability. In contrast, neutralizing Ab (red, blue) against Ad vector pre-dominantly target highly variable regions (depicted in red and blue) in the hexon and to a lower extent in the fiber. These nAbs are unable to neutralize Ad from different serotypes. (B) Cross-reactive (gray) Ad specific CD8 (top) and CD4 (bottom) T cells are generated upon Ad vector immunization. CD8 T cells mediate killing of Ad transduced APC and secrete inflammatory cytokines, while Ad specific CD4 T cell secrete various cytokines and provide help to Ad specific CD8 and B cells.
Cellular responses
In addition to nAbs, Ad specific T cells also decrease vector efficacy. Despite the fact that passive transfer of either serum or T cells from HAd5 immune mice can significantly decrease cellular and humoral response against the insert after HAd5 immunization,16 less attention has been given to the role of Ad specific T cells in pre-existing Ad vector immunity. Although, passive transfer of nAbs induced a greater inhibition of HAd5 induced immune response, they did not inhibit Ad immune stimulation to levels observed in Ad5 pre-immune mice,16 pointing at the contribution of Ad specific T cells in dampening HAd immunogenicity in pre-immune subjects. Depletion of T cells in Ad immune animals would provide a more accurate evaluation of T cell contribution as both circulating and tissue-resident T cells will be taken into account.
Ad specific T cell inhibition of vector efficacy is important due to their cross-reactivity. Indeed, unlike nAbs, Ad specific T cells are able to recognize a broad range of HAd from different subgroups (Fig. 1B). Several groups have shown that human CD4 and CD8 T cells predominantly recognize conserved epitopes in the adenovirus hexon, especially in the C-terminal region,27-31 with a limited number of T cells targeting the fiber and penton region.29,30 In addition to being widely cross-reactive, Ad specific T cells are detected in the great majority of tested individuals. Ad5 reactive CD4 or CD8 T cells were detected in 80% to 100% of subjects in Amsterdam, Netherlands30,32 and in all subjects in Philadelphia, USA.29,33,34 It is worth noting that these individuals were not screened for pre-existing Ad immunity using nAbs.
Due to the wide distribution of Ad reactive T cells, pre-existing Ad immunity might be more widespread than previously thought. Indeed, most studies solely rely on the presence of nAbs against Ad to assess seroprevalence. However in a few studies, both nAbs and cellular response against Ad were assessed and a higher proportion of individuals possessed T cell responses compare with nAbs against Ad. For example, in a study by Calcedo and colleagues, only 28% of tested individuals had neutralizing Ab against HAd5 whereas all of these individuals had Ad specific T cell responses as measured by Enzyme-Linked ImmunoSpot (ELISPOT).33 Similarly, a higher proportion of individuals had Ad specific T cell responses (64%) compared with neutralizing Ab against HAd5 (55%) in another study.35
In addition to being widely distributed and cross-reactive, Ad specific T cells were mainly effector memory T cells that could readily be reactivated in vitro28 and were polyfunctional.33,34 This high potency may result from frequent reactivation by infection with different Ad serotypes.28,33,34 Taken together, due to their ability to cross-react with Ad from different sub-groups, their wide-distribution in the human population and their high potency, the impact of Ad specific T cells cannot be overlooked when developing future Ad vectors.
Innate immune responses
In addition to Ad specific T and B cells, innate immune responses to Ad vectors can also dampen Ad efficacy, especially in the context of pre-existing immunity. The innate immune response does not possess immunological memory, with the exception of NK cells when previously activated by haptens36 or cytokines.37 However, despite this lack of “conventional” memory, Ad induced innate immune responses can regulate Ad immunogenicity.
Ad vectors, like other viral vectors can activate the innate immune response upon injection. Various pattern recognition receptors (PRR) including retinoic acid-inducible gene 1 (RIG-I), nucleotide oligomerization domain (NOD)-like receptors (NLR), factor X, and Toll-like receptors (TLR) 4 and 9 are involved in Ad recognition. In addition, Ad binding to the Coxsackievirus and Adenovirus receptor (CAR), as well as integrins, are also involved in this process.38-41 Upon activation, the above PRR molecules trigger signaling pathways such as NF-KB, which can induce the production of numerous pro-inflammatory mediators including cytokines (interferon (IFN) α/ β, interleukin (IL)-1α/ β, tumor necrosis factor (TNF)a, and IL-6) and chemokines (IL-8, macrophage inflammatory protein (MIP)-1α/β, IP-10, RANTES, and monocyte chemoattractant protein (MCP)-1).38-40 Among these pro-inflammatory molecules, IL-1 and its receptor IL-1R1, as well as type I IFN, play a central role as they induce positive feed-back loops that in turn, produce additional pro-inflammatory mediators.42-44
The role of innate immunity on antigen expression and vector efficacy after Ad immunization was revealed by comparing different HAd of various immunogenicities. It was first observed that when compared with the benchmark HAd5, both in vitro and in vivo, HAd vectors with lower immunogenicity such HAd35, HAd26 and HAd48,12,45,46 induce greater inflammatory responses, as illustrated by higher levels of inflammatory cytokines (IFNα, IFNγ, IL-6) and chemokines (IP-10, I-TAC, MIP1α/β).47,48 In addition, HAd35 and HAd28 also induced stronger NK cells activation. Stronger IFNα dependent NK cells activation can lead to lower transgene expression and persistence in vitro, due to NK killing of Ad transduced monocytes.48
The inverse relation between NK cell activation, type I IFN production and transgene expression were confirmed in vivo. For example, in rodents, high type I IFN levels were associated with lower transgene expression. Indeed, compared with HAd5, ChAd68 induces a higher level of type I IFN but lower transgene expression in vivo.49,50 Furthermore, higher transgene expression was detected after ChAd68 and HAd5 injection in IFN-α/β-R knockout mice when compared with the same vectors in wild type mice.42,50,51 Similarly, NK depletion prior to HAd5 immunization increases transgene expression level while NK transplantation had the opposite effect.51 The above studies indicate that Ad vectors inducing higher inflammation and NK activation have lower transgene expression and persistence. Of note, type I IFN and NK cells are also able to control the expressed level of antigen, which influences the T cell response in mice models of mouse cytomegalovirus (MCMV) infection.52,53 In individuals or animals with pre-existing Ad immunity, high inflammatory responses will further reduce transgene expression and persistence due to Ad specific CD8 T cell recruitment to the site of inflammation and subsequent killing of Ad transduced antigen presenting cells (APC) (Fig. 2).
Figure 2.
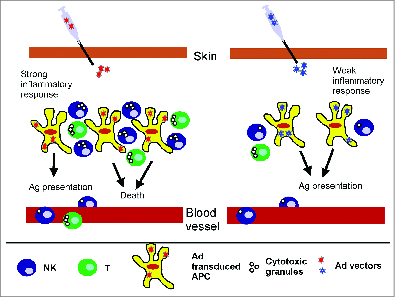
Strong innate immunity. Strong Ad induced inflammatory responses result in higher levels of chemokines that in turn recruit APC, NK cells, and CD8 T cells, among other lymphocytes. Recruited NK cells are activated by the cytokine milieu. Activated NK cells, as well as Ad specific CD8 T cells, mediate lysis of Ad transduced APCs leading to lower and shorter transgene expression as well as lower cellular and humoral response against the transgene. Milder inflammatory responses result in lower chemokine levels and lower NK cell recruitment and activation. Concomitantly, lower numbers of Ad specific CD8 T cells are recruited. As a result, Ad transduced APCs are not killed, transgene expression is higher and persists for longer period of time, resulting in higher cellular and humoral response against the transgene.
It is worth noting that although excessive innate immune responses are detrimental to Ad vector immunogenicity, inflammatory responses are still required to trigger a robust adaptive response and therefore a good vaccine-derived immune response. The requirement for inflammatory responses was illustrated with IFN-α/β-R knockout mice. In these studies, depending on the immunogenicity of the transgene, knockout mice failed to mount cellular responses42,51 or mounted lower responses after Ad5 immunization.49,50
Solutions to overcome pre-existing HAd immunity
Ad vectors based on alternative adenoviruses
Low seroprevalence human Ad
Due to the importance of nAbs in dampening Ad vector efficacy, Ad vectors were developed using human Ad with low seroprevalence in the human population. Among these vectors, both HAd35 and HAd26 vectors have been tested and shown to be safe in phase I clinical trials.54-56 However, compared with HAd5, human serotype vectors based on low seroprevalence, including Ad35 and Ad26, have shown lower immunogenicity12,46, 57-59 and protection efficacy in the non-human primate (NHP) model of Ebola virus infection,45 therefore questioning their clinical utility. In addition to lower immunogenicity, the protection efficacy of vectors based on low seroprevalence serotypes will probably also suffer from pre-existing Ad specific T cells. Although HAd5 specific neutralizing Ab do not cross-react with HAd35,60 HAd2 specific T cells, which are common in the human population, were able to cross-react with HAd35. Furthermore, HAd35 cross-reactive T cells were detected in individuals that lack nAbs against HAd35.61
Animal derived adenoviruses
Due to the lower immunogenicity of low seroprevalence HAd derived vectors, attention shifted toward the development of vectors based on animal Ad. Among these, chimpanzee derived Ad vector (ChAd) are the most studied. NAbs against simian Ad vectors are relatively rare. For example, nAbs against ChAd7 are detected in less than 15% of American, European, Chinese and African populations. The frequency of nAbs against ChAd6 are similar in these populations except in Africa where it peaks below 40%.14,46,62 Furthermore, unlike rare human Ad serotypes, ChAd have demonstrated great efficacy in pre-clinical studies including ChAd7 in a mouse model of EBOV infection,63 ChAd68 (SAd-24) in a NHP model of rabies,64,65 ChAd63, ChAd7 or ChAd9 in combination with modified vaccinia Ankara (MVA) boost in a malaria mouse model,66,67 and ChAdOx1 in a rodent model of Rift Valley Fever (RVF).68 In addition, in Phase I clinical trials against Malaria and HCV, ChAd6346 and ChAd3,69 respectively, were found to be safe and able to generate robust, broad and polyfunctional T cell responses against the transgene.
Although ChAd vectors appear very promising, cross-reacting pre-existing cellular immunity against Ad should not be overlooked. Indeed, in vitro, HAd specific T cells cross-react with ChAd vectors, such as ChAd6 and 7.34 Similarly, Ad specific T cells from a human cohort recognize HAd5, HAd4 as well as simian derived Ad (SAd-24/ChAd68 and SAd-32) to a similar extent.33 This could be expected due to the close phylogenetic proximity between human and chimpanzee Ad,46,70 more specifically to the highly conserved hexon C-terminal sequences that are recognized by Ad cross-reactive T cells.27-30, 33 The inhibitory role of Ad specific T cells on ChAd vectors was confirmed in animal models. In rare occasion, such as in the mouse model of malaria infection, pre-existing immunity to HAd5 reduced protection mediated by ChAd7 or ChAd 9 immunization.66 However, in most animal models, although ChAd induced protection was not impacted by pre-existing HAd5 immunity,46,63,65,71 ChAd induced immune responses tend to be lower in the presence of pre-existing immunity to HAd5. It is worth noting that the animal models mentioned above might underestimate the importance of cellular immunity. In pre-clinical studies, pre-existing immunity is usually generated by a single injection with a replication deficient Ad vector.46,63,65,71 This artificial pre-existing immunity may not recapitulate the potent Ad specific T cell response observed in humans following repeated exposure to replication competent Ad that initiate productive infections. Indeed, in primates, multiple exposures of replication competent Ad over time gives rise to polyfunctional and highly potent T cells.
In addition to chimpanzee Ad vectors, bovine, porcine, ovine, canine and fowl derived Ad vectors are currently in preclinical stages of development.72 NAbs against these vectors, including porcine Ad serotype 3 (PAd3), bovine Ad serotype 3 (BAd3) and Ovine Ad serotype 7 (OAd7), have not been detected in the human population.73,74 Similar to simian Ad vectors, cross-nAbs against PAd3 and BAd3 were not generated by mice immunized with HAd5.25 Furthermore, little to no CD4 and CD8 T cells cross-reactive against PAd3 and BAd3 were detected in HAd5 immunized mice.75 Finally, immunization with either BAd3 or Pad3-based vaccines protected both naïve and HAd5 pre-immune mice against influenza virus challenge. Both humoral and cellular immune responses were not impacted by pre-existing HAd5 immunity.76,77 Similarly, transgene expression and cellular responses were not affected in HAd5 pre-immune mice after OAd7 and OAdV injection, respectively.73,78 However, although promising results were obtained in rodents using non-primate Ad-derived vectors including BAd, PAd and OAd, their usefulness needs to be ultimately confirmed in humans. Whether Ad cross-reactive T cells can react with BAd and Pad still needs to be assessed as the cellular immune response is heavily influenced by MHC alleles, which are different in mice and humans.75
Varying immunization routes
In addition to using alternative Ad serotypes, pre-existing immunity has been overcome by varying the route of immunization. Indeed, mice with pre-existing HAd5 immunity generated by intranasal or intramuscular immunization did not experience reduced humoral responses upon subsequent oral immunization.79 Similarly, in the NHP model of Ebola virus, HAd5 mediated protection after intranasal/intratracheal immunization was not impacted by pre-existing HAd5 immunity generated by intramuscular (IM) injection of an irrelevant HAd5 vector.80 The lack of impact despite pre-existing immunity may be due to evasion from tissue resident Ad specific T cells.
Tissue resident memory T cells (TRM) are memory CD8 T cells which, unlike effector memory CD8 T (TEM) cells that circulate between blood and extra-lymphoid organs, do not circulate and remain confined to the tissue where the original infection occurred (For reviews see refs.81-83). TRM have been shown to induce better protection compared with TEM. Using parabiotic mice, skin resident T cells were shown to confer better protection against vaccinia virus skin infection compared with circulating memory T cells.84 Similarly, TRM induced a greater viral load reduction after skin and vaginal infection with Herpes simplex virus (HSV) compared with TEM. 85 In addition, TRM were still detectable in tissues after effector memory T cells were no longer visible in circulation.86
Tissue specificity of TRM is mainly determined during priming by tissue derived migratory dendritic cells (DC). Migratory DCs from different tissues induce TRM with different homing molecules and therefore different tissue specificity.83 Various immunization routes result in antigen (Ag) presentation by distinct tissue derived migratory DC and therefore, generation of TRM with different tissue specificity (Fig. 3). In the Richardson and colleagues study, IM injection probably did not generate TRM in the lung. Subsequent airway immunizations (intranasal/intratracheal) would be affected by the previously generated Ad specific TEM in the circulation but not by TRM, which would be absent from the lung. The ability to detect Ad specific T cells in human colon biopsies as well as in NHP gut (Ileum, colon and rectum) is supporting the existence of Ad specific TRM.33
Figure 3.
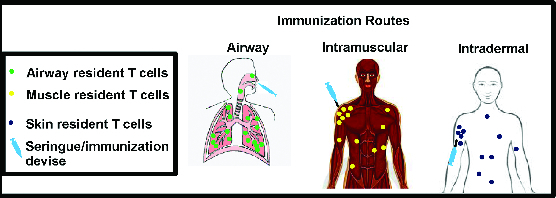
Model of tissue resident T cells induction by various vaccination routes. The majority of vector and insert specific tissue resident T cells will reside close to the site of immunization, in the tissue where the antigen was first encountered. Some of these resident T cells migrate away from the injection site while remaining within the same tissue. Depending on the immunization routes, the generated tissue resident T cells will reside in different tissues. Of note, circulating T cells are not depicted.
However, there are limitations in using different immunization routes to circumvent Ad pre-existing immunity. There are limited routes of immunization including intradermal, oral, intranasal/intratracheal, IM, sublingual and intravenous (IV). The latter may not be the best route for Ad vector immunization as one fatality has been reported in a gene therapy clinical trial.87 Furthermore, the route of natural Ad infections has to be considered in order to determine where putative Ad specific TRM are to be located in humans. Natural Ad infections mainly target the respiratory tract, and to lower extend the eye, gut and urinary tract of infected individuals.9,40 Therefore, Ad specific TRM may be present in the lung and gastro-intestinal tract of Ad immune individuals, which may affect intranasal/intratracheal and oral immunizations, respectively. This will need to be evaluated experimentally in the future.
After infection or vaccination, Abs can readily migrate throughout the body, as demonstrated by systemic administration of monoclonal Ab to treat various solid cancers that are located in different tissues such as breast, colorectal, gastric and kidney.88,89 However, Abs do not persist in all organs. Interestingly, nAbs against HAd5 could not be detected in the airway of a cohort of 51 volunteers naturally exposed to HAd, including those that possessed high levels of circulating HAd5 specific nAbs in the blood.90 This is likely due to the rapid turnover of immune cells and IgA type antibodies in the airway, making Ad-mediated vaccination at this site promising.
Prime-boost regimens
Prime-boost regimens may also help to overcome pre-existing Ad immunity. Prime-boost regimens do not bypass pre-existing Ad immunity per se. Immune responses induced by prime-boost regimens are still decreased by pre-existing immunity; however they are of sufficient magnitude to still confer protection. Indeed, more robust immune responses are obtained after heterologous prime-boost regimens compared with single vaccination or homologous prime-boost immunizations.91,92 This rule also holds true for heterologous Ad prime/boost regimens.12,45, 93-96
The usefulness of prime-boost regimens in overcoming pre-existing Ad immunity was demonstrated more than a decade ago using several DNA primes followed by a single Ad boost. Yang and colleagues demonstrated that cellular responses generated by DNA primes and HAd boost (DNA/HAd5) were not affected by pre-existing HAd5 immunity. In addition, although generated humoral responses were significantly reduced by pre-existing immunity, the antibody titers obtained were similar to those generated in naïve mice immunized with homologous HAd5 prime-boost.97 The ability of a DNA prime to circumvent pre-existing immunity was further suggested in a clinical trial. In tested individuals, pre-existing HAd5 immunity did not affect T cell responses induced by a DNA prime, HAd5 boost regimen, while pre-existing HAd5 immunity decreases cellular responses generated after a single HAd5 injection.98
In addition to DNA/HAd, heterologous prime-boost regimens involving Ad from different serotypes may also prove protective in HAd5 immune subjects. Pre-clinical studies involving ChAd68/ChAd1 prime-boost immunizations have shown encouraging signs. Indeed, although compare with naïve animals immune responses generated after ChAd68/ChAd1 prime-boost immunization were decreased in HAd5 pre-immune NHPs, stronger humoral responses were obtained compared with HAd5 homologous prime-boost.99 Finally, it will be a great interest to determine whether prime-boost regimens including Ad vectors in combination with other vaccine platforms such as modified vaccinia virus ankara (MVA)are also able to provide protection despite pre-existing immunity. Indeed in pre-clinical studies, regimens involving MVA and Ad encoding plasmodium berghei CS protein or severe acute respiratory syndrome (SARS) S protein were able to induce very robust T cell and Ab responses of higher magnitude than Ad/DNA regimens expressing the same insert.93,100 ChAd63/MVA prime-boost are even being tested in various stages of clinical trials against Malaria, HIV and HCV.101 Results from these trials will be crucial in terms of determining the ability of ChAd/MVA prime-boost regimens to overcome pre-existing HAd immunity. It is worth noting that prime-boost immunization has also been used to overcome pre-existing MVA immunity.102
One attractive feature of developing prime-boost regimens to circumvent pre-existing immunity is that it does not require the development of additional Ad vectors. Existing Ad vectors, especially those with robust immunogenicity can be used. However, determining which of the numerous vaccine platforms available, including DNA, VSV, AAV, and MVA, are best suited for priming or boosting of Ad vectors will be crucial. In addition, prime-boost regimens are time consuming thus precluding the rapid induction of protective immunity, which can be desirable in emergency vaccination situations, for example against pathogens that emerge or re-emerge sporadically in unpredictable locations.
Conclusions
Tremendous effort has been made in circumventing pre-existing Ad immunity in the human population. An important focus has been on the development of new animal based Ad vectors, especially Ad vectors of simian origin. While most of these vectors are in pre-clinical phases of development, some, including ChAd3 and ChAd63, have reached clinical trials. Selection of alternative Ad serotypes has been mainly driven by the absence of neutralizing Ab against these serotypes in the human population. However, the impact of Ad specific T cells on the efficacy of these vectors has been mainly overlooked. This is in part due to the complex nature of T cell assays that are designed to detect anti-Ad T cell responses. Compared with nAbs assays, T cell assays require complex stimulatory conditions and/or multi-parametric flow cytometry. The study of Ad specific T cell responses are further complicated by the presence of TRM that do not circulate in the blood. Isolation of these T cells usually requires mechanical and/or enzymatic disruption of the tissue of interest. These isolation procedures can affect T cell viability and function, which further complicates analysis of TRM cells. In addition, for obvious ethical reasons, isolation of TRM cells is restricted to animal models for most tissues except the skin and gut. Although harder to obtain, information regarding both Ad specific circulating and tissue resident T cells is necessary for a full understanding of pre-existing immunity against Ad vectors and to select the most appropriate Ad vector for clinical use. Therefore, the ability of Ad specific T cells in the target population to cross-react with the Ad vector being developed (chimpanzee, porcine, bovine, ovine) should be systematically investigated.
In pre-clinical studies, alternating the immunization route has bypassed pre-existing Ad immunity. Immunization through the airway holds promise and may turn out to be a delivery method of choice for inducing potent and long lasting protective immune responses from an Ad vector. While the airway may be a viable solution to bypass pre-existing immunity, several remaining questions will need to be addressed. Despite the relatively rapid turnover of resident immune cells and IgA in the lungs, the effect of immune stimulation following natural Ad exposure or vaccination will be critical to determine whether the same or other Ad vectors can be re-administer in the airway, at what frequency, and according to what regimen (single dose vs. prime-boost). This knowledge should ultimately inform on the number of independent vaccines one antigenic Ad backbone could be used for. Ultimately, this will have to be determined in clinical studies.
In addition, heterologous prime-boost regimens involving Ad vectors should be further optimized. The immune response generated by prime-boost regimens may be sufficient to induce full protection despite pre-existing Ad immunity. Developing prime-boost regimens using Ad vectors where limited to no nAbs or cross-reactive T cells in the target population should further increase the chance of success. However, optimization of prime-boost regimens will be labor intensive due to the increasing number of available vaccine platforms. Useful data regarding the ability of prime-boost regimens to overcome pre-existing HAd immunity will certainly be generated by clinical trials involving ChAd/MVA prime-boost. Careful analysis of these trials should provide crucial protection information in the presence or absence of pre-existing Ad immunity. In addition, future clinical trials involving Ad vectors should also determine whether Ad-based vaccine regimens impact HIV acquisition. Although HAd5 immunization increased HIV acquisition in participants with pre-existing HAd5 immunity in the STEP trial, whether this trend holds true for other Ad vectors expressing different inserts needs to be systematically evaluated. Upcoming clinical trials performed in populations at high risk of HIV infection will address this critical issue.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Amanna IJ, Messaoudi I, Slifka MK. Protective immunity following vaccination: how is it defined? Hum Vaccin 2008; 4:316-9; PMID:18398296; http://dx.doi.org/ 10.4161/hv.4.4.5751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, Orenstein WA. Measles antibody: reevaluation of protective titers. J Infect Dis 1990; 162:1036-42; PMID:2230231; http://dx.doi.org/ 10.1093/infdis/162.5.1036 [DOI] [PubMed] [Google Scholar]
- 3.Mason RA, Tauraso NM, Spertzel RO, Ginn RK. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Appl Microbiol 1973; 25:539-44; PMID:4633476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tatsis N, Ertl HCJ. Adenoviruses as vaccine vectors. Mol Ther 2004; 10:616-29; PMID:15451446; http://dx.doi.org/ 10.1016/j.ymthe.2004.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, Mulangu S, Hu Z, Andrews CA, Sheets RA, et al.; VRC 205 Study Team. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine 2010; 29:304-13; PMID:21034824; http://dx.doi.org/ 10.1016/j.vaccine.2010.10.037 [DOI] [PubMed] [Google Scholar]
- 6.Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, Mlisana K, Metch B, de Bruyn G, Latka MH, et al.; HVTN 503/Phambili study team. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis 2011; 11:507-15; PMID:21570355; http://dx.doi.org/ 10.1016/S1473-3099(11)70098-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al.; Step Study Protocol Team. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008; 372:1881-93; PMID:19012954; http://dx.doi.org/ 10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2007–an update. J Gene Med 2007; 9:833-42; PMID:17721874; http://dx.doi.org/ 10.1002/jgm.1100 [DOI] [PubMed] [Google Scholar]
- 9.Kremer EJ, Perricaudet M. Adenovirus and adeno-associated virus mediated gene transfer. Br Med Bull 1995; 51:31-44; PMID:7767647 [DOI] [PubMed] [Google Scholar]
- 10.Echavarría M. Adenoviruses in immunocompromised hosts. Clin Microbiol Rev 2008; 21:704-15; PMID:18854488; http://dx.doi.org/ 10.1128/CMR.00052-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nwanegbo E, Vardas E, Gao W, Whittle H, Sun H, Rowe D, Robbins PD, Gambotto A. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin Diagn Lab Immunol 2004; 11:351-7; PMID:15013987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, et al. . Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol 2007; 81:4654-63; PMID:17329340; http://dx.doi.org/ 10.1128/JVI.02696-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, et al. . International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 2010; 28:950-7; PMID:19925902; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.145 [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Xiang ZQ, Li Y, Kurupati RK, Jia B, Bian A, Zhou DM, Hutnick N, Yuan S, Gray C, et al. . Adenovirus-based vaccines: comparison of vectors from three species of adenoviridae. J Virol 2010; 84:10522-32; PMID:20686035; http://dx.doi.org/ 10.1128/JVI.00450-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman A, Tsai V, Goudreau A, Shinoda JY, Wen SF, Ramachandra M, Ralston R, Maneval D, LaFace D, Shabram P. Specific depletion of human anti-adenovirus antibodies facilitates transduction in an in vivo model for systemic gene therapy. Mol Ther 2001; 3:768-78; PMID:11356081; http://dx.doi.org/ 10.1006/mthe.2001.0316 [DOI] [PubMed] [Google Scholar]
- 16.Sumida SM, Truitt DM, Kishko MG, Arthur JC, Jackson SS, Gorgone DA, Lifton MA, Koudstaal W, Pau MG, Kostense S, et al. . Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J Virol 2004; 78:2666-73; PMID:14990686; http://dx.doi.org/ 10.1128/JVI.78.6.2666-2673.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gahéry-Ségard H, Farace F, Godfrin D, Gaston J, Lengagne R, Tursz T, Boulanger P, Guillet JG. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J Virol 1998; 72:2388-97; PMID:9499099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy S, Clawson DS, Calcedo R, Lebherz C, Sanmiguel J, Wu D, Wilson JM. Use of chimeric adenoviral vectors to assess capsid neutralization determinants. Virology 2005; 333:207-14; PMID:15721355; http://dx.doi.org/ 10.1016/j.virol.2004.12.029 [DOI] [PubMed] [Google Scholar]
- 19.Pichla-Gollon SL, Drinker M, Zhou X, Xue F, Rux JJ, Gao GP, Wilson JM, Ertl HC, Burnett RM, Bergelson JM. Structure-based identification of a major neutralizing site in an adenovirus hexon. J Virol 2007; 81:1680-9; PMID:17108028; http://dx.doi.org/ 10.1128/JVI.02023-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumida SM, Truitt DM, Lemckert AA, Vogels R, Custers JH, Addo MM, Lockman S, Peter T, Peyerl FW, Kishko MG, et al. . Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol 2005; 174:7179-85; PMID:15905562; http://dx.doi.org/ 10.4049/jimmunol.174.11.7179 [DOI] [PubMed] [Google Scholar]
- 21.Roberts DM, Nanda A, Havenga MJ, Abbink P, Lynch DM, Ewald BA, Liu J, Thorner AR, Swanson PE, Gorgone DA, et al. . Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 2006; 441:239-43; PMID:16625206; http://dx.doi.org/ 10.1038/nature04721 [DOI] [PubMed] [Google Scholar]
- 22.Roy S, Shirley PS, McClelland A, Kaleko M. Circumvention of immunity to the adenovirus major coat protein hexon. J Virol 1998; 72:6875-9; PMID:9658137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gall J, Kass-Eisler A, Leinwand L, Falck-Pedersen E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J Virol 1996; 70:2116-23; PMID:8642632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toogood CI, Crompton J, Hay RT. Antipeptide antisera define neutralizing epitopes on the adenovirus hexon. J Gen Virol 1992; 73:1429-35; PMID:1376769; http://dx.doi.org/ 10.1099/0022-1317-73-6-1429 [DOI] [PubMed] [Google Scholar]
- 25.Moffatt S, Hays J, HogenEsch H, Mittal SK. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology 2000; 272:159-67; PMID:10873758; http://dx.doi.org/ 10.1006/viro.2000.0350 [DOI] [PubMed] [Google Scholar]
- 26.Pichla-Gollon SL, Lin SW, Hensley SE, Lasaro MO, Herkenhoff-Haut L, Drinker M, Tatsis N, Gao GP, Wilson JM, Ertl HC, et al. . Effect of preexisting immunity on an adenovirus vaccine vector: in vitro neutralization assays fail to predict inhibition by antiviral antibody in vivo. J Virol 2009; 83:5567-73; PMID:19279092; http://dx.doi.org/ 10.1128/JVI.00405-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olive M, Eisenlohr L, Flomenberg N, Hsu S, Flomenberg P. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum Gene Ther 2002; 13:1167-78; PMID:12133270; http://dx.doi.org/ 10.1089/104303402320138952 [DOI] [PubMed] [Google Scholar]
- 28.Leen AM, Sili U, Vanin EF, Jewell AM, Xie W, Vignali D, Piedra PA, Brenner MK, Rooney CM. Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood 2004; 104:2432-40; PMID:15265797; http://dx.doi.org/ 10.1182/blood-2004-02-0646 [DOI] [PubMed] [Google Scholar]
- 29.Tang J, Olive M, Pulmanausahakul R, Schnell M, Flomenberg N, Eisenlohr L, Flomenberg P. Human CD8+ cytotoxic T cell responses to adenovirus capsid proteins. Virology 2006; 350:312-22; PMID:16499941; http://dx.doi.org/ 10.1016/j.virol.2006.01.024 [DOI] [PubMed] [Google Scholar]
- 30.Veltrop-Duits LA, Heemskerk B, Sombroek CC, van Vreeswijk T, Gubbels S, Toes RE, Melief CJ, Franken KL, Havenga M, van Tol MJ, et al. . Human CD4+ T cells stimulated by conserved adenovirus 5 hexon peptides recognize cells infected with different species of human adenovirus. Eur J Immunol 2006; 36:2410-23; PMID:16933360; http://dx.doi.org/ 10.1002/eji.200535786 [DOI] [PubMed] [Google Scholar]
- 31.Leen AM, Christin A, Khalil M, Weiss H, Gee AP, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J Virol 2008; 82:546-54; PMID:17942545; http://dx.doi.org/ 10.1128/JVI.01689-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heemskerk B, Veltrop-Duits LA, van Vreeswijk T, ten Dam MM, Heidt S, Toes RE, van Tol MJ, Schilham MW. Extensive cross-reactivity of CD4 +adenovirus-specific T cells: implications for immunotherapy and gene therapy. J Virol 2003; 77:6562-6; PMID:12743315; http://dx.doi.org/ 10.1128/JVI.77.11.6562-6566.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calcedo R, Vandenberghe LH, Roy S, Somanathan S, Wang L, Wilson JM. Host immune responses to chronic adenovirus infections in human and nonhuman primates. J Virol 2009; 83:2623-31; PMID:19116257; http://dx.doi.org/ 10.1128/JVI.02160-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutnick NA, Carnathan D, Demers K, Makedonas G, Ertl HC, Betts MR. Adenovirus-specific human T cells are pervasive, polyfunctional, and cross-reactive. Vaccine 2010; 28:1932-41; PMID:20188249; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther 1999; 6:1574-83; PMID:10490767; http://dx.doi.org/ 10.1038/sj.gt.3300994 [DOI] [PubMed] [Google Scholar]
- 36.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol 2006; 7:507-16; PMID:16617337; http://dx.doi.org/ 10.1038/ni1332 [DOI] [PubMed] [Google Scholar]
- 37.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A 2009; 106:1915-9; PMID:19181844; http://dx.doi.org/ 10.1073/pnas.0813192106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartman ZC, Appledorn DM, Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res 2008; 132:1-14; PMID:18036698; http://dx.doi.org/ 10.1016/j.virusres.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaci B, Ulasov IV, Wainwright DA, Lesniak MS. The challenge for gene therapy: innate immune response to adenoviruses. Oncotarget 2011; 2:113-21; PMID:21399236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fejer G, Freudenberg M, Greber UF, Gyory I. Adenovirus-triggered innate signalling pathways. Eur J Microbiol Immunol (Bp) 2011; 1:279-88; PMID:24516734; http://dx.doi.org/ 10.1556/EuJMI.1.2011.4.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doronin K, Flatt JW, Di Paolo NC, Khare R, Kalyuzhniy O, Acchione M, Sumida JP, Ohto U, Shimizu T, Akashi-Takamura S, et al. . Coagulation factor X activates innate immunity to human species C adenovirus. Science 2012; 338:795-8; PMID:23019612; http://dx.doi.org/ 10.1126/science.1226625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol 2007; 81:3170-80; PMID:17229689; http://dx.doi.org/ 10.1128/JVI.02192-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fejer G, Drechsel L, Liese J, Schleicher U, Ruzsics Z, Imelli N, Greber UF, Keck S, Hildenbrand B, Krug A, et al. . Key role of splenic myeloid DCs in the IFN-alphabeta response to adenoviruses in vivo. PLoS Pathog 2008; 4:e1000208; PMID:19008951; http://dx.doi.org/ 10.1371/journal.ppat.1000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA, Papayannopoulou T, Shayakhmetov DM. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity 2009; 31:110-21; PMID:19576795; http://dx.doi.org/ 10.1016/j.immuni.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geisbert TW, Bailey M, Hensley L, Asiedu C, Geisbert J, Stanley D, Honko A, Johnson J, Mulangu S, Pau MG, et al. . Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J Virol 2011; 85:4222-33; PMID:21325402; http://dx.doi.org/ 10.1128/JVI.02407-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, Siani L, Naddeo M, Grazioli F, Esposito ML, et al. . Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med 2012; 4:ra2; PMID:22218691; http://dx.doi.org/ 10.1126/scitranslmed.3002925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teigler JE, Iampietro MJ, Barouch DH. Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. J Virol 2012; 86:9590-8; PMID:22787208; http://dx.doi.org/ 10.1128/JVI.00740-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson MJ, Björkström NK, Petrovas C, Liang F, Gall JG, Loré K, Koup RA. Type I interferon-dependent activation of NK cells by rAd28 or rAd35, but not rAd5, leads to loss of vector-insert expression. Vaccine 2014; 32:717-24; PMID:24325826; http://dx.doi.org/ 10.1016/j.vaccine.2013.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hensley SE, Giles-Davis W, McCoy KC, Weninger W, Ertl HCJ. Dendritic cell maturation, but not CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J Immunol 2005; 175:6032-41; PMID:16237098; http://dx.doi.org/ 10.4049/jimmunol.175.9.6032 [DOI] [PubMed] [Google Scholar]
- 50.Hensley SE, Cun AS, Giles-Davis W, Li Y, Xiang Z, Lasaro MO, Williams BR, Silverman RH, Ertl HC. Type I interferon inhibits antibody responses induced by a chimpanzee adenovirus vector. Mol Ther 2007; 15:393-403; PMID:17235319; http://dx.doi.org/ 10.1038/sj.mt.6300024 [DOI] [PubMed] [Google Scholar]
- 51.Zhu J, Huang X, Yang Y. A critical role for type I IFN-dependent NK cell activation in innate immune elimination of adenoviral vectors in vivo. Mol Ther 2008; 16:1300-7; PMID:18443600; http://dx.doi.org/ 10.1038/mt.2008.88 [DOI] [PubMed] [Google Scholar]
- 52.Mitrović M, Arapović J, Traven L, Krmpotić A, Jonjić S. Innate immunity regulates adaptive immune response: lessons learned from studying the interplay between NK and CD8+ T cells during MCMV infection. Med Microbiol Immunol 2012; 201:487-95; PMID:22965169; http://dx.doi.org/ 10.1007/s00430-012-0263-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welsh RM, Waggoner SN. NK cells controlling virus-specific T cells: Rheostats for acute vs. persistent infections. Virology 2013; 435:37-45; PMID:23217614; http://dx.doi.org/ 10.1016/j.virol.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keefer MC, Gilmour J, Hayes P, Gill D, Kopycinski J, Cheeseman H, Cashin-Cox M, Naarding M, Clark L, Fernandez N, et al. . A phase I double blind, placebo-controlled, randomized study of a multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults. PLoS One 2012; 7:e41936; PMID:22870265; http://dx.doi.org/ 10.1371/journal.pone.0041936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, Patel A, Johnson JA, Kleinjan J, Yanosick KE, Perry J, et al. . First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis 2013; 207:240-7; PMID:23125444; http://dx.doi.org/ 10.1093/infdis/jis670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barouch DH, Liu J, Peter L, Abbink P, Iampietro MJ, Cheung A, Alter G, Chung A, Dugast AS, Frahm N, et al. . Characterization of humoral and cellular immune responses elicited by a recombinant adenovirus serotype 26 HIV-1 Env vaccine in healthy adults (IPCAVD 001). J Infect Dis 2013; 207:248-56; PMID:23125443; http://dx.doi.org/ 10.1093/infdis/jis671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, et al. . Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol 2004; 172:6290-7; PMID:15128818; http://dx.doi.org/ 10.4049/jimmunol.172.10.6290 [DOI] [PubMed] [Google Scholar]
- 58.Lemckert AAC, Sumida SM, Holterman L, Vogels R, Truitt DM, Lynch DM, Nanda A, Ewald BA, Gorgone DA, Lifton MA, et al. . Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-ad5 immunity. J Virol 2005; 79:9694-701; PMID:16014931; http://dx.doi.org/ 10.1128/JVI.79.15.9694-9701.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nanda A, Lynch DM, Goudsmit J, Lemckert AA, Ewald BA, Sumida SM, Truitt DM, Abbink P, Kishko MG, Gorgone DA, et al. . Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J Virol 2005; 79:14161-8; PMID:16254351; http://dx.doi.org/ 10.1128/JVI.79.22.14161-14168.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Béthune MP, Kostense S, Penders G, Helmus N, Koudstaal W, et al. . Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol 2003; 77:8263-71; PMID:12857895; http://dx.doi.org/ 10.1128/JVI.77.15.8263-8271.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flomenberg P, Piaskowski V, Truitt RL, Casper JT. Characterization of human proliferative T cell responses to adenovirus. J Infect Dis 1995; 171:1090-6; PMID:7751682; http://dx.doi.org/ 10.1093/infdis/171.5.1090 [DOI] [PubMed] [Google Scholar]
- 62.Jian L, Zhao Q, Zhang S, Huang W, Xiong Y, Zhou X, Jia B. The prevalence of neutralising antibodies to chimpanzee adenovirus type 6 and type 7 in healthy adult volunteers, patients with chronic hepatitis B and patients with primary hepatocellular carcinoma in China. Arch Virol 2014; 159:465-70; PMID:24057756; http://dx.doi.org/ 10.1007/s00705-013-1828-y [DOI] [PubMed] [Google Scholar]
- 63.Kobinger GP, Feldmann H, Zhi Y, Schumer G, Gao G, Feldmann F, Jones S, Wilson JM. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology 2006; 346:394-401; PMID:16356525; http://dx.doi.org/ 10.1016/j.virol.2005.10.042 [DOI] [PubMed] [Google Scholar]
- 64.Zhou D, Cun A, Li Y, Xiang Z, Ertl HCJ. A chimpanzee-origin adenovirus vector expressing the rabies virus glycoprotein as an oral vaccine against inhalation infection with rabies virus. Mol Ther 2006; 14:662-72; PMID:16797238; http://dx.doi.org/ 10.1016/j.ymthe.2006.03.027 [DOI] [PubMed] [Google Scholar]
- 65.Xiang ZQ, Greenberg L, Ertl HC, Rupprecht CE. Protection of non-human primates against rabies with an adenovirus recombinant vaccine. Virology 2014; 450-451:243-9; PMID:24503087; http://dx.doi.org/ 10.1016/j.virol.2013.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reyes-Sandoval A, Sridhar S, Berthoud T, Moore AC, Harty JT, Gilbert SC, Gao G, Ertl HC, Wilson JC, Hill AV. Single-dose immunogenicity and protective efficacy of simian adenoviral vectors against Plasmodium berghei. Eur J Immunol 2008; 38:732-41; PMID:18266272; http://dx.doi.org/ 10.1002/eji.200737672 [DOI] [PubMed] [Google Scholar]
- 67.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, Colloca S, Cortese R, Hill AV. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect Immun 2010; 78:145-53; PMID:19858306; http://dx.doi.org/ 10.1128/IAI.00740-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Warimwe GM, Lorenzo G, Lopez-Gil E, Reyes-Sandoval A, Cottingham MG, Spencer AJ, Collins KA, Dicks MD, Milicic A, Lall A, et al. . Immunogenicity and efficacy of a chimpanzee adenovirus-vectored Rift Valley fever vaccine in mice. Virol J 2013; 10:349; PMID:24304565; http://dx.doi.org/ 10.1186/1743-422X-10-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, et al. . Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med 2012; 4:ra1; PMID:22218690; http://dx.doi.org/ 10.1126/scitranslmed.3003155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roy S, Vandenberghe LH, Kryazhimskiy S, Grant R, Calcedo R, Yuan X, Keough M, Sandhu A, Wang Q, Medina-Jaszek CA, et al. . Isolation and characterization of adenoviruses persistently shed from the gastrointestinal tract of non-human primates. PLoS Pathog 2009; 5:e1000503; PMID:19578438; http://dx.doi.org/ 10.1371/journal.ppat.1000503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiang Z, Gao G, Reyes-Sandoval A, Cohen CJ, Li Y, Bergelson JM, Wilson JM, Ertl HC. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol 2002; 76:2667-75; PMID:11861833; http://dx.doi.org/ 10.1128/JVI.76.6.2667-2675.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bangari DS, Mittal SK. Development of nonhuman adenoviruses as vaccine vectors. Vaccine 2006; 24:849-62; PMID:16297508; http://dx.doi.org/ 10.1016/j.vaccine.2005.08.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hofmann C, Löser P, Cichon G, Arnold W, Both GW, Strauss M. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J Virol 1999; 73:6930-6; PMID:10400791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bangari DS, Shukla S, Mittal SK. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem Biophys Res Commun 2005; 327:960-6; PMID:15649439; http://dx.doi.org/ 10.1016/j.bbrc.2004.12.099 [DOI] [PubMed] [Google Scholar]
- 75.Sharma A, Tandon M, Ahi YS, Bangari DS, Vemulapalli R, Mittal SK. Evaluation of cross-reactive cell-mediated immune responses among human, bovine and porcine adenoviruses. Gene Ther 2010; 17:634-42; PMID:20164856; http://dx.doi.org/ 10.1038/gt.2010.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh N, Pandey A, Jayashankar L, Mittal SK. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus. Mol Ther 2008; 16:965-71; PMID:18301400; http://dx.doi.org/ 10.1038/mt.2008.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel A, Tikoo S, Kobinger G. A porcine adenovirus with low human seroprevalence is a promising alternative vaccine vector to human adenovirus 5 in an H5N1 virus disease model. PLoS One 2010; 5:e15301; PMID:21179494; http://dx.doi.org/ 10.1371/journal.pone.0015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wüest T, Both GW, Prince AM, Hofmann C, Löser P. Recombinant ovine atadenovirus induces a strong and sustained T cell response against the hepatitis C virus NS3 antigen in mice. Vaccine 2004; 22:2717-21; PMID:15246602; http://dx.doi.org/ 10.1016/j.vaccine.2004.01.048 [DOI] [PubMed] [Google Scholar]
- 79.Xiang ZQ, Gao GP, Reyes-Sandoval A, Li Y, Wilson JM, Ertl HC. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J Virol 2003; 77:10780-9; PMID:14512528; http://dx.doi.org/ 10.1128/JVI.77.20.10780-10789.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richardson JS, Pillet S, Bello AJ, Kobinger GP. Airway delivery of an adenovirus-based Ebola virus vaccine bypasses existing immunity to homologous adenovirus in nonhuman primates. J Virol 2013; 87:3668-77; PMID:23302894; http://dx.doi.org/ 10.1128/JVI.02864-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 2009; 10:524-30; PMID:19305395; http://dx.doi.org/ 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- 82.Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends Immunol 2013; 34:27-32; PMID:23036434; http://dx.doi.org/ 10.1016/j.it.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 83.Krzysiek R, de Goër de Herve M-G, Yang H, Taoufik Y. Tissue Competence Imprinting and Tissue Residency of CD8 T Cells. Front Immunol 2013; 4:283; PMID:24062749; http://dx.doi.org/ 10.3389/fimmu.2013.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 2012; 483:227-31; PMID:22388819; http://dx.doi.org/ 10.1038/nature10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A 2012; 109:7037-42; PMID:22509047; http://dx.doi.org/ 10.1073/pnas.1202288109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 2011; 477:216-9; PMID:21841802; http://dx.doi.org/ 10.1038/nature10339 [DOI] [PubMed] [Google Scholar]
- 87.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 2003; 80:148-58; PMID:14567964; http://dx.doi.org/ 10.1016/j.ymgme.2003.08.016 [DOI] [PubMed] [Google Scholar]
- 88.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010; 10:317-27; PMID:20414205; http://dx.doi.org/ 10.1038/nri2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science 2013; 341:1192-8; PMID:24031011; http://dx.doi.org/ 10.1126/science.1241145 [DOI] [PubMed] [Google Scholar]
- 90.Richardson JS, Abou MC, Tran KN, Kumar A, Sahai BM, Kobinger GP. Impact of systemic or mucosal immunity to adenovirus on Ad-based Ebola virus vaccine efficacy in guinea pigs. J Infect Dis 2011; 204(Suppl 3):S1032-42; PMID:21987739; http://dx.doi.org/ 10.1093/infdis/jir332 [DOI] [PubMed] [Google Scholar]
- 91.Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol 2004; 25:98-104; PMID:15102369; http://dx.doi.org/ 10.1016/j.it.2003.11.009 [DOI] [PubMed] [Google Scholar]
- 92.Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol 2009; 21:346-51; PMID:19500964; http://dx.doi.org/ 10.1016/j.coi.2009.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gilbert SC, Schneider J, Hannan CM, Hu JT, Plebanski M, Sinden R, Hill AV. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine 2002; 20:1039-45; PMID:11803063; http://dx.doi.org/ 10.1016/S0264-410X(01)00450-9 [DOI] [PubMed] [Google Scholar]
- 94.Barouch DH, McKay PF, Sumida SM, Santra S, Jackson SS, Gorgone DA, Lifton MA, Chakrabarti BK, Xu L, Nabel GJ, et al. . Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J Virol 2003; 77:8729-35; PMID:12885892; http://dx.doi.org/ 10.1128/JVI.77.16.8729-8735.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park S-H, Yang SH, Lee CG, Youn JW, Chang J, Sung YC. Efficient induction of T helper 1 CD4+ T-cell responses to hepatitis C virus core and E2 by a DNA prime-adenovirus boost. Vaccine 2003; 21:4555-64; PMID:14575768; http://dx.doi.org/ 10.1016/S0264-410X(03)00499-7 [DOI] [PubMed] [Google Scholar]
- 96.Pinto AR, Fitzgerald JC, Giles-Davis W, Gao GP, Wilson JM, Ertl HC. Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J Immunol 2003; 171:6774-9; PMID:14662882; http://dx.doi.org/ 10.4049/jimmunol.171.12.6774 [DOI] [PubMed] [Google Scholar]
- 97.Yang ZY, Wyatt LS, Kong WP, Moodie Z, Moss B, Nabel GJ. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J Virol 2003; 77:799-803; PMID:12477888; http://dx.doi.org/ 10.1128/JVI.77.1.799-803.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kibuuka H, Kimutai R, Maboko L, Sawe F, Schunk MS, Kroidl A, Shaffer D, Eller LA, Kibaya R, Eller MA, et al. . A phase 1/2 study of a multiclade HIV-1 DNA plasmid prime and recombinant adenovirus serotype 5 boost vaccine in HIV-Uninfected East Africans (RV 172). J Infect Dis 2010; 201:600-7; PMID:20078213; http://dx.doi.org/ 10.1086/650299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin SW, Li Y, Giles-Davis W, Cun A, Zhou D, et al. . Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J Virol 2007; 81:6594-604; PMID:17428852; http://dx.doi.org/ 10.1128/JVI.02497-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ba L, Yi CE, Zhang L, Ho DD, Chen Z. Heterologous MVA-S prime Ad5-S boost regimen induces high and persistent levels of neutralizing antibody response against SARS coronavirus. Appl Microbiol Biotechnol 2007; 76:1131-6; PMID:17581748; http://dx.doi.org/ 10.1007/s00253-007-1073-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Capone S, D’Alise AM, Ammendola V, Colloca S, Cortese R, Nicosia A, Folgori A. Development of chimpanzee adenoviruses as vaccine vectors: challenges and successes emerging from clinical trials. Expert Rev Vaccines 2013; 12:379-93; PMID:23560919; http://dx.doi.org/ 10.1586/erv.13.15 [DOI] [PubMed] [Google Scholar]
- 102.Gudmundsdotter L, Nilsson C, Brave A, Hejdeman B, Earl P, Moss B, Robb M, Cox J, Michael N, Marovich M, et al. . Recombinant Modified Vaccinia Ankara (MVA) effectively boosts DNA-primed HIV-specific immune responses in humans despite pre-existing vaccinia immunity. Vaccine 2009; 27:4468-74; PMID:19450644; http://dx.doi.org/ 10.1016/j.vaccine.2009.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]


