Abstract
Pseudomonas aeruginosa is an opportunistic bacterial pathogen that causes fatal acute lung infections in critically ill individuals. Its pathogenesis is associated with bacterial virulence conferred by the type III secretion system (TTSS), through which P. aeruginosa causes necrosis of the lung epithelium and disseminates into the circulation, resulting in bacteremia, sepsis, and mortality. TTSS allows P. aeruginosa to directly translocate cytotoxins into eukaryotic cells, inducing cell death. The P. aeruginosa V-antigen PcrV, a homolog of the Yersinia V-antigen LcrV, is an indispensable contributor to TTS toxin translocation. Vaccination against PcrV ensures the survival of challenged mice and decreases lung inflammation and injury. Both the rabbit polyclonal anti-PcrV antibody and the murine monoclonal anti-PcrV antibody, mAb166, inhibit TTS toxin translocation. mAb166 IgG was cloned, and a molecular engineered humanized anti-PcrV IgG antigen-binding fragment, KB001, was developed for clinical use. KB001 is currently undergoing Phase-II clinical trials for ventilator-associated pneumonia in France and chronic pneumonia in cystic fibrosis in USA. In these studies, KB001 has demonstrated its safety, a favorable pharmacokinetic profile, and promising potential as a nonantibiotic strategy to reduce airway inflammation and damage in P. aeruginosa pneumonia.
Keywords: antibody, PcrV, Pseudomonas aeruginosa, type III secretion system, V-antigen
Abbreviations
- CF
cystic fibrosis
- Fab
fragment antigen binding
- Fc
fragment crystallizable region
- immunoglobulin G
IgG
- mAb
monoclonal antibody
- MDR
multidrug resistant
- MDRP
multidrug resistant Pseudomonas aeruginosa
- P. aeruginosa
Pseudomonas aeruginosa
- TTSS
type III secretion system
- TTS
type III secretory
- VAP
ventilator-associated pneumonia
Introduction
Pseudomonas aeruginosa is an opportunistic bacterial pathogen that causes fatal acute lung infections in critically ill individuals.1-4 Its pathogenesis is frequently associated with the development of septic shock and multiple organ failure, because certain strains of P. aeruginosa have the ability to cause necrosis of the lung epithelium and disseminate into the circulation.5,6 Damage to the lung epithelium is associated with the expression of toxins that are directly translocated into eukaryotic cells through the type III secretion system (TTSS).7,8 The P. aeruginosa V-antigen PcrV, a homolog of the Yersinia V-antigen LcrV, is an indispensable contributor to the process of TTS toxin translocation. Vaccination against PcrV ensures the survival of challenged mice and decreases lung inflammation and injury (Table 1).9 Both the rabbit polyclonal anti-PcrV antibody and the murine monoclonal anti-PcrV antibody, mAb166, inhibit the TTS toxin translocation.10,11 Till date, the therapeutic effects of anti-PcrV antibodies are the subject of most published studies on P. aeruginosa infections in animal models. Previously, we cloned mAb166 from a hybridoma, and humanized this monoclonal antibody for potential clinical use.12 This humanized anti-PcrV IgG antigen-binding (Fab’) fragment, KB001, is currently undergoing Phase-II clinical trials for ventilator-associated pneumonia (VAP) in France and chronic pneumonia in cystic fibrosis (CF) in USA (Table 1).13,14 In this review, we summarize the development and characterization of anti-PcrV antibodies, including the early outcomes of their Phase-II clinical trials. Figure 1
Figure 1.
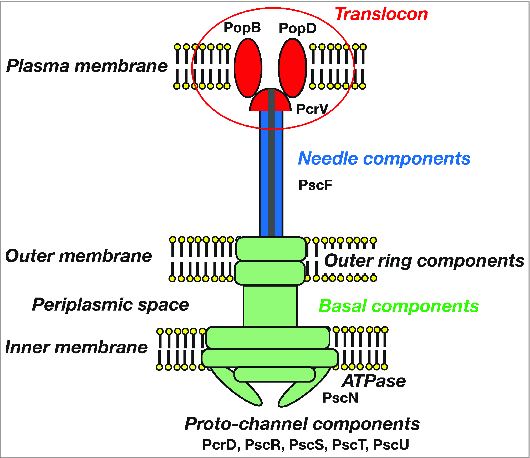
The type III secretory apparatus of Pseudomonas aeruginosa: A basal component, a needle structure, and a translocon. The type III secretory (TTS) apparatus also known as the injectisome comprises many protein components. The basal component comprises an outer ring PscC, periplasmic joint PscJ, ATPase PscN, and more, and is the mechanism by which TTS toxins pass through the bacterial cell membrane. The needle structure comprises approximately 140 PscF proteins. The translocon comprises PcrV, PopB, and PopD, and is the mechanism by which TTS toxins translocate through the eukaryotic cell membrane. PcrV forms a cap structure on the tip of the secretory needle rod.
TTSS and P. Aeruginosa PcrV
Understanding the precise mechanism of acute lung injury caused by P. aeruginosa is key to identifying new therapeutic targets. Reportedly, the ability of this bacterium to cause epithelial injury, disseminate into the circulation, and avoid host innate immune responses is due to TTSS.7,8,15,16 Although most toxins produced by bacteria are secreted into the surrounding extracellular environment via classical type I or II secretion systems, recent studies in gram-negative bacteria have identified a specific group of toxins that are injected directly into adjacent host cells. This protein secretion mechanism is termed TTSS. TTSS is found in a wide variety of pathogenic strains of gram-negative bacteria, including Salmonella, Shigella, and Yersinia. 17,18 Figure 2
Figure 2.
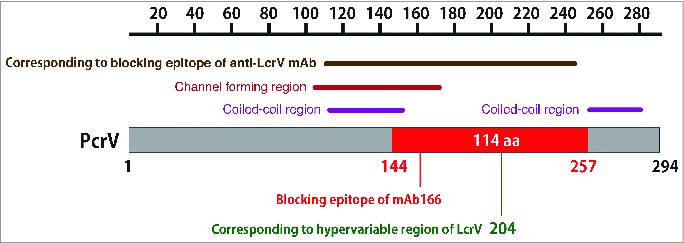
The mAb166 blocking epitope of PcrV. PcrV consists of 294 amino acids. The C-terminal center region between amino acids 144–257, consisting of 114 amino acids and located between 2 coiled-coil regions, is the blocking epitope for the monoclonal anti-PcrV IgG antibody, mAb166. This blocking epitope overlaps with both the channel-forming region for erythrocyte hemolysis and the blocking region of the homologous Yersinia LcrV, based on a sequence alignment between PcrV and LcrV. mAb166 binds to this epitope and blocks the translocation of type III secretory toxins. Amino acid 204 corresponds to the hypervariable region of Yersinia LcrV.
TTSS was shown to contribute to the virulence of P. aeruginosa. 7,8 In addition, many in-vitro clinical reports have recently shown that patients infected with strains of P. aeruginosa expressing TTSS have a higher risk of mortality than those infected with strains not expressing TTSS.15,19-22 In our in vitro cell culture experiments, P. aeruginosa strains exhibited cytotoxicity if they produced TTS products.23,24 In our animal models of P. aeruginosa pneumonia, airspace instillation of cytotoxic P. aeruginosa strains caused consistent alveolar epithelial injury, progressive bacteremia, and septic shock in sheep, rabbits, rats, and mice.25-29 In contrast, airspace instillation of noncytotoxic strains unable to produce TTS toxins did not cause a systemic inflammatory response or septic shock, despite a potent inflammatory response in the lung.16 Therefore, blocking TTSS-associated virulence is key to improving acute lung injury and mortality caused by cytotoxic P. aeruginosa. However, neutralizing TTS toxins using a specific antibody is ineffective, because these toxins are translocated from the bacterial cytosol directly into the cytosol of target eukaryocytes through TTSS without entering the extracellular environment. Therefore, it is necessary to target the toxin secretion and/or translocation system to disrupt toxin translocation and abrogate virulence. PcrV, a translocational component of the P. aeruginosa TTSS, is reportedly a homolog of the Yersinia V-antigen LcrV.9,30 Immunoprotective effects of Yersinia V-antigen have been reported in animal models of pneumonic plague.31 In our previous study, active immunization with Escherichia coli recombinant PcrV was protective in mice infected with lethal doses of P. aeruginosa and the mortality of the infected mice decreased following the administration of an antibody to PcrV.10 Figure 3
Figure 3.
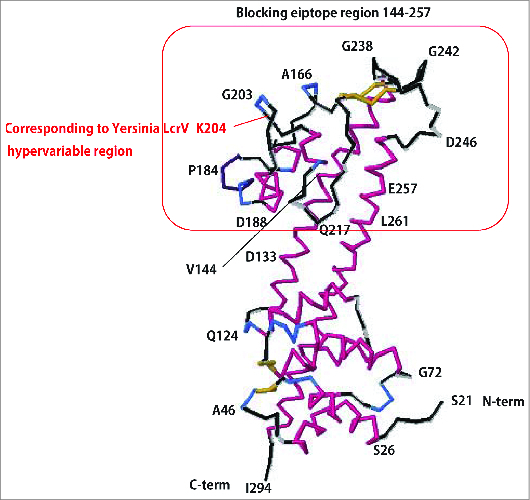
Predicted tertiary structure of PcrV. Based on the structural information for LcrV (Swissplot IR6F), the tertiary structure of PAO1 PcrV was predicted by the protein structure prediction server RaptorX.58 PcrV, consisting of 294 amino acids, forms a central shaft structure, with coiled-coil double strands and 2 globular domains at either end. The C-terminal globular domain includes amino acid 204, corresponding to the hypervariable region of Yersinia LcrV, and amino acids 144–257, recognized as the mAb166 blocking epitope.
The Molecular Mechanism of The TTS Toxin Translocation
The process of toxin translocation across the eukaryotic plasma membrane and directly into the cytosol via TTSS has been extensively studied in Yersinia over the last 25 years.32,33 The translocator proteins LcrV, YopB, and YopD are involved in the process of translocation via the Yersinia TTSS, and lcrV-null mutant Yersinia is unable to translocate TTS toxins.34,35 The homologous translocator proteins PcrV, PopB, and PopD are involved in toxin translocation in P. aeruginosa. 36 Isogenic mutant, P. aeruginosa, lacking pcrV and PcrV expression cannot translocate toxins and is unable to affect eukaryotic cells.10 Figure 4
Figure 4.
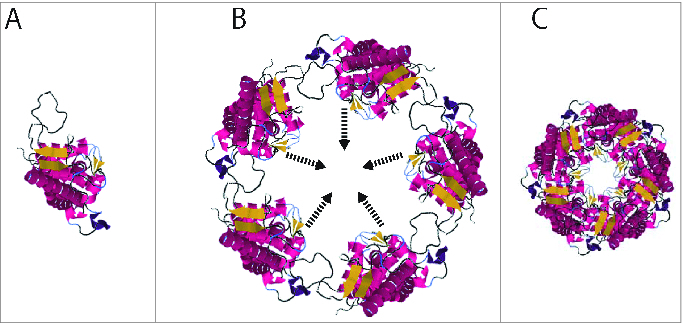
PcrV forms a ring structure at the tip of the secretion needle. A. Monomeric PcrV. B. Five PcrV aligned in a circle. C. Top view of the ring structure formed by pentameric PcrV.43,44
The supramolecular structure of the TTS apparatus, also known as the injectisome, has been well studied over the last 15 years.37-39 The injectisome is evolutionarily associated with bacterial flagella, and comprises:
a basal body, anchored in the bacterial outer and inner membranes;
a needle structure, extending from the outer surface of the membrane; and
translocon proteins, including a needle cap structure and proteins that form pores in the plasma membrane of the target eukaryotic cell.
In Yersinia, the needle has a helical structure and comprises oligomers of more than 100 YscF subunits, and LcrV, which forms a distinct mushroom-like structure at the needle tip acts as a bridge during the translocation of TTSS effectors between the YscF secretion needle and the translocational pore, composed of YopB and YopD, on the eukaryotic cell membrane.40 An oligomeric donut-like LcrV-protein assembly structure, with approximate internal and external diameters of 3–4 and 8–10 nm, respectively, was observed in vitro, and in the atomic model of the tip complex, the N-terminal globular domain of LcrV forms the base, the central globular domain forms the head, and the tip complex itself is likely formed by pentameric LcrV.41-45 For translocating TTS, the formation of a translocational pore on the eukaryotic plasma membrane, involving a cap protein such as LcrV or PcrV, was demonstrated.46-49 In Yersinia, the pore associated with LcrV was approximately 3 nm in diameter, whereas in P. aeruginosa, the homologous PcrV protein induces pores of approximately 2 nm in diameter.41 The regions important for translocational pore size were localized to amino acids 127–195 in LcrV and 106–173 in PcrV.9,11,35 The size of the translocational pore correlates with its ability to translocate Yop effectors into host cells, suggesting that LcrV is a size-determining structural component of the Yop translocon.41 The mechanism of translocational blockade by specific antibodies against LcrV or PcrV remains poorly elucidated. However, specific antibodies probably interfere with the toxin passage through the translocational pore formed by LcrV or PcrV, because the binding sites of such antibodies overlap with the pore-forming regions of LcrV and PcrV.9
The Anti-PcrV Polyclonal Antibody
Immunization with PcrV
The in-vitro inhibition of translocation by anti-PcrV IgG suggested that anti-PcrV or immunization with PcrV may protect against the lethal effects of cytotoxic P. aeruginosa. 10 Mice were immunized with endotoxin-free rPcrV or the recombinant TTS toxin ExoU, and subsequently challenged via airspace instillations of a lethal dose of the cytotoxic P. aeruginosa strain PA103. When survival was measured, both vaccines protected the mice to some degree but animals immunized with rPcrV had significantly fewer bacteria in their lungs. Next, to determine whether prophylactic intervention was possible, mice were passively immunized with preimmune rabbit IgG or rabbit anti-PcrV IgG or anti-ExoU IgG prior to airspace instillation of PA103. Anti-PcrV IgG provided complete protection to a lethal infection, whereas anti-ExoU IgG provided partial protection, which was significantly different from the administration of control IgG.10 If PcrV is accessible for neutralization, then concomitant administration of the bacterial inoculum with anti-PcrV IgG should completely protect against lung injury and lethality. Then, either anti-PcrV IgG or control IgGs were mixed with the inoculum (at a 10-fold higher dose than the lethal inoculum) prior to instilling the bacteria into the lung and the survival was examined. Only anti-PcrV IgG was protective against this extreme infection.
In the next series of experiments, studies were performed in mice with lethal pulmonary P. aeruginosa infections to examine the therapeutic effects of rabbit polyclonal anti-PcrV IgG on survival after this infection.50 Nonspecific control IgG, anti-PcrV IgG, or anti-ExoU IgG were administered intravenously after airspace instillation of a lethal dose of PA103. All mice that received anti-PcrV IgG 4 h after airspace instillation survived, but fewer than 20% of mice that received either control IgG or anti-ExoU IgG 1 or 4 h after infection survived only for more than 3 days. Anti-PcrV IgG significantly decreased inflammation and lung injury, even when administered 4 h after infection, whereas increased recruitment of neutrophils and destruction of the alveolar structure were observed in the lungs of mice treated with nonspecific control IgG.50
The mechanism of anti-PcrV blockade
In vitro study, anti-PcrV IgG demonstrated no bactericidal activity and did not affect bacterial growth.50 It did not affect the other toxic exoproducts such as elastase, exotoxin A, alkaline protease, and pigments. We still don't know whether anti-PcrV blockade affect the biofilm production or not while it was reported that biofilms and type III secretion are not mutually exclusive in P. aeruginosa. 51 In the co-culture of eukaryotic cells such as macrophages or lung epithelial cells with cytotoxic P. aeruginosa, anti-PcrV IgG prevented the eukaryotic cells from bacterial intoxication. Therefore, to directly test whether anti-PcrV IgG can inhibit type III translocation, the TTS toxin translocation was quantified by measuring cyclic AMP levels in macrophages infected with an isogenic mutant of P. aeruginosa that secretes only ExoY, adenylate cyclase type III secretory toxin. As a result, neutralization of PcrV by the antibody significantly decreased ExoY translocation and prevents or inhibits the intoxication of eukaryotic cells. In addition, anti-PcrV IgG restored phagocytic function of macrophages.10
Use of the anti-PcrV polyclonal antibody against sepsis
Studies on the pathogenesis of septic shock following P. aeruginosa pneumonia have shown that airspace instillation of cytotoxic PA103 into rabbits caused consistent alveolar epithelial injury, progressive bacteremia, and septic shock.16 However, instilling a noncytotoxic, isogenic mutant strain (PA103ΔUT), unable to produce TTS toxins, did not cause either a systemic inflammatory response or septic shock, despite a potent inflammatory response in the lung.16 These results suggest that injury to the alveolar epithelium caused by TTSS-associated virulence allows the release of proinflammatory mediators into the circulation that are primarily responsible for septic shock. This demonstrates the importance of the compartmentalization of inflammatory mediators in the lung and the crucial role of bacterial cytotoxins in causing alveolar epithelial damage in the pathogenesis of acute septic shock in P. aeruginosa pneumonia.
To analyze the therapeutic effects of anti-PcrV IgG in a lung infection associated with sepsis, a rabbit model in which acute septic shock was induced by airspace instillation of P. aeruginosa was utilized.50 Rabbits receiving airspace instillation of PA103 consistently exhibit septic physiology within 8 h.50 By monitoring parameters of septic shock, including hemodynamics, metabolic acidosis, bacteremia, and the production of inflammatory cytokines, the therapeutic effects of the anti-PcrV IgG were analyzed in detail. In these experiments, either anti-PcrV IgG or control IgG was administered intravenously or intratracheally 1 h after airspace instillation of PA103. Rabbits receiving control IgG developed septic shock regardless of the route of administration. These rabbits exhibited a decrease in cardiac output to less than 70% of the baseline; mean arterial pressures decreased to approximately 60% of the baseline, and they developed severe metabolic acidosis. The rabbits that received control IgG intratracheally died from septic shock and severe hypoxemia at 8 h. Lung edema, as assessed by the lung water:dry weight ratio, was severe and progressive lung epithelial injury, quantified by the efflux of an alveolar protein tracer, was detected in rabbits that received control IgG. Significant increases in bacteremia and plasma tumor necrosis factor (TNF)-α concentrations were also observed in rabbits that received control IgG. In contrast, rabbits receiving anti-PcrV IgG intravenously or intratracheally exhibited stable hemodynamics and did not develop metabolic acidosis. Lung damage was also significantly less severe in these rabbits, particularly in those given IgG intratracheally. Bacteremia was significantly reduced from 10- to 100-fold compared with that in control IgG groups and developed later in the course of infection. The plasma TNF-α concentration was also significantly lower (10–20 pg/ml), compared with that in the control IgG groups (>100 pg/ml). Finally, the number of bacteria cultured from the instilled lung was also significantly decreased by intravenous anti-PcrV IgG. These findings indicate that anti-PcrV IgG not only has significant therapeutic effects against acute lung injury but also decreases systemic bacteremia and TNF-α concentrations and prevents septic shock.
Anti-PcrV F(ab’)2 protects animals comparably to whole anti-PcrV immunoglobulin G
To further explore the mechanism of the effect of anti-PcrV IgG, the administration of a F(ab’)2 fragment of anti-PcrV IgG was examined in infected animals.50 One hundred micrograms of anti-PcrV F(ab’)2 administered intravenously 1 h after airspace instillation of PA103 resulted in an 80% survival rate at 1 week. An examination of the histology of the lungs of animals that received anti-PcrV F(ab’)2 therapy revealed significantly decreased inflammation and lung injury, similar to that evident after anti-PcrV IgG therapy. Next, the effects of anti-PcrV F(ab’)2 were examined in a rabbit model of septic shock. Anti-PcrV F(ab’)2 (3 mg/kg) was administered intratracheally 1 h after instillation of PA103 50. This treatment provided statistically significant protection from lung damage, bacteremia, and septic shock. These effects were comparable to those achieved with whole anti-PcrV IgG molecules.
Use of the anti-PcrV polyclonal antibody in burned mice
Because P. aeruginosa is a major cause of infection in burned patients, passive anti-PcrV treatment was examined in burned mice that received a fatal P. aeruginosa challenge.52 Mice were given a third-degree burn, challenged with a lethal dose of P. aeruginosa, and treated intraperitoneally with either anti-PcrV IgG or control IgG. Protection against 3 different P. aeruginosa isolates was tested. Genotyping by PCR and phenotyping by immunoblotting showed all P. aeruginosa strains to all be of the invasive TTS toxin phenotype: ExoS+ and/or ExoT+, ExoU−, and ExoY+. Against all strains, the anti-PcrV treatment yielded significantly better survival than the control IgG treatment. Therefore, passive anti-PcrV immunization could protect burned mice against a fatal challenge with P. aeruginosa of an invasive TTS phenotype. These findings suggest that immunotherapy represents a possible treatment for a P. aeruginosa infection in burned hosts.
Use of the anti-PcrV polyclonal antibody against chronic pneumonia
P. aeruginosa is one of the most important pathogens in patients with chronic airway conditions, such as CF and diffuse panbronchiolitis. The effects of anti-PcrV IgG were evaluated in a mouse model of chronic P. aeruginosa infection.53 In this model, a chronic airway infection was induced in mice by placing a plastic catheter infected with P. aeruginosa 6073, a human corneal isolate, in the main bronchus of the mouse. The analysis of bronchoalveolar lavage fluid (BALF) revealed that total cell numbers and neutrophil ratios in anti-PcrV IgG-treated groups were lower than those in the control group. In addition, macrophage inflammatory protein-2, TNF-α, and interleukin (IL)-1β concentrations in the BALF were lower in anti-PcrV IgG-treated groups than in that in controls. These data suggest that anti-PcrV IgG reduces the inflammatory reaction caused by chronic P. aeruginosa respiratory infection and may be useful in treating respiratory diseases. In this experiment, the changes in anti-PcrV IgG titer in mouse plasma were evaluated. For 3 weeks after administration of anti-PcrV IgG (100 μg/mouse), a significant concentration was maintained. Pro-inflammatory cytokine levels were significantly lower in the anti-PcrV IgG twice-treated group, but not in the once-treated group; therefore, pro-inflammatory cytokines appear to be beneficial in maintaining higher serum anti-PcrV titer.
Use of affinity purified antibody and truncated vaccines in neutropenic mice
Rabbit-derived polyclonal anti-PcrV IgG was affinity purified using rPcrV covalently conjugated to sepharose beads.54 In the case of passive immunization, the administration of affinity purified anti-PcrV IgG against either PcrV1–294 or PcrV139-258 showed a significantly higher efficacy against lethal P. aeruginosa infection than that of unpurified anti-PcrV IgG. The increased efficacy of affinity purified anti-PcrV IgG implies that more potent anti-PcrV strategies are possible. The results of this study are crucial for the development of both an effective PcrV vaccine for active immunization and an appropriate-blocking anti-PcrV antibody against P. aeruginosa infection in humans.
Monoclonal Anti-PcrV Antibody
Development of an anti-PcrV monoclonal blocking antibody
Because polyclonal anti-PcrV antibodies inhibit the delivery of TTS toxins and enhance the clearance of bacteria during acute lung infections, the next issue was whether a specific monoclonal antibody against PcrV could demonstrate the same blocking capacity as that of rabbit anti-PcrV IgG. To generate blocking monoclonal clones against PcrV, approximately 80 clones from a hybridoma library derived from the spleen cells of a PcrV-vaccinated mouse were tested in a protection assay against TTSS-mediated intoxication.11 Of the initial 80 cell lines, only 2 hybridomas, mAb166 and mAb179, demonstrated protective activity. mAb166 (IgG2bK) and mAb179 (IgG1K) were purified and tested for protection in an acute lung infection model. Groups of mice that received mAb166 had smaller decrements in their body temperatures, and all infected mice survived.
Anti-PcrV monoclonal antibody mAb166 and the blocking epitope
Monoclonal antibodies were tested for their ability to affect the survival of mice infected with cytotoxic PA103.11 The survival of each group of animals was monitored for 1 week. Only mAb166 demonstrated significant protection in this assay. The effects of mAb166 on survival were further characterized by testing different doses in co-instillation experiments. The addition of 0.5 μg or 1 μg of mAb166 IgG to the bacterial inoculum (at a 4-fold lethal dose) appeared to have a slight effect on survival within the first 48 h of infection. Higher doses of antibody, 10 and 50 μg, resulted in 80% and 90% survival, respectively. In another series of experiments, the ability of mAb166 to passively protect against PA103 infection was compared with that of polyclonal rabbit IgG.11 When the antibodies were administered by an intraperitoneal injection 1 h before bacterial lung instillation (at a 4-fold lethal dose), mAb166 and rabbit polyclonal anti-PcrV were protective at a dose of 100 μg. Different doses of PA103 were prepared and mixed with a fixed amount of mAb166 IgG for co-instillation. Fifty micrograms of mAb166 protected 100% of the animals against infection with 4-, 8-, and 10-fold lethal doses of strain PA103. Seventy percent of the animals were protected at a 20-fold lethal dose of PA103 with 50 μg of mAb166. Survival was diminished to nominal levels as the bacterial instillation was increased to 30-fold the lethal dose. Although only 20% of the animals survived this severe infection, co-instillation with mAb166 seemed to delay mortality by 1–2 days.
Next, to determine whether the protective properties of mAb166 were dependent on its functional fragment crystallizable (Fc) region, Fab fragments from mAb166 were purified. Purified fragments (10 μg) were premixed and co-instilled with a 4-fold lethal dose of strain PA103, and survival was assessed.11 The administration of mAb166 Fab fragments resulted in a high level of protection (80%), comparable to the effect of whole IgG. These data indicate that the Fc portion of both the polyclonal and monoclonal antibodies is unnecessary to mediate protection in vivo.
Full-length PcrV comprises 294 amino acids. To define the amino acid residues bound by mAb166, truncated rPcrV tagged with glutathione S-transferase was constructed, and mAb166 binding with truncated rPcrV was tested by immunoblotting or the dot blot technique.11 mAb166 failed to bind to expression clones encoding the N terminus of PcrV or to one internal deletion of PcrV, but it did bind to a construct encoding the C terminus of PcrV. The smallest epitope recognized by mAb166 is located between amino acids 144–257 of PcrV.
The therapeutic effects of the monoclonal anti-PcrV antibody mAb166
The therapeutic effects of mAb166 on acute lung injury induced by P. aeruginosa were analyzed in a rat model.55 In this model, lung injury was induced by instilling the P. aeruginosa strain PA103 directly into the left lungs of anesthetized rats. One hour after bacterial instillation, either rabbit polyclonal anti-PcrV IgG, murine monoclonal anti-PcrV IgG mAb166, or mAb166 Fab fragments (4 mg/kg, respectively) were administered intratracheally directly into the lungs. The degree of alveolar epithelial injury, amount of lung edema, decrease in oxygenation, and extent of lung inflammation were histologically evaluated as independent parameters of acute lung injury. Rats that received phosphate-buffered saline alone 1 h after bacterial instillation showed a significant increase in lung epithelial injury and lung edema after 4 h. Arterial blood pressure gradually decreased to 80 mmHg over the experimental period. Arterial blood oxygenation remained significantly decreased. Severe metabolic acidosis developed over 4 h. Rats that received either rabbit polyclonal anti-PcrV IgG, murine monoclonal anti-PcrV IgG mAb166, or mAb166 Fab fragments intratracheally showed significant improvement of alveolar epithelial injury and lung edema 4 h after bacterial instillation. The protective effect of mAb166 Fab fragments on lung epithelial injury was the most significant among the 3 antibodies, whereas rabbit polyclonal and murine monoclonal anti-PcrV IgGs were better at improving lung edema than mAb166 Fab fragments. Hypotension did not develop in any group. Arterial oxygenation in the 3 treated groups was significantly improved compared with that in the untreated rats. Although mild metabolic acidosis did develop in rats that received rabbit polyclonal anti-PcrV IgG and mAb166 Fab fragments, those that received mAb166 did not become acidotic. In this series of experiments, lung histology was compared between rats treated with mAb166 and those treated with control IgG. Whereas rats that received control IgG 1 h after bacterial instillation exhibited severe neutrophil recruitment and destruction of alveolar structures; those that received mAb166 had almost no neutrophils in their airspaces and normal alveolar structure was preserved. Therefore, the therapeutic administration of mAb166 showed comparable effects to that of rabbit polyclonal anti-PcrV IgG in preventing acute lung injury and subsequent systemic distress.
Recently, Song et al.56 examined whether the combined administration of mAb166 and an antibiotic could further improve the survival of P. aeruginosa-infected mice. Three clinically relevant antibiotics (ciprofloxacin, tobramycin, and ceftazidime) were used in this study. The administration of mAb166 with an antibiotic significantly improved the survival of mice infected with 3 times the lethal dose (LD90) of PA103 compared with that of either antibiotic or antibody alone. This synergistic effect was because of enhanced bactericidal effects and protection against lung injury, which prevented bacterial dissemination to other organs. Therefore, combined mAb166 and antibiotic administration provides a new, more effective strategy against P. aeruginosa airway infection, particularly when large numbers of highly virulent strains are present.
Wang et al.57 recently investigated the level of TTSS expression in clinically isolated multidrug resistant P. aeruginosa (MDRP) strains and the effects of anti-PcrV antibody on an MDR isolate-induced acute lung injury. The level of TTSS expression was quantified in 53 isolates, comprising 25 MDR strains and 28 susceptible strains. They also investigated the effect of anti-PcrV antibody using a murine model created by instilling an MDRP strain intratracheally into the left lung through and showed that the level of TTSS expression in MDRP strains is comparable to that of susceptible strains. Anti-PcrV ensured the survival of challenged mice, reduced bacterial numbers and attenuated lung inflammation and injury. Therefore, these results suggest that anti-PcrV may be a potentially effective strategy against an MDRP-induced acute lung injury.
A Humanized Anti-PcrV Monoclonal Antibody
Cloning anti-PcrV antibody genes
We cloned the blocking murine monoclonal anti-PcrV antibody mAb166 and generated a murine–human chimeric monoclonal antibody to PcrV, chimera166, which is protective against P. aeruginosa when co-instilled with the bacterial inoculum or intraperitoneally transferred into mice. As a first step in characterizing the structural features of mAb166 underlying its interactions with PcrV, we sequenced both the heavy- and light-chain variable regions of mAb166 IgG mRNA. The predicted amino acid sequence of the κ light-chain is shown in Table 2. This κ variable region is a Class II mouse κ variable region. Although its sequence is not identical to any germline-variable region present in the data bank, the DNA sequence of the 5′-untranslated region and V-region in the k light-chain showed highest homology to the germline IGKV12-41 (IGKV Subgroup 12; Accession Number: AJ235953). The DNA sequence in the J-region was identical to the germline IGKJ2 (Accession Number: V00777). The predicted amino acid sequence of the mAb166 heavy-chain region is shown in Table 2. The DNA sequence of the 5′-untranslated region and V-region segments in the heavy-chain containing complementarity determining regions (CDRs) 1 and 2 is identical, with the exception of 2 amino acids in the Frame 3 region, to the germline IGHV2S2 (IGHV Subgroup 2, VH101; Accession Number: J00502). The V-region sequence also showed the same level of homology with the reported pseudogene IGHV2S5 (Accession Number: M21165). The DNA sequence in the J-region was identical to the germline IGHJ4 (Accession Number: V00770). The unique CDR3 sequence includes an Arg–Gly–Asp sequence known to function as a recognition sequence for adhesion receptors in many adhesive proteins, including fibrinogen, fibronectin, von Willebrand factor, and vitronectin.
Table 1.
Reports on antibodies against Pseudomonas aeruginosa PcrV
| Authors | Antibody | Species | Target | Country | Year | Ref |
|---|---|---|---|---|---|---|
| Sawa T, et al. | Rabbit polyclonal IgG | mice | pneumonia | USA | 1999 | 10 |
| Shime N, et al. | Rabbit polyclonal IgG & F(ab)’ | rabbit | sepsis | USA | 2001 | 50 |
| Frank DW, et al. | Murine monoclonal IgG (mAb166) | mice | pneumonia | USA | 2002 | 11 |
| Faure K, et al. | Murine monoclonal IgG (mAb166), and Fab | rat | pneumonia | USA | 2003 | 54 |
| Neely AN, et al. | Rabbit polyclonal IgG | mice | burned | USA | 2005 | 51 |
| Imamura K, et al. | Rabbit polyclonal IgG | mice | chronic pneumonia | Japan | 2007 | 52 |
| Baer M, et al. | Humanized Fab’ (KB001) | mice | pneumonia | USA | 2008 | 12 |
| Moriyama K, et al. | Rabbit polyclonal IgG (affinity purified) | mice | pneumonia (immunocompromised) | USA | 2009 | 53 |
| Song Y, et al. | Murine monoclonal (mAb166) | mice | pneumonia | USA | 2012 | 55 |
| François B, et al. | Humanized Fab’ (KB001) | human | Ventilator-associated pneumonia | France | 2012 | 13 |
| Milla CE, et al. | Humanized Fab’ (KB001) | human | cystic fibrosis | USA | 2013 | 14 |
| Wang Q, et al. | Monoclonal (mAb166) | mice | pneumonia with MDRP | China | 2014 | 56 |
Table 2.
Primary sequences of the Anti-PcrV monoclonal antibody, mAb166
| Chain | Type | Region | Amino Acid Sequence | KB001 modification |
|---|---|---|---|---|
| heavy chain | IgG2b | Sig-pep | MAVLGLLFCLVTFPSCVLS | |
| FR1 | QVQLKQSGPGLVQPSQSLSITCTVSGFSLT | |||
| CDR1 | SYGVH | |||
| FR2 | WVRQSPGKGLEWLG | |||
| CDR2 | VIWSGGDTDYNAAFIS | |||
| FR3 | RLSISKDNSKSQLFFKMNSLRATDTAIYYCAR | |||
| CDR3 | NRGDIYYDFTYAMDY | NRGDIYYDFTYAXDZ | ||
| (X = M/F, Z = I/S/Q) | ||||
| FR4 | WGQGTSVTVSS | |||
| CH1 | AKTTPPSVYPLAPGCGDTTGSSVTLGCLVKGYFPESVTVTWNSGSLSSSVHTFPALLQSGLYTMSSSVTVPSSTWPSQTVTCSVAHPASSTTVDKKLEPSGPISTINPCPPCKECHKCPAPNLEGGPSVFIFPPNIKDVLMISLTPKVTCVVVDVSEDDPDVQISWFVNNVEVHTAQTQTHREDYNSTIRVVSTLPIQHQDWMSGKEFKCKVNNKDLPSPIERTISKIKGLVRAPQVYILPPPAEQLSRKDVSLTCLVVGFNPGDISVEWTSNGHTEENYKDTAPVLDSDGSYFIYSKLNMKTSKWEKTDSFSCNVRHEGLKNYYLKKTISRSPGK | |||
| light chain | κ | Sig-pep | MSVLTQVLALLLLWLTGARC | |
| FR1 | DIQMTQSPASLSASVGETVTITC | |||
| CDR1 | RASGNIQNYLA | |||
| FR2 | WYQQTQGKSPQLLVY | |||
| CDR2 | SAKTLAD | |||
| FR3 | GVPSRFSGSGSGTQYSLKINSLQPEDFGSYYC | |||
| CDR3 | QHFWSTPYT | QQFWXTPYT (X = S/G) | ||
| FR4 | FGGGTKLEIKR | FGGGTKLTVLR | ||
| CL | ADAAPTVSIFPPSSEQLTSGGASVVCFLNNFYPKDINVKWKIDGSERQNGVLNSWTDQDSKDSTYSMSSTLTLTKDEYERHNSYTCEATHKTSTSPIVKSFNRNEC |
An engineered human anti-PcrV antibody Fab fragment
An engineered human antibody Fab fragment that binds to the P. aeruginosa PcrV protein with a high affinity has been identified, and it has potent in-vitro neutralization activity against TTSS.12 Instillation of a single dose of Fab into the lungs of mice provided protection against a lethal pulmonary challenge with P. aeruginosa, leading to a substantial reduction in viable bacterial cell counts in the lungs. These results demonstrate that blocking TTSS with a Fab fragment that lacks antibody Fc-mediated effector functions is sufficient for the effective clearance of a pulmonary P. aeruginosa infection.
Clinical trial for ventilator-associated pneumonia (VAP)
In France, a multicenter, Phase-IIa clinical trial of the recombinant, PEGylated, engineered, human Fab’ anti-PcrV fragment KB001 involving 10 intensive care units was undertaken.13 The purpose of the study was to determine the safety and pharmacokinetics of KB001, and its ability to prevent P. aeruginosa VAP. In this trial, 39 mechanically ventilated patients, who were colonized by P. aeruginosa but not infected, were randomized at a 1:1:1 ratio to receive a single intravenous 3 mg/kg or 10 mg/kg infusion of KB001 or a placebo. KB001 was well tolerated and not immunogenic. The respective maximum serum concentrations of the 3- and 10-mg/kg groups were 5–8 × 104 and 12–28 × 104 ng/ml, with mean elimination half-lives of 8.1 and 9.3 days, respectively. KB001 was detected in the endotracheal aspirates of all patients receiving it as early as day 1 and up to 28 days. Patients who received either 3 or 10 mg/kg of KB001 developed P. aeruginosa pneumonia less frequently (33% and 31%, respectively) than placebo recipients (60%). In this study, KB001 demonstrated its safety, a favorable pharmacokinetic profile, and a promising potential for reducing the incidence of P. aeruginosa pneumonia in mechanically ventilated patients colonized with the bacterium in intensive care. The administration of 3 mg/kg of KB001 appears to be promising to keep the effective serum titer which is high enough to neutralize PcrV of P. aeruginosa in the bronchial secretes at least for a month and prevent the ventilated patients from development of pneumonia.
Clinical trial for chronic pneumonia in cystic fibrosis
In USA, the safety and pharmacokinetic and pharmacodynamic properties of KB001 were evaluated in CF patients with a chronic P. aeruginosa infection.14 In this study, 27 CF patients with a sputum P. aeruginosa density >105 CFU/g received a single intravenous dose of KB001 (3 mg/kg or 10 mg/kg) or a placebo. After administration, KB001 demonstrated a mean serum half-life of 11.9 days. At day 28, there was a trend toward a dose-dependent reduction in sputum myeloperoxidase, IL-1β, and IL-8, and there were significant overall differences in the change in sputum neutrophil elastase and neutrophil counts favoring the KB001 10 mg/kg group versus the control group, suggesting that targeting the P. aeruginosa TTSS with KB001 as a nonantibiotic strategy reduces airway inflammation and damage in CF patients with a chronic P. aeruginosa infection.
Summary
The observations described in this review support the hypothesis that anti-PcrV strategies have potential as nonantibiotic immunostrategies for cytotoxic P. aeruginosa infections. Clinical trials of the humanized anti-PcrV IgG Fab’ fragment, KB001, which was molecularly engineered from the monoclonal anti-PcrV IgG mAb166 are currently ongoing for patients with VAP and CF. In preliminary reports from these clinical trials, KB001 has demonstrated its safety, a favorable pharmacokinetic profile, and a promising potential as a nonantibiotic strategy to reduce airway inflammation and damage in P. aeruginosa pneumonia.14 Further analyses of the role of PcrV in TTSS and the mechanism of its antibody mediated disruption are critical for developing improved anti-V-antigen strategies. Up to date, there is no report about the effects of anti-PcrV on Staphylococcus aureus and other potentially drug resistant pathogens in polymicrobial environment. Therefore, in further clinical trials, an evaluation of the efficacy of antibody mediated therapies including the analysis of anti-PcrV in the polymicrobial environment which is frequently observed in the cystic fibrosis airways, will also strengthen these strategies.
Antibodies to other bacterial V-antigen homologs may protect hosts from other infections such as diarrheal enterocolitis and sepsis caused by Vibrio parahaemolyticus and Aeromonas sp.9 This strategy could also be used to protect fish and shrimp from massive mortality caused by A. salmonicida or V. harveyi infections at fish and shrimp farms. V-antigen-associated virulence is linked to lethal infections caused by pathogenic gram-negative bacteria at fish and shrimp farms, in potential plagues caused by bioterrorism, and in opportunistic infections in humans.
Disclosure of Potential Conflicts of Interest
With regard to the content of the manuscript, Teiji Sawa has a patent fee from the Regent of the University of California, USA.
Acknowledgments
The authors would like to thank Dr. Jeanine P. Wiener-Kronish, Anesthetist-in-Chief; Henry Isaiah Dorr, Professor of Anesthesia; and Harvard Medical School for critical support of the authors’ research.
Funding
This work was supported by grants from a Grant-in-Aid for Scientific Research (KAKENHI No. 24390403) and The Ministry of Education, Culture, Sports, Science and Technology, Japan to Teiji Sawa.
References
- 1.Parker CM, Kutsogiannis J, Muscedere J, Cook D, Dodek P, Day AG, Heyland DK, Canadian critical care trials G. ventilator-associated pneumonia caused by multidrug-resistant organisms or Pseudomonas aeruginosa: prevalence, incidence, risk factors, and outcomes. J Crit Care 2008; 23:18-26; PMID:18359417; http://dx.doi.org/ 10.1016/j.jcrc.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 2.Bassetti M, Taramasso L, Giacobbe DR, Pelosi P. Management of ventilator-associated pneumonia: epidemiology, diagnosis and antimicrobial therapy. Expert Rev Anti Infect Ther 2012; 10:585-96; PMID:22702322; http://dx.doi.org/ 10.1586/eri.12.36 [DOI] [PubMed] [Google Scholar]
- 3.Grgurich PE, Hudcova J, Lei Y, Sarwar A, Craven DE. Management and prevention of ventilator-associated pneumonia caused by multidrug-resistant pathogens. Expert Rev Respir Med 2012; 6:533-55; PMID:23134248; http://dx.doi.org/ 10.1586/ers.12.45 [DOI] [PubMed] [Google Scholar]
- 4.Barbier F, Andremont A, Wolff M, Bouadma L. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med 2013; 19:216-28; PMID:23524477; http://dx.doi.org/ 10.1097/MCP.0b013e32835f27be [DOI] [PubMed] [Google Scholar]
- 5.Sawa T, Wiener-Kronish JP. A therapeutic strategy against the shared virulence mechanism utilized by both Yersinia pestis and Pseudomonas aeruginosa. Anesthesiol Clin North Am 2004; 22:591-606, viii-ix; PMID:15325721 [DOI] [PubMed] [Google Scholar]
- 6.Wiener-Kronish JP, Frank DW, Sawa T. Mechanisms of lung epithelial cell Injury by acute by Pseudomonas aeruginosa. In: Clark RSB, Carcillo JA, eds. Molecular Biology of Acute Lung Injury Boston, USA: Kluwer Academic Publishers, 2001:149-61; http://dx.doi.org/ 10.1007/978-1-4615-1427-5_10 [DOI] [Google Scholar]
- 7.Sawa T. The molecular mechanism of acute lung injury caused by Pseudomonas aeruginosa: from bacterial pathogenesis to host response. J Intensive Care 2014; 2:10; http://dx.doi.org/ 10.1186/2052-0492-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol 2009; 7:654-65; PMID:19680249; http://dx.doi.org/ 10.1038/nrmicro2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawa T, Katoh H, Yasumoto H. V-antigen homologs in pathogenic gram-negative bacteria. Microbiol Immunol 2014; 58, 267-85; PMID:24641673; http://dx.doi.org/ 10.1111/1348-0421.12147 [DOI] [PubMed] [Google Scholar]
- 10.Sawa T, Yahr TL, Ohara M, Kurahashi K, Gropper MA, Wiener-Kronish JP, Frank DW. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat Med 1999; 5:392-8; PMID:10202927 [DOI] [PubMed] [Google Scholar]
- 11.Frank DW, Vallis A, Wiener-Kronish JP, Roy-Burman A, Spack EG, Mullaney BP, Megdoud M, Marks JD, Fritz R, Sawa T. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J Infect Dis 2002; 186:64-73; PMID:12089663 [DOI] [PubMed] [Google Scholar]
- 12.Baer M, Sawa T, Flynn P, Luehrsen K, Martinez D, Wiener-Kronish JP, Yarranton G, Bebbington C. An engineered human antibody fab fragment specific for Pseudomonas aeruginosa PcrV antigen has potent antibacterial activity. Infect Immun 2009; 77:1083-90; PMID:19103766; http://dx.doi.org/ 10.1128/IAI.00815-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francois B, Luyt CE, Dugard A, Wolff M, Diehl JL, Jaber S, Forel JM, Garot D, Kipnis E, Mebazaa A, et al. . Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized,double-blind, placebo-controlled trial. Crit Care Med 2012; 40:2320-6; PMID:22622405; http://dx.doi.org/ 10.1097/CCM.0b013e31825334f6 [DOI] [PubMed] [Google Scholar]
- 14.Milla CE, Chmiel JF, Accurso FJ, Vandevanter DR, Konstan MW, Yarranton G, Geller DE, for the KBSG. Anti-PcrV antibody in cystic fibrosis: A novel approach targeting Pseudomonas aeruginosa airway infection. Pediatr Pulmonol 2013; 49:650–8 PMID:24019259; http://dx.doi.org/ 10.1002/ppul.22890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser AR, Cobb E, Bodi M, Mariscal D, Valles J, Engel JN, Rello J. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med 2002; 30:521-8; PMID:11990909 [DOI] [PubMed] [Google Scholar]
- 16.Kurahashi K, Kajikawa O, Sawa T, Ohara M, Gropper MA, Frank DW, Martin TR, Wiener-Kronish JP. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest 1999; 104:743-50; PMID:15107296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hueck CJ. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev 1998; 62:379-433; PMID:9618447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coburn B, Sekirov I, Finlay BB. Type III secretion systems and disease. Clin Microbiol Rev 2007; 20:535-49; PMID:17934073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong-Beringer A, Wiener-Kronish J, Lynch S, Flanagan J. Comparison of type III secretion system virulence among fluoroquinolone-susceptible and -resistant clinical isolates of Pseudomonas aeruginosa. Clin Microbiol Infect 2008; 14:330-6; PMID:18190571; http://dx.doi.org/ 10.1111/j.1469-0691.2007.01939.x [DOI] [PubMed] [Google Scholar]
- 20.Agnello M, Wong-Beringer A. Differentiation in quinolone resistance by virulence genotype in Pseudomonas aeruginosa. PLOS one 2012; 7:e42973; PMID:22905192; http://dx.doi.org/ 10.1371/journal.pone.0042973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Solh AA, Hattemer A, Hauser AR, Alhajhusain A, Vora H. Clinical outcomes of type III Pseudomonas aeruginosa bacteremia. Crit Care Med 2012; 40:1157-63; PMID:22080633; http://dx.doi.org/ 10.1097/CCM.0b013e3182377906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howell HA, Logan LK, Hauser AR. Type III secretion of ExoU is critical during early Pseudomonas aeruginosa pneumonia. MBio 2013; 4:e00032-13; PMID:23481600; http://dx.doi.org/ 10.1128/mBio.00032-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleiszig SM, Wiener-Kronish JP, Miyazaki H, Vallas V, Mostov KE, Kanada D, Sawa T, Yen TS, Frank DW. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun 1997; 65:579-86; PMID:9009316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, Sawa T, Frank DW, Wiener-Kronish JP. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis 2001; 183:1767-74; PMID:11372029 [DOI] [PubMed] [Google Scholar]
- 25.Wiener-Kronish JP, Broaddus VC, Albertine KH, Gropper MA, Matthay MA, Staub NC. Relationship of pleural effusions to increased permeability pulmonary edema in anesthetized sheep. J Clin Invest 1988; 82:1422-9; PMID:3170750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiener-Kronish JP, Sakuma T, Kudoh I, Pittet JF, Frank D, Dobbs L, Vasil ML, Matthay MA. Alveolar epithelial injury and pleural empyema in acute P. aeruginosa pneumonia in anesthetized rabbits. J Appl Physiol 1993; 75:1661-9; PMID:8282618 [DOI] [PubMed] [Google Scholar]
- 27.Kudoh I, Wiener-Kronish JP, Hashimoto S, Pittet JF, Frank D. Exoproduct secretions of Pseudomonas aeruginosa strains influence severity of alveolar epithelial injury. Am J Physiol 1994; 267:L551-6; PMID:7977765 [DOI] [PubMed] [Google Scholar]
- 28.Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SM, Wu C, Mende-Mueller L, Frank DW. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol 1997; 25:547-57; PMID:9302017 [DOI] [PubMed] [Google Scholar]
- 29.Ernst EJ, Hashimoto S, Guglielmo J, Sawa T, Pittet JF, Kropp H, Jackson JJ, Wiener-Kronish JP. Effects of antibiotic therapy on Pseudomonas aeruginosa-induced lung injury in a rat model. Antimicrob Agents Chemother 1999; 43:2389-94; PMID:10508012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato H, Frank DW. Multi-functional characteristics of the Pseudomonas aeruginosa type III needle-tip protein, PcrV; comparison to orthologs in other gram-negative bacteria. Frontiers in Microbiol 2011; 2:142; PMID:21772833; http://dx.doi.org/ 10.3389/fmicb.2011.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brubaker RR. The V antigen of yersiniae: an overview. Contrib Microbiol Immunol 1991; 12:127-33; PMID:1935079 [PubMed] [Google Scholar]
- 32.Cornelis GR, Wolf-Watz H. The yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol 1997; 23:861-7; PMID:9076724 [DOI] [PubMed] [Google Scholar]
- 33.Mueller CA, Broz P, Cornelis GR. The type III secretion system tip complex and translocon. Mol Microbiol 2008; 68:1085-95; PMID:18430138; http://dx.doi.org/ 10.1111/j.1365-2958.2008.06237.x [DOI] [PubMed] [Google Scholar]
- 34.Fields KA, Straley SC. LcrV of Yersinia pestis enters infected eukaryotic cells by a virulence plasmid-independent mechanism. Infect Immun 1999; 67:4801-13; PMID:10456934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettersson J, Holmstrom A, Hill J, Leary S, Frithz-Lindsten E, von Euler-Matell A, Carlsson E, Titball R, Forsberg A, Wolf-Watz H. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol Microbiol 1999; 32:961-76; PMID:10361299 [DOI] [PubMed] [Google Scholar]
- 36.Schoehn G, Di Guilmi AM, Lemaire D, Attree I, Weissenhorn W, Dessen A. Oligomerization of type III secretion proteins PopB and PopD precedes pore formation in Pseudomonas. EMBO J 2003; 22:4957-67; PMID:14517235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol 2006; 4:811-25; PMID:17041629 [DOI] [PubMed] [Google Scholar]
- 38.Cornelis GR. The type III secretion injectisome, a complex nanomachine for intracellular ‘toxin’ delivery. Biol Chem 2010; 391:745-51; PMID:20482311; http://dx.doi.org/ 10.1515/BC.2010.079 [DOI] [PubMed] [Google Scholar]
- 39.Erhardt M, Namba K, Hughes KT. Bacterial nanomachines: the flagellum and type III injectisome. Cold Spring Harb Perspect Biol 2010; 2:a000299; PMID:20926516; http://dx.doi.org/ 10.1101/cshperspect.a000299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller CA, Broz P, Muller SA, Ringler P, Erne-Brand F, Sorg I, Kuhn M, Engel A, Cornelis GR. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 2005; 310:674-6; PMID:16254184 [DOI] [PubMed] [Google Scholar]
- 41.Holmstrom A, Olsson J, Cherepanov P, Maier E, Nordfelth R, Pettersson J, Benz R, Wolf-Watz H, Forsberg A. LcrV is a channel size-determining component of the Yop effector translocon of Yersinia. Mol Microbiol 2001; 39:620-32; PMID:11169103 [DOI] [PubMed] [Google Scholar]
- 42.Derewenda U, Mateja A, Devedijiev Y, Routzahn KM, Evodokimov AG, Derewenda ZS, Waugh DS. The structure of Yersinia pestis V-antigen, an essential virulence factor and mediator of immunity against plague. Structure 2004; 12:301-6; PMID:11169103 [DOI] [PubMed] [Google Scholar]
- 43.Broz P, Mueller CA, Muller SA, Philippsen A, Sorg I, Engel A, Cornelis GR. Function and molecular architecture of the Yersinia injectisome tip complex. Mol Microbiol 2007; 65:1311-20; PMID:17697254 [DOI] [PubMed] [Google Scholar]
- 44.Blocker AJ, Deane JE, Veenendaal AK, Roversi P, Hodgkinson JL, Johnson S, Lea SM. What's the point of the type III secretion system needle? Proc Nat Acad of Sci USA 2008; 105:6507-13; PMID:18458349; http://dx.doi.org/ 10.1073/pnas.0708344105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caroline G, Eric F, Bohn YS, Sylvie E, Attree I. Oligomerization of PcrV and LcrV, protective antigens of Pseudomonas aeruginosa and Yersinia pestis. J Biol Chem 2008; 283:23940-9; PMID:18583342; http://dx.doi.org/ 10.1074/jbc.M803146200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee VT, Tam C, Schneewind O. LcrV, a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J Biol Chem 2000; 275:36869-75; PMID:10930402 [DOI] [PubMed] [Google Scholar]
- 47.Dacheux D, Goure J, Chabert J, Usson Y, Attree I. Pore-forming activity of type III system-secreted proteins leads to oncosis of Pseudomonas aeruginosa-infected macrophages. Mol Microbiol 2001; 40:76-85; PMID:11298277 [DOI] [PubMed] [Google Scholar]
- 48.Hoiczyk E, Blobel G. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc Nat Acad of Sci USA 2001; 98:4669-74; PMID:11287645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goure J, Pastor A, Faudry E, Chabert J, Dessen A, Attree I. The V antigen of Pseudomonas aeruginosa is required for assembly of the functional PopB/PopD translocation pore in host cell membranes. Infect Immun 2004; 72:4741-50; PMID:15271936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shime N, Sawa T, Fujimoto J, Faure K, Allmond LR, Karaca T, Swanson BL, Spack EG, Wiener-Kronish JP. Therapeutic administration of anti-PcrV F(ab’)(2) in sepsis associated with Pseudomonas aeruginosa. J Immunol 2001; 167:5880-6; PMID:11698464 [DOI] [PubMed] [Google Scholar]
- 51.Imamura Y, Yanagihara K, Fukuda Y, Kaneko Y, Seki M, Izumikawa K, Miyazaki Y, Hirakata Y, Sawa T, Wiener-Kronish JP, et al. . Effect of anti-PcrV antibody in a murine chronic airway Pseudomonas aeruginosa infection model. Eur Respir J 2007; 29:965-8; PMID:17301098 [DOI] [PubMed] [Google Scholar]
- 52.Mikkelsen H, Bond NJ, Skindersoe ME, Givskov M, Lilley KS, Welch M. Biofilms and type III secretion are not mutually exclusive in Pseudomonas aeruginosa. Microbiology. 2009; 155:687-98; PMID:19246740; http://dx.doi.org/ 10.1099/mic.0.025551-0 [DOI] [PubMed] [Google Scholar]
- 53.Neely AN, Holder IA, Wiener-Kronish JP, Sawa T. Passive anti-PcrV treatment protects burned mice against Pseudomonas aeruginosa challenge. Burns 2005; 31:153-8; PMID:15683685 [DOI] [PubMed] [Google Scholar]
- 54.Moriyama K, Wiener-Kronish JP, Sawa T. Protective effects of affinity-purified antibody and truncated vaccines against Pseudomonas aeruginosa V-antigen in neutropenic mice. Microbiol Immunol 2009; 53:587-94; PMID:19903258; http://dx.doi.org/ 10.1111/j.1348-0421.2009.00165.x [DOI] [PubMed] [Google Scholar]
- 55.Faure K, Fujimoto J, Shimabukuro DW, Ajayi T, Shime N, Moriyama K, Spack EG, Wiener-Kronish JP, Sawa T. Effects of monoclonal anti-PcrV antibody on Pseudomonas aeruginosa-induced acute lung injury in a rat model. J Immune Based Ther Vaccines 2003; 1:2; PMID:12943554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song Y, Baer M, Srinivasan R, Lima J, Yarranton G, Bebbington C, Lynch SV. PcrV antibody-antibiotic combination improves survival in Pseudomonas aeruginosa-infected mice. Eur J Clin Microbiol Infect Dis 2012; 31:1837-45; PMID:22187351; http://dx.doi.org/ 10.1007/s10096-011-1509-2 [DOI] [PubMed] [Google Scholar]
- 57.Wang Q, Li H, Zhou J, Zhong M, Zhu D, Feng N, Liu F, Bai C, Song Y. PcrV antibody protects multi-drug resistant Pseudomonas aeruginosa induced acute lung injury. Respir Physiol Neurobiol 2014; 193:21-8; PMID:24418353; http://dx.doi.org/ 10.1016/j.resp2014.01.001 [DOI] [PubMed] [Google Scholar]
- 58.Källberg M, Wang H, Wang S, Peng J, Wang Z, Lu H, Xu J. Template-based protein structure modeling using the RaptorX web server. Nature Protocols 2012; 7:1511-22; PMID:22814390; http://dx.doi.org/ 10.1007/978-1-4939-0366-5_2 [DOI] [PMC free article] [PubMed] [Google Scholar]


