Abstract
Although recent control measures have significantly reduced malaria cases and deaths in many endemic areas, an effective vaccine will be essential to eradicate this parasitic disease. Malaria vaccine strategies developed to date focus on different phases of the parasite's complex life cycle in the human host and mosquito vector, and include both subunit-based and whole-parasite vaccines. This review focuses on the 3 live-attenuated malaria vaccination strategies that have been tested in humans to date, and discusses their progress, challenges and the immune correlates of protection that have been identified.
Keywords: attenuation, Malaria, P. falciparum, pre-erythrocytic, whole-parasite vaccines
Abbreviations
- CPS
Chemoprophylaxis and Sporozoite immunization
- CQ
chloroquine
- CSP
circumsporozoite protein
- GAP
Genetically Attenuated Parasite
- i.d.
intradermal
- ITV
Immunization-Treatment-Vaccination
- i.v.
intravenous
- PfSPZ
P. falciparum sporozoite vaccine
- RAS
Radiation Attenuated Sporozoites
- s.c.
subcutaneous
Introduction
Malaria constitutes one of the most severe public health problems worldwide, killing 473,000- 789,000 of the 135-287 million infected people in 2012.1 Five species of Plasmodium parasites cause malaria in humans, but most cases of severe disease and deaths are caused by P. falciparum, although P. vivax is widely prevalent and causes severe disease and relapses. The recent scale-up of control measures such as drugs and insecticide-treated bednets have significantly reduced malaria morbidity and mortality, but a vaccine that stops transmission of the parasite between the mosquito vector and the human host is widely considered to be essential for eradication.
The design of effective malaria vaccines has been hindered by multiple hurdles, including the fact that the parasite expresses different sets of antigens as it progresses from the sporozoites inoculated in the skin through the liver and blood stages, and its ability to undergo antigenic variation. In addition, individuals naturally exposed to malaria fail to develop sterile protection, although the severity of their symptoms diminishes after repeated blood stage infections (at least in areas of high transmission), and this partial immunity wanes over time in the absence of regular exposure.2
Several lines of evidence suggest that, in spite of these challenges, the development of malaria vaccines is feasible. Although naturally acquired immunity does not protect against re-infection, it does result in progressively better clinical outcomes. Also, early experiments in the 1960s showed that transfer of serum from individuals with naturally acquired anti-malaria immunity to acutely-ill children controlled their symptoms.3 Subsequently, a few experimental vaccines yielded encouraging results, as described below. However, to date none have accomplished the World Health Organization strategic criteria that must be met prior to widespread distribution of new malaria vaccines, which include inducing at least 50% protection against severe malaria that lasts a minimum of one year; and, in the longer term, induction of >80% protection for 4 years against clinical disease caused by both P. vivax and P. falciparum.4
P. falciparum malaria vaccine design strategies
Many different malaria vaccine strategies have been tested in mouse models of malaria, and several have progressed to human clinical trials. They can be classified in terms of the stage of the parasite's life cycle that they target, namely pre-erythrocytic vaccines that block parasite development before the symptomatic blood stages, blood stage vaccines that aim to inhibit erythrocyte invasion and reduce symptoms; and transmission blocking vaccines that prevent formation of viable oocysts in the mosquito. Regarding the antigens they employ, while some approaches use live-attenuated whole parasites, the majority consist of one or a few antigenic proteins selected on the basis of their biological and immunogenic properties.
Subunit vaccines
The most advanced malaria vaccine is RTS,S, a pre-erythrocytic, subunit based vaccine currently in Phase III clinical trials (reviewed in ref. 5). It is based on a C-terminal fragment of the circumsporozoite protein (CSP), the main sporozoite surface protein, and contains known B and T cell epitopes. After initial studies showed that RTS,S could protect 6/7 malaria-naïve volunteers against challenge with the homologous parasite strain,6,7 field studies in semi-immune adults from endemic regions achieved 34% vaccine efficacy, although protection waned with time.8,9 These studies led to pediatric efficacy trials,10-16 in which significant reductions in the risk of uncomplicated malaria (30%), severe malaria (58%) and P. falciparum infection (37%) were achieved in children ages 1-4 years10 that persisted for >3 years,11,12 but waned by year 5.13 Later studies in infants produced short-term anti-infection vaccine efficacies of ∼65% with 40-60% reductions in the risk of clinical disease14,15 that dropped substantially by month 18.16 The most recent field studies reported ∼50% reduction in the rate of P. falciparum infection and the incidence of clinical malaria in young children that lasted ∼1 year,17,18 however protection in infants was only ∼30%.19 Although very promising, RTS,S has not achieved the levels of protection and longevity thought necessary for malaria eradication. Other antigens are also being investigated, but RTS,S is the most advanced in clinical development.20,21
Whole-parasite approaches
Approaches that use whole, attenuated live parasites have achieved 100% protection. Here, we focus on 3 such strategies, in which parasites are attenuated either by irradiation, coverage with anti-erythrocytic malaria drugs during immunization, or genetic modification, resulting in parasites that arrest during liver stage development or that fail to complete blood stages, preventing symptomatic blood stage infections (Fig. 1).
Figure 1.
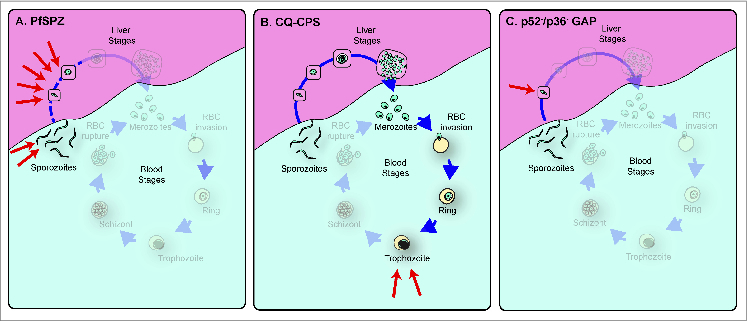
Live attenuated pre-erythrocytic malaria vaccines tested in human volunteers. (A) In the PfSPZ strategy, parasites are irradiated while in the mosquito vector, and arrest at different times during early development (red arrows) depending on the specific mutations induced. Late liver stages are absent, and no merozoites are released (indicated in the figure by the fact that those stages are greyed out). (B) In CPS under chloroquine (CQ) coverage, parasites complete liver stage development, and erythrocyte-infective merozoites are released, but the presence of CQ prevents development past the intra-erythrocytic trophozoite stage (red arrows). (C) The p52−/p36− GAP knockout results in very early liver stage arrest before formation of the parasitophorous vacuole (red arrow). Modified from ref. 45.
Attenuation by Irradiation
Attenuation of malaria parasites by irradiation was the first of these strategies to be developed: proof-of-concept experiments in mouse models in the late 1960s showed that i.v. immunization with sporozoites obtained from γ-irradiated mosquitoes induced sterile protection against challenge with a lethal strain of P. berghei.22,23 It was later shown that volunteers bitten by γ-irradiated mosquitoes infected with human Plasmodium parasites were protected against challenge with either homologous and heterologous wild-type parasites strains.24-28 However, the fact that at least 1,000 mosquito bites were required for protection, and the impracticality of using live irradiated mosquitoes under field conditions casted doubts on the feasibility of developing this Radiation Attenuated Sporozoites (RAS) strategy into a viable vaccine. Nevertheless, this approach was extensively explored in the ensuing years (reviewed in ref. 29), providing invaluable data of the role of humoral and cellular responses in protection against malaria.30-32 These studies also showed that protection could last at least 9 months,29,33 established the number of bites required for protection, and a radiation dose that yielded consistent attenuation, allowing parasites to invade and transform inside hepatocytes but not to form infectious merozoites, thus preventing breakthrough infections.29,34,35
Technical advances by Sanaria allowing the production of aseptic, cryopreserved irradiated sporozoites that could be delivered by needle significantly advanced this strategy as a viable malaria vaccine candidate, known as PfSPZ.36,37 The first results from a dose escalation study in human volunteers were not encouraging: although PfSPZ was safe and well tolerated, only 2/44 immunized volunteers were protected against challenge, a result that was ascribed to inefficient i.d. and s.c. administration of sporozoites resulting in suboptimal immunogenicity.37 However, the same study reported that i.v. administration of PfSPZ to non-human primates induced long-lasting T cell and antibody responses,37 and led to a clinical trial to deliver PfSPZ by i.v. injection, which protected, respectively, 60% and 100% of volunteers immunized with 4 or 5 doses of 135,000 cryopreserved PfSPZ sporozoites.38 The main caveat to this very promising approach is that protection required several large doses of cryopreserved sporozoites, which are currently expensive and laborious to produce. In addition, it remains to be demonstrated whether the protection is long lasting, and whether such large sporozoite doses delivered by i.v. are safe for children, the primary target population of any malaria vaccine. Nevertheless, these results are a significant advance toward an attenuated live parasite vaccine, and will be instrumental in understanding the immune correlates of protection afforded by irradiated sporozoites.
Attenuation by Drug Coverage
Initial work conducted in a mouse model of malaria in the late 1970s showed that sterile protection could also be achieved by infecting animals with wild type sporozoites under the coverage of the anti-malarial drug chloroquine (CQ), which prevents completion of the intraerythrocytic cycle of the parasite.39 Subsequent studies designed to characterize the immune response elicited by this immunization strategy, alternatively known as Chemoprophylaxis and Sporozoite (CPS) immunization or Immunization-Treatment-Vaccination (ITV), showed that sterile protection could be achieved with very few mosquito bites, suggesting that CPS/ITV might be more efficient than RAS.40-42 Human clinical trials conducted in the last 5 years have confirmed these results: 3 series of 15 P. falciparum-infected mosquito bites under CQ coverage (for a total of 36-45 bites) conferred 100% protection against challenge with 5 wild-type P. falciparum-infected mosquitoes 2 months after the last immunization.43 Importantly, the protection was long lasting, since 4 of 6 volunteers rechallenged >2 years later were still protected, while the other 2 displayed a 3-6 day delay in the onset of patency.44
Since CQ kills late blood stages but has no effect on sporozoites, liver stages or early blood stages,45,46 it is possible that CQ-CPS/ITV could induce cross-stage immunity against blood stage parasites by exposing the host's immune system to blood stage antigens expressed by late liver stage and early blood stage parasites. However, a recent human trial to identify which stage of the parasite's life cycle is targeted by CQ-CPS/ITV induced protective immunity showed that volunteers were protected against challenge with P. falciparum sporozoites, but not blood stages.47 In contrast, similar studies in the mouse model of malaria infection have suggested that CQ-CPS/ITV can induce cross-stage protection.45,48 This discrepancy between the human and mouse studies can be attributed to the fact that in the human trials, volunteers were under constant CQ treatment and developed only submicroscopic parasitemia (as detected by PCR) after the first course of immunization,43,47 whereas in the animal studies cross-stage protection was shown to be dependent on low-grade, transient parasitemia that developed after CQ had been withdrawn,45,48 and was lost when CQ treatment was maintained throughout the experiment.48 While CQ-CPS/ITV constitutes an effective approach, widespread CQ resistance prevents its use in malaria endemic regions. A recent study in mice by our group showed that CPS/ITV under artesunate (AS) coverage, which eliminates early blood stage parasites and does not cause transient low-grade parasitemia after drug withdrawal, efficiently elicits anti-sporozoite protection but does not yield significant cross-stage protection.45 Although AS-CPS/ITV has not yet been tested in humans, it will be interesting to determine whether it can induce sterile protection against sporozoite challenge. The use of AS could be advantageous because it would prevent the subpatent parasitemia observed in CQ treated individuals, reducing side effects, as well as the possibility of breakthrough infections. CPS/ITV strategies using other anti-malarial drugs have also been tested in rodent models, and suggest that the most efficient approaches are those that result in progression through the liver stages but do not allow any blood stage development.49-52
Genetic Attenuation
Immunization of mice with rodent Plasmodium sporozoites carrying deletions of specific genes that cause the arrest of the parasite during early liver stage development (including UIS3, UIS4, p36, p52 and sap1) can elicit long-lasting sterile protection from sporozoite challenge.53-57 An attempt to transfer this Genetically Attenuated Parasite (GAP) approach to human experimental vaccination studies led to the generation of p52 and p36/p52 knockouts in P. falciparum, which were able to infect mosquitoes and invade hepatocytes, but arrested during the liver stage.58,59 These promising results led to a dose escalation human clinical safety trial of the p36/p52 double knockout.60 This parasite was determined to be safe and well tolerated, and no breakthroughs were observed after the bite of 5 infected mosquitoes. However, 1 out of 6 volunteers experienced blood stage parasitemia after a second dose of 200 infective bites, suggesting that this mutant was not completely attenuated.60 In consequence, a triple knockout parasite in which sap1 is deleted in addition to p36 and p52 was constructed, and recently shown to be completely attenuated in a humanized mouse model with human hepatocytes and red blood cells.61 It will be interesting to compare the protection elicited by this strategy in human volunteers to those that use irradiated and chemically attenuated parasites. Because the deleted genes arrest parasite development during the early liver stage, it is likely that this GAP mutant will behave like RAS, although it may require lower doses to induce protection, since the vast majority of GAP parasites will invade and develop within hepatocytes until the missing functions that would have been provided by the deleted genes prevent further development. On the other hand, both of these strategies expose the immune system of the host to a more restricted range of antigens than CPS/ITV, which has been shown to induce better protection than irradiated sporozoites. A late arresting GAP could provide the optimal vaccination strategy by producing controlled and consistent arrest of a parasite that expresses a broader range of antigens than early-arresting GAPs. Deletion of the P. yoelii FabB/f gene leads to late arrest during liver stage development in the mouse model of malaria, and has been shown to result in enhanced protection as compared to both RAS and early arresting GAP.62 Unfortunately, the deletion of the P. falciparum ortholog of this gene abrogates sporozoite production,63 and thus the identification of other late-arresting GAPs will be critical in the development of whole-parasite vaccines.
Immunity elicited by whole-parasite vaccination
Although natural exposure to malaria parasites does not lead to sterile immunity, several of the strategies described above have shown that it is possible to elicit high levels of protection that persist for significant periods of time. Although the reasons for this difference are still unclear, the use of experimental vaccination can provide important clues to understand the development of anti-malaria immunity.
The RTS,S vaccine was designed around known B and CD4+ T cell CSP epitopes, and induces strong antibody responses to CSP, which have been associated with protection from infection in several clinical trials and have been shown to persist for months to years post-vaccination.64,65 In addition, there is evidence that protection also correlates with CSP-specific CD4+ T cells that produce functional cytokines, including IL2, IFNγ, TNF, and CD40L.64,66
Antibodies have also been shown to play a role in protection against malaria induced by whole-parasite vaccination approaches. Early studies showed that immunization with irradiated sporozoites induced antibody responses to whole sporozoites and CSP, which inhibited invasion of hepatocytes by sporozoites in vitro and correlated with protection.30,32,33 These results were recapitulated by i.v. immunization with PfSPZ of both non-human primates and humans, which also demonstrated the dose-dependent nature of protective antibody responses.37,38 Initial analyses of antibody responses elicited by CPS/ITV showed that they are mostly focused on pre-erythocytic antigens,43 in particular CSP and LSA-1.67 Although both antibodies and memory B cells were elicited by exposure to the parasite, they did not correlate with sterile protection.68 Functional studies showed that high concentrations of IgG from protected volunteers provided only minimal inhibition of gliding motility and traversal, although in vivo studies using immunized mice reduced liver stage burden by over 90%.69 In volunteers immunized with GAP, it was shown that exposure to 200 bites induced antibody responses against CSP but not LSA-1 or blood stage antigens.60 In addition, we showed that volunteer sera collected 3 months after exposure to GAP inhibited sporozoite invasion of hepatocytes at levels comparable to those observed in sera collected 2 weeks after a series of high doses of PfSPZ, and these levels were strongly correlated with antibody titers against CSP.70 Further studies will be needed to establish whether these functional antibodies correlate with protection against malaria.
The identification of other antigens targeted by humoral responses elicited by whole-parasite vaccination will help guide future vaccine design. Protection elicited by RAS immunization was shown to be associated with antibody responses against both previously identified and novel antigens, although no single antigen could be associated with protection.71 A similar approach using sera from volunteers immunized via the CPS/ITV strategy showed that protection was associated with the recognition of variable numbers of pre-erythrocytic antigens.67 Whether responses against any of these individual proteins were responsible for protection remains to be determined.
Protection elicited by whole-parasite vaccination in human volunteers has also been linked to IFNγ-producing CD8+ T cells, but more recently CD4+ T cells, γδ T cells and NK cells have also been implicated. Mouse studies suggested that cytotoxic CD8+ T cells can kill parasite-infected hepatocytes through direct contact,72,73 and their depletion lead to significant loss of protection (reviewed in ref. 74). Although the mechanism of action of these cells in human immunity remains unknown, early studies showed that humans produce CSP-specific cytotoxic T lymphocytes when exposed to malaria sporozoites via irradiated mosquitoes,31 and serum from these individuals contained high levels of IFNγ and CRP.75 Later studies of non-human primates immunized with PfSPZ by i.v. showed persistent induction of IFNγ- and TNF-producing, sporozoite-specific central and effector memory CD8+ T cells, and IFNγ-producing CD8+ T cells were shown to be more abundant in liver than peripheral blood.37 More recently, PfSPZ clinical studies have reported dose-dependent levels of IFNγ-producing CD8+ T cells that correlated with protection.38 These studies also showed that long-lasting cytotoxic central and effector memory CD4+ cells were induced by immunization with irradiated sporozoites,33,76 and that these cells can lyse autologous B cells pulsed with CSP peptides.76 They also produce IFNγ, IL2 and TNF after sporozoite stimulation, although cytokine levels did not correlate with protection.37,38 Few targets of CD8+ and CD4+ cells besides CSP and LSA-1 have been characterized to date, although some studies have identified larger numbers of potential targets.77,78 The establishment of definitive correlations between protection and T cell responses to specific antigens will be important for future anti-malarial vaccine design. Finally, although no differences were observed in the amount of IFNγ produced before and after immunization, protected volunteers exposed to high doses of PfSPZ displayed increased proportions of γδ T cells.38
Initial data from CPS/ITV vaccinated humans showed that protection associated with multifunctional effector memory T cells that produced IFNγ, IL2 and TNF upon stimulation with parasite-infected erythrocytes.43,79 CD4+ T cells, as well as uncharacterized CD4− CD8− T cells were major contributors to these cytokine levels, while CD8+ cells did not produce any detectable cytokines.43 More detailed analyses showed that in addition to CD4+ T cells, γδ T cells, NK and NKT cells also contributed to IFNγ production up to 400 days post-challenge.79 Stimulation with sporozoites confirmed that CD4+ (but not CD8+) T cells were major contributors to IFNγ production.79 However, experiments addressing the mechanism of action of T cells in the CPS/ITV model determined that protection was associated with the expression of degranulating marker CD107a+ by CD4+ T cells and with production of granzyme B by CD8+ T cells, but not with production of IFNγ by either CD4+, CD8+ or γδ T cells, confirming the role of cytotoxic T cells in protection against experimental malaria infection.80
Although the lack of protection data for GAP-vaccinated volunteers limits the interpretation of the results, initial analyses suggest that CD4+ T cells generated IFNγ, IL2 and TNF in response to sporozoite stimulation, whereas CD8+ T cells mainly produced IFNγ.60 Stimulation with individual antigens also showed that CD8+ T cells were mostly responsible for production of IFNγ, while TNF was mostly produced by CD4+ T cells.
The human experimental vaccination studies described above have provided many clues regarding factors that contribute to anti-malarial immunity, but they have not yet yielded a clear picture of the immunological correlates of protection. Both RAS and CPS/ITV report malaria-specific induction of IFNγ, but the differ in the cell types responsible for the production of this cytokine, as well as the degree to which it correlates with protection. These apparently conflicting data could result from intrinsic differences among the whole-parasite strategies that can be explained by mouse studies, which suggest a prominent role for IFNγ-producing CD8+ T cells in protection against liver stages, whereas CD4+ T cells seem to be important during blood stages. Thus, the CD4+ response observed in CPS/ITV could result from the fact that they allow the expression of late liver stage and early blood stage antigens, while the correlation between protection and induction of IFNγ-producing CD8+ T cells observed in RAS could result from the earlier arrest of these parasites during the liver stage. Alternatively, it is possible that variations in the experimental techniques used by the different research groups could influence these observations. Side by side comparison of human samples generated by the various whole-parasite vaccine approaches would better define the contribution of cellular immunity to protection against malaria infection.
Conclusion
Several anti-malaria vaccination strategies show promise but each one has caveats that must be addressed before they can be deployed. Subunit vaccines could be easier to implement, but the best candidate identified to date has shown less than the desired efficacy in field studies. In contrast, whole-parasite approaches show high efficacy in naïve individuals, but present safety, manufacturing, and delivery challenges, and their efficacy in the field has not been determined. Whether any of these candidates will be able to surpass their current limitations remains to be established, but nevertheless, they can all provide important clues to understand malaria immunity. Progress in malaria vaccine development will depend on the integration of the data generated by current and future studies using advanced immunobiology systems analyses to identify immune correlates of protection elicited by whole parasite vaccines, as well as antigenic profiling, which could lead to the design of subunit vaccines with improved efficacy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.World Health Organization World Malaria Report. 2013 http://www.who.int/malaria/publications/world_malaria_report_2013/en/ World Health Organization, 2013. Available from.
- 2.Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev 2009; 22:13-36; PMID:19136431; http://dx.doi.org/ 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature 1961; 192:733-7; PMID:13880318; http://dx.doi.org/ 10.1038/192733a0 [DOI] [PubMed] [Google Scholar]
- 4.Malaria Vaccine Funders Group Malaria Vaccine Technology Roadmap. Malaria Vaccine Funders Group, 2013. Available from http://www.who.int/immunization/topics/malaria/vaccine_roadmap/en/. [Google Scholar]
- 5.Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum Vaccin 2010; 6:90-6; PMID:19806009; http://dx.doi.org/ 10.4161/hv.6.1.9677 [DOI] [PubMed] [Google Scholar]
- 6.Gordon DM, McGovern TW, Krzych U, Cohen JC, Schneider I, LaChance R, Heppner DG, Yuan G, Hollingdale M, Slaoui M, et al. . Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J Infect Dis 1995; 171:1576-85; PMID:7769295; http://dx.doi.org/ 10.1093/infdis/171.6.1576 [DOI] [PubMed] [Google Scholar]
- 7.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garcon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med 1997; 336:86-91; PMID:8988885; http://dx.doi.org/ 10.1056/NEJM199701093360202 [DOI] [PubMed] [Google Scholar]
- 8.Bojang KA, Milligan PJ, Pinder M, Vigneron L, Alloueche A, Kester KE, Ballou WR, Conway DJ, Reece WH, Gothard P, et al. . Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 2001; 358:1927-34; PMID:11747915; http://dx.doi.org/ 10.1016/S0140-6736(01)06957-4 [DOI] [PubMed] [Google Scholar]
- 9.Doherty JF, Pinder M, Tornieporth N, Carton C, Vigneron L, Milligan P, Ballou WR, Holland CA, Kester KE, Voss G, et al. . A phase I safety and immunogenicity trial with the candidate malaria vaccine RTS,S/SBAS2 in semi-immune adults in The Gambia. Am J Trop Med Hyg 1999; 61:865-8; PMID:10674660 [DOI] [PubMed] [Google Scholar]
- 10.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, Mandomando I, Spiessens B, Guinovart C, Espasa M, et al. . Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 2004; 364:1411-20; PMID:15488216; http://dx.doi.org/ 10.1016/S0140-6736(04)17223-1 [DOI] [PubMed] [Google Scholar]
- 11.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Aide P, Sigauque B, Milman J, Mandomando I, Bassat Q, et al. . Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 2005; 366:2012-8; PMID:16338450; http://dx.doi.org/ 10.1016/S0140-6736(05)67669-6 [DOI] [PubMed] [Google Scholar]
- 12.Sacarlal J, Aide P, Aponte JJ, Renom M, Leach A, Mandomando I, Lievens M, Bassat Q, Lafuente S, Macete E, et al. . Long-term safety and efficacy of the RTS,S/AS02A malaria vaccine in Mozambican children. J Infect Dis 2009; 200:329-36; PMID:19569964; http://dx.doi.org/ 10.1086/600119 [DOI] [PubMed] [Google Scholar]
- 13.Campo JJ, Sacarlal J, Aponte JJ, Aide P, Nhabomba AJ, Dobano C, Alonso PL. Duration of vaccine efficacy against malaria: 5th year of follow-up in children vaccinated with RTS,S/AS02 in Mozambique. Vaccine 2014; 32:2209-16; PMID:24631081; http://dx.doi.org/ 10.1016/j.vaccine.2014.02.042 [DOI] [PubMed] [Google Scholar]
- 14.Aponte JJ, Aide P, Renom M, Mandomando I, Bassat Q, Sacarlal J, Manaca MN, Lafuente S, Barbosa A, Leach A, et al. . Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet 2007; 370:1543-51; PMID:17949807; http://dx.doi.org/ 10.1016/S0140-6736(07)61542-6 [DOI] [PubMed] [Google Scholar]
- 15.Abdulla S, Oberholzer R, Juma O, Kubhoja S, Machera F, Membi C, Omari S, Urassa A, Mshinda H, Jumanne A, et al. . Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N Engl J Med 2008; 359:2533-44; PMID:19064623; http://dx.doi.org/ 10.1056/NEJMoa0807773 [DOI] [PubMed] [Google Scholar]
- 16.Abdulla S, Salim N, Machera F, Kamata R, Juma O, Shomari M, Kubhoja S, Mohammed A, Mwangoka G, Aebi T, et al. . Randomized, controlled trial of the long term safety, immunogenicity and efficacy of RTS,S/AS02(D) malaria vaccine in infants living in a malaria-endemic region. Malar J 2013; 12:11; PMID:23297680; http://dx.doi.org/ 10.1186/1475-2875-12-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, Mshamu S, Lang T, Gould J, Dubois MC, et al. . Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med 2008; 359:2521-32; PMID:19064627; http://dx.doi.org/ 10.1056/NEJMoa0807381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmuller B, et al. . First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011; 365:1863-75; PMID:22007715; http://dx.doi.org/ 10.1056/NEJMoa1102287 [DOI] [PubMed] [Google Scholar]
- 19.RTS,S Clinical Trials Partnership , Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, et al. . A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 2012; 367:2284-95; PMID:23136909; http://dx.doi.org/ 10.1056/NEJMoa1208394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz L, Brown GV, Genton B, Moorthy VS. A review of malaria vaccine clinical projects based on the WHO rainbow table. Malar J 2012; 11:11; PMID:22230255; http://dx.doi.org/ 10.1186/1475-2875-11-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arama C, Troye-Blomberg M. The path of malaria vaccine development: challenges and perspectives. J Intern Med 2014; 275:456-66; PMID:24635625; http://dx.doi.org/ 10.1111/joim.12223 [DOI] [PubMed] [Google Scholar]
- 22.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature 1967; 216:160-2; PMID:6057225; http://dx.doi.org/ 10.1038/216160a0 [DOI] [PubMed] [Google Scholar]
- 23.Nussenzweig R, Vanderberg J, Most H. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. IV. Dose response, specificity and humoral immunity. Mil Med 1969; 134:1176-82; PMID:4987036; . [PubMed] [Google Scholar]
- 24.Clyde DF, McCarthy VC, Miller RM, Hornick RB. Specificity of protection of man immunized against sporozoite-induced falciparum malaria. Am J Med Sci 1973; 266:398-403; PMID:4590095; http://dx.doi.org/ 10.1097/00000441-197312000-00001 [DOI] [PubMed] [Google Scholar]
- 25.Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci 1973; 266:169-77; PMID:4583408; http://dx.doi.org/ 10.1097/00000441-197309000-00002 [DOI] [PubMed] [Google Scholar]
- 26.Rieckmann KH, Carson PE, Beaudoin RL, Cassells JS, Sell KW. Letter: Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans R Soc Trop Med Hyg 1974; 68:258-9; PMID:4608063; http://dx.doi.org/ 10.1016/0035-9203(74)90129-1 [DOI] [PubMed] [Google Scholar]
- 27.Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg 1975; 24:397-401; PMID:808142 [DOI] [PubMed] [Google Scholar]
- 28.Rieckmann KH, Beaudoin RL, Cassells JS, Sell KW. Use of attenuated sporozoites in the immunization of human volunteers against falciparum malaria. Bull World Health Organ 1979; 57(Suppl 1):261-5; PMID:120773 [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, et al. . Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis 2002; 185:1155-64; PMID:11930326; http://dx.doi.org/ 10.1086/339409 [DOI] [PubMed] [Google Scholar]
- 30.Egan JE, Hoffman SL, Haynes JD, Sadoff JC, Schneider I, Grau GE, Hollingdale MR, Ballou WR, Gordon DM. Humoral immune responses in volunteers immunized with irradiated Plasmodium falciparum sporozoites. Am J Trop Med Hyg 1993; 49:166-73; PMID:8357078 [DOI] [PubMed] [Google Scholar]
- 31.Malik A, Egan JE, Houghten RA, Sadoff JC, Hoffman SL. Human cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. Proc Natl Acad Sci U S A 1991; 88:3300-4; PMID:1707538; http://dx.doi.org/ 10.1073/pnas.88.8.3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrington D, Davis J, Nardin E, Beier M, Cortese J, Eddy H, Losonsky G, Hollingdale M, Sztein M, Levine M, et al. . Successful immunization of humans with irradiated malaria sporozoites: humoral and cellular responses of the protected individuals. Am J Trop Med Hyg 1991; 45:539-47; PMID:1951863 [DOI] [PubMed] [Google Scholar]
- 33.Edelman R, Hoffman SL, Davis JR, Beier M, Sztein MB, Losonsky G, Herrington DA, Eddy HA, Hollingdale MR, Gordon DM, et al. . Long-term persistence of sterile immunity in a volunteer immunized with X-irradiated Plasmodium falciparum sporozoites. J Infect Dis 1993; 168:1066-70; PMID:8376823; http://dx.doi.org/ 10.1093/infdis/168.4.1066 [DOI] [PubMed] [Google Scholar]
- 34.Silvie O, Semblat JP, Franetich JF, Hannoun L, Eling W, Mazier D. Effects of irradiation on Plasmodium falciparum sporozoite hepatic development: implications for the design of pre-erythrocytic malaria vaccines. Parasite Immunol 2002; 24:221-3; PMID:12010486; http://dx.doi.org/ 10.1046/j.1365-3024.2002.00450.x [DOI] [PubMed] [Google Scholar]
- 35.Mellouk S, Lunel F, Sedegah M, Beaudoin RL, Druilhe P. Protection against malaria induced by irradiated sporozoites. Lancet 1990; 335:721; PMID:1969073; http://dx.doi.org/ 10.1016/0140-6736(90)90832-P [DOI] [PubMed] [Google Scholar]
- 36.Lyke KE, Laurens M, Adams M, Billingsley PF, Richman A, Loyevsky M, Chakravarty S, Plowe CV, Sim BK, Edelman R, et al. . Plasmodium falciparum malaria challenge by the bite of aseptic Anopheles stephensi mosquitoes: results of a randomized infectivity trial. PLoS One 2010; 5:e13490; PMID:21042404; http://dx.doi.org/ 10.1371/journal.pone.0013490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, et al. . Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science 2011; 334:475-80; PMID:21903775; http://dx.doi.org/ 10.1126/science.1211548 [DOI] [PubMed] [Google Scholar]
- 38.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, et al. . Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013; 341:1359-65; PMID:23929949; http://dx.doi.org/ 10.1126/science.1241800 [DOI] [PubMed] [Google Scholar]
- 39.Beaudoin RL, Strome CP, Mitchell F, Tubergen TA. Plasmodium berghei: immunization of mice against the ANKA strain using the unaltered sporozoite as an antigen. Exp Parasitol 1977; 42:1-5; PMID:324783; http://dx.doi.org/ 10.1016/0014-4894(77)90054-6 [DOI] [PubMed] [Google Scholar]
- 40.Belnoue E, Costa FT, Frankenberg T, Vigario AM, Voza T, Leroy N, Rodrigues MM, Landau I, Snounou G, Renia L. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J Immunol 2004; 172:2487-95; PMID:14764721; http://dx.doi.org/ 10.4049/jimmunol.172.4.2487 [DOI] [PubMed] [Google Scholar]
- 41.Belnoue E, Voza T, Costa FT, Gruner AC, Mauduit M, Rosa DS, Depinay N, Kayibanda M, Vigario AM, Mazier D, et al. . Vaccination with live Plasmodium yoelii blood stage parasites under chloroquine cover induces cross-stage immunity against malaria liver stage. J Immunol 2008; 181:8552-8; PMID:19050274; http://dx.doi.org/ 10.4049/jimmunol.181.12.8552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orjih AU, Cochrane AH, Nussenzweig RS. Comparative studies on the immunogenicity of infective and attenuated sporozoites of Plasmodium berghei. Trans R Soc Trop Med Hyg 1982; 76:57-61; PMID:7043807; http://dx.doi.org/ 10.1016/0035-9203(82)90019-0 [DOI] [PubMed] [Google Scholar]
- 43.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, et al. . Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 2009; 361:468-77; PMID:19641203; http://dx.doi.org/ 10.1056/NEJMoa0805832 [DOI] [PubMed] [Google Scholar]
- 44.Roestenberg M, Teirlinck AC, McCall MB, Teelen K, Makamdop KN, Wiersma J, Arens T, Beckers P, van Gemert G, van de Vegte-Bolmer M, et al. . Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 2011; 377:1770-6; PMID:21514658; http://dx.doi.org/ 10.1016/S0140-6736(11)60360-7 [DOI] [PubMed] [Google Scholar]
- 45.Peng X, Keitany GJ, Vignali M, Chen L, Gibson C, Choi K, Huang F, Wang R. Artesunate versus chloroquine Infection-Treatment-Vaccination defines stage-specific immune responses associated with prolonged sterile protection against both pre-erythrocytic and erythrocytic Plasmodium yoelii infection. J Immunol 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White NJ. Qinghaosu (artemisinin): the price of success. Science 2008; 320:330-4; PMID:18420924; http://dx.doi.org/ 10.1126/science.1155165 [DOI] [PubMed] [Google Scholar]
- 47.Bijker EM, Bastiaens GJ, Teirlinck AC, van Gemert GJ, Graumans W, van de Vegte-Bolmer M, Siebelink-Stoter R, Arens T, Teelen K, Nahrendorf W, et al. . Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci U S A 2013; 110:7862-7; PMID:23599283; http://dx.doi.org/ 10.1073/pnas.1220360110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doll KL, Butler NS, Harty JT. CD8 T cell independent immunity after single dose infection-treatment-vaccination (ITV) against Plasmodium yoelii. Vaccine 2014; 32:483-91; PMID:24321740; http://dx.doi.org/ 10.1016/j.vaccine.2013.11.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friesen J, Borrmann S, Matuschewski K. Induction of antimalaria immunity by pyrimethamine prophylaxis during exposure to sporozoites is curtailed by parasite resistance. Antimicrob Agents Chemother 2011; 55:2760-7; PMID:21444698; http://dx.doi.org/ 10.1128/AAC.01717-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friesen J, Matuschewski K. Comparative efficacy of pre-erythrocytic whole organism vaccine strategies against the malaria parasite. Vaccine 2011; 29:7002-8; PMID:21787828; http://dx.doi.org/ 10.1016/j.vaccine.2011.07.034 [DOI] [PubMed] [Google Scholar]
- 51.Friesen J, Silvie O, Putrianti ED, Hafalla JC, Matuschewski K, Borrmann S. Natural immunization against malaria: causal prophylaxis with antibiotics. Sci Transl Med 2010; 2:40ra9; http://dx.doi.org/ 10.1126/scitranslmed.3001058 [DOI] [PubMed] [Google Scholar]
- 52.Putrianti ED, Silvie O, Kordes M, Borrmann S, Matuschewski K. Vaccine-like immunity against malaria by repeated causal-prophylactic treatment of liver-stage Plasmodium parasites. J Infect Dis 2009; 199:899-903; PMID:19434915; http://dx.doi.org/ 10.1086/597121 [DOI] [PubMed] [Google Scholar]
- 53.Labaied M, Harupa A, Dumpit RF, Coppens I, Mikolajczak SA, Kappe SH. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect Immun 2007; 75:3758-68; PMID:17517871; http://dx.doi.org/15699336 10.1128/IAI.00225-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, Matuschewski K, Kappe SH. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci U S A 2005; 102:3022-7; PMID:15699336; http://dx.doi.org/ 10.1073/pnas.0408442102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 2005; 433:164-7; PMID:15580261; http://dx.doi.org/ 10.1038/nature03188 [DOI] [PubMed] [Google Scholar]
- 56.van Dijk MR, Douradinha B, Franke-Fayard B, Heussler V, van Dooren MW, van Schaijk B, van Gemert GJ, Sauerwein RW, Mota MM, Waters AP, et al. . Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc Natl Acad Sci U S A 2005; 102:12194-9; PMID:16103357; http://dx.doi.org/ 10.1073/pnas.0500925102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aly AS, Mikolajczak SA, Rivera HS, Camargo N, Jacobs-Lorena V, Labaied M, Coppens I, Kappe SH. Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection. Mol Microbiol 2008; 69:152-63; PMID:18466298; http://dx.doi.org/ 10.1111/j.1365-2958.2008.06271.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.VanBuskirk KM, O'Neill MT, De La Vega P, Maier AG, Krzych U, Williams J, Dowler MG, Sacci JB, Jr., Kangwanrangsan N, Tsuboi T, et al. . Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc Natl Acad Sci U S A 2009; 106:13004-9; PMID:19625622; http://dx.doi.org/ 10.1073/pnas.0906387106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Schaijk BC, Janse CJ, van Gemert GJ, van Dijk MR, Gego A, Franetich JF, van de Vegte-Bolmer M, Yalaoui S, Silvie O, Hoffman SL, et al. . Gene disruption of Plasmodium falciparum p52 results in attenuation of malaria liver stage development in cultured primary human hepatocytes. PLoS One 2008; 3:e3549; PMID:18958160; http://dx.doi.org/ 10.1371/journal.pone.0003549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spring M, Murphy J, Nielsen R, Dowler M, Bennett JW, Zarling S, Williams J, de la Vega P, Ware L, Komisar J, et al. . First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine 2013; 31:4975-83; PMID:24029408; http://dx.doi.org/ 10.1016/j.vaccine.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 61.Mikolajczak SA, Lakshmanan V, Fishbaugher M, Camargo N, Harupa A, Kaushansky A, Douglass AN, Baldwin M, Healer J, O'Neill M, et al. . A next-generation genetically attenuated Plasmodium falciparum parasite created by triple gene deletion. Mol Ther 2014; PMID:24827907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, Harty JT. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 2011; 9:451-62; PMID:21669394; http://dx.doi.org/ 10.1016/j.chom.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Schaijk BC, Kumar TR, Vos MW, Richman A, van Gemert GJ, Li T, Eappen AG, Williamson KC, Morahan BJ, Fishbaugher M, et al. . Type II fatty acid biosynthesis is essential for Plasmodium falciparum sporozoite development in the midgut of Anopheles mosquitoes. Eukaryot Cell 2014; 13:550-9; PMID:24297444; http://dx.doi.org/ 10.1128/EC.00264-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, Schwenk R, Nielsen RA, Debebe Z, Pinelis E, et al. . Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis 2009; 200:337-46; PMID:19569965; http://dx.doi.org/ 10.1086/600120 [DOI] [PubMed] [Google Scholar]
- 65.Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG, Hall T, Krzych U, Delchambre M, Voss G, Dowler MG, et al. . Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J Infect Dis 2001; 183:640-7; PMID:11170991; http://dx.doi.org/ 10.1086/318534 [DOI] [PubMed] [Google Scholar]
- 66.White MT, Bejon P, Olotu A, Griffin JT, Riley EM, Kester KE, Ockenhouse CF, Ghani AC. The relationship between RTS,S vaccine-induced antibodies, CD4(+) T cell responses and protection against Plasmodium falciparum infection. PLoS One 2013; 8:e61395; PMID:23613845; http://dx.doi.org/ 10.1371/journal.pone.0061395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Felgner PL, Roestenberg M, Liang L, Hung C, Jain A, Pablo J, Nakajima-Sasaki R, Molina D, Teelen K, Hermsen CC, et al. . Pre-erythrocytic antibody profiles induced by controlled human malaria infections in healthy volunteers under chloroquine prophylaxis. Sci Rep 2013; 3:3549; PMID:24351974; http://dx.doi.org/ 10.1038/srep03549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nahrendorf W, Scholzen A, Bijker EM, Teirlinck AC, Bastiaens GJ, Schats R, Hermsen CC, Visser LG, Langhorne J, Sauerwein RW. Memory B-cell and antibody responses induced by Plasmodium falciparum sporozoite immunization. J Infect Dis 2014; PMID:24970846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Behet MC, Foquet L, van Gemert GJ, Bijker EM, Meuleman P, Leroux-Roels G, Hermsen CC, Scholzen A, Sauerwein RW. Sporozoite immunization of human volunteers under chemoprophylaxis induces functional antibodies against pre-erythrocytic stages of Plasmodium falciparum. Malar J 2014; 13:136; PMID:24708526; http://dx.doi.org/ 10.1186/1475-2875-13-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finney OC, Keitany GJ, Smithers H, Kaushansky A, Kappe S, Wang R. Immunization with genetically attenuated P. falciparum parasites induces long-lived antibodies that efficiently block hepatocyte invasion by sporozoites. Vaccine 2014; 32:2135-8; PMID:24582635; http://dx.doi.org/ 10.1016/j.vaccine.2014.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trieu A, Kayala MA, Burk C, Molina DM, Freilich DA, Richie TL, Baldi P, Felgner PL, Doolan DL. Sterile protective immunity to malaria is associated with a panel of novel P. falciparum antigens. Mol Cell Proteomics 2011; 10:M111 007948; http://dx.doi.org/ 10.1074/mcp.M111.007948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trimnell A, Takagi A, Gupta M, Richie TL, Kappe SH, Wang R. Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes. J Immunol 2009; 183:5870-8; PMID:19812194; http://dx.doi.org/ 10.4049/jimmunol.0900302 [DOI] [PubMed] [Google Scholar]
- 73.Cockburn IA, Amino R, Kelemen RK, Kuo SC, Tse SW, Radtke A, Mac-Daniel L, Ganusov VV, Zavala F, Menard R. In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages. Proc Natl Acad Sci U S A 2013; 110:9090-5; PMID:23674673; http://dx.doi.org/ 10.1073/pnas.1303858110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Braeckel-Budimir N, Harty JT. CD8 T-cell-mediated protection against liver-stage malaria: lessons from a mouse model. Front Microbiol 2014; 5:272; PMID:24936199; http://dx.doi.org/ 10.3389/fmicb.2014.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harpaz R, Edelman R, Wasserman SS, Levine MM, Davis JR, Sztein MB. Serum cytokine profiles in experimental human malaria. Relationship to protection and disease course after challenge. J Clin Invest 1992; 90:515-23; PMID:1644922; http://dx.doi.org/ 10.1172/JCI115889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moreno A, Clavijo P, Edelman R, Davis J, Sztein M, Herrington D, Nardin E. Cytotoxic CD4+ T cells from a sporozoite-immunized volunteer recognize the Plasmodium falciparum CS protein. Int Immunol 1991; 3:997-1003; PMID:1721837; http://dx.doi.org/ 10.1093/intimm/3.10.997 [DOI] [PubMed] [Google Scholar]
- 77.Doolan DL, Southwood S, Freilich DA, Sidney J, Graber NL, Shatney L, Bebris L, Florens L, Dobano C, Witney AA, et al. . Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc Natl Acad Sci U S A 2003; 100:9952-7; PMID:12886016; http://dx.doi.org/ 10.1073/pnas.1633254100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krzych U, Lyon JA, Jareed T, Schneider I, Hollingdale MR, Gordon DM, Ballou WR. T lymphocytes from volunteers immunized with irradiated Plasmodium falciparum sporozoites recognize liver and blood stage malaria antigens. J Immunol 1995; 155:4072-7; PMID:7561118 [PubMed] [Google Scholar]
- 79.Teirlinck AC, McCall MB, Roestenberg M, Scholzen A, Woestenenk R, de Mast Q, van der Ven AJ, Hermsen CC, Luty AJ, Sauerwein RW. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog 2011; 7:e1002389; PMID:22144890; http://dx.doi.org/ 10.1371/journal.ppat.1002389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bijker EM, Teirlinck AC, Schats R, van Gemert GJ, van de Vegte-Bolmer M, van Lieshout L, IntHout J, Hermsen CC, Scholzen A, Visser LG, et al. . Cytotoxic markers associate with protection against malaria in human volunteers immunized with Plasmodium falciparum sporozoites. J Infect Dis 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


