Abstract
Objective: The aim of the study was to estimate long-term cost‑effectiveness of a hepatitis B vaccination catch-up program among children born between 1994 and 2001 (when they were 8‑15 y old) in Shandong province, China, to provide information for nationwide evaluation and future policy making.
Methods: We determined the cost-effectiveness of the catch-up program compared with the status quo (no catch-up program). We combined a Decision Tree model and a Markov model to simulate vaccination and clinical progression after hepatitis B virus (HBV) infection. Parameters in the models were from the literature, a field survey, program files, and the National Notifiable Disease Reporting System (NNDRS). The incremental cost‑effectiveness ratio (ICER) was used to compare the 2 alternative strategies. One-way sensitivity analysis, 2-way sensitivity analysis, and probability sensitivity analysis were used to assess parameter uncertainties.
Results: The catch-up program was dominant compared with the status quo. Using a total of 5.53 million doses of vaccines, the catch-up program could prevent 21,865 cases of symptomatic acute hepatitis B, 3,088 carrier states with positive hepatitis B surface antigen (HBsAg), and 812 deaths due to HBV infection. The catch-up program could add 28,888 quality-adjusted life years (QALYs) and save $192.01 million in the targeted population in the future. The models were robust, considering parameter uncertainties.
Conclusion: The catch-up program in Shandong province among children born between 1994 and 2001 was ‘very cost-saving.’ It could save life years and reduce total future costs. Our study supported the desirability and impact of such a catch-up program throughout China.
Keywords: catch-up program, Cost-effectiveness Analysis, hepatitis B virus, ICER, QALY, vaccination
Abbreviations
- HBV
Hepatitis B Virus
- HBsAg
Hepatitis B Surface Antigen
- GAVI
Global Alliance on Vaccines and Immunization
- HepB3
3-dose Coverage of Hepatitis B Vaccine
- MOH
Ministry of Health
- NNDRS
National Notifiable Diseases Reporting System
- CHB
Chronic Hepatitis B
- CC
Compensated Cirrhosis
- DC
Decompensated Cirrhosis
- HCC
Hepatocellular Carcinoma
- LT-1
the Year of Liver Transplantation
- LT-2
Years after Liver Transplantation
- QALYs
Quality-Adjusted Life Years
- HRQoL
Health-Related Quality of Life
- Anti-HBs
Antibody to Hepatitis B Surface Antigen
- ICER
Incremental Cost-Effectiveness Ratio
- GDP
Gross Domestic Product
- BCR
Benefit-Cost Ratio
China has the greatest burden of hepatitis B virus (HBV) disease and liver cancer in the world.1 Data from the 2006 national hepatitis B sero-epidemiologic survey showed that about 7.18% (93 million people) of China's population carried hepatitis B surface antigen (HBsAg).2
China added hepatitis B vaccine into its national immunization program in 2002, allowing the vaccine to be provided free to all newborns, nationwide. With support of the Global Alliance on Vaccines and Immunization (GAVI), hepatitis B vaccination in China's western region and economically weaker areas increased rapidly.3,4 According to China's Surveillance System of Information in the National Immunization Program, reported 3-dose coverage of hepatitis B vaccine (HepB3) exceeded 90% after 2003, compared with less than 60% before 1998 and 20% in 1994. These data indicated that a large proportion of children born between 1994 and 2001 were not vaccinated with hepatitis B vaccine and remained at risk of HBV infection.
In order to increase HepB3 coverage among children born between 1994 and 2001 (then aged 8‑15 years), the Ministry of Health (MOH) initiated a nationwide hepatitis B vaccine catch-up program for these children that lasted from 2009 to 2011. During the catch-up program, approximately 68 million children who had not been fully vaccinated were caught up on the doses of hepatitis B vaccine that they needed, based on their vaccination history.
Traditionally, catch-up vaccination for unvaccinated children has not been included in the global strategy to control hepatitis B. Therefore, very few studies are available on such a catch-up strategy, especially studies on a national scale using data from completed campaigns.5 There was a study of this catch-up strategy based on a hypothesized situation, and this study supported such a strategy for China.6 Considering the rapidly changing and complicated economic situation and society in China, including the downward trend of HBsAg prevalence, more precise evaluations and analyses are needed for future decision-making. In our study we focused on the economic benefits of a completed catch-up program using original data collection. We selected Shandong province for the study because it has a vigorous economy and a good research infrastructure. Cost‑effectiveness information on the catch-up program in Shandong province will provide information for more comprehensive evaluations nationwide.
Methods
In our cost-effective analysis,7 we used the Shandong catch-up program and the status quo (i.e., no catch-up program) as 2 alternative strategies for comparison. All children born between 1994 and 2002 without full hepatitis B immunization took part in the catch-up program. Children with local residency and without previous vaccination, children with one previous dose, and children with 2 previous doses would receive 3 doses, 2 doses and one dose of hepatitis B vaccine, respectively. We categorized the children into 8 cohorts based on their birth year, and each cohort consisted of 3 groups. Immunization histories were confirmed by vaccination certificates, without serological tests. Decision Tree and Markov models were used in the analysis.
Data and resources
Data in our study were from a field survey, program files, the National Notifiable Diseases Reporting System (NNDRS), and published literature. After completion of the catch-up program, the government of Shandong province summarized the program in several files. These files included descriptions of the age cohorts and population vaccinated, program costs, and program achievements. In order to improve the accuracy and reliability of the model parameters, we gave priority preference to the field survey, the program files, and to NNDRS. If data were not available from these sources, we performed a literature review of studies in both Chinese and English. Studies set in Shandong province or with a similar population in China, or neighboring districts with a relatively large sample size were preferred. If we found that there was limited published literature, we chose the most appropriate study to be the point estimation for each parameter, and other studies were used to form confidence intervals for sensitivity analysis.
Model construction
A Decision Tree model and a Markov model were combined to simulate the catch-up strategy and the clinical consequences in the target population of HBV infection (Fig. 1). The Decision Tree model was constructed using data from the program files, while the Markov model was based on published literature.1,7-9 The Markov model consisted of 10 infectious conditions: immune, susceptible, carriers, chronic hepatitis B (CHB), compensated cirrhosis (CC), decompensated cirrhosis (DC), hepatocellular carcinoma (HCC), the year of liver transplantation (LT-1), years after liver transplantation (LT-2), and death. We distinguished between carriers and CHB patients using standards from the Chinese Medical Association.10 Since we measured health and economic outcomes for all children over their lifetimes, the Markov model cycled in yearly increments for 100 y. The construction of models was accomplished in TreeAge Pro 2012.
Figure 1.
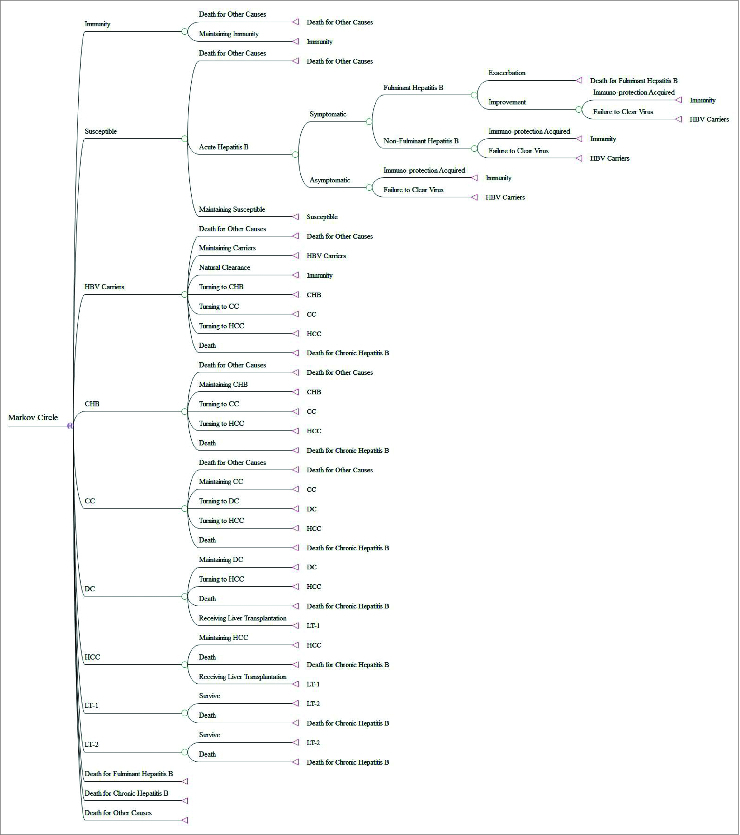
The Markov Model.
The Markov model was based on several assumptions11,12: (1) the targeted population would be protected from HBV infection if the serum anti-HBs was positive after administration of HBV vaccine; (2) the population with no response or a low response to HBV vaccine incurred costs, but were assumed to have no protection from HBV infection; and (3) since the vaccine was known to be safe, costs and loss of quality-adjusted life years (QALYs) due to adverse reaction were neglected.
Parameters
The parameters used in the model were categorized into 4 groups: (1) transition probabilities for disease progression; (2) costs of the catch-up program and of treatment of patients with HBV infection; (3) health-related quality of life (HRQoL) of patients with HBV infection; (4) other parameters (Table 1).
Table 1.
Point and Interval Estimation of Parameters in the Markov Model
| Parameters | Point Estimation (%) | Interval Estimation (%) | Ref. | |
|---|---|---|---|---|
| Transition Probabilities in Disease Progress | ||||
| Proportion of Symptomatic Acute Hepatitis B in total Acute Hepatitis B | 30.00 | — | 11 | |
| Proportion of Fulminant Hepatitis B in Symptomatic Acute Hepatitis B | Age<20 | 0.10 | 0.10–0.60 | 8, 35 |
| Age≥20 | 0.50 | 0.10–0.60 | ||
| Mortality of Fulminant Hepatitis B | Age<15 | 63.00 | — | 8, 35 |
| Age<45 | 80.00 | |||
| Age≥45 | 92.00 | |||
| Proportion of Fulminant Hepatitis B becaming Carriers | 6.25 | — | 22 | |
| Proportion of Acute Hepatitis B became Carriers | — | 23 | ||
| Natural Clearance of HBV Carriers | 1.80 | 1.47–1.80 | 24, 25 | |
| Probability of HBV Carriers became CHB | 0.38 | 0.38–3.00 | 24, 25 | |
| Probability of HBV Carriers became CC | 0.25 | — | 26 | |
| Probability of HBV Carriers became HCC | 0.36 | — | 27 | |
| Mortality of HBV Carriers due to HBV Infection | 0.73 | — | 25 | |
| Natural Clearance of CHB | 1.20 | — | 28 | |
| Probability of CHB became CC | 1.16 | 0.84–3.25 | 29 | |
| Probability of CHB became HCC | 0.68 | 0.45–0.68 | 30, 31 | |
| Mortality of CHB due to HBV Infection | 0.77 | 0.77–1.58 | 32, 33 | |
| Probability of CC became DC | 3.56 | 2.51–4.98 | 33 | |
| Probability of CC became HCC | 0.28 | 0.06–3.25 | 32, 33 | |
| Mortality of CC due to HBV Infection | 3.52 | 2.43–4.87 | 32, 33 | |
| Probability of DC became HCC | 0.82 | 0.34–3.25 | 32, 33 | |
| Mortality of DC due to HBV Infection | 15.96 | 15.96–39.32 | 32, 34 | |
| Probability of DC receiving Liver Transplantation | 0.15 | 0.00–0.40 | 35 | |
| Mortality of HCC Due to HBV Infection | 80.35 | 48.52–80.35 | 34, 36 | |
| Probability of HCC Receiving Liver Transplantation | 0.08 | 0.08–0.10 | 35 | |
| Mortality of LT-1 | 22.42 | 22.42–28.32 | 37 | |
| Mortality of LT-2 | 13.78 | 13.78–13.92 | 37 | |
| Costs of Treatments After HBV Infection (Per Year Per Person) | ||||
| HBV Carriers | 110.23 | — | 38 | |
| Symptomatic Acute HBV Infection | 2632.15 | 2632.15–5285.22 | 38–40 | |
| Fulminant Hepatitis B | 11163.49 | — | 39 | |
| CHB | 3051.69 | 730.52–3051.69 | 38–40 | |
| CC | 4699.74 | 2224.89–4699.74 | 38, 39 | |
| DC | 7176.04 | 4528.10–7176.04 | 38, 39 | |
| HCC | 10568.54 | 6493.13–10568.54 | 38–40 | |
| LT-1 | 38659.00 | — | 41 | |
| LT-2 | 3381.46 | — | 41 | |
| Costs of the Catch-up Program (Per Dose)* | ||||
| Vaccines and Single-Use Syringe | 0.34 | — | Program files | |
| Surveillance | 0.29 | — | 12 | |
| Staff Remuneration, Training, Supervision, Administration | 0.18 | Program files | ||
| Transportation | 0.23 | — | 12 | |
| Publicity | 0.12 | — | 12 | |
| Cold Chain | 0.09 | — | 12 | |
| Other Equipment | 0.13 | — | 12 | |
| HRQoL of the Targeted Population | ||||
| General Population | 0.848 | 17 | ||
| HBV Carriers | 0.813 | — | 15 | |
| Symptomatic Acute Hepatitis B | 0.739 | — | 19 | |
| CHB | 0.789 | — | 15 | |
| Liver Transplantation | 0.766 | — | 42 | |
| CC | 0.763 | — | 15 | |
| HCC | 0.699 | — | 15 | |
| DC | 0.661 | — | 15 | |
| Other Parameters | ||||
| Prevalence Proportion with Previous Vaccination | 4.30 | 2.69–5.91 | 20 | |
| Prevalence Proportion without Previous Vaccination | 4.95 | 1.27–8.63 | 20 | |
| Natural Immunization Proportion | 36.75 | 31.26–43.24 | 20 | |
| Anti-HBs Positive Conversion Rate with 1 Dose | 14.50 | — | 43 | |
| Anti-HBs Positive Conversion Rate with 2 Doses | 81.00 | 79.5–82.9 | 43, 44 | |
| Anti-HBs Positive Conversion Rate with 3 Doses | 98.10 | — | 45 | |
| Proportion of HBV Susceptible Persons with 0 Previous Dose | 58.30 | 31.51–67.47 | — | |
| Proportion of HBV Susceptible Persons with 1 Previous Doses | 50.40 | 43.48–56.47 | — | |
| Proportion of HBV Susceptible Persons with 2 Previous Doses | 11.20 | 9.66-12.55 | — | |
| Proportion of HBV Susceptible Persons with 3 Previous Doses | 1.12 | 9.66–12.55 | — | |
| All-Cause Mortality | — | — | 46 | |
| Discount Rate | 0.03 | 0–0.07 | 6 | |
| The proportion of HBV Susceptible Persons Becoming HBV Infectious Each Year | 0.000756 | 0.00020–0.00500 | NNDRS | |
CC: Compensated cirrhosis; CHB: Chronic hepatitis B; DC: Decompensated cirrhosis; HBs: Hepatitis B surface; HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma; HRQoL: Health-Related Quality of Life; LT-1: The year of liver transplantation; LT-2: Years after liver transplantation; NNDRS: National Notifiable Disease Reporting System.
Transition Probabilities of Disease Progression: Susceptible persons were at risk of HBV infection. The probability of becoming a carrier after infection varied with age at infection. DC and HCC were the major causes of death for patients with HBV; however, some patients could recuperate following liver transplantation.11,12 Parameters for this group were primarily obtained from the published literature (Table 1).
Cost Parameters: We used the societal perspective to calculate costs. Costs of the catch-up program consisted of the implementation costs of the program and of the treatment costs for patients with HBV infection. Costs of the status quo strategy group included only the treatment costs.
Costs of the catch-up program were subdivided into 10 parts (Table 1). Parameters from the program files were prioritized to be used in the model, including costs of vaccines and single-use syringes, staff remuneration, training, supervision, and administration. The costs of vaccines and single-use syringes were obtained from the central government; others were obtained from provincial, municipal, and county governments. If no data were available in files, we used data from a study13 of the costs of a national immunization program in regions of China, including Shandong province.
Treatment costs of patients with HBV infection were primarily obtained from published literature. Both outpatient and inpatient costs were included in the study in which only direct and indirect medical costs were used, whereas other costs such as loss in productivity and intangible costs were embodied in HRQoL.7 All costs were adjusted with respect to the cost of US dollar in 2013 according to the consumer price index and the exchange rate.14,15
Effectiveness Parameters: Effectiveness was quantified by QALY in the form of HRQoL. We performed a field survey using EQ-5D-5L, the 5-level version of EQ-5D to determine the HRQoL of the targeted population in Shandong province in August, 2013.16 We first confirmed the applicability of EQ-5D-5L in the targeted population. HRQoL of 5 HBV infectious conditions, including carriers, CHB, CC, DC, and HCC, were obtained from a field survey. HRQoL of patients with liver transplantation were obtained from the published literature. Since different general instruments using the same population may result in different outcomes and lead to opposite conclusions,17 only studies using EQ-5D as the survey instrument were included in our model.
Since there was no study of HRQoL of acute hepatitis B patients using EQ-5D, we estimated this parameter by comparing 2 instruments. According to a model used in Japan, the value by EQ-5D ranged from −0.106 to 0.848,18 while for SF-6D, the value ranged from 0.30 to 1.00.19 Therefore, HRQoL for acute hepatitis B patients was estimated as 0.766.20
Other Parameters: Information on the targeted cohorts was extracted from program files, including the number of children in each cohort and the number of doses received previously in each group (Table 2).
Table 2.
Information on the Targeted Cohorts
| Vaccination Histories |
||||
|---|---|---|---|---|
| Birth Year | 1 Dose Needed | 2 Doses Needed | 3 Doses Needed | Total |
| 1994 | 70,219 | 58,426 | 172,279 | 300,924 |
| 1995 | 72,944 | 61,006 | 193,212 | 327,162 |
| 1996 | 76,791 | 62,866 | 188,935 | 328,592 |
| 1997 | 81,622 | 61,994 | 185,395 | 329,011 |
| 1998 | 80,154 | 64,795 | 172,589 | 317,538 |
| 1999 | 77,230 | 56,143 | 155,918 | 289,291 |
| 2000 | 75,041 | 51,651 | 138,871 | 265,563 |
| 2001 | 68,219 | 45,955 | 127,286 | 241,460 |
| Total | 602,220 | 462,836 | 1,334,485 | 2,399,541 |
Since there were no serological tests available, and since it was not possible to trace targeted children by the information collected during the program, the proportion of HBV susceptible persons in each cohort was estimated based on a sero-epidemiologic survey by age groups,21 using a formula given below:
Ps represents the proportion of HBV susceptible persons in each group and P1 and P2 represent the HBV prevalence proportion and natural immunization proportion in each group, respectively. P3 represents the antibody to hepatitis B surface antigen (Anti-HBs) positive conversion rate after different doses of vaccination.
The proportion of HBV susceptible persons becoming infected each year was estimated using data from NNDRS. NNDRS data showed that the incidence of acute hepatitis B in Shandong province was 3.58/100,000 in 2008. We assumed that 30% of the patients with acute hepatitis B would have clinical symptoms; 31.51% of the total population was susceptible for HBV infection; and that 50% of patients were hospitalized for treatment after onset of symptoms. We therefore assumed that the proportion of HBV susceptible persons becoming infected each year could be estimated as 75.6/100,000. Since the estimate of this proportion has varied considerably in studies,7,9 we used a wide interval from 20/100,000 to 500/100,000 in the sensitivity analysis.
Model analysis
We used the Incremental Cost-Effectiveness Ratio (ICER) to compare the 2 alternative strategies. The ICER was determined by calculating the difference in costs of the 2 strategies divided by the difference in health effects. According to WHO, an intervention can be considered 'very cost-effective' if the ICER is lower than the annual per capita Gross Domestic Product (GDP) or 3 times the annual per capita GDP.47 These values were $5,414 and $16,242,14,15 respectively. We used one-way sensitivity, 2-way sensitivity, and probabilistic sensitivity analysis based on Monte-Carlo simulation with 10,000 iterations to evaluate the impact of parameter uncertainty on the ICER. Since the distributions of parameters were unclear due to limited literature-based estimates, all parameters were assigned triangular distributions.7
Results
Base case: The catch-up program could not only increase the QALYs of the targeted population, but could also save costs related to hepatitis B. This indicated that the status quo was dominated (inferior), and the catch-up program was ‘cost-saving.' According to our estimate, the cost to provide a single dose was $1.42. A total of 5.53 million doses of vaccines were used in the catch-up program, so the implementation costs of the program in Shandong province totaled to $7.89 million. The costs of the status quo was only the treatment costs ($208.26 million), while the costs of the catch-up strategy were composed of the implementation costs ($7.89 million) and the reduced treatment costs ($8.38 million). The saved treatment costs of $199.88 million were greater than the implementation costs of the vaccination program ($7.89 million).
The catch-up program dominated in all the cohorts,indicating that the program was not sensitive to the age parameter of the targeted population (Table 3). By using a total of 5.53 million doses of vaccines, the catch-up program could prevent 21,865 cases with symptomatic acute hepatitis B, 3,088 carriers with positive HBsAg, and 812 deaths due to HBV infection compared with the status quo (Table 4). The catch-up program could increase 28,888 QALYs and reduce $192.01 million in the future.
Table 3.
Costs and Effectiveness Outcomes by Age Cohort
| Cost ($) (Per Person)* |
Effectiveness |
||||||
|---|---|---|---|---|---|---|---|
| Birth Year | Catch-up Vaccination | Status Quo | Incremental Cost | Catch-up Vaccination | Status Quo | Incremental Effectiveness | Whether Cost-effective or Not |
| 1994 | 6.58 | 78.89 | −72.30 | 22.24 | 22.23 | 0.01 | Very Cost-saving |
| 1995 | 6.71 | 82.17 | −75.47 | 22.36 | 22.35 | 0.01 | Very Cost-saving |
| 1996 | 6.73 | 83.93 | −77.21 | 22.46 | 22.45 | 0.01 | Very Cost-saving |
| 1997 | 6.75 | 85.57 | −78.83 | 22.57 | 22.56 | 0.01 | Very Cost-saving |
| 1998 | 6.79 | 88.18 | −81.39 | 22.67 | 22.66 | 0.01 | Very Cost-saving |
| 1999 | 6.84 | 90.23 | −83.39 | 22.77 | 22.75 | 0.02 | Very Cost-saving |
| 2000 | 6.88 | 92.38 | −85.50 | 22.86 | 22.85 | 0.01 | Very Cost-saving |
| 2001 | 6.99 | 96.36 | −89.37 | 22.96 | 22.94 | 0.02 | Very Cost-saving |
| Total | 6.78 | 86.79 | −80.02 | 22.59 | 22.58 | 0.01 | Very Cost-saving |
*All costs were adjusted with respect to the cost of dollar in 2013.
Table 4.
Numbers of Infections and Deaths Prevented by the Catch-up Vaccination
| Symptomatic Acute Infections |
HBsAg Carriers |
Deaths |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Birth Year | Status Quo | Catch-up Vaccination | Infections Prevented | Status Quo | Catch-up Vaccination | Carriers Prevented | Status Quo | Catch-up Vaccination | Deaths Prevented |
| 1994 | 2728 | 66 | 2,662 | 327 | 9 | 318 | 86 | 2 | 84 |
| 1995 | 3044 | 72 | 2,972 | 380 | 10 | 370 | 100 | 3 | 97 |
| 1996 | 3068 | 74 | 2,994 | 403 | 11 | 392 | 106 | 3 | 103 |
| 1997 | 3071 | 74 | 2,997 | 422 | 11 | 411 | 111 | 3 | 108 |
| 1998 | 2987 | 72 | 2,915 | 433 | 12 | 421 | 114 | 3 | 111 |
| 1999 | 2721 | 66 | 2,655 | 414 | 11 | 403 | 109 | 3 | 106 |
| 2000 | 2491 | 62 | 2,429 | 403 | 11 | 392 | 106 | 3 | 103 |
| 2001 | 2298 | 57 | 2,241 | 391 | 10 | 381 | 103 | 3 | 100 |
| Total | 22408 | 543 | 21,865 | 3173 | 85 | 3088 | 835 | 23 | 812 |
Sensitivity analysis: According to the one-way sensitivity analysis (Table 5), none of the single parameters could change the conclusion that the program was 'cost-saving.' The model was robust to the uncertainty of any single parameter. Parameters that showed the greatest impact on the ICER were the probability of HBV carriers becoming CHB, the proportion of HBV susceptible persons becoming HBV infectious each year, and the discount rate. We conducted 2-way sensitivity analyses using these parameters, and no combination could change the conclusion of being ‘cost-saving.' Figure 2 shows the results of the probabilistic sensitivity analysis. All the points are in the fourth quadrant, indicating that all the samples derived supported our conclusion that the catch-up program was always dominant with reduced costs and increased QALYs. Taking all parameter uncertainties into account, the model remained robust and reliable.
Table 5.
Results of One-way Sensitivity Analysis
| ICER Vaccination vs. Status quo |
|||
|---|---|---|---|
| Parameters | Minimum Value | Maximum Value | |
| Proportion of Fulminant Hepatitis B in Symptomatic Acute Hepatitis B | Age< 20 | Cost-saving | Cost-saving |
| Age≥ 20 | Cost-saving | Cost-saving | |
| Natural Clearance of HBV Carriers | Cost-saving | Cost-saving | |
| Probability of HBV Carriers became CHB | Cost-saving | Cost-saving | |
| Probability of CHB became CC | Cost-saving | Cost-saving | |
| Probability of CHB became HCC | Cost-saving | Cost-saving | |
| Mortality of CHB due to HBV Infection | Cost-saving | Cost-saving | |
| Probability of CC became DC | Cost-saving | Cost-saving | |
| Probability of CC became HCC | Cost-saving | Cost-saving | |
| Mortality of CC due to HBV Infection | Cost-saving | Cost-saving | |
| Probability of DC became HCC | Cost-saving | Cost-saving | |
| Mortality of DC due to HBV Infection | Cost-saving | Cost-saving | |
| Probability of DC receiving Liver Transplantation | Cost-saving | Cost-saving | |
| Mortality of HCC Due to HBV Infection | Cost-saving | Cost-saving | |
| Probability of HCC Receiving Liver Transplantation | Cost-saving | Cost-saving | |
| Mortality of LT-1 | Cost-saving | Cost-saving | |
| Mortality of LT-2 | Cost-saving | Cost-saving | |
| Symptomatic Acute HBV Infection | Cost-saving | Cost-saving | |
| Costs of Treatment of CHB | Cost-saving | Cost-saving | |
| Costs of Treatment of CC | Cost-saving | Cost-saving | |
| Costs of Treatment of DC | Cost-saving | Cost-saving | |
| Costs of Treatment of HCC | Cost-saving | Cost-saving | |
| Prevalence Proportion with Previous Vaccination | Cost-saving | Cost-saving | |
| Prevalence Proportion without Previous Vaccination | Cost-saving | Cost-saving | |
| Natural Immunization Proportion | Cost-saving | Cost-saving | |
| Anti-HBs Positive Conversion Rate with 2 Doses | Cost-saving | Cost-saving | |
| Proportion of HBV Susceptible Persons with 0 Previous Dose | Cost-saving | Cost-saving | |
| Proportion of HBV Susceptible Persons with 1 Previous Doses | Cost-saving | Cost-saving | |
| Proportion of HBV Susceptible Persons with 2 Previous Doses | Cost-saving | Cost-saving | |
| Proportion of HBV Susceptible Persons with 3 Previous Doses | Cost-saving | Cost-saving | |
| Discount Rate | Cost-saving | Cost-saving | |
| The proportion of HBV Susceptible Persons Becoming HBV Infectious Each Year | Cost-saving | Cost-saving | |
Figure 2.

Results of Probabilistic Sensitivity Analysis
Discussion
In recent years, China has made great efforts to control and prevent HBV infection. But before the extensive use of vaccines, many newborns were not vaccinated and remained susceptible to HBV infection. According to WHO, implementation of catch-up immunization in such populations will produce broad immunity to HBV infection and eventually prevent transmission among all age groups.10
A review showed that in regions with intermediate or high levels of HBV epidemic, universal vaccination of newborns would be cost-effective,5 and this conclusion extends to Taiwan,8 Gambia,9 and India.48 Many studies using cost‑effectiveness analysis or cost-benefit analysis in small populations showed that vaccination of newborns,49-52 and of some specific group (soldiers,53,54 workers,55 or middle school students,56 or children under 15 y of age57) in mainland China, can also be economically acceptable. In our study, we examined a nationwide catch-up program using original data collection to improve the accuracy and reliability of parameters and obtain credible results. Although there were differences in benefit-cost ratio (BCR) and ICER between studies, the conclusion always remained that universal vaccination of newborns and catch-up programs for specific groups was a cost-saving strategy.
Our study showed that the catch-up program not only increased the QALYs of the targeted population, but also reduced total costs substantially. Although the incremental cost and incremental effectiveness varied in different cohorts, the program was always ‘cost-saving' for all the age groups. Since most parameters from the literature had uncertainties of their true values, these uncertainties might influence model results. We used sensitivity analysis to explore the direction and degree of effects from each and all parameters. The results showed that even within a wide range, no single parameter could reverse the conclusion of program of being ‘cost-saving.' The models were always robust, considering the combination of all uncertainties from parameters. Our study provided evidence to support the economic benefits of the catch-up program in Shandong province. Recently, China has experienced rapid economic progress, and its annual per capita GDP has increased as well. It can be inferred that in coming years such catch-up programs may be necessary and affordable.
In previous research on this catch-up strategy, the annual incidence of acute HBV infection was the only parameter could reverse the conclusion.6 Additionally, the discount rate and the transition rate from carrier to CHB were among the critical parameters35 that we found in our study. According to different populations and vaccination strategies, there are differences in the contribution of parameter uncertainties. Since there were not sufficient data on the distribution of parameters, we applied triangular distributions in our models,7 while previous studies used other parameter distributions.8,50 Compared with prior work6 arriving at the same conclusion, the accuracy and reliability of parameters have been improved by our study. It was the first time in mainland China that EQ-5D-5L was used as the instrument of HRQoL, and that data from program files was used directly as a resource in a cost‑effectiveness analysis. We received data and support from NNDRS managed by Chinese Center for Disease Control and Prevention to form parameters in the Markov model.
We conducted our study in Shandong province, while the program was implemented nationally. We believe that the results of a nationwide cost‑effectiveness study would not differ greatly from our study because: (1) the cost, effectiveness, and transition parameters were obtained primarily from national literature rather than being restricted in Shandong province and, (2) our sensitivity analysis showed that the influence of parameter uncertainty was rather limited. In addition, data from NNDRS showed that the acute incidence of hepatitis B in Shandong province was only 3.5753/100,000, compared with 6.8295/100,000 nationwide. Since the incidence had a positive correlation with cost-effective, it could be inferred that if we conducted a cost‑effectiveness analysis across the country, the conclusion would be even more cost-saving than in Shandong province.
Our study had some limitations. Although we tried to improve the accuracy of the parameters, limited availability of literature about the target population still existed. Our estimate was based on current treatments and costs, and since CHB could last a long time, treatment improvement in future might reduce treatment costs and change the natural disease progression. Such evolution would be against the efficacy and feasibility of a catch-up program. Progression and symptoms of hepatitis B are complicated, and in our model, we used simplified assumptions to fit the model and make the analysis clearer and more practical. However, this may results in biased estimate.
In conclusion, the catch-up program in Shandong province among children born between 1994 and 2001 was 'cost-saving.' The catch-up program could save life years and reduce total future costs. Our study supports the feasibility and efficacy of such a catch-up program on hepatitis B, and therefore provides information for a more comprehensive evaluation nationwide and evidence for policy-makers to consider.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to express the special thanks to Dr. Lance Rodewald, who provided comments and suggestions on the manuscript.
Funding
This study was sponsored by National Health and Family Planning Commission and Minister of Science and Technology (No.2012ZX10002001).
References
- 1.Wu B, Li T, Chen H, Shen J. Cost-effectiveness of nucleoside analog therapy for hepatitis B in China: a Markov analysis. Value Health 2010; 13(5):592-600; PMID:20561341; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 2.Qi XQ, Wang Y, Yu JJ. Report on national population hepatitis B sero-epidemiologic survey. Beijing: The People's Medical Publishing House [Google Scholar]
- 3.Averhoff F, Kolwaite A, Ward JW. The role of the GAVI Alliance in improving childhood hepatitis B vaccination in China: Successes, lessons learned, and future global challenges. Vaccine 2013; 31 Suppl 9:J5-7. PMID:23623862 10.1016/j.Vaccine.2013.04.022 [DOI] [PubMed] [Google Scholar]
- 4.Cui FQ, Gong XH, Chen YS. Evaluation on impact of hepatitis B vaccine integrated into routine immunization in the areas of ministry of health/global alliance for vaccine and immunization (gavi) cooperation project P.R. China. Chinese J Vaccines Immun 2009; 15(4):289-93; PMID:20077723 [PubMed] [Google Scholar]
- 5.Beutels P. Economic evaluations of hepatitis B immunization: a global review of recent studies (1994-2000). Health Econ 2001; 10(8):751-74; PMID:11747055; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 6.Hutton DW, So SK, Brandeau ML. Cost-effectiveness of nationwide hepatitis B catch-up vaccination among children and adolescents in China. Hepatology 2010; 51(2):405-14; PMID:19839061; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muennig P. Cost-effectiveness Analysis in HealthA Practical Approach. Second Edition San Francisco, CA: Jossey-Bass, 2008. [Google Scholar]
- 8.Hung HF, Chen TH. Probabilistic cost-effectiveness analysis of the long-term effect of universal hepatitis B vaccination: an experience from Taiwan with high hepatitis B virus infection and Hepatitis B e Antigen positive prevalence. Vaccine 2009; 27(48):6770-6; PMID:19735755; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 9.Kim SY, Salomon JA, Goldie SJ. Economic evaluation of hepatitis B vaccination in low-income countries: using cost-effectiveness affordability curves. Bull World Health Organ 2007; 85(11):833-42; PMID:18038073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinese Society of Hepatology, Chinese Society of Infectious Diseases The guideline of prevention and treatment for chronic hepatitis B (2010 Version). Chinese J Gastroenterol 2011; 16(6):351-66. [Google Scholar]
- 11.Hepatitis B vaccines: WHO position paper-recommendations Vaccine 2010; 28(3):589-90; PMID:19896455; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 12.Plotkin SA, Orenstein WA, Offit PA. Vaccines. London: Elsevier Inc, 2008. [Google Scholar]
- 13.Yu WZ, Yu JJ, Cui G, Jin SG. Study on the reasonable cost of national immunization program in some regions of China. Chinese J Vaccines Immun 2006; 12(4):280-5. [Google Scholar]
- 14.National Bureau of Statistics of People's Republic of China Annual Data. 2013. http://data.stats.gov.cn/workspace/index?m=hgnd [Google Scholar]
- 15.People's Bank of China Statistical Data. 2013. http://www.pbc.gov.cn/publish/zhengcehuobisi/637/index.html [Google Scholar]
- 16.Jia YX, Cui FQ, Li L, Zhang DL, Zhang GM, Gong XH, Zheng H, Wu ZH, Miao N, Sun XJ, et al. . Comparison between the EQ-5D-5L and the EQ-5D-3L in patients with hepatitis B. Qual Life Res 2014. Mar 14; 23(8):2355-63; PMID:24627090; http://dx.doi.org/ 10.1007/s11136-014-0670-3; Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 17.Sach TH, Barton GR, Jenkinson C, Doherty M, Avery AJ, Muir KR. Comparing cost-utility estimates: does the choice of EQ-5D or SF-6D matter? Med Care 2009; 47(8):889-94; PMID:19584759; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 18.Tsuchiya A, Ikeda S, Ikegami N, Nishimura S, Sakai I, Fukuda T, Hamashima C, Hisashige A, Tamura M. Estimating an EQ-5D population value set: the case of Japan. Health Econ 2002; 11(4):341-53; PMID:12007165; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 19.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002; 21(2):271-92; PMID:11939242; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 20.Jacobs RJ, Saab S, Meyerhoff AS. The cost effectiveness of hepatitis immunization for US college students. J Am Coll Health 2003; 51(6):227-36; PMID:14510025; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 21.Zhang L. Sero-epidemiological and molecular epidemiological studies for viral hepatitis B after 20 years from the introduction of hepatitis B vaccine in Shandong province: Shandong University; 2009. [Google Scholar]
- 22.Karvountzis GG, Redeker AG, Peters RL. Long term follow-up studies of patients surviving fulminant viral hepatitis. Gastroenterology 1974; 67(5):870-7; PMID:4426491 [PubMed] [Google Scholar]
- 23.Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci 1993; 253(1337):197-201; PMID:8397416; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 24.Li GW, Shao JR. Long-term follow up study on 40 cases of children with asymptomatic hepatitis B surface antigen. Chinese Commun Doctors 2009; 11(1):53. [Google Scholar]
- 25.Hong DQ, Li JS, Dai HM. A follow-up study on 103 HBsAg carriers during 16~21 years. Chinese J Prevent Med 1994; 20(5): 287-90; PMID:7842894 [PubMed] [Google Scholar]
- 26.Chu CM, Liaw YF. Incidence and risk factors of progression to cirrhosis in inactive carriers of hepatitis B virus. Am J Gastroenterol 2009; 104(7):1693-9; PMID:19455130; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 27.Chen JG, Lu JH, Zhu YR, Zhu J, Zhang YH. A thirty-one year prospective follow-up program on the HBsAg carrier state and primary liver cancer in Qidong, China. Chinese J Epidemiol 2010; 31(7):721-6. [PubMed] [Google Scholar]
- 28.Wu GC, Zhou WP, Zhao YR, Guo SH, Wang ZY, Zhang DF. Study on the long-term clearance rate of HBeAg and HBsAg of chronic hepatitis B. J Chongqing Med Univ 2003; 28(2):169-71. [Google Scholar]
- 29.Guo F, Ma H, Wei L, Sun M, Wang H. Nine-year study of HBeAg-negative chronic hepatitis B. Chinese J Exp Clin Virol 2006; 20(4):370-2. [Google Scholar]
- 30.Li T, Zhang C, Li S, Wen W. Long-term follow up of patients with chronic hepatitis B. Chinese J Gastroenterol Hepatol 2006; 15(3):242-3, 248. [Google Scholar]
- 31.Wu G, Zhou W, Zhao Y, Guo S, Wang Z, Zou S, Zhang Q, Ren H, Huang A, Zhang D. Study on the natural history of chronic hepatitis B. Chinese J Hepatol 2002;10(1):46-8; PMID:11856503 [PubMed] [Google Scholar]
- 32.Yang S, Xie H, Zhong C. Research progress on follow-up study on patients with chronic hepatitis B virus infection. Chinese J Mod Nurs 2009; 15(34):3700-2. [Google Scholar]
- 33.Xu B, Hu C, Rosenberg D, Jiang Q, Lin X, Lu J, Li X. A retrospective cohort study on the natural history of chronic hepatitis B in Shanghai, China. Chinese J Internal Med 2002; 41(6):384-7; PMID:12137600 [PubMed] [Google Scholar]
- 34.Wang S, Wang J, Chen J, Giri R, Chen M. Natural history of liver cirrhosis in south China based on a large cohort study in one center: a follow-up study for up to 5 years in 920 patients. Chin Med J (Engl) 2012; 125(12):2157-62; PMID:22884146 [PubMed] [Google Scholar]
- 35.Lu SQ, McGhee SM, Xie X, Cheng J, Fielding R. Economic evaluation of universal newborn hepatitis B vaccination in China. Vaccine 2013; 31(14):1864-9; PMID:23384752; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 36.Chen JG, Shen ZC, Yao YH, Li WG. Population based cancer survival: an analysis of 16 922 cases. Chinese J Oncol 1998; 20(03): 202-206. [PubMed] [Google Scholar]
- 37.Fan J, Yang G, Fu Z, Peng Z, Xia Q, Peng C, Qian JM, Zhou J, Xu Y, Qiu SJ, et al. . Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi-center experience in Shanghai, China. J Cancer Res Clin Oncol 2009; 135(10):1403-12; PMID:19381688; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 38.Qiao F, Wu M. A study on direct economic burden of diseases related to hepatitis B viral infection in Xicheng district of Beijing. Capital J Public Health 2011; 5(6):247-250. [Google Scholar]
- 39.Liang S, Zhang S, Ma Q, Xiao H, Lv Q, Xie X, Mei SJ, Hu DS, Zhou BP, Li B, et al. . Financial burden of hepatitis B-related diseases and factors influencing the costs in Shenzhen, China. Chinese J Epidemiol 2010; 31(12):1340-5; PMID:21223660 [PubMed] [Google Scholar]
- 40.Hu M, Chen W. Assessment of total economic burden of chronic hepatitis B (CHB)-related diseases in Beijing and Guangzhou, China. Value Health 2009; 12(Suppl 3):S89-92; PMID:20586991; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 41.Chen D, Yao G, Chen W. Economic evaluation of peginterferon α-2a and lamivudine in the treatment of HBeAg-positive chronic hepatitis B. Chinese J Infect Dis 2007; 12(3):164-7. [Google Scholar]
- 42.McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making 2008; 28(4):582-92; PMID:18424560; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 43.Gao E, Yang P, Che Y. The random clinical comparison of two gene engineering hepatitis B vaccine. China Public Health 2001; 17(4):315-6. [Google Scholar]
- 44.Yang L, Wang D, Xi J, Zhang S, Jin S. Three year's observation on the protection of two schedules of hepatitis B recombinant DNA vaccine. Disease Surveillance 1999; 14(3):88-90. [Google Scholar]
- 45.Lv J, Yan B, Zhang L, Liu J, Feng Y, Xu A, et al. . Study on the immune response and influencing factors of primary immunization by 5μg hepatitis B vaccine made by recombinant dexyribonucleic acid techniques in saccharomyces Cerevisiae Yeast among infants. Chinese J Vaccines Immun 2012(04):320-4. [Google Scholar]
- 46.Census Office of the State Council, Population and Employment Statistics Division of the National Bureau of statistics The 2012 Census in China. Beijing: China Statistics Press, 2012. [Google Scholar]
- 47.WHO WHO guide for standardization of economic evaluations of immunization programmes. Switzerland: Word Health Organization Press, 2005. [Google Scholar]
- 48.Aggarwal R, Ghoshal UC, Naik SR. Assessment of cost-effectiveness of universal hepatitis B immunization in a low-income country with intermediate endemicity using a Markov model. J Hepatol 2003; 38(2):215-22; PMID:12547411; http://dx.doi.org/ 10.1111/j.1524-4733.2010.00733.x [DOI] [PubMed] [Google Scholar]
- 49.Guan X, Yang C, Wang J, Yang P, Wu X, Liao A, et al. . The impact and health economic evaluation of hepatitis B vaccination for the infant population in Sichuan province. Chinese J Vaccines Immun 2010; 16(5):447-52. [Google Scholar]
- 50.Zhang J, Luo Y, Li J, Cai H, Yang W, Peng Z. Cost-effective analysis of administering hepatitis B vaccine to newborns for 10 years in Guangdong province. South China J Prevent Med 2006; 32(3):12-5. [Google Scholar]
- 51.Qi Y, Wang F, Gong X, Pan L, Zeng X, Li H. Analysis on cost-benefit for infant hepatitis B vaccine immunization strategy in Beijing. China Public Health 2004; 20(9):1067-9. [Google Scholar]
- 52.Wu G, Gong Y, Yu S, Shao R, Qin H. Study on the cost-effectiveness, benefit and utility analysis on the infant inoculation hepatitis B vaccine in Shanghai. Chinese J Epidemiol 2004; 25(6):474-8; PMID:15231120 [PubMed] [Google Scholar]
- 53.Wang S, Xi Y, Wang H, Li X. Cost benefit analysis of hepatitis a and hepatitis b vaccination in army crowds in South Region. J Preventive Med Information 2003; 19(2):102-3. [Google Scholar]
- 54.Hu R, Cao W, Zhang X. Cost effectiveness analysis of hepatitis B vaccination in People's Liberation Army. Chinese J Epidemiol 2001; 22(2):142-5; PMID:11860866 [PubMed] [Google Scholar]
- 55.Jiang L, Yin X. Cost and benefit study on hepatitis B vaccination among adults in JiangSu oil field. Occup Health 2005; 21(5):649-50 [Google Scholar]
- 56.Feng Z. Cost and saved health-related life year analysis on hepatitis B vaccination among boarding middle school students. Hebei Med J 2009; 31(18):2482-4. [Google Scholar]
- 57.Si L, Jiang Q. Cost-effectiveness analysis of a catch-up hepatitis B vaccination among the children in China. Chinese Health Econ 2012; 31(06):74-6. [Google Scholar]


