Abstract
Enterovirus 71 (EV71) and Coxsackievirus A16 (CVA16) have caused severe epidemics of hand, foot and mouth disease (HFMD) in the Asia Pacific in recent years, particularly in infants and young children. This disease has become a serious public health problem, as no vaccines or antiviral drugs have been approved for EV71 and CA16 infections. In this study, we compared four monovalent vaccines, including formalin-inactivated EV71 virus (iEV71), EV71 virus-like particles (VLPs) (vEV71), formalin-inactivated CVA16 virus (iCVA16) and CVA16 VLPs (vCVA16), along with two bivalent vaccines, including equivalent doses of formalin-inactivated EV71+CVA16 virus (iEV71+iCVA16) and EV71+CVA16 VLPs (vEV71+vCVA16). The IgG titers and neutralization antibodies titers demonstrated that there are no immune interference exists between the two immunogens of EV71 and CVA16. IgG subclass isotyping revealed that IgG1 and IgG2b were induced primarily in all vaccine groups. Furthermore, cross-neutralization antibodies were elicited in mouse sera against other sub-genotypes of EV71 and CVA16. In vivo challenge experiments showed that the immune sera from vaccinated animals could confer passive protection to newborn mice against lethal challenge with 14 LD50 of EV71 and 50 LD50 of CVA16. Our results indicated that bivalent vaccination is promising for HFMD vaccine development. With the advantage of having a better safety profile than inactivated virus vaccines, VLPs should be used to combine both EV71 and CVA16 antigens as a candidate vaccine for prevention of HFMD virus transmission.
Keywords: HFMD, Enterovirus 71, Coxsackievirus A16, monovalent vaccine, bivalent vaccines, pseudovirus
Introduction
Hand, foot and mouth disease (HFMD) is a common illness in infants and children. As the major causative agents of HFMD, Enterovirus 71 (EV71) and Coxsackievirus A16 (CVA16) have a single positive-stranded RNA, non-enveloped viruses which belong to the family Picornaviridae with the genome of approximately 7,410 nucleotides, including a long single open reading frame (ORF) flanked by a 5′-UTR and a 3′-UTR. The ORF encodes a single poly-protein P1, which is cleaved by viral proteases (3CD) into viral capsid (VP4, VP2, VP3 and VP1) and nonstructural (P2 and P3) proteins. VP1, VP2 and VP3 are exposed to immune pressure at the surface of the viral capsid, whereas VP4 is located inside the capsid.1
HFMD disease has become a serious public health problem, with outbreaks occurring periodically throughout the world. In recent years, HFMD has occurred in Japan, Malaysia, Singapore, Vietnam, Mainland China, England and Australia.2-8 And data reported by the Western Pacific Regional Office of the World Health Organization showed that over two million cases were diagnosed in 2012 in asian countries, including China (2,198,442 cases, 569 deaths), Vietnam (148,366 cases, 45 deaths), Japan (70,682 cases), and Singapore (37,276 cases).9 EV71 has a propensity to cause severe neurological diseases during acute infection. The most severe forms of EV71-associated diseases of the central nervous system (CNS) that can even result in death include aseptic meningitis, brainstem encephalitis and acute flaccid paralysis, which is indistinguishable from poliomyelitis. By contrast, most CVA16 infections present only mild symptoms, such as fever, mouth ulcers, rashes and blisters on the surface of the hands and feet.10-15 However, a recent study reported that CVA16 may be more virulent in children and has caused a number of deaths and severe cases of neurological complications.16,17 An epidemiological survey showed that, out of 92 severe HFMD cases with neurological complications, 19 cases were caused by CVA16 infection.17 Importantly, the co-circulation of CVA16 and EV71 has resulted in co-infections, and recombination between the two viruses, which can cause more serious clinical symptoms compared with a single viral infection, making it more complex and difficult to control HFMD epidemics.18-20 As no approved antiviral drugs or vaccines are available for HFMD, increasing the pace of vaccine development therefore has become a priority. Previous vaccine research for HFMD has focused only on EV71, and formalin-inactivated vaccine for this virus has been evaluated through a phase III clinical trial in Mainland China.21 CVA16 and EV71 were the major agents of HFMD, therefore, bivalent vaccines against both EV71 and CVA16 should be considered for HFMD vaccine development.
The development of HFMD vaccines has focused on several different forms, including live-attenuated, DNA, polypeptide, subunit, virus-like particle (VLP) and inactivated whole-virus vaccines.22 The EV71 live-attenuated and DNA vaccines have shown high levels of immunogenicity and protection, but safety concerns have hindered their development. Two synthetic polypeptide vaccines, SP70 and SP55, could induce low levels of antigen-specific antibodies, and both of them were found to protect newborn mice against EV71 virus but not CVA16 virus. The VP1 subunits of CVA16 and EV71 are not suitable as vaccine candidates due to their low immunogenicity and lack of spatial structure that would be found on virions. Because VLPs and inactivated whole-virus vaccines theoretically present epitopes in spatial structure, they are thought to be good vaccine candidates. VLPs have a greater safety profile than inactivated vaccines since they do not contain the virus genome. Currently, several experimental CVA16 and EV71 vaccines are under development, including inactivated CVA16,23-25 and CVA16 VLPs derived from insect cells26 and Saccharomyces cerevisiae.27 Among these, the EV71 inactivated vaccine has been evaluated and passed through a phase III clinical trial, while the development of EV71 VLPs in insect cells and S. cerevisiae is still ongoing.28,29 However, until now these vaccines have not been evaluated in bivalent composition.
In the present study, we first compared the monovalent vaccines with the bivalent vaccines in ICR mice. The monovalent vaccines included formalin-inactivated EV71 virus (iEV71), EV71 VLPs (vEV71), formalin-inactivated CVA16 virus (iCVA16) and CVA16 VLPs (vCVA16), whereas the bivalent vaccines contained VLPs (vEV71+vCVA16) and formalin-inactivated (iEV71+iCVA16) The induction of neutralizing antibodies was monitored using a pseudovirus-luciferase (PVA) assay system, and the ICR neonatal mouse model was used to evaluate the efficacy of the monovalent or bivalent vaccines after EV71 or CVA16 challenge.
Results
Purification of VLPs and virions
EV71 and CVA16 VLPs were produced from the baculovirus expression system and purified by CsCl gradient as demonstrated previously.28,30 EV71 and CVA16 viruses were produced from the RDS cell line due to its high susceptibility to infection.31 Both VLPs and virions were characterized by SDS-PAGE and western blot using the corresponding rabbit anti-VLP (EV71 or CVA16) antibody (Fig. 1). The VLPs contained the major structural proteins, VP0, VP1 and VP3. Virions preparations typically consist of both mature and empty particles. The empty particle contains VP1, VP3 and VP0 structural proteins, while the mature particle contains VP2 and VP4, which have been cleaved from VP0, as well as the viral genome inside the capsid.32 The TEM image of the sample showed that the purified VLPs and virions had similar morphologies (Fig. 2).Table 1.
Figure 1.
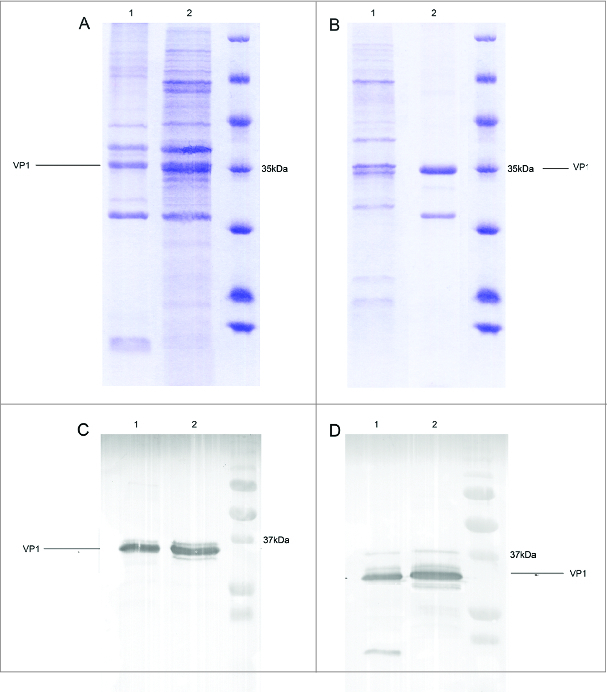
Characterization of purified CVA16 VLPs, CVA16 virions, EV71 VLPs and EV71 virions. CVA16 VLPs and EV71 VLPs produced from insect cells and purified using CsCl gradient. CVA16 and EV71 virions were produced from RDS cells and purified with a sucrose gradient. (A, C) EV71 VLPs (lane 1) and EV71 virions (lane 2) were analyzed by SDS-PAGE and western blot using an anti-EV71 VP1 antibody. (B, D) CVA16 VLPs (lane 1) and CVA16 virions (lane 2) were detected by SDS-PAGE and western blot using an anti-CVA16 VP1 antibody.
Figure 2.
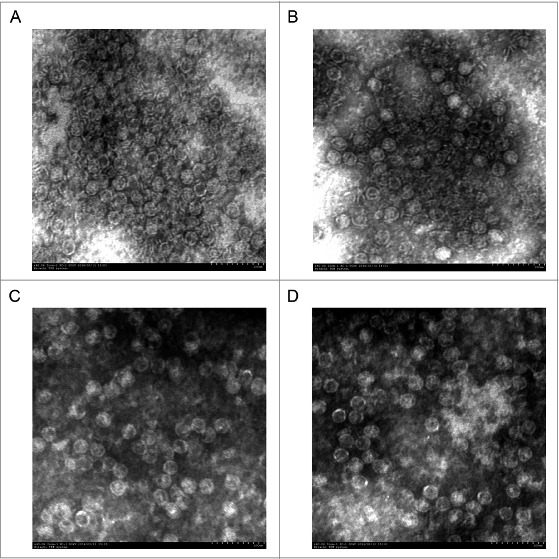
TEM image of VLPs and virions. (A) EV71 VLPs, (B) EV71 virions, (C) CVA16 VLPs and (D) CVA16 virions. Both VLPs and virions are approximately 30 nm in diameter. The morphology of VLPs resembles that of the authentic virus. Bar, 100 nm.
Table 1.
Different Genotypes of EV71 and CVA16 to construct the plasmid, for obtaining the Pseudoviruses
| Genotype | Accession number | Genotype | Accession number |
|---|---|---|---|
| EV71-B1 | AB482183.1 | CVA16-A | U05876.1 |
| EV71-B2 | U22522 | CVA16-B1a | AF177911.1 |
| EV71-B3 | AB550334 | CVA16-B1b | EU262658.1 |
| EV71-B4 | AF316321 | CVA16-B2 | AY895127.1 |
| EV71-B5 | EU527985 | ||
| EV71-C1 | AB575937.1 | ||
| EV71-C2 | AF304457.1 | ||
| EV71-C2like | HM622392.1 | ||
| EV71-C3 | DQ341356.1 | ||
| EV71-C4b | GQ994988 | ||
| EV71-C5 | AM490161.1 |
Detection of total IgG, cross-reactive IgG and IgG subtypes
Total IgG was measured by ELISA. Sera from immunized mice were collected every two weeks. The results demonstrated that IgG antibodies reached the plateau at week 6 after the primary immunization. The bivalent vaccines induced slightly high antibody levels at week 6 (Fig. 3). Intriguingly, IgG antibodies elicited by vCVA16 and iCVA16 showed low cross-reactivity. Similar results were observed in EV71-immunized groups (Fig. 4). The IgG subtypes also were detected. The results demonstrated that the monovalent vaccine and bivalent vaccines induced higher levels of IgG1 and IgG2b than those of IgG2a and IgG3 (Table 2A, 2B).
Figure 3.
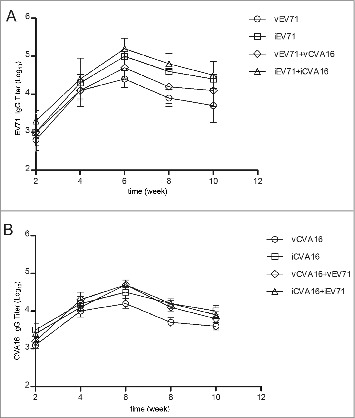
Antigen-specific titers in sera samples of mice immunized with vEV71, iEV71, vCVA16, iCVA16, vCVA16+vEV71 or iCVA16+iEV71. (A) anti-vEV71, iEV71, vEV71+vCVA16 and iEV71+iCVA16 titer. (B) anti-vCVA16, iCVA16, vCVA16+vEV71 and iCVA16+iEV71 IgG titer. Results are presented as the mean ± SD of three independent experiments.
Figure 4.
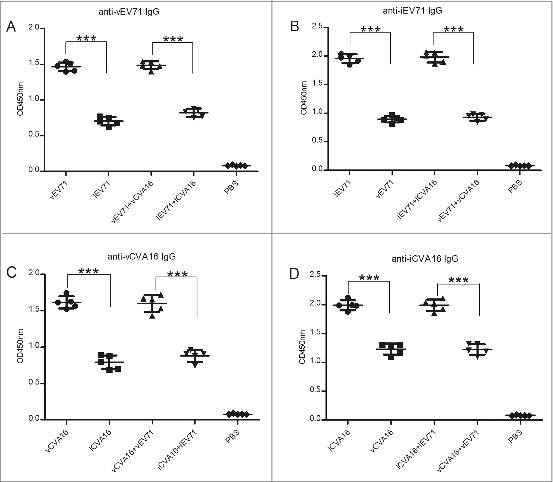
Cross-reactive IgG titers were analyzed. The sera of the 6th week (1,000-fold dilution) were detected using vEV71 antigen or iEV71 antigen as coating antigen. And the same method was used to investigate the cross-reactive IgG titers between vCVA16 antigen and iCVA16 antigen. (A) Anti-vEV71 IgG, (B) anti-iEV71 IgG, (C) anti-vCVA16 IgG and (D) anti-iCVA16 IgG.
Table 2A.
Distribution of specific anti-EV71 IgG subtypes in ICR immunized mice with vEV71 and iEV71 antigens
| OD405nm of ELISA for EV71-specific IgG |
|||||
|---|---|---|---|---|---|
| Immunogen | IgG1 OD (S.D.) | IgG2a OD (S.D.) | IgG2b OD (S.D.) | IgG3 OD (S.D.) | IgG1:IgG2a |
| vEV71 | 0.31 (0.05) | 0.08 (0.02) | 0.26 (0.006) | 0.09 (0.01) | 4:1 |
| vEV71+vCVA16 | 0.20 (0.04) | 0.07 (0.01) | 0.22 (0.002) | 0.08 (0.02) | 3:1 |
| iEV71 | 0.18 (0.04) | 0.05 (0.02) | 0.22 (0.01) | 0.07 (0.02) | 3:1 |
| iEV71+iCVA16 | 0.21 (0.02) | 0.06 (0.02) | 0.19 (0.01) | 0.06 (0.02) | 3:1 |
Groups of mice received vEV71, iEV71, vCVA16+vEV71 or iCVA16+iEV71. Mouse sera were collected by tail vein bleeding at the 6th week post-immunization and assayed for specific anti-EV71 IgG subtypes by ELISA. Results are presented as the mean ± SD of three independent experiments.
Table 2B.
Distribution of specific anti-CVA16 IgG subtypes in ICR immunized mice with vCA16 and iCVA16 antigens
| OD405nm of ELISA for CVA16-specific IgG |
|||||
|---|---|---|---|---|---|
| Immunogen | IgG1 OD (S.D.) | IgG2a OD (S.D.) | IgG2b OD (S.D.) | IgG3 OD (S.D.) | IgG1:IgG2a |
| vCVA16 | 0.29 (0.02) | 0.07 (0.02) | 0.36 (0.04) | 0.17 (0.01) | 4:1 |
| vCVA16+vEV71 | 0.20 (0.02) | 0.06 (0.02) | 0.3 (0.03) | 0.13 (0.02) | 3:1 |
| iCVA16 | 0.20 (0.04) | 0.05 (0.02) | 0.32 (0.03) | 0.10 (0.02) | 4:1 |
| iCVA16+iEV71 | 0.20 (0.003) | 0.05 (0.01) | 0.28 (0.02) | 0.10 (0.01) | 4:1 |
Groups of mice received vCVA16, iCVA16, vCVA16+vEV71 or iCVA16+iEV71. Mouse sera were collected by tail vein bleeding at the 6th week post-immunization and assayed for specific anti-CVA16 IgG subtypes by ELISA. Results are presented as the mean ± SD of three independent experiments.
Neutralization and cross-reactivity
To determine whether these vaccines could elicit neutralization antibodies, mice were immunized intraperitoneally (i.p.) with vEV71, iEV71, vCVA16, iCVA16, vEV71+vCVA16 or iEV71+iCVA16. Each group received three injections via i.p. at week 0, 2 and 4, and sera samples were collected from the tail vein at week 2, 4, 6, 8 and 10. The neutralization antibody titer against EV71 (C4a pseudovirus) from the vEV71, iEV71, vEV71+vCVA16 and iEV71+iCVA16 groups increased rapidly after the first immunization and peaked at week 6 (Fig. 5A). However, the neutralization antibody titers against CVA16 (B1a pseudovirus) were undetectable at 2 wk after the first immunization except in the iCVA16-immunized group (Fig. 5B).
Figure 5.
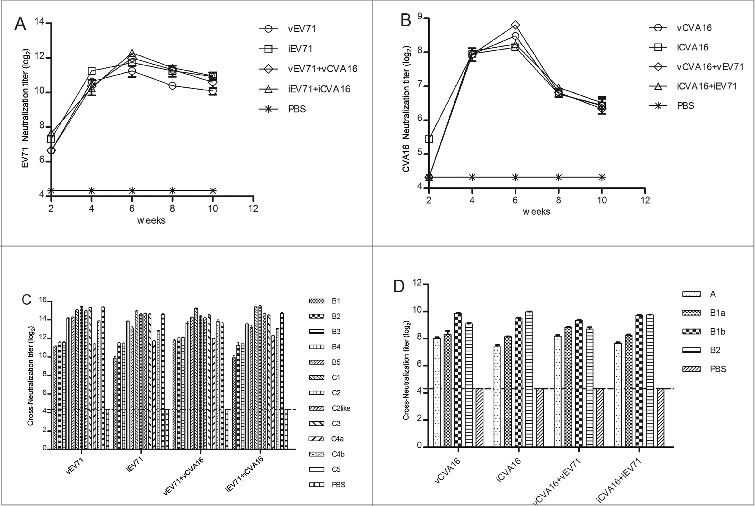
Neutralization titers of sera antibodies from mice immunized against EV71 and CVA16 were analyzed by pseudovirus-based assay. Sera collected from the immunized mice (n = 5 per group) at week 2, 4, 6, 8 and 10 were serially diluted (31 to 39) after an initial 20-fold dilution and mixed with the EV71 or CVA16 pseudoviruses before measuring luciferase activity. (A) EV71 neutralization titer; (B) CVA16 neutralization titer; (C) crossneutralizati- on titer of EV71 sera at the 6th week against genogroups B (B1-B5) and C (C1-C5); (D)cross-neutralization titer of CVA16 sera at the 6th week against genogroups A and B (B1a, B1b and B2).
Mizuta et al. reported that a single EV71 agent could elicit cross-neutralizing antibodies against other genotypes of EV71.33 Similarly, the pseudovirus-luciferase assay (PVA) in this study showed that cross-neutralizing antibodies were induced in sera from animals immunized with EV71, either a monovalent vaccine (vEV71 and iEV71) or a bivalent vaccine (vEV71+vCVA16 and iEV71+iCVA16) (Fig. 5C). Sera of CVA16-immunized groups were also used to detect cross-neutralizing activities among the four genotypes by the CVA16 pseudovirus system (Fig. 5D).
Neutralization antibody titers were also detected using the CPE-based micro-neutralization assay to compare with results obtained by the PVA. The neutralization titer in the micro-neutralization assay is expressed as the reciprocal of the highest dilution where over 50% of the wells show a complete inhibition of CPE. Compared with the 50% inhibition ratio of the PVA, no significant difference was found (P > 0.05) (Fig. 6A and B).
Figure 6.
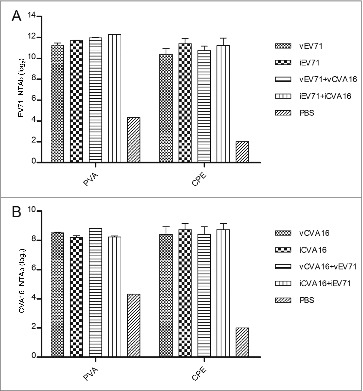
Comparison of sera neutralization titers using micro-neutralization assay (CPE) and PVA method for EV71 and CVA16 viruses. Diluted the 6th week sera samples (50 μL) and virus stock (50 μL) containing 100 TCID50 EV71 or CVA16 were mixed, and incubated in microplates with RDS cells at 37 °C for 4 d. The neutralizing antibody titer was defined as the highest dilution of sera that prevented the occurrence of CPE. (A) EV71 neutralization antibody, comparison CPE and PVA. (B) CVA16 neutralization antibody, comparison CPE and PVA.
Protection against lethal challenge with EV71 and CVA16 in suckling mice
In vivo protective efficacy against lethal challenge with EV71 and CVA16 was evaluated using the newborn mouse model.29,34 The immune sera obtained from inoculation with monovalent and bivalent vaccines to EV71 were heat-treated at 56°C for 30 min, serially diluted from 1:10 to 1:1,000 and then incubated with 14 LD50 of EV71 virus. Pups aged <24 h were injected i.c. with the sera-virus mixture. Control pups treated with sera from the PBS group showed clinical symptoms five days after inoculation and died at week 8. The pups inoculated with 1:10 and 1:100 diluted sera-virus mixtures of the monovalent and bivalent vaccine groups had 100% survival rates, while the pups inoculated with 1:1,000 diluted sera from both monovalent and bivalent EV71 vaccine groups all died (Fig. 7A and B). The CVA16 sera were processed in the same manner as the EV71 sera and were incubated with 50 LD50 of CVA16 virus. All of the control pups showed clinical symptoms after four days and died by day 7. Pups that were inoculated with the 1:10 or 1:100 diluted iCVA16 sera-virus mixture as well as with the 1:10 diluted vCVA16 sera-virus mixture had 100% survival rates. The survival rates of groups given iCVA16 at 1:1,000 dilution and vCVA16 at 1:100 and 1:1,000 dilutions were 80%, 80% and 40%, respectively (Fig. 7C). For sera of animals given bivalent vaccines, the CVA16 VLP immune sera diluted at 1:10 and 1:100 resulted in 100% survival of the pups, and the same results were found with the iCVA16 immune sera. Meanwhile, survival rates of pups administered 1:1,000 diluted sera from animals vaccinated with VLPs and formalin-inactivated virus were 60% and 80%, respectively (Fig. 7D).
Figure 7.
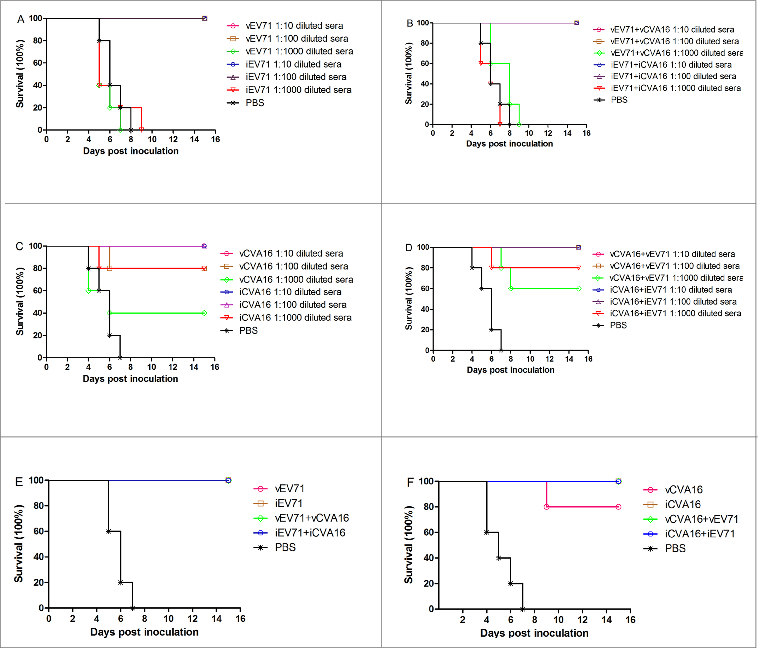
Passive protection against lethal EV71 and CVA16 challenge in newborn mice. 10-fold serially diluted antisera from mice immunized with monovalent, bivalent vaccines or PBS control group were incubated with an equal volume of 14 LD50 of EV71 or 50 LD50 of CVA16 at 37 °C for 1.5 h. One-day-old ICR mice (n = 6–9 per group were i.c. inoculated with the mixture described above. (A, B) Protection against lethal EV71 challenge by antisera from mice immunized with monovalent or bivalent EV71 vaccines. (C, D) Protection against lethal CVA16 challenge by antisera from mice immunized with monovalent and bivalent CVA16 vaccines. In addition, Pups (age < 24 h, n = 6–9 per group) were i.p. inoculated with 50 μL of sera from mice immunized with monovalent vaccines, bivalent vaccines or PBS. Within 2 h of inoculation, the pups were i.c. challenged with 14 LD50 of EV71 or 50 LD50 of CVA16. (E) Protection against lethal EV71 challenge by anti-EV71 sera; (F) protection against lethal CVA16 challenge by anti-CVA16 sera.
Another set of pups were used to test passive protection by the immune sera against EV71 and CVA16 challenge. In this experiment, the immune sera of animals given monovalent and bivalent EV71 vaccines were injected i.p. into neonatal mice, which were then challenged i.c. with 14 LD50 of EV71 within 2 h. The results (Fig. 7E) showed that the control mice (injected with sera from PBS control mice) died at day 7, while the mice injected with sera from vaccinated mice were all 100% survival rates. The CVA16 immune sera, processed in the same manner as the EV71 immune sera, could provide 100% protection against CVA16 challenge at a dose of 50 LD50, except for the sera from vCVA16-immunized mice, which yielded 80% protection (Fig. 7F). These results demonstrated that the sera induced by both the monovalent and bivalent vaccines could protect against EV71 and CVA16 infections in mice. Although the protection of sera derived from vaccination with vCVA16 was lower than that with iCVA16, the sera from animals receiving bivalent EV71 and CVA16 vaccines could confer full protection to the newborn mice against CVA16 challenge.
Discussion
The current lack of an effective drug to treat HFMD highlights the importance of developing vaccines to prevent transmission of the causative agents EV71 and CVA16. The focus of vaccine development in this field has been based mainly on inactivated virus and VLPs. Although three candidate inactivated EV71 vaccine(Mainland China) have been evaluated through a phase III clinical trial,21 CVA16 vaccine development has lagged further behind. Many research groups have reported that inactivated virus and VLP vaccines retain their antigenicity and immunogenicity.24,26,35 A formalin-inactivated EV71 vaccine was recently shown to induce either low or no cross-neutralization activity against CVA16 and vice versa.35 Therefore, a bivalent EV71 and CVA16 vaccine should be considered as a candidate for HFMD. Comparing VLPs with inactivated vaccine, the VLPs have the advantage of a higher safety profile than inactivated vaccine, since they do not contain the viral genome, while possessing the same morphological characteristics, protein composition, capsid conformation structure and epitopes present on the surface of the particles. In recent years, many VLP-based vaccines have been evaluated for many viruses, such as human immunodeficiency virus, Norwalk virus, JC virus and rotavirus,36 as well as influenza, HBV and human papillomavirus.37-39
In this study, we found that both monovalent and bivalent vaccines could induce specific antibodies as measured by ELISA (Fig. 4). The bivalent vaccines also were able to elicit IgG antibodies at levels similar to that induced by the monovalent vaccines. These results were consistent with those of a previous study.40 However, low cross-reactivity was observed between VLPs and formalin-inactivated vaccines, which could have resulted from the slightly different spatial structure of VLPs as compared with the authentic virus. Based on the crystal structure, a conformational change is triggered by receptor binding after virus attachment to the host cell to form the empty particles (E-particle) and native mature virus (F-particle).41 A recent cryo-EM report demonstrated that before the viruses successfully infect the host cell and release mature virus, the procapsid particle, an intermediate particle forms an expanded, modifiable structure.42 Furthermore, the subunit protein of the procapsid particle containing VP0, VP3 and VP1 is more like the VLPs produced from insect cells and the S. cerevisiae system. However, the fundamental reason for the low cross-reactivity of the induced IgG remains to be determined.
VP1 is the major antigen for human enteroviruses. Based on the evolution of VP1, EV71 is classified into three genotypes (genotype A; genotype B, B1 to B5; genotype C, C1 to C5),43-46 while CVA16 has two genotypes (genotype A; genotype B, B1a, B1b and B2).47-49 Detection of neutralization antibodies by the pseudovirus method previously has been described and evaluated by comparison with the traditional CPE method.50,51 The pseudovirus method used in our study could detect the neutralizing antibodies elicited by vaccination of ICR mice. The results proved that the titers of neutralizing antibodies between monovalent vaccine and bivalent vaccines have the similar response. It demonstrated that there are no immune interference exists between these two immunogens of EV71 and CVA16. Furthermore, no significant differences were observed when comparing results of the pseudovirus and CPE assays (Fig. 6A and B) (P > 0.05). The various vaccines could induce cross-neutralizing antibodies among 12 sub-genotypes of EV71 and 4 sub-genotypes of CVA16. Neutralizing antibodies to C4a were only slightly lower than those against other sub-genotypes of EV71, except B1, B2 and B3 (Fig. 5C). Epidemiological surveys in Mainland China confirmed that sub-genotypes C4a and C4b of EV71 have been predominant since 1998,52-54 the phase III clinical trial demonstrated that the neutralizing antibody titer of 1:32 could be used as a surrogate of protection against EV71-associated disease. Therefore, we predict that vaccination can prevent epidemics of the EV71 sub-genotypes above (Fig. 5C). Epidemiological studies have indicated B genotypes of CVA16 to be predominant.47,48 While neutralizing titers raised against CVA16 were lower than those against EV71, likely reflecting the nature of the antigens, nevertheless, our data suggested that cross-neutralization among CVA16 sub-genotypes can be elicited by the vaccines in this study (Fig. 5D). For assessing the cellular immune response is also highly important for determining the efficacy of a vaccine. Therefore, we will evaluate and compare cellular immune responses induced by monovalent and bivalent vaccines in the future.
In vivo protective efficacy demonstrated that with the sera dilution, the EV71 groups (monovalent and bivalent) have the similar protection against lethal doses of EV71 (Fig. 7A and B). And the CVA16 groups, the protection of monovalent inactivated vaccine groups proved high survival rates than VLPs groups (Fig. 7C), but the bivalent VLPs vaccines could provide a high protection than monovalent VLPs vaccine (Fig. 7D), this effect may due to the synergistic protection between EV71 and CVA16 vaccines; and the virus challenge in the CVA16 VLPs groups were conducted with heterologous strains of CVA16 virus. Meanwhile, the passive transfer of antisera to EV71 and CVA16 also proved our hypothesis (Fig. 7E and F). Though these two methods had proved that the bivalent vaccine could against the EV71 and CVA16 viruses challenge, in future studies, the maternal-neonatal protection should be evaluated.
In summary, the 10 μg VP1 protein/dose of monovalent or 20 μg VP1 protein/dose of bivalent vaccines against EV71 and CVA16 was selected to immunize ICR mice. The induction of neutralizing antibodies by these vaccines was evaluated, and their ability to confer passive protection to newborn mice against lethal doses of EV71 and CVA16 was determined. Both monovalent and bivalent vaccines (VLP or inactivated virus) elicited high levels of neutralizing antibodies, which could protect mice against lethal challenge with both viruses. Furthermore, the bivalent vaccines were shown to provide higher protective efficacy in the newborn mice than monovalent vaccines. We speculate that the EV71 and CVA16 antigens may provide synergistic protection to immunized mice. Thus, a potentially effective approach to interrupting viral transmission is to develop a vaccine containing both EV71 and CVA16 antigens. However, the lowest effective dose should be evaluated in mouse or rhesus monkey in the further studies.. As VLPs vaccines are deemed safer than inactivated virus vaccines, a VLP-based vaccine with bivalent EV71 and CVA16 antigens should be considered for HFMD vaccine development.
Materials and Methods
Cells and viruses
The RD-SCARB2 (RDS) cell line stably overexpressing hSCARB2 has been described previously.31 Briefly, CVA16 (JQ180468.1) and EV71C4 (KJ508817) strains were propagated in RDS cells and cultured with Dulbecco's modified Eagle's medium (DMEM, Sigma, St. Louis, MO) containing 10% FBS (Gibco), supplemented with puromycin (0.5 μg/mL; Clontech). The virus stocks were collected from the supernatants of infected RDS cells at 3–4 d post-infection (DPI). The virus titers were determined by a plaque assay. Anther, the P1 and 3CD gene sequences of CVA16 (AF177911.1) and EV71C4 (EU703814.1) were synthesized and inserted into the pFastBacTM Dual vector. Sf-9 insect (ATCC CRL-171) cells were cultured in shake flasks at 27 °C using SFX-insect medium (HyCyclone).
Purification of EV71 and CVA16 inactivated vaccines
RDS cells were infected with EV71 or CVA16. After 3–4 DPI, supernatants were harvested and subjected to three to four freeze-thaw cycles and purified by centrifugation at 3,000 × g for 20 min at 4°C. Viral particles were then concentrated by polyethylene glycol (PEG) precipitation (20% PEG 8000-PBS) overnight at 4°C. Finally, viral particles were concentrated by centrifugation at 8,000 × g for 40 min (R25.50 Rotor Beckman) at 4°C. The sediment was loaded on a 20–50% discontinuous sucrose gradient at 100,000 × g for 4.5 h (SW 28 Rotor Beckman). The virus was separated from the PEG by using a 10 kD filter with PBS buffer and then inactivated by formaldehyde at 37°C for 72 h.55 The inactivated viruses were analyzed by SDS-PAGE and were identified by western blotting.
Production and purification of EV71 and CVA16 VLPs
EV71 and CVA16 VLPs were produced from Sf-9 cells that were cultured in SFX-insect medium with the BAC-EV71 and BAC-CVA16 recombinant baculoviruses infection, respectively.26,28,30 Briefly, the Sf-9 cells were cultured in 1L shake flasks with 400 mL medium at a density of 2.5 × 106 cells/mL, then the recombinant baculoviruses infected cells at the multiplicity of infection (M.O.I.) of 1. After 4 d of infection, the cells were harvested by centrifugation at 1,000 × g for 20 min, (JA10 Rotor, Beckman), followed by two washes with 200 mL PBS buffer. The cells were suspended in PBS buffer and lysed by sonication for 5 min and then centrifuged at 20,000 × g for 30 min (R25.50 Rotor, Beckman). The sample was loaded on a 30% sucrose cushion and centrifuged at 100,000 × g for 3.5 h (SW28 Rotor, Beckman). The pellets were suspended in PBS buffer and loaded onto a discontinuous CsCl gradient 20–50% and ultracentrifuged at 100,000 × g for 24 h (SW40 Rotor, Beckman). The milky white bands were collected, and CsCl was removed by ultracentrifugation for 4 h using PBS buffer. The purified VLPs were analyzed by SDS-PAGE and were identified by western blotting.
Transmission electron microscopy (TEM)
The purified CVA16 and EV71 virions and VLPs were examined by TEM (JEM-1220; JEOL Datum Tokyo, Japan).
Immunization and sera sample collection
Female ICR mice (purchased from Changchun Institute of Biological Products) aged 6–8 wk were randomly divided into 7 groups (n = 5 per group). The concentration of VP1 was quantified using an Odyssey Imaging System. Each mouse was immunized with 10 μg of VP1 protein for the monovalent vaccines (vEV71, vCVA16, iEV71 and iCVA16) and 20 μg VP1 protein (10 μg for each) for the bivalent vaccines (vEV71+vCVA16, iEV71+iCVA16). PBS was used for the control group. The samples were adsorbed on a suitable amount of aluminum hydroxide adjuvant (Accurate Chemical and Scientific Corporation) at room temperature for 3 h. The vaccines were administrated intraperitoneally (i.p.) at week 0, 2 and 4. Sera samples were collected from the tail vein at week 2, 4, 6, 8 and 10. Blood samples were stand at 37°C for 1h before centrifugation at 3,000 × g, 4°C for 30 min. The sera were inactivated at 56°C for 30 min and stored at −20°C until use. University Committee on the Use and Care of Animals of Jilin University approved all animal studies.
Total specific IgG antibodies and cross-reactive IgG antibodies
Levels of total anti-EV71 and anti-CVA16 IgG in sera samples from mice immunized with the monovalent and bivalent vaccines were measured by ELISA using vEV71, iEV71, vCVA16 and iCVA16 proteins in carbonate buffer (pH 9.6) as coating antigens. Briefly, each well of the 96-well plate was coated with a suitable amount of antigen and incubated at 4°C overnight. After blocking with PBS-3% BSA at 37°C for 2 h, the plates were washed with PBS-T (0.5% Tween-20) three times. Sera samples with PBS-0.3% BSA were added into the wells and incubated at 37°C for 2 h. Following the incubation, the plates were washed five times and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (H+L) with PBS-0.1% BSA for 1 h at 37°C and then washed five times. TMB substrate (100 μl) was added into each well and developed in the dark at room temperature for 15 min. The reaction was stopped by adding 100 μl of 2 M H2SO4, and the optical density was read at 450 nm.55 For titration of total IgG, the positive cut-off value was defined as 1.5 times the optical density of the normal mouse sera used as the negative control.
Based on the total IgG detection method, cross-reactive IgG antibodies were detected. By using the protein of vEV71 as a coating antigen, we detected the 6th week sera at dilution 1:1000, which immunized the monovalent and bivalent vaccines of EV71. Changing the coating antigen into iEV71, vCVA16 or iCVA16, we also detected the cross-reactive of the monovalent and bivalent vaccines of EV71 and CAV16.
IgG subtype assay
IgG subtype was tested by ELISA with the vEV71, iEV71, vCVA16 and iCVA16 as the coating proteins as described above. After the incubation of sera samples, capture antibodies for IgG1, IgG2b, IgG2a and IgG3 (Sigma) were added and incubated for 30 min. After three washes, the substrate buffer was added and incubated in the dark for 10 min at room temperature. The reaction was stopped by 1 M NaOH, and the optical density was read at 405 nm.55
Neutralization assay
Neutralization antibodies were detected by the PVA method as described previously.50 The EV71 C4a and CVA16 B1a pseudoviruses were used to detect neutralization antibody titers in sera samples at week 2, 4, 6, 8 and 10. Sera at the 6th week were selected to detect the potential induction of cross-neutralization antibodies using pseudoviruses of 12 sub-genotypes of EV71 and 4 sub-genotypes of CVA16 as listed in Table 1. The ability of the PVA to detect neutralization antibodies was compared with that of the traditional cytopathic effect (CPE) method by treating and assaying the sera at the 6th week against EV71 C4 virus and CVA16 B1a virus.
Passive protection assays
Neonatal mice have been utilized as animal models for EV71 and CVA16 infection.24,26,34,56,57 The virus titer of EV71 strain (KJ508817) 4.5 × 105 PFU/ml and CVA16 strain (JQ180468.1) 6.6 × 106 PFU/ml were determined using a plaque assay on RDS cells. The newborn mice (age < 24 h) were selected (n = 10–20 per group) and intracerebrally (i.c.) inoculated with 4-fold serial dilutions of EV71 strain (0.14 × 105, 0.28 × 105, 1.25 × 105, and 4.5 × 105 PFU/mouse) and CVA16 strain (2.25, 9, 3.6 × 10, 1.44 × 102, 5.76 × 102 and 2.3 × 103) respectively. The control mice were injected an uninfected cultured medium. Mice were observed daily for clinical illness and death until 15 d post-inoculation. The LD50 was calculated by Reed and Muench.58
For the passive protection assays the 14LD50 of EV71 strain and 50LD50 of CVA16 strain were selected and incubated in ICR/mice (one-day-age). Briefly, sera samples of mice immunized with monovalent vaccines, bivalent vaccines of EV71 or PBS were diluted from 10- to 1,000-fold and incubated with 14 LD50 of the EV71 strain (KJ508817) at 37°C for 90 min. The pups were challenged i.c. with the sera-virus mixture. Another pups of the CVA16 groups were challenged i.c. with 50 LD50 of CVA16 (JQ180468.1) with the sera-virus mixture. All mice were monitored daily within 15 d after challenge.
To further evaluate the protective efficacy of sera antibodies raised by vaccination, newborn mice (one-day-old) were inoculated i.p. with sera from groups inoculated with the monovalent vaccines, bivalent vaccine of EV71 or PBS. At 2 h after the inoculation, the pups were challenged i.c. with 14 LD50 of EV71. The groups of CVA16 were processed in the same manner as the EV71. All mice were monitored daily within 15 d after challenge.
Statistical analysis
All results were obtained with at least three replicates and expressed as the mean ± standard deviation (SD). All statistical analyses were performed with the GraphPad Prism software package. Groups were compared by using Student`s t test, and P values <0.05 were considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Putnak JR, Phillips BA. Picornaviral structure and assembly. Microbiol Rev 1981; 45:287-315; PMID:7022155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimoto T, Chikahira M, Yoshida S, Ebira H, Hasegawa A, Totsuka A, Nishio O. Outbreak of central nervous system disease associated with hand, foot, and mouth disease in Japan during the summer of 2000: detection and molecular epidemiology of enterovirus 71. Microbiol Immunol 2002; 46:621-7; PMID:12437029; http://dx.doi.org/ 10.1111/j.1348-0421.2002.tb02743.x [DOI] [PubMed] [Google Scholar]
- 3.Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF, Wang JR, Shih SR; Taiwan Enterovirus Epidemic Working Group An epidemic of enterovirus 71 infection in Taiwan. N Engl J Med 1999; 341:929-35; PMID:10498487; http://dx.doi.org/ 10.1056/NEJM199909233411301 [DOI] [PubMed] [Google Scholar]
- 4.Bendig JW, Fleming DM. Epidemiological, virological, and clinical features of an epidemic of hand, foot, and mouth disease in England and Wales. Commun Dis Rep CDR Rev 1996; 6:R81-6; PMID:8664928 [PubMed] [Google Scholar]
- 5.Gilbert GL, Dickson KE, Waters MJ, Kennett ML, Land SA, Sneddon M. Outbreak of enterovirus 71 infection in Victoria, Australia, with a high incidence of neurologic involvement. Pediatr Infect Dis J 1988; 7:484-8; PMID:2841639; http://dx.doi.org/ 10.1097/00006454-198807000-00007 [DOI] [PubMed] [Google Scholar]
- 6.Shekhar K, Lye MS, Norlijah O, Ong F, Looi LM, Khuzaiah R, Marzuki I, Hussein I, Wong SL, Mohan J, et al. . Deaths in children during an outbreak of hand, foot and mouth disease in Peninsular Malaysia–clinical and pathological characteristics. Med J Malaysia 2005; 60:297-304; PMID:16379183 [PubMed] [Google Scholar]
- 7.Zhao SW, Yin WD, Cun JP, Gao Q, Yin J, Liu GJ, Xu W. [Identification and characterization of enterovirus 71 isolated in Yunnan province]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2013; 27:263-5; PMID:24579470 [PubMed] [Google Scholar]
- 8.Zhang Q, MacDonald NE, Smith JC, Cai K, Yu H, Li H, Lei C. Severe enterovirus type 71 nervous system infections in children in the Shanghai region of China: clinical manifestations and implications for prevention and care. Pediatr Infect Dis J 2014; 33:482-7; PMID:24732390; http://dx.doi.org/ 10.1097/INF.0000000000000194 [DOI] [PubMed] [Google Scholar]
- 9.WHO WPRO Hand, Foot and Mouth Disease Situation Update, 08 January 2013. http://www.wpro.who.int/emerging_diseases/HFMD.Report.8Jan2013.pdf; Accessed 2013 Sptember 23. [Google Scholar]
- 10.McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev 2002; 26:91-107; PMID:12007645; http://dx.doi.org/ 10.1111/j.1574-6976.2002.tb00601.x [DOI] [PubMed] [Google Scholar]
- 11.Iwai M, Masaki A, Hasegawa S, Obara M, Horimoto E, Nakamura K, Tanaka Y, Endo K, Tanaka K, Ueda J, et al. . Genetic changes of coxsackievirus A16 and enterovirus 71 isolated from hand, foot, and mouth disease patients in Toyama, Japan between 1981 and 2007. Jpn J Infect Dis 2009; 62:254-9; PMID:19628900 [PubMed] [Google Scholar]
- 12.Li L, He Y, Yang H, Zhu J, Xu X, Dong J, Zhu Y, Jin Q. Genetic characteristics of human enterovirus 71 and coxsackievirus A16 circulating from 1999 to 2004 in Shenzhen, People's Republic of China. J Clin Microbiol 2005; 43:3835-9; PMID:16081920; http://dx.doi.org/ 10.1128/JCM.43.8.3835-3839.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ang LW, Koh BK, Chan KP, Chua LT, James L, Goh KT. Epidemiology and control of hand, foot and mouth disease in Singapore, 2001-2007. Ann Acad Med Singapore 2009; 38:106-12; PMID:19271036 [PubMed] [Google Scholar]
- 14.Wang CY, Li Lu F, Wu MH, Lee CY, Huang LM. Fatal coxsackievirus A16 infection. Pediatr Infect Dis J 2004; 23:275-6; PMID:15014311; http://dx.doi.org/ 10.1097/01.inf.0000115950.63906.78 [DOI] [PubMed] [Google Scholar]
- 15.Yu DS, Zhang Y, Chen JH, Duan LP, Zhao XH, Li XL, Sun Q, Chen X, Liu JF, Zheng YH, et al. . [Epidemiological features and pathogenic characteristics of hand, foot and mouth disease in Gansu Province, China during 2008-2012]. Bing Du Xue Bao 2014; 30:25-32; PMID:24772894 [PubMed] [Google Scholar]
- 16.Goto K, Sanefuji M, Kusuhara K, Nishimura Y, Shimizu H, Kira R, Torisu H, Hara T. Rhombencephalitis and coxsackievirus A16. Emerg Infect Dis 2009; 15:1689-91; PMID:19861078; http://dx.doi.org/ 10.3201/eid1510.090594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu W, Liu CF, Yan L, Li JJ, Wang LJ, Qi Y, Cheng RB, Xiong XY. Distribution of enteroviruses in hospitalized children with hand, foot and mouth disease and relationship between pathogens and nervous system complications. Virol J 2012; 9:8; PMID:22230340; http://dx.doi.org/ 10.1186/1743-422X-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Z. Severe hand, foot, and muoth disease caused by mixed infection of enterovirus 71 and coxsackie A16: report of 6 cases. Chin Gen 2011; Practice 14(10):3341-3346. [Google Scholar]
- 19.Yang F, Zhang T, Hu Y, Wang X, Du J, Li Y, Sun S, Sun X, Li Z, Jin Q. Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virol J 2011; 8:508; PMID:22054534; http://dx.doi.org/ 10.1186/1743-422X-8-508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, Wu S, Xiong Y, Li T, Wen Z, Yan M, Qin K, Liu Y, Wu J. Co-circulation and genomic recombination of coxsackievirus A16 and enterovirus 71 during a large outbreak of hand, foot, and mouth disease in Central China. PLoS One 2014; 9:e96051; PMID:24776922; http://dx.doi.org/ 10.1371/journal.pone.0096051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li JX, Mao QY, Liang ZL, Ji H, Zhu FC. Development of enterovirus 71 vaccines: from the lab bench to Phase III clinical trials. Expert Rev Vaccines 2014; 13:609-18; PMID:24621093; http://dx.doi.org/ 10.1586/14760584.2014.897617 [DOI] [PubMed] [Google Scholar]
- 22.Liang Z, Mao Q, Gao F, Wang J. Progress on the research and development of human enterovirus 71 (EV71) vaccines. Front Med 2013; 7:111-21; PMID:23247645; http://dx.doi.org/ 10.1007/s11684-012-0237-z [DOI] [PubMed] [Google Scholar]
- 23.Cai Y, Liu Q, Huang X, Li D, Ku Z, Zhang Y, Huang Z. Active immunization with a Coxsackievirus A16 experimental inactivated vaccine induces neutralizing antibodies and protects mice against lethal infection. Vaccine 2013; 31:2215-21; PMID:23499596; http://dx.doi.org/ 10.1016/j.vaccine.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 24.Mao Q, Wang Y, Gao R, Shao J, Yao X, Lang S, Wang C, Mao P, Liang Z, Wang J. A neonatal mouse model of coxsackievirus A16 for vaccine evaluation. J Virol 2012; 86:11967-76; PMID:22951825; http://dx.doi.org/ 10.1128/JVI.00902-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang E, Cheng C, Zhang Y, Wang J, Che Y, Pu J, Dong C, Liu L, He Z, Lu S, et al. . Comparative study of the immunogenicity in mice and monkeys of an inactivated CA16 vaccine made from a human diploid cell line. Hum Vaccin Immunother 2014; 10:10; PMID:24583556; http://dx.doi.org/ 10.4161/hv.28083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q, Yan K, Feng Y, Huang X, Ku Z, Cai Y, Liu F, Shi J, Huang Z. A virus-like particle vaccine for coxsackievirus A16 potently elicits neutralizing antibodies that protect mice against lethal challenge. Vaccine 2012; 30:6642-8; PMID:22959985; http://dx.doi.org/ 10.1016/j.vaccine.2012.08.071 [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Li HY, Han JF, Deng YQ, Li YX, Zhu SY, He YL, Qin ED, Chen R, Qin CF. Virus-like particles produced in Saccharomyces cerevisiae elicit protective immunity against Coxsackievirus A16 in mice. Appl Microbiol Biotechnol 2013; 97:10445-52; PMID:24085395; http://dx.doi.org/ 10.1007/s00253-013-5257-3 [DOI] [PubMed] [Google Scholar]
- 28.Chung YC, Ho MS, Wu JC, Chen WJ, Huang JH, Chou ST, Hu YC. Immunization with virus-like particles of enterovirus 71 elicits potent immune responses and protects mice against lethal challenge. Vaccine 2008; 26:1855-62; PMID:18329759; http://dx.doi.org/ 10.1016/j.vaccine.2008.01.058 [DOI] [PubMed] [Google Scholar]
- 29.Li HY, Han JF, Qin CF, Chen R. Virus-like particles for enterovirus 71 produced from Saccharomyces cerevisiae potently elicits protective immune responses in mice. Vaccine 2013; 31:3281-7; PMID:23726823; http://dx.doi.org/ 10.1016/j.vaccine.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 30.Chung YC, Huang JH, Lai CW, Sheng HC, Shih SR, Ho MS, Hu YC. Expression, purification and characterization of enterovirus-71 virus-like particles. World J Gastroenterol 2006; 12:921-7; PMID:16521221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Fan P, Jin J, Su W, An D, Xu L, Sun S, Zhang Y, Meng X, Gao F, et al. . Establishment of cell lines with increased susceptibility to EV71/CA16 by stable overexpression of SCARB2. Virol J 2013; 10:250; PMID:23919614; http://dx.doi.org/ 10.1186/1743-422X-10-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curry S, Fry E, Blakemore W, Abu-Ghazaleh R, Jackson T, King A, Lea S, Newman J, Stuart D. Dissecting the roles of VP0 cleavage and RNA packaging in picornavirus capsid stabilization: the structure of empty capsids of foot-and-mouth disease virus. J Virol 1997; 71:9743-52; PMID:9371640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuta K, Aoki Y, Suto A, Ootani K, Katsushima N, Itagaki T, Ohmi A, Okamoto M, Nishimura H, Matsuzaki Y, et al. . Cross-antigenicity among EV71 strains from different genogroups isolated in Yamagata, Japan, between 1990 and 2007. Vaccine 2009; 27:3153-8; PMID:19446185; http://dx.doi.org/ 10.1016/j.vaccine.2009.03.060 [DOI] [PubMed] [Google Scholar]
- 34.Bek EJ, Hussain KM, Phuektes P, Kok CC, Gao Q, Cai F, Gao Z, McMinn PC. Formalin-inactivated vaccine provokes cross-protective immunity in a mouse model of human enterovirus 71 infection. Vaccine 2011; 29:4829-38; PMID:21550375; http://dx.doi.org/ 10.1016/j.vaccine.2011.04.070 [DOI] [PubMed] [Google Scholar]
- 35.Chong P, Guo MS, Lin FH, Hsiao KN, Weng SY, Chou AH, Wang JR, Hsieh SY, Su IJ, Liu CC. Immunological and biochemical characterization of coxsackie virus A16 viral particles. PLoS One 2012; 7:e49973; PMID:23226233; http://dx.doi.org/ 10.1371/journal.pone.0049973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol 2003; 108:241-7; PMID:14499247; http://dx.doi.org/ 10.1016/S1521-6616(03)00120-7 [DOI] [PubMed] [Google Scholar]
- 37.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, et al.; HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161-70; PMID:17602732; http://dx.doi.org/ 10.1016/S0140-6736(07)60946-5 [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Ma Y, Wang H, Liu Q. Quantitative evaluation of the effect of the hepatitis B vaccine based on the HBsAg- and anti-HBs-positive rates in the Chinese population over the last 33 years. Vaccine 2012; 30:3483-7; PMID:22433962; http://dx.doi.org/ 10.1016/j.vaccine.2012.02.053 [DOI] [PubMed] [Google Scholar]
- 39. http://www.clinicaltrials.gov (Identifier NCT01897701). [Google Scholar]
- 40.Cai Y, Ku Z, Liu Q, Leng Q, Huang Z. A combination vaccine comprising of inactivated enterovirus 71 and coxsackievirus A16 elicits balanced protective immunity against both viruses. Vaccine 2014; 32:2406-12; PMID:24657161; http://dx.doi.org/ 10.1016/j.vaccine.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Peng W, Ren J, Hu Z, Xu J, Lou Z, Li X, Yin W, Shen X, Porta C, et al. . A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat Struct Mol Biol 2012; 19:424-9; PMID:22388738; http://dx.doi.org/ 10.1038/nsmb.2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shingler KL, Yoder JL, Carnegie MS, Ashley RE, Makhov AM, Conway JF, Hafenstein S. The enterovirus 71 A-particle forms a gateway to allow genome release: a cryoEM study of picornavirus uncoating. PLoS Pathog 2013; 9:e1003240; PMID:23555253; http://dx.doi.org/ 10.1371/journal.ppat.1003240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt NJ, Lennette EH, Ho HH. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis 1974; 129:304-9; PMID:4361245; http://dx.doi.org/ 10.1093/infdis/129.3.304 [DOI] [PubMed] [Google Scholar]
- 44.Brown BA, Oberste MS, Alexander JP, Jr., Kennett ML, Pallansch MA. Molecular epidemiology and evolution of enterovirus 71 strains isolated from 1970 to 1998. J Virol 1999; 73:9969-75; PMID:10559310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang YP, Lin TL, Kuo CY, Lin MW, Yao CY, Liao HW, Hsu LC, Yang CF, Yang JY, Chen PJ, et al. . The circulation of subgenogroups B5 and C5 of enterovirus 71 in Taiwan from 2006 to 2007. Virus Res 2008; 137:206-12; PMID:18706461; http://dx.doi.org/ 10.1016/j.virusres.2008.07.015 [DOI] [PubMed] [Google Scholar]
- 46.Shimizu H, Utama A, Onnimala N, Li C, Li-Bi Z, Yu-Jie M, Pongsuwanna Y, Miyamura T. Molecular epidemiology of enterovirus 71 infection in the Western Pacific Region. Pediatr Int 2004; 46:231-5; PMID:15056257; http://dx.doi.org/ 10.1046/j.1442-200x.2004.01868.x [DOI] [PubMed] [Google Scholar]
- 47.Urquhart GE. A survey of coxsackie A16 virus antibodies in human sera. J Hyg (Lond) 1984; 93:205-12; PMID:6094661; http://dx.doi.org/ 10.1017/S002217240006472X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Wang D, Yan D, Zhu S, Liu J, Wang H, Zhao S, Yu D, Nan L, An J, et al. . Molecular evidence of persistent epidemic and evolution of subgenotype B1 coxsackievirus A16-associated hand, foot, and mouth disease in China. J Clin Microbiol 2010; 48:619-22; PMID:20018819; http://dx.doi.org/ 10.1128/JCM.02338-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei W, Guo H, Li J, Ren S, Wei Z, Bao W, Hu X, Zhao K, Zhang W, Zhou Y, et al. . Circulating HFMD-associated coxsackievirus A16 is genetically and phenotypically distinct from the prototype CV-A16. PLoS One 2014; 9:e94746; PMID:24736564; http://dx.doi.org/ 10.1371/journal.pone.0094746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin J, Xu L, Guo S, Sun S, Zhang S, Zhu C, Kong W, Jiang C. Safe and Objective Assay of Enterovirus 71 Neutraliziong Antibodies Via Pseudovirus. CHEM RES CHINESE UNIVERSITIES 28, 91-95 2012. [Google Scholar]
- 51.Wu X, Mao Q, Yao X, Chen P, Chen X, Shao J, Gao F, Yu X, Zhu F, Li R, et al. . Development and evaluation of a pseudovirus-luciferase assay for rapid and quantitative detection of neutralizing antibodies against enterovirus 71. PLoS One 2013; 8:e64116; PMID:23755115; http://dx.doi.org/ 10.1371/journal.pone.0064116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Tan XJ, Wang HY, Yan DM, Zhu SL, Wang DY, Ji F, Wang XJ, Gao YJ, Chen L, et al. . An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J Clin Virol 2009; 44:262-7; PMID:19269888; http://dx.doi.org/ 10.1016/j.jcv.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 53.Yang F, Ren L, Xiong Z, Li J, Xiao Y, Zhao R, He Y, Bu G, Zhou S, Wang J, et al. . Enterovirus 71 outbreak in the People's Republic of China in 2008. J Clin Microbiol 2009; 47:2351-2; PMID:19439545; http://dx.doi.org/ 10.1128/JCM.00563-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan X, Huang X, Zhu S, Chen H, Yu Q, Wang H, Huo X, Zhou J, Wu Y, Yan D, et al. . The persistent circulation of enterovirus 71 in People's Republic of China: causing emerging nationwide epidemics since 2008. PLoS One 2011; 6:e25662; PMID:21980521; http://dx.doi.org/ 10.1371/journal.pone.0025662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martín J, Crossland G, Wood DJ, Minor PD. Characterization of formaldehyde-inactivated poliovirus preparations made from live-attenuated strains. J Gen Virol 2003; 84:1781-8; PMID:12810872; http://dx.doi.org/ 10.1099/vir.0.19088-0 [DOI] [PubMed] [Google Scholar]
- 56.Ong KC, Devi S, Cardosa MJ, Wong KT. Formaldehyde-inactivated whole-virus vaccine protects a murine model of enterovirus 71 encephalomyelitis against disease. J Virol 2010; 84:661-5; PMID:19864378; http://dx.doi.org/ 10.1128/JVI.00999-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong C, Liu L, Zhao H, Wang J, Liao Y, Zhang X, Na R, Liang Y, Wang L, Li Q. Immunoprotection elicited by an enterovirus type 71 experimental inactivated vaccine in mice and rhesus monkeys. Vaccine 2011; 29:6269-75; PMID:21722686; http://dx.doi.org/ 10.1016/j.vaccine.2011.06.044 [DOI] [PubMed] [Google Scholar]
- 58.Krah DL. A simplified multiwell plate assay for the measurement of hepatitis A virus infectivity. Biologicals 1991; 19:223-7; PMID:1659431; http://dx.doi.org/ 10.1016/1045-1056(91)90039-M [DOI] [PubMed] [Google Scholar]


