Abstract
E. coli O111 strains are responsible for outbreaks of blood diarrhea and hemolytic uremic syndrome throughout the world. Because of their phenotypic variability, the development of a vaccine against these strains which targets an antigen that is common to all of them is quite a challenge. Previous results have indicated, however, that O111 LPS is such a candidate, but its toxicity makes LPS forbidden for human use. To overcome this problem, O111 polysaccharides were conjugated either to cytochrome C or to EtxB (a recombinant B subunit of LT) as carrier proteins. The O111-cytochrome C conjugate was incorporated in silica SBA-15 nanoparticles and administered subcutaneously in rabbits, while the O111-EtxB conjugate was incorporated in VaxcineTM, an oil-based delivery system, and administered orally in mice. The results showed that one year post-vaccination, the conjugate incorporated in silica SBA-15 generated antibodies in rabbits able to inhibit the adhesion of all categories of O111 E. coli to epithelial cells. Importantly, mice immunized orally with the O111-EtxB conjugate in VaxcineTM generated systemic and mucosal humoral responses against all categories of O111 E. coli as well as antibodies able to inhibit the toxic effect of LT in vitro. In summary, the results obtained by using 2 different approaches indicate that a vaccine that targets the O111 antigen has the potential to prevent diarrhea induced by O111 E. coli strains regardless their mechanism of virulence. They also suggest that a conjugated vaccine that uses EtxB as a carrier protein has potential to combat diarrhea induced by ETEC.
Keywords: conjugated vaccine, E. coli, O111 polysaccharide
Abbreviations
- E. coli
Escherichia coli
- LT
heat labile toxin of ETEC
- HUS
hemolytic uremic syndrome
- EPEC
enteropathogenic E. coli
- STEC
shiga-producing toxins E. coli
- EAEC
enteroaggregative E. coli
- LPS
lipopolysaccharide
- SBA-15
Santa Barbara Amorphous-15
- aEPEC
atypical EPEC
- t-EPEC
typical EPEC
- CT
cholera toxin
- EtxB
non-toxic B subunit of LT
- PAGE
polyacrylamide gel electrophoresis
- SDS
sodium dodecyl sulfate
- EHEC
enterohemorrhagic E. coli
Introduction
Diarrheal diseases kill more children than do malaria or tuberculosis, 6 times more than armed conflicts and 5 times more than AIDS.1 Annually, nearly 5 billion cases of diarrhea are reported around the world leading to 760 thousand deaths per year in under-fives.2 Approximately 20 to 60 % of travelers to developing countries contract diarrheal disorders, Escherichia coli being the etiological agent responsible for most of them.3 In addition, a surveillance study in Mexico, Brazil and South Africa demonstrated that diarrheagenic strains of E. coli are responsible for approximately 40% of all cases of diarrhea, in some places exceeding the numbers induced by rotavirus.4
Only a few serogroups of E. coli are responsible for the majority of diarrheal diseases, including outbreaks of blood diarrhea and hemolytic uremic syndrome (HUS) in developed countries.5-7 One of these serogroups is O111,5-9 whose strains can be categorized as enteropathogenic E. coli (EPEC), shiga-producing toxins E. coli (STEC) and enteroaggregative E. coli (EAEC), reflecting the fact that E. coli O111 strains themselves have a variety of different mechanisms of virulence.10,11 Furthermore, several strains of E. coli O111 are considered emerging pathogens with the potential to cause serious outbreaks.12-19 Also needing to be taken into consideration is the fact that these pathogens can survive in cattle stools for up to 8 weeks in temperatures ranging from 5°C to 28°C.20 This is a situation that is of great concern since cattle are the main reservoir of these pathogens.21
Despite the economic burden that E. coli O111 inflicts on governmental funds and the severe repercussions caused by them on public health, there is no vaccine available against these pathogens.
It has been shown previously that the O111 LPS is a promising antigen candidate for the formulation of a vaccine against O111 pathogens since antibodies raised against them are able to recognize and inhibit the adhesion of all 3 categories of O111 E. coli to human epithelial cells.22 However, there are problems associated with the use of LPS as an antigen in vaccine formulations23-25 relating to the high level of toxicity of this material. Therefore, intact LPS is not appropriate for human use.
Table 1.
Strains and categories of diarrheagenic E. coli
| SOROTYPE | PATHOTYPE | REFERENCE |
|---|---|---|
| O127:H6 | t-EPEC | 10 |
| O111:H- | EHEC | 10 |
| O111:H12 | EAEC | 10 |
| O111:H25 | a-EPEC | 10 |
| O111:H2 | t-EPEC | 10 |
An alternative route is to use the polysaccharide part of the LPS as an antigen. However, because most B cells of children under 2 y old are immature, their immune response to polysaccharides is weak with no immunological memory 26. In spite of this, response to polysaccharides in young children can be achieved by the conjugation of polysaccharides to a carrier protein. However, to improve the efficacy of polysaccharide-conjugated vaccines in newborns, the use of an adjuvant may be required.27 Unfortunately, alum, the adjuvant most commonly used in routine medical practice, does not have a major adjuvant effect against Type II independent antigens such as carbohydrates and polysaccharides antigens.28 In addition, alum can increase IgE responses and induce local reactions such as granulomas.29,30 Therefore, there is a continued search to find an adjuvant that can increase the antibody response induced by polysaccharide conjugated vaccines in young children.
In order to select an adjuvant appropriate for a given vaccine formulation, certain factors such as the route of administration and specific properties of the adjuvant must be considered. It has been demonstrated that several adjuvants such as the mesoporous silica SBA-15 nanoparticles exhibit very high adjuvant property via the parenteral route.31,32 Although such materials are highly efficacious when injected, in the case of enteric pathogens such as O111 E. coli, the preferred method of administration of the vaccine is the oral route, since this may be easier to distribute and administer in populations in developing countries, and can stimulate strong immunity in the intestine itself. However, oral antigen administration can lead to tolerance, for this reason, it is necessary to include in the oral vaccine formulation an adjuvant able to generate mucosal and systemic antibody responses against the co-administered antigen.33 The most potent mucosal adjuvants described so far are LT, the Heat Labile Toxin from enterotoxigenic E. coli and CT, Cholera toxin from V. cholerae, but in their native form they are too toxic and consequently forbidden for human use. Therefore, mutants of CT and LT with low or no toxicity have being formulated. A good example of such a molecule is EtxB, a recombinant B subunit of LT which is able to induce mucosal and systemic immune response to the co-administered antigen via the oral route.34
Another important aspect of oral vaccines is the requirement to incorporate the antigen into a delivery system, in order to protect it during its passage through the gastro-intestinal tract. Liposomes or oil-based carriers have been commonly used as oral antigen delivery systems.35,36 One oil based carrier that has been successfully used to deliver antigens via the oral route is VaxcineTM. This delivery system has 2 potentially important features, (i) it can be taken up by the M cells of the Peyer's patches which are the immune competent sites of the intestine and it can protect antigens from attack by degradative actions of the gut milieu such as proteases.35
Despite all the technology accessible for the construction of oral vaccines against enteric pathogens, only a few products are so far available in the market. One of them is Dukoral, for prevention of cholera.37 It has been found however, that Dukoral can also be used to prevent traveler's diarrhea induced by enterotoxigenic E. coli,37 and this gives encouragement that the analogous approach employed here may be successful in protecting against other E. coli pathogenicities.
In view of the fact that there is no vaccine available against the highly virulent O111 E. coli strains, this work was conducted in order to determine whether conjugated polysaccharide vaccines have the ability to generate mucosal and systemic immune response against these pathogens, and the results obtained demonstrate that this is indeed the case.
Results
Analysis of the conjugates
In order to confirm that O111 polysaccharides were bound to the carrier proteins, both conjugates (O111-cytochrome C and O111-EtxB) were analyzed by SDS-PAGE and Western-blotting techniques. The results demonstrated that there was formation of matrix type complexes of O111 polysaccharides and cytochrome C with different molecular mass. The same was not observed in the free cytochrome c, free EtxB and O111-EtxB conjugate samples (Figs. 1A, C) The results also showed that antibodies against O111 polysaccharides were able to recognize only the conjugate samples and the native O111 LPS used as control in place of O111 detoxified polysaccharide whose molecular mass obtained by SDS-PAGE was very low ≤20 kDa. However, the antibodies against O111 polysaccharides were not able to recognize the carrier protein samples (Figs. 1A, C). Size-exclusion chromatography results showed, in the cytochrome C conjugate sample, the presence of molecules with molecular mass higher than those observed in the O111-ADH polysaccharide sample. (Fig. 1B). The analyses of the EtxB- conjugate sample showed that a large portion of free O111-ADH polysaccharides was eliminated from the O111-EtxB conjugate after its purification on a 30.000 MW cut-off Minicon centrifugal concentrator (Fig. 1D).
Figure 1.
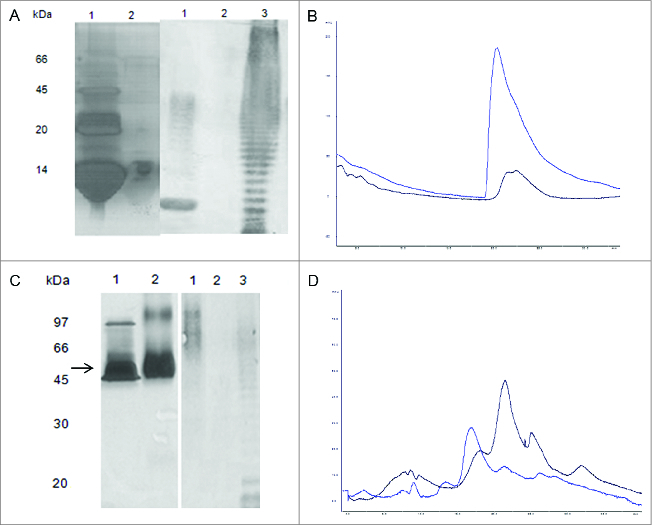
Characterization of the conjugates. (A): 15 % SDS PAGE analysis of O111-cytochrome C conjugate (Lane 1); in comparison with horse heart derived cytochrome C (Lane 2); Immunoblot analysis of O111-cytochrome C conjugate (Lane 3), in comparison with horse heart derived cytochrome C. (Lane 4); O111 LPS extract (Lane 5). The bands were recognized by serum from rabbits immunized against purified O111 LPS. (B) Analysis of the O111-cytochrome C conjugate by size exclusion chromatography in a TSK gel Super SW2000 (TOSOH Bioscience 4,6 mm x 30,0 cm) using absorbance wavelength of 220 nm. O111-cytochrome C conjugate (blue), O111-ADH polysaccharide (black). (C) 15 % SDS PAGE analysis of O111-EtxB conjugate (Lane 2) in comparison with recombinant EtxB, (Lane 1). Immunoblot analysis of O111-EtxB conjugate (Lane 3) in comparison with recombinant EtxB (Lane 4), O111 LPS extract (Lane 5). The bands were recognized by serum from rabbits immunized against purified O111 LPS. (D) Analysis of the O111-EtxB conjugate by size exclusion chromatography was performed as described above. O111-ADH polysaccharides (black); O111-EtxB conjugate after purification in centricon 30 MW cut off (dark blue),
Immune response generated in rabbits after subcutaneous immunization with O111-cytochrome C conjugate incorporated in Sílica SBA-15 nanoparticles.
Results obtained by ELISA showed that one year after immunization, the immune response induced in rabbits by the O111-cytochrome C conjugate incorporated in silica SBA-15 nanoparticles was equivalent to the response generated in rabbits immunized either with whole formalinized bacteria or with intact LPS extract (Fig. 2A).
Figure 2.

Humoral response induced by the O111-cytochrome C conjugate in rabbits. (A) Antibody detection. Rabbits were immunized 6 times by the subcutaneous route either with O111-cytochrome C conjugate (incorporated or not in silica SBA-15 nanoparticles) or formalinized O111:H2 E. coli or intact O111:H2 LPS extract or O111 polysaccharide in silica SBA-15. Serum samples collected before immunization and one year after the last injection were tested by ELISA for the presence of IgG antibodies against O111 E. coli. The optical density is extrapolated from a 1/100 dilution. (B) Recognition of live E. coli by O111 polysaccharide antibodies as determined by the test-tube agglutination assay.41 Different dilutions of O111 polysaccharide antibodies generated in rabbits by immunization either with O111-cytochrome C conjugate incorporated in Silica SBA-15 nanoparticles, or formalinized O111:H2 E. coli or intact LPS extract of O111:H2 E. coli or O111 polysaccharide incorporated in silica SBA-15 were incubated with different categories of live E. coli samples. The titer was determined as the last serum dilution which visually showed a positive reaction. The error bar is related to the mean of the ELISA determinations, which were performed in triplicate
The results also demonstrated that rabbits immunized with the conjugate O111-cytochrome C in PBS or with detoxified O111 polysaccharide alone incorporated in silica SBA-15 did not generate antibody response against O111 polysaccharide (Fig. 2A).
Results obtained using the agglutination tube assay showed that the IgG antibodies generated in rabbits by the conjugate were able to recognize live O111 E. coli strains with mechanisms of virulence differing from each other (Fig. 2B). Furthermore, they were also able to inhibit the adhesion of all categories of O111 E. coli (EHEC, EPEC e EAEC) to human epithelial cells (Fig. 3).
Figure 3.
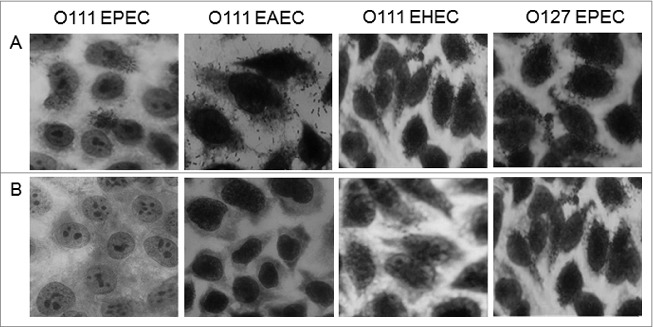
Determination of the capacity of the antibodies generated by the O111-cytochrome C conjugate to inhibit bacterial adhesion. Hep-2 cells were incubated for 3 hours with bacterial sample either alone (a) or in the presence of serum from rabbits immunized with the O111-cytochrome C conjugate incorporated in silica SBA-15 nanoparticles (b). Ocular 10 Objective (100 ×).
Immune response generated in mice after oral immunization with the conjugates incorporated in Vaxcine
Since successful results were obtained by subcutaneous immunization of rabbits with the O111-cytochrome C conjugate, this conjugate was incorporated in Vaxcine, a carrier which has been proved to be an effective vehicle as an oral antigen delivery system in vaccine formulations.35,36 However, the results obtained from mice immunized orally with O111-cytochrome C conjugate incorporated into VaxcineTM showed that this formulation generated neither systemic nor mucosal humoral immune responses against O111 polysaccharides (Fig. 4). Therefore, O111 polysaccharides were conjugated to EtxB, since it has been demonstrated previously that this recombinant protein has the property to abrogate oral tolerance to co-administered antigens.34 Subsequently, the O111-EtxB conjugate was incorporated in Vaxcine and administered orally to mice. ELISA results demonstrated that mice immunized orally with the O111-EtxB conjugate either free or incorporated in Vaxcine generated IgG and IgA responses against O111 polysaccharides detected in the blood and stools; however, the level of both isotypes was higher in animals immunized with the conjugate incorporated in Vaxcine (Fig. 4).
Figure 4.
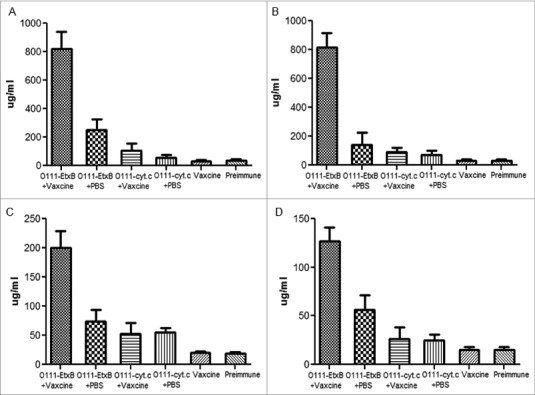
Antibody response against O111 polysaccharides after oral immunization. Mice were immunized orally 3 times either with the O111-EtxB conjugate or O111-Cytochrome C conjugate (free in PBS or incorporated in Vaxcine). Ten days after the last immunization, blood (A, B) and stool (C, D) samples were collected and analyzed by ELISA for the presence of IgG (A, C) and IgA (B, D) antibodies against O111 polysaccharides. To calculate the absolute concentration (μg/ml) of IgG and IgA in the blood and stools against O111 polysaccharides, a standard curve was created by coating wells with different concentrations of mouse IgG and IgA. The error bars are standard deviations of the mean of 5 mice per group.
The results also showed that antibodies present in the stools and serum of mice immunized orally with the conjugate O111-EtxB incorporated in Vaxcine were able to recognize all categories of O111 E. coli tested. In contrast, they were not able to recognize an E. coli strain derived from an unrelated serogroup (O127H6) (Fig. 5).
Figure 5.

Recognition of live O111 E. coli by antibodies generated by oral immunization with the O111-EtxB conjugate. Different dilutions of serum from mice orally immunized with the O111-EtxB conjugate incorporated in Vaxcine were incubated with different categories of live E. coli samples as determined by the test-tube agglutination method.41 The titer was determined as the last serum dilution which visually showed a positive reaction. As control, the bacterial sample were incubated with serum from mice immunized with O111 polysaccharide incorporated in Vaxcine or immunized with Vaxcine alone. This experiment was performed in triplicate, repeated on 3 subsequent occasions and similar results were obtained.
It was also observed that the O111-EtxB conjugate generated in mice an antibody immune response against EtxB higher than the one generated in the group immunized with the conjugate in PBS (Fig. 6). These antibodies were also able to inhibit the cytotoxic effect of LT in Y-1 cells (Fig. 6).
Figure 6.
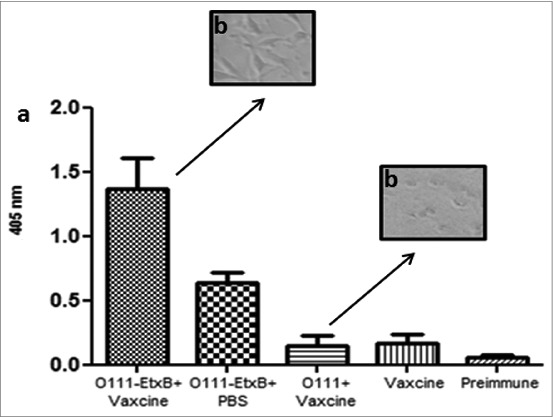
Antibody response against EtxB. (A) Detection of IgG against EtxB in the blood after oral immunization. Balb/c female mice were immunized 3 times by gavage with the conjugate O111-EtxB incorporated in Vaxcine. Blood samples were collected before immunization and 21 d after the last one. EtxB. (B) Determination of the capacity of the antibodies generated by the O111-EtxB conjugate to inhibit the cytopathic effect of LT. Y-1 cells were incubated in 96 well plates for 1 hour with 1 μg/ml (100 μl/well) of LT either in the presence of serum from mice immunized with the O111-EtxB conjugate incorporated in Vaxcine or in the presence of serum from mice immunized with the O111 polysaccharide in Vaxcine. Ocular 10 Objective (100 ×).
Discussion
It has been demonstrated previously that the O111 polysaccharide is an excellent candidate to be used as an antigen in a universal vaccine formulation against all categories of O111 E. coli. 22 However, children under 2 y old, who are the ones most affected by diarrhea induced by these pathogens, do not produce antibodies against polysaccharides efficiently.26 In addition, even mature B cells can become unresponsive or anergic through excessive receptor cross-linking in the presence of high concentrations of polysaccharides, whereas at too low a concentration, there is insufficient receptor cross-linking to activate the cells.26 To overcome these problems and induce an effective antibody response against O111 polysaccharides, they have to be conjugated to a carrier protein. However, the method utilized to obtain detoxified O111 polysaccharides from native LPS has to be chosen carefully, since usual treatments such as the use of acetic acid can remove some of the colitose, which is the major antigenic determinant of the molecule.38 Accordingly, the O111 LPS used in the present work was detoxified by alkaline hydrolyses,39 which removes ester-linked fatty acids from lipid A and eliminates many toxic effects of LPS.
Another aspect that must be taken into account is the method for conjugation, since it can interfere with the final structure of the conjoined molecules. For instance, it has been demonstrated by Gupta and co-workers that the use of ADH as a linker for the conjugation of detoxified O111 LPS with tetanus toxoid gives better results than the use of SPDP.38 They observed that using ADH as a linker, TT binds throughout the polysaccharide chain, whereas using SPDP as a linker, the attachment of TT was only through the terminal amino group at the nonreducing end of the polysaccharide. For this reason, ADH was used as a linker in the present work.
However, in order to prevent the formation of large matrix-type polysaccharide-protein complexes when using cytochrome c as a carrier protein, the polysaccharides were not oxidized with periodic acid to produce aldehydes.
For several reasons, in the present work, cytochrome C was the first carrier protein utilized for conjugation. First, it is a commercially available well-characterized monomer, with 18 lysine and 13 carboxyl residues on its surface which are easily accessible to modifying agents. In addition, because of its orange color it can be visually tracked and assayed spectrophotometrically.
In the present work, the results obtained by staining the SDS-PAGE gel for polysaccharides showed that during conjugation there was the formation of polymeric cytochrome c and polysaccharide matrix type complexes, as is commonly observed in preparations that use cytochrome C as a carrier protein.40 The presence of O111 polysaccharides and cytochrome C matrix type complexes in the conjugate has an advantage because cytochrome c polymers are much more immunogenic than their monomeric forms.41 Despite that, O111 polysaccharides are still poor immunogens. For this reason the O111-cytochrome C conjugate was incorporated in silica SBA-15 nanoparticles as an adjuvant. The results obtained from subcutaneously immunized rabbits showed that in the presence of SBA-15 nanoparticles, the conjugate induced a humoral immune response against the polysaccharide after the second immunization (data not shown). The response increased after the third (data not shown) and remained the same up to one year after the last (sixth) immunization maintaining its ability to recognize and inhibit the adhesion of O111 (EPEC, EHEC and EAEC) to human epithelial cells. These results are of great significance in terms of vaccination for 2 reasons: first, bacterial adherence and colonization precedes invasion; second, a persistent humoral immune response is fundamental to protection of children under 2 y old against capsulated bacteria regardless of the presence of immunological memory.42-44
In the case of O111 E. coli, the ability of the conjugate to generate antibodies able to inhibit the adhesion of pseudo-capsulated strains to epithelial cells is also extremely important, since it has been shown that antibodies generated by membrane O111 polysaccharides do not recognize effectively pseudo-capsulated O111 E. coli, notwithstanding the fact that the pseudo-capsule of these pathogens has the same constituents as the O-chain unit of the LPS present on their membrane.45
Although the results obtained with the O111-cytochrome c conjugate in rabbits were positive, the antibodies were generated by parenteral immunization, which in the case of enteric pathogens is not considered the best route for vaccination, because it does not induce a protective immune response in the mucosa against diarrhea disease-causing agents.46 Nevertheless, there are available on the market 2 parenteral vaccines against enteric pathogens licensed for human use, Typherix (Glaxo SmithKline) and Typhim Vi (Sanofi Pasteur Pty Ltd), both against Typhoid fever.47,48 However, despite their efficacy, they are not recommended for children under 2 years old given that they are not conjugated vaccines.46 To overcome this problem, the possibility has been raised of vaccinating pregnant mothers in order to transfer protection against enteric pathogens by breast feeding. Guidance from Departments of Health in UK and US has confirmed that maternal immunityagainst diseases such as influenza can protect newborns.49-52
All the other vaccines against enteric pathogens approved for human use such as cholera, ETEC, Shigella and rotavirus are administered orally, which is accepted as the ideal route.53 Accordingly, another conjugate was constructed, using EtxB as a carrier protein, since it has been proved that EtxB is able to abrogate oral tolerance and generate systemic and mucosal immune responses against the co-administered antigen after oral immunization34
The results obtained by oral immunization of mice with the O111-EtxB conjugate demonstrated that the conjugate, either free or incorporated in Vaxcine as an oral delivery system, was able to abrogate oral tolerance and induce systemic and mucosal antibody responses against O111 E. coli. However, the presence of VaxcineTM resulted in a significant increase in the antibody response. This adjuvant effect of VaxcineTM is probably related to its ability to protect the conjugate from degradation during its passage through the gastric intestinal system and its potential for targeting the M cells.35,36
It was also observed that the antibodies generated by the O111-EtxB conjugate in the presence of Vaxcine were able to recognize all 3 categories of O111 E. coli. In addition, they were able to inhibit, the cytotoxic effect of LT on Y1-cells, indicating that a conjugated vaccine that uses EtxB as a carrier protein is also able to generate protection against ETEC, as is the case with DUKORAL that uses CTB (B subunit of Cholera Toxin) in its formulation.37
It is worth noting that the O111-EtxB conjugate was the only one among several others tested by ourselves with the ability to induce both systemic and mucosal humoral responses against O111 E. coli. These results indicate that the O111-EtxB conjugate is able to generate 2 lines of defense against O111 E. coli, one at the local site and another that mediates the elimination of the pathogen that breaches the mucosal barrier. In terms of protection, a systemic humoral immune response is extremely important, since it seems that the majority of intestinal IgG is derived from blood transudate.54 In the case of shiga-producing toxin strains, this humoral immune response reinforcement is very significant, given that there is no treatment available against hemolytic uremic syndrome induced by these pathogens.55
In addition, the following aspects are worth emphasizing: Firstly, all the components utilized in VaxcineTM as a delivery system are GRAS-listed or pharmacopeial; secondly, EtxB has been used in human vaccination trials against Neisseria meningitidis group B (NmB)63; finally, a protocol for the utilization of silica SBA-15 nanoparticles as an adjuvant has been submitted for a phase 1clinical trial. Thus, we consider that in the near future the findings presented in this work have the potential to be translated into a human testable vaccine against O111 E. coli which is capable of preventing the establishment of infection by inhibiting local bacterial adherence to epithelial cells and by reinforcing the immune response with a second line of defense represented by the systemic immune response.
Material and Methods
Material
intact purified O111 LPS extract from O111:B4 E. coli (L30–24), purified detoxified O111 LPS from O111:B4 E. coli (L3023), ADH (Adipic Acid Dihydrazide) (217824), JandaJel -1-(3 dimethylaminopropyl)-3-ethylcarbodiimide, (EDAC resin) (587248), Cytochrome C (C7752), Bovine Serum Albumin (BSA) (A2153), Sephadex G25 column (G25150), Goat anti-rabbit IgG alkaline phosphatase conjugate (A3812), Goat anti-mouse IgG alkaline phosphatase conjugate (A2179), Goat anti-mouse IgA alkaline phosphatase conjugate (A4937), ELISA alkaline phosphatase substrate (N2640), SIGMA FAST BCIP/NBT substrate (B5655), Bicinconinic Acid (B9643), copper sulfate solution (C2284), BSA Protein Standard (P0914), were all purchased from Sigma. Tryptic Soy Broth (211825), LB-Agar (244520), LB Broth (244620), were obtained from Becton Dickenson. Inactivated Fetal Bovine Serum and DMEM without antibiotics (D0017) were purchased from Cultilab. Giemsa (1092041002) and May-Grunwald's eosin methylene Blue solution (1014241002) were purchased from Merck and agarose was obtained from Invitrogen. The mesoporous silica SBA-15 nanoparticles were obtained from Dr. Osvaldo A. Santana in the Immunochemistry Laboratory of the Butantan Institute. Recombinant EtxB was provided by Prof. Neil Williams, Department of Cellular and Molecular Medicine, Bristol University. The LT toxin was kindly donated by Dr John Clements from Tulane University Health Sciences Center (USA). The Vaxcine(TM) oil-delivery carrier was provided by Dr Roger New at Proxima Concepts, London, UK.
Bacterial strains
The strains used in this study are listed in Table 1. Stocks derived from the E. coli collection of the Instituto Butantan, laboratory of bacteriology, São Paulo, Brazil were utilized in this work.
Cell line
The HEp-2 and Y1 cell lines used in this study were obtained from the Instituto Adolfo Lutz, São Paulo, Brazil. They were previously acquired from the American Type Culture Collection (CCL 2). The cells were grown in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% calf serum, 1 mM L-glutamine.
Animals
Swiss male rabbits (60 d old) and Balb/c female mice (6–8 weeks old) were supplied by The Animal Research Facilities of the Butantan Institute. All procedures involving the use of animals were performed according to the Care and Use of Laboratory Animal Guidelines (1996) and were approved by the Ethical Committee of the Butantan Institute (certificate 663/09).
'Polysaccharide O-antigen isolation'
O111 LPS detoxified by alkaline reaction39 was obtained from Sigma (L3023). According to the product specification only traces of lipid A were detected (≤1000 EU/mg).
Polysaccharide – cytochrome C conjugation
Detoxified LPS polysaccharide derived from O111:B4 E. coli was conjugated to the carrier protein via multiple ligation points using ADH as cross-linking agent. Briefly, 2 mg of O111 detoxified polysaccharides was dissolved in phosphate buffer pH 7.5 (0.4 ml). Subsequently, 44 mg of ADH was added to the polysaccharide solution and incubated for 30 minutes at room temperature in low speed rotation on a tube roller. After incubation, the polysaccharide was purified by passing through a Sephadex G25 column swollen with distilled water to get rid of free ADH molecules. The purified polysaccharide solution was lyophilized for 18 h. The lyophilized material was then weighed and added to 200 μl solution containing 7 mg of Cytochrome C in 0.2 M phosphate buffer pH 7.5. EDAC resin (10 mg) was added to the solution and incubated for 3 h at room temperature at low rotation in a tube mixer. The EDAC resin was then removed, and the conjugate kept at 4°C until use.
Polysaccharide – EtxB conjugation
Detoxified LPS polysaccharides were conjugated to the carrier protein by multiple ligation points using ADH as cross-linking agent. Briefly, 2 mg of O111 detoxified polysaccharide derived from O111:B4 E. coli was dissolved in phosphate buffer pH 7.5 (0.4 ml). Subsequently, 44 mg of ADH was added to the polysaccharide solution and then incubated overnight at 60°C. After incubation, the polysaccharide solution was purified in a Sephadex G25 column with distilled water to get rid of free ADH molecules. EtxB (2 mg) and EDAC resin (20 mg) were added to the polysaccharide solution and subsequently incubated for 4 hours at room temperature at low rotation in a tube mixer. To separate both EDAC and unbound polysaccharides from the protein-polysaccharide conjugate, the conjugate solution was diluted in 15 ml of PBS and concentrated on a 30,000 MWt cut-off Minicon centrifugal concentrator. After discarding the filtrate, the concentrated conjugate solution was diluted once more in 15 ml of PBS and concentrated again. The purified conjugate solution was then stored at 4°C until use.
Protein quantification
The protein concentration of EtxB and LT was determined by the bicinchoninic acid methodology using bovine serum albumin as standard.56
Electrophoresis profile of the conjugates
Electrophoresis was performed accordingly to Laemmli et al, 1970.57 and the gel was stained with silver either for polysaccharide visualization58 or for protein visualization.59
Western Blotting
Western Blotting was performed according to Towbin and coworkers.60
Size-exclusion chromatographic analysis of the O111 polysaccharide-EtxB conjugate
In order to determine the conjugate molecular mass profile liquid chromatography (AKTA purifier GE Healthcare, Sweden) was used, employing a Sepharose TSKgel TOSOH BIOCIENCE column of 4.6mm × 3.6 cm. The column was eluted at a constant flow rate of 0.2 mL min−1 with 0.2 M phosphate buffer over 40 min. The column eluents were monitored by a Shimadzu SPDM20A PDA detector at 280 and 490 nm.
Formalinized bacterial suspension for immunization
To generate IgG antibodies against O111 polysaccharide, rabbits were immunized with a O111:H2 EHEC strain sample. For immunization, bacterial colonies grown overnight in LB agar were homogenized in 0.5 ml of 0.5% formol saline solution (85%) to fix the capsulated material. The fixed bacterial suspension was then centrifuged in an Eppendorf 5804 centrifuge (rotor number F 34–6–38) for 20 min at 5,000× g, and the supernatant was discarded. The pellet containing the encapsulated bacteria was resuspended in saline to achieve a concentration of 9 × 108 cells/ml on the McFarland scale.
Processing of LPS extracts for immunization
LPS extracts were prepared according to the methodology described by Hitchcock and Brown58 with a few modifications. Samples of O111:H2 E. coli were grown in 3 ml of LB broth at 37°C for 18 h. After incubation, 1 ml of each culture was added to 5 ml of LB broth and kept in agitation at 37°C until an optical density of 0.4 at 530 nm was achieved. Subsequently, 1.5 ml of each culture was centrifuged in a Hitachi CR21E centrifuge (rotor 46) at 12000 rpm for 5 min. The pellets were resuspended in 50 μl of lysis buffer (0.5 M Tris-HCl [pH 6.8]–4% SDS–2 ml mercaptoethanol–0.05% bromophenol blue in double-distilled water to a final volume of 100 μl) and incubated for 10 min at 100°C. After incubation, the samples were run in a SDS-PAGE 15% gel. The gel was cut into strips of one cm in diameter each. Subsequently, each strip was macerated in 2 ml of PBS.
Preparation of Vaxcine(TM) formulation
The incorporation of the conjugates into oil was performed by using the Vaxcine(TM) methodology that allows hydrophilic molecules and other complexes to be incorporated stably in droplets of oil, either in the form of reverse micelles or as water-in-oil microemulsions.61 In this case, a self-emulsifying preparation of mineral oil containing a combination of non-ionic and negatively-charged pharmacopoeial amphiphiles was combined with antigen in aqueous solution in a volume-volume ratio of 20:1 oil/water. A clear single phase microemulsion preparation was obtained. The antigen concentration was adjusted so that 10 μg was contained in 0.2ml of oil.
Immunization of Rabbits
Two rabbits were immunized subcutaneously 6 times within a period of one year with 5 μg/ml of O111-Cytochrome C conjugate incorporated in Sílica SBA-15 nanoparticles (1/25), to obtain serum against O111 polysaccharides. Four other rabbits divided in groups of 2 each were immunized 6 times within a period of one year with either formalinized bacterial suspension or intact O111 LPS. As controls, 2 rabbits were also immunized 6 times within a period of one year with either the conjugate O111-cytochrome C in PBS or with O111 detoxified polysaccharides incorporated in silica SBA-15. For immunization, the animals were shaved on the back, and independently injected with the samples (2ml/per animal) at 4 different sites on the shaved area.
Blood samples were collected before immunization, 30 d after the first one, 10 d after each subsequent immunization and one year after the last one.
Immunization of mice
Twenty BALB/c female mice (6–7 weeks old) divided in groups of 5 mice each were immunized orally with 0.2 ml of O111-EtxB conjugate either in PBS or in VaxcineTM, and control animals were immunized with 0.2 ml of either the O111 polysaccharide in Vaxcine or with Vaxcine alone. The animals were immunized 3 times with an interval of 30 d between each immunization. Blood samples were collected before and 10 d after the last immunization.
Collection of blood samples
Murine and rabbit blood samples were collected by tail or ear vein puncture respectively into Eppendorf tubes. The samples from each group were collected individually centrifuged at 500 g for 10 minutes in an Eppendorf 5804 R centrifuge and the sera were then stored at −20°C until use.
Antibody detection
Antibodies were detected by enzyme-linked immunosorbent assay (ELISA). For the detection of antibodies against O111 polysaccharides, plates (100 μl/well) were coated overnight at 4°C with a 1/10 dilution in Tryptic Soy Broth (TSB) of an O111:H21 E. coli culture previously grown in TSB for 18 hours at 37°C. The following day the bacterial cells were fixed by emptying the plates, filling each well with 100 μl of methanol and incubating for 1 hour at room temperature. After incubation the plates were emptied and blocked for 2 hours at 37°C by incubating the wells with a solution of 3% BSA in PBS (0.2 ml/well). The plates were then washed 3 times with PBS containing 0.05% Tween 20. After washing the wells, serum samples were dispensed in triplicate into individual wells of the plates and diluted in doubling dilutions starting from 1/100. The samples were then incubated overnight at 4°C. After incubation the plates were washed again and goat anti-mouse IgG alkaline phosphatase conjugate in PBS with 1 % BSA (1/5000 dilution) was added to the plates (100μl/well) and incubated for 90 minutes at 37°C. The plates were washed once more, and then the enzymatic reaction was developed with 5 mg/ml of p-nitrophenyl phosphate in diethanolamine buffer (0.1 ml/well).
The optical density was read at 405 nm in a Titertek plate reader after 15 and 30 minutes of incubation at room temperature.
To calculate the absolute concentration (μg/ml) of IgG and IgA in the blood and stools against O111 polysaccharides, a standard curve was created by coating wells with different concentrations of mouse IgG and IgA, which were then incubated with anti-Ig enzyme conjugate. The values of the samples were read off from the regression line obtained from the standard curve.
For the detection of antibodies against EtxB, the same procedure described above was used, except for the fact that the plates were coated with 5 μg/ml (100 μl/well) of EtxB in PBS instead of 100 μl/well of O111:H21 E. coli culture.
Agglutination assay
The titers of rabbit and murine antibodies against different strains of live O111 E. coli were determined by the test tube agglutination method as described by Ewing and coworkers.62 The titer was determined as the last serum dilution which induced visible agglutination. This test was performed in triplicate.
Inhibition of bacterial adhesion to epithelial cells
HEp-2 cells were grown to 70% confluence on circular coverslips in wells of 24-well tissue culture plates in the presence of DMEM without antibiotics. In parallel, 40 μl of O111 and O127 E. coli samples at a concentration of 105/ml were incubated for 1 h at 37°C with 1 ml of rabbit serum samples diluted 1/10 in 1 ml of DMEM without antibiotics containing 2% fetal bovine serum. After incubation, the samples were added in triplicate to the wells and incubated for 3 h at 37°C in 5% CO2. As a positive control for bacterial adhesion, the cells were incubated only with bacteria in the absence of antibodies. After incubation, the monolayers were washed 6 times with sterile PBS and then fixed with 100% methanol for 10 min, stained for 5 min with May–Grunwald stain diluted 1:2 in Sorensen buffer, and finally stained for 20 min with Giemsa stain diluted 1:3 in Sorensen buffer. The excess stain was discarded, and the coverslips with the stained cells were affixed to microscope slides for visualization by light microscopy (eyepiece, ×10; objective, ×100).
Inhibition of the cytotoxic effect of LT on Y-1 cells
Y-1 cells were grown to 60% confluence on 96-well tissue culture plates in the presence of DMEM without antibiotics. In parallel, 1 μg/ml of LT was incubated for 1 hour with serum of mice immunized orally with the conjugated O111-EtxB incorporated in Vaxcine or serum of mice immunized orally with O111 polysaccharides incorporated in Vaxcine. After incubation, LT pre-incubated with the antibodies was added in triplicate to the plates (100 μl/well) and incubated for 1 hour at 37°C in a CO2 incubator. As a control, 1 μg/ml of LT in DMEM (100 μl/well) was added in triplicate to the plates. After incubation, cells were visualized by light microscopy and pictures were taken after 1 hour of incubation (eyepiece, ×10; objective, ×100).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo), Brazil, Grant 2012/11325–5 and is part of the FAPESP CeTICS (Project 2013/07467–1) and Cristália Produtos Químicos Famacêuticos Ltda, Brazil. OA Sant’Anna is researcher of CNPq – Brazil.This research is under the scope of the Patents WO07030901, IN248654, ZA2008/02277, KR1089400, MX297263, JP5091863 and CN101287491.
References
- 1. Children: reducing mortality. World Health Organization (WHO). Updated September 2013. http://www.who.int/mediacentre/factsheets/fs178/en/index.html.AccessedDec20,2013. [Google Scholar]
- 2. Diarrhoeal disease. World health organization (WHO). April 2013. http://www.who.int/mediacentre/factsheets/fs330/en/index.html.AccessedMay02,2013. [Google Scholar]
- 3.Hill DR, Beeching NJ. Travelers’ diarrhea. Curr Opin Infect Dis 2010; 23(5):481-7; PMID:20683261; http://dx.doi.org/ 10.1097/QCO.0b013e32833dfca5. [DOI] [PubMed] [Google Scholar]
- 4.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev 1998; 11(2):142-201; PMID:9457432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma VK. Detection and quantitation of enterohemorrhagic Escherichia coli O157, O111, and O26 in beef and bovine feces by real-time polymerase chain reaction. J Food Prot 2002; 65(9):1371-80; PMID:12233845 [DOI] [PubMed] [Google Scholar]
- 6.Bettelheim KA. The non-O157 Shiga-toxigenic (verocytotoxigenic) Escherichia coli; under-rated pathogens. Crit Rev Microbiol 2007;33:67-87; PMID:17453930; http://dx.doi.org/ 10.1080/10408410601172172 [DOI] [PubMed] [Google Scholar]
- 7.Brooks JT, Sowers EG, Wells JG, Greene KD, Griffin PM, Hoekstra RM, Strockbine NA. Non -O157 Shiga Toxin–producing Escherichia coli infections in the united states, 1983–2002. J Infect Dis 2005; 192:1422-1429; PMID:16170761; http://dx.doi.org/ 10.1086/466536 [DOI] [PubMed] [Google Scholar]
- 8.Piercefield EW, Bradley KK, Coffman RL, Mallonee SM. Hemolytic uremic syndrome after an Escherichia coli O111 outbreak. Arch Intern Med 2010; 170(18):1656-63; PMID:20937925; http://dx.doi.org/ 10.1001/archinternmed.2010.346 [DOI] [PubMed] [Google Scholar]
- 9.Bradley KK, Williams JM, Burnsed LJ, Lytle MB, McDermott MD, Mody RK, Bhattarai A, Mallonee S, Piercefield EW, McDonald-Hamm CK, Smithee LK. Epidemiology of a large restaurant-associated outbreak of Shiga toxin-producing Escherichia coli O111:NM. Epidemiol Infect 2012; 140(9):1644-54; PMID:22117135; http://dx.doi.org/ 10.1017/S0950268811002329 [DOI] [PubMed] [Google Scholar]
- 10.Campos LC, Whittam TS, Gomes TA, Andrade JR, Trabulsi LR. Escherichia coli serogroup O111 includes several clones of diarrheagenic strains with different virulence properties. Infect Immun 1994; 62:3282-8; PMID:8039899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campos LC, Franzolin MR, Trabulsi LR. Diarrheagenic Escherichia coli categories among the traditional enteropathogenic E.coli O serogroups- a review. Mem Inst Oswaldo Cruz 2004; 99:545-52; PMID:15558161; http://dx.doi.org/ 10.1590/S0074-02762004000600001 [DOI] [PubMed] [Google Scholar]
- 12.Boudailliez B, Berquin P, Mariani-Kurkdjian P, Ilef D, Cuvelier B, Capek I, Tribout B, Bingen E, Piussan C. Possible person-to-person transmission of Escherichia coli O111-associated hemolytic uremic syndrome. Pediatr Nephrol 1997;11(1):36-9; PMID:9035170; http://dx.doi.org/ 10.1007/s004670050229 [DOI] [PubMed] [Google Scholar]
- 13.Morabito S, Karch H, Mariani-Kurkdjian P, Schmidt H, Minelli F, Bingen E, Caprioli A. Enteroaggregative, Shiga toxin-producing Escherichia coli O111:H2 associated with an outbreak of hemolytic-uremic syndrome. J Clin Microbiol 1998; 36(3):840-2; PMID:9508328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morabito S, Karch H, Schmidt H, Minelli F, Mariani-Kukdjian P, Allerberger F, Bettelheim KA, Caprioli A. Molecular characterisation of verocytotoxinproducing Escherichia coli of serogroup 0111 from different countries. J. Med. Microbiol 1999; 48, 891-6; PMID:10510965; http://dx.doi.org/ 10.1099/00222615-48-10-891 [DOI] [PubMed] [Google Scholar]
- 15.Brooks JT, Bergmire-Sweat D, Kennedy M, Hendricks K, Garcia M, Marengo L, Wells J, Ying M, Bibb W, Griffin PM, et al. . Outbreak of Shiga toxin-producing Escherichia coli O111:H8 infections among attendees of a high school cheerleading camp. Clin Infect Dis. 2004; 38(2):190-8; PMID:14699450; http://dx.doi.org/ 10.1086/380634 [DOI] [PubMed] [Google Scholar]
- 16. Outbreak of Shiga Toxin–Producing Escherichia coli O111 Infections Associated with a Correctional Facility Dairy — Colorado, 2010. Centers for Disease Control and Prevention- Morbidity and Mortality Weekly Report. Morbidity and Mortality Weekly Report. March 2012. http://www.cdc.gov/mmwr/pdf/wk/mm6109.pdf.AccessedDec15,2013. [PubMed] [Google Scholar]
- 17. Four Deaths in E. coli O111 Outbreak in Japan. Food Safety News- Breaking news for everyone's consumption. May 2011. http://www.foodsafetynews.com/2011/05/two-deaths-in-e-coli-o111-outbreak-in-japan/#.UzlU_PldV8E.AccessedDec15,2013. [Google Scholar]
- 18. Non-O157 Shiga toxin-producing E. coli (STEC) outbreaks, United States. Public Health Service. Department of Health & Human Services. Centers for Disease Control and Prevention (CDC). May 2010. http://blogs.cdc.gov/publichealthmatters/files/2010/05/nono157stec_obs_052110.pdf.AccessedJan20,2013. [Google Scholar]
- 19.Caprioli A, Morabito S, Brugère H, Oswald E. Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet Res 2005; 36(3):289-311; PMID:15845227; http://dx.doi.org/ 10.1051/vetres:2005002. [DOI] [PubMed] [Google Scholar]
- 20.Fukushima H, Hoshina K, Gomyoda M. Long-term survival of shiga toxin-producing Escherichia coli O26, O111, and O157 in bovine feces. Appl Environ Microbiol 1999; 65(11):5177-81; PMID:10543842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JH, Hur J, Stein BD. Occurrence and characteristics of enterohemorrhagic Escherichia coli O26 and O111 in calves associated with diarrhea. Vet J. 2008;176(2):205-9; PMID:17400008; http://dx.doi.org/ 10.1016/j.tvjl.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Santos MF, New RR, Andrade GR, Ozaki CY, Sant'Anna OA, Mendonça-Previato L, Trabulsi LR, Domingos MO. Lipopolysaccharide as an antigen target for the formulation of a universal vaccine against Escherichia coli O111 strains. Clin. Vacc. Immunol 2010; 17:1772-80; http://dx.doi.org/ 10.1128/CVI.00232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris M, Li L. Molecular mechanisms and pathological consequences of endotoxin tolerance and priming. Arch Immunol Ther Exp (Warsz) 2012; 60(1):13-8; PMID:22143158; http://dx.doi.org/ 10.1007/s00005-011-0155-9. [DOI] [PubMed] [Google Scholar]
- 24.Munford RS. Murine responses to endotoxin: another dirty little secret? J Infect Dis. 2010; 201(2):175-7; PMID:20001601; http://dx.doi.org/ 10.1086/649558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivapalaratnam S, Farrugia R, Nieuwdorp M, Langford CF, van Beem RT, Maiwald S, Zwaginga JJ, Gusnanto A, Watkins NA, Trip MD, et al. . Identification of candidate genes linking systemic inflammation to atherosclerosis; results of a human in vivo LPS infusion study. BMC Med Genomics. 2011; 4:64; PMID:21827714; http://dx.doi.org/ 10.1186/1755-8794-4-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology, 5th edition. The Immune System in Health and Disease. New York: Garland Science; 2001. [Google Scholar]
- 27.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009; 9(3):185-94; PMID:19240757; http://dx.doi.org/ 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 28.González-Fernández A, Faro J, Fernández C. Immune responses to polysaccharides: lessons from humans and mice. Vaccine. 2008; 26(3):292-300; http://dx.doi.org/ 10.1016/j.vaccine.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 29.Lindblad EB. Aluminium compounds for use in vaccines. Immunology and Cell Biology. 2004; 82:497-505; PMID:15479435; http://dx.doi.org/ 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 30.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004; 82(5):488-96; PMID:15479434; http://dx.doi.org/ 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 31.Mercuri LP, Carvalho LV, Lima FA, Quayle C, Fantini MC, Tanaka GS, Cabrera WH, Furtado MF, Tambourgi DV, Matos Jdo R, et al. . Ordered mesoporous silica SBA-15: a new effective adjuvant to induce antibody response. Small 2006; 2(2):254-6; PMID:17193031; http://dx.doi.org/ 10.1002/smll.200500274. [DOI] [PubMed] [Google Scholar]
- 32.Carvalho LV, Ruiz Rde C, Scaramuzzi K, Marengo EB, Matos JR, Tambourgi DV, Fantini MC, Sant'Anna OA. Immunological parameters related to the adjuvant effect of the ordered mesoporous silica SBA-15. Vaccine 2010; 28(50):7829-36; PMID:20937318; http://dx.doi.org/ 10.1016/j.vaccine.2010.09.087. [DOI] [PubMed] [Google Scholar]
- 33.Woodrow KA, Bennett KM, Lo DD. Mucosal vaccine design and delivery. Annu Rev Biomed Eng 2012; 14:17-46; PMID:22524387; http://dx.doi.org/ 10.1146/annurev-bioeng-071811-150054. [DOI] [PubMed] [Google Scholar]
- 34.Plant A, Williams R, Jackson ME, Williams NA. The B subunit of Escherichia coli heat labile enterotoxin abrogates oral tolerance, promoting predominantly Th2-type immune responses. Eur J Immunol. 2003; 33(11):3186-95; PMID:14579287; http://dx.doi.org/ 10.1002/eji.200324154. [DOI] [PubMed] [Google Scholar]
- 35.Domingos MO, Lewis DJ, Jansen T, Zimmerman DH, Williamson ED, New RRC. A new oil-based antigen delivery formulation for both oral and parenteral vaccination. Open Drug Deliv J 2008; 2:52-60; http://dx.doi.org/ 10.2174/1874126600802010052. [DOI] [Google Scholar]
- 36.Prabakaran M, Madhan S, Prabhu N, Geng GY, New R, Kwang J. Reverse micelle-encapsulated recombinant baculovirus as an oral vaccine against H5N1 infection in mice. Antiviral Res. 2010; 86(2):180-7; PMID:20153776; http://dx.doi.org/ 10.1016/j.antiviral.2010.02.315. [DOI] [PubMed] [Google Scholar]
- 37. Dukoral. European Medicines Agency- Science Medicines Health; 2010. http://www.ema.europa.eu/docs/pt_PT/document_library/EPAR_-_Summary_for_the_public/human/000476/WC500037569.pdf.AccessedNov15,2013. [Google Scholar]
- 38.Gupta RK, Egan W, Bryla DA, Robbins JB, Szu SC. Comparative immunogenicity of conjugates composed of Escherichia coli O111 O-specific polysaccharide, prepared by treatment with acetic acid or hydrazine, bound to tetanus toxoid by two synthetic schemes. Infect Immun 1995; 63(8):2805-10; PMID:7542631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding HF, Nakoneczna I, Hsu HS. Protective immunity induced in mice by detoxified salmonella lipopolysaccharide. J Med Microbiol. 1990; 31(2):95-102; PMID:2406449 [DOI] [PubMed] [Google Scholar]
- 40.Chu YH, Whitesides GM. Preparation of conjugates of proteins with amyloses by elongation of covalently attached primers using glycogen phosphorylase a1. Bioorg Chem 1993; 21:319-29. [Google Scholar]
- 41.Reichlin M, Nisonoff A, Margoliash E. Immunological activity of cytochrome c. Three. enhancement of antibody detection and immune response initiation by cytochrome c polymers. J Biol Chem. 1970; 245(5):947-54; PMID:4190484 [PubMed] [Google Scholar]
- 42.McVernon J, MacLennan J, Pollard AJ, Oster P, Wakefield MJ, Danzig L, Moxon ER. Immunologic memory with no detectable bactericidal antibody response to a first dose of meningococcal serogroup C conjugate vaccine at four years. Pediatr Infect Dis J 2003; 22(7):659-61; PMID:12886896 [PubMed] [Google Scholar]
- 43.Balnchard-Rohner G, Pollard AJ. Long-term protection after immunization with protein-polysaccharide conjugate vaccines in infancy. Expert Rev Vaccines 2011; 10(5):673-84; PMID:21604987 [DOI] [PubMed] [Google Scholar]
- 44.Truck J, Pollard AJ. Challenges in immunization against bacterial infection in children. Early Hum Dev 2010; 86(11):695-701; PMID:20851537 [DOI] [PubMed] [Google Scholar]
- 45.Goldman RC, White D, Orskov F, Orskov I, Rick PD, Lewis MS, Bhattacharjee AK, Leive L. A surface polysaccharide of Escherichia coli O111 contains O-antigens and inhibits agglutination of cells by O-antiserum. J Bacteriol 1982; 151(3):1210-21; PMID:6179923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langridge W, Odumosu O, Nandi S, Rodriguez R, DeLeon M, Cordero-MacIntyre Z. Mucosal vaccination against enteric pathogens in the developing world. Br J Med Med Res 2012; 2(3):260-291. [Google Scholar]
- 47.Clemens J. Evaluation of vaccines against enteric infections: a clinical and public health research agenda for developing countries. Philos Trans R Soc Lond B Biol Sci 2011; 366(1579):2799-805; PMID:21893543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Australian Government Department of Health. The Australian Immunization Handbook. 10th Edition 2013. (Updated January 2014) http://www.health.gov.au/internet/immunise/publishing.nsf/Content/EE1905BC65D40BCFCA257B26007FC8CA/$File/handbook-Jan2014v2.pdf.AccessedJan10,2014. [Google Scholar]
- 49. Whooping cough vaccination in pregnancy. NHS- choices your health, your choices. September 2012. http://www.nhs.uk/conditions/pregnancy-and-baby/pages/whooping-cough-vaccination-pregnant.aspx?tabname=Your%20newborn#Why.AcessedFeb02,2014. [Google Scholar]
- 50.Lloyd KL. Protecting pregnant women, newborns, and families from pertussis. J Midwifery Womens Health 2013, 58(3):288-96. [DOI] [PubMed] [Google Scholar]
- 51.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vázquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis. 2010; 51(12):1355-61; PMID:21058908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Preidt R. Flu shots for pregnant women also protect newborns. health day- news for helthier living. October 2011. http://consumer.healthday.com/women-s-health-information-34/birth-health-news-61/flu-shots-for-pregnant-women-also-protect-newborns-657993.htmlAccessedFeb02,2014. [Google Scholar]
- 53.Dougan G, Huett A, Clare S. Vaccines against human enteric bacterial pathogens. Br Med Bull 2002; 62:113-23; PMID:12176854 [DOI] [PubMed] [Google Scholar]
- 54.Meckelein B, Externest D, Schmidt MA, Frey A. Contribution of serum immunoglobulin transudate to the antibody immune status of murine intestinal secretions: influence of different sampling procedures. Clin Diagn Lab Immunol 2003; 10(5):831-4; PMID:12965913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis TK, McKee R, Schnadower D, Tarr PI. Treatment of Shiga toxin-producing Escherichia coli infections. Infect Dis Clin North Am 2013; 27(3):577-97; PMID:24011831 [DOI] [PubMed] [Google Scholar]
- 56.Redinbaugh MG, Turley RB. Adaptation of the bicinchoninic acid protein assay for use with microtiter plates and sucrose gradient fractions. Anal Biochem 1986; 153(2):267-71; PMID:3706710 [DOI] [PubMed] [Google Scholar]
- 57.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227(5259):680-5; PMID:5432063 [DOI] [PubMed] [Google Scholar]
- 58.Hitchcock PJ, Brown TM. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol 1983; 154(1):269-77; PMID:6187729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987; 8:93-99. [Google Scholar]
- 60.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications.Proc Natl Acad Sci U S A 1979; 76(9):4350-4; PMID:388439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.New RRC, Kirby CJ. Solubilisation of hydrophilic drugs in oily formulations. Adv Drug Deliv Rev 1997; 25:59-69. [Google Scholar]
- 62.Ewing WH. Edwards and Ewing's identification of Enterobacteriaceae, 4th ed. New York, NY: Elsevier Science Publishing Co, 1986. [Google Scholar]


