Abstract
Several ChimeriVax-Dengue (CYD)-based vaccination strategies were investigated as potential alternatives to vaccination with tetravalent CYD vaccine (CYD-TDV) in this phase IIa trial conducted in 2008–9 in 150 healthy adults. Participants were randomized and vaccinated on D0 and D105 (± 15 days). One group received bivalent CYD vaccine against serotypes 1 and 3 (CYD-1;3) on day 0 and CYD-2;4 on day 105 (±15 days). Two groups received an injection at each timepoint of a tetravalent blend of CYD-1;3;4 and a VERO cell derived, live attenuated vaccine against serotype 2 (VDV-2), or the reference CYD-TDV. A fourth group received Japanese encephalitis (JE) vaccine on days -14, -7 and 0, followed by CYD-TDV on day 105. Viraemia was infrequent in all groups. CYD-4 viraemia was most frequent after tetravalent vaccination, while CYD-3 viraemia was most frequent after the first bivalent vaccination. Immunogenicity as assessed by 50% plaque reduction neutralisation test on D28 was comparable after the first injection of either tetravalent vaccine, and increased after the second injection, particularly with the blended CYD-1;3;4/ VDV-2 vaccine. In the bivalent vaccine group, immune response against serotype 3 was highest and the second injection elicited a low immune response against CYD 2 and 4. Immune responses after the first injection of CYD-TDV in the JE-primed group were in general higher than after the first injection in the other groups. All tested regimens were well tolerated without marked differences between groups. Bivalent vaccination showed no advantage in terms of immunogenicity.
Clinical trial registration number: NCT00740155
Keywords: dengue, flavivirus, immunogenicity, Japanese encephalitis, safety, vaccine
Abbreviations
- ADE
antibody-dependent enhancement
- AE
adverse event
- ALT
aspartate aminotransferase
- AST
alanine aminotransferase
- CBA
cytometric bead array
- CI
confidence interval
- CPK
creatine phosphokinase
- CYD-TDV
CYD tetravalent dengue vaccine
- GMT
geometric mean titres
- ICS
intracellular cytokine staining
- IFN
interferon
- JE
Japanese encephalitis
- LLOQ
lower limit of quantitation
- MOI
multiplicity of infection
- MedDRA
medical dictionary for regulatory activities
- PBMC
peripheral blood mononuclear cells
- PFU
plaque forming unit
- PRNT
plaque reduction neutralization test
- RT-PCR
reverse transcriptase-polymerase chain reaction
- TCID
tissue culture infectious dose
- VDV
vero-cell adapted attenuated dengue vaccine
- YF
yellow fever
Introduction
Dengue disease is endemic in more than 100 countries in Asia and Latin America and data show that the burden of disease is steadily increasing.1,2 The disease is caused by infection with any of the 4 serotypes of this mosquito-borne flavivirus. The 4 dengue virus serotypes (DENV1–4) are closely related, but antigenically distinct, and infection with one serotype is thought to confer immunity to subsequent infection with the same serotype, but not long-lasting immunity to the other serotypes.3 Upon secondary infection with a different serotype, patients are at increased risk of developing potentially fatal severe dengue disease, such as dengue hemorrhagic fever, which can be fatal.4
While no dengue vaccine is licensed for commercial use, several candidates are in development, as reviewed elsewhere,5 one of which—the live, attenuated, CYD tetravalent dengue vaccine (CYD-TDV)—is currently undergoing clinical phase III evaluation with a 3-dose, 0–6–12 month vaccination regimen. A proof-of-concept efficacy study, completed in 2012 showed that this vaccine candidate provides protection against disease caused by 3 of the 4 dengue serotypes, but showed a lack of protection against the circulating DENV-2 virus.6 This vaccine comprises 4 monovalent viruses, one per serotype (CYD-1–4), each of which was produced by replacing the genes for pre-membrane and envelope proteins of the attenuated yellow fever (YF) 17D vaccine strain with the corresponding sequences from wild-type dengue viruses.7 The resulting recombinant dengue/YF viruses displays the respective dengue envelope antigens on the surface, and in the core contains the non-structural proteins and replication machinery of the well-characterized, attenuated YF17D vaccine virus.
One of the challenges of developing a single live vaccine against 4 viruses is the potential intrinsic immunodominance of serotype-specific epitopes due to differences in initial replication and subsequent presentation to the immune system, which can lead to preferential immune response against one or more dominant serotypes.8,9
In a phase II study we evaluated the immunogenicity, viremia and safety of several CYD-based vaccination strategies as potential alternatives to vaccination with tetravalent CYD vaccine (CYD-TDV). These strategies were based in part on previous studies performed in monkeys some of which were seen to be promising.9 We also assessed the priming potential of Japanese encephalitis (JE) vaccination with JE-VAX® on the immunogenicity, viremia and safety of CYD-TDV was assessed.
Results
Between August and November 2008, 215 volunteers were screened and 155 were enrolled and randomized to one of the 5 groups, of which 124 were randomized to one of the 4 groups reported here (Fig. 1).
Figure 1.
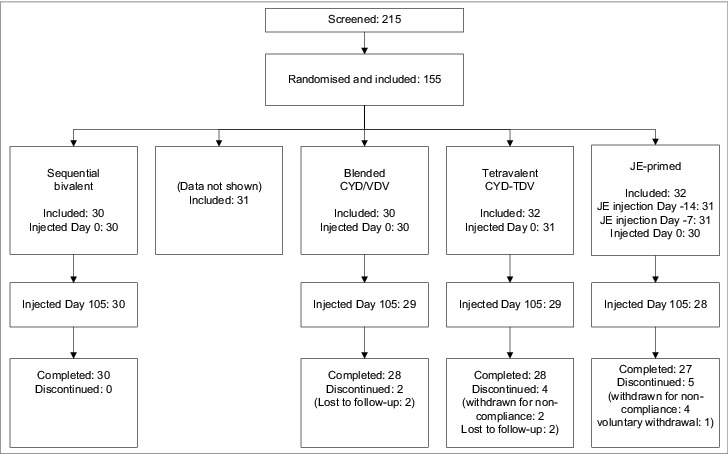
Study flowchart describing the 4 groups included in the report.
At day 0, the first injection was administered to 31 participants in the sequential bivalent group, 29 in the CYD/VDV group, and 31 in the CYD-TDV group. The third JE vaccine dose was administered to 30 subjects in the JE-primed group at day 0. One participant in the CYD/VDV blended formulation group actually received the vaccination assigned to the sequential bivalent group, and so was included in the sequential bivalent group analyses. Twelve participants discontinued before the end of the study either for non-compliance (n = 7), or withdrawal of consent (n = 1) or were lost to follow-up (n = 4). No participant withdrew due to adverse events.
At screening there were more females than males and the number of females was higher in the bivalent and JE-primed groups than in the other groups (Table 1). The mean age ranged from 27 y in the concomitant bivalent group to 32 y in the CYD/VDV blended formulation group. The body mass index was similar across the groups.
Table 1.
Demographic characteristics at baseline
| Sequential Bivalent | Blended CYD/VDV | Tetravalent CYD-TDV | JE-primed | |
|---|---|---|---|---|
| N | 31 | 29 | 31 | 30 |
| N male:female | 9:22 | 15:14 | 15:16 | 10:20 |
| Age at screening (years) mean (±SD) (range) |
28 (6.7) (19–44) |
32 (6.5) (18–44) |
30 (6.3) (20–41) |
31 (7.1) (19–44) |
| Weight (kg) mean (±SD) (range) |
64 (9.4) (44–86) |
70 (12.3) (45–93) |
65 (11.7) (46–99) |
67 (14.1) (43–105) |
| Height (cm) mean (±SD) (range) |
159 (8.0) (149–185) |
162 (9.9) (144–179) |
162 (10.3) (145–183) |
161 (12.1) (137–190) |
| BMI (kg/m2) mean (±SD) (range) |
26 (4.6) (16–38) |
27 (3.7) (19–34) |
25 (4.2) (18–41) |
26 (3.1) (16–41) |
Safety and reactogenicity
There were no marked differences between the groups with regard to either injection site or systemic reactogenicity after either the first or second vaccination (Table 2). In each group, the incidence of injection site reactions was comparable after the first and second injection, although there was a trend for more injection site reactions after the second injection in the bivalent group and the blended CYD/VDV group. Solicited systemic reactions were less common after the second than the first injection.
Table 2.
Injection site and systemic reactogenicity after the first (a) and second (b) vaccinations (number and percentage of participants experiencing at least one reaction during the reporting period) (a)
| Sequential bivalent | Blended CYD/VDV | Tetravalent CYD-TDV | JE-primed | |
|---|---|---|---|---|
| Vaccine(s) injected | CYD-1;3 | CYD-1;3;4/VDV-2 | CYD-TDV | JE |
| N | 31 | 29 | 31 | 29 |
| Injection site reactions (day 0–7) | ||||
| Any | 7 (23%) 95%CI: 9.6;41.1 |
7 (24%) 95%CI: 10.3;43.5 |
10 (32%) 95%CI: 16.7;51.4 |
8 (28%) 95%CI: 12.7;47.2 |
| Pain | 7 (23%) | 7 (24%) | 8 (26%) | 6 (21%) |
| Erythema | 1 (3%) | 1 (3%) | 4 (13%) | 2 (7%) |
| Edema | 0 | 1 (3%) | 0 | 0 |
| Systemic reactions (day 0–21) | ||||
| Any | 28 (90%) 95%CI: 74.2;98.0 |
23 (79%) 95%CI: 60.3;92.0 |
26 (84%) 95%CI: 66.3;94.5 |
24 (83%) 95%CI: 64.2;94.2 |
| Fever | 6 (19%) | 7 (24%) | 4 (13%) | 5 (17%) |
| Headache | 20 (65%) | 18 (62%) | 21 (68%) | 15 (52%) |
| Malaise | 13 (42%) | 11 (38%) | 7 (23%) | 13 (45%) |
| Myalgia | 16 (52%) | 11 (38%) | 10 (32%) | 13 (45%) |
| Asthenia | 16 (52%) | 10 (35%) | 13 (42%) | 15 (52%) |
| (b) | ||||
| Vaccine(s) injected | CYD-2;4 | CYD-1;3;4/VDV-2 | CYD-TDV | CYD-TDV |
|
N |
31 |
28 |
28 |
28 |
| Injection site reactions (day 0–7) | ||||
| Any | 12 (39%) 95%CI: 21.8;57.8 |
10 (36%) 95%CI: 18.6;55.9 |
9 (32%) 95%CI: 15.9;52.4 |
7 (25%) 95%CI: 10.7;44.9 |
| Pain | 10 (32%) | 7 (25%) | 8 (29%) | 5 (18%) |
| Erythema | 6 (19%) | 5 (18%) | 4 (14%) | 3 (11%) |
| Edema | 4 (13%) | 1 (4%) | 3 (11%) | 2 (7%) |
| Systemic reactions (day 0–21) | ||||
| Any | 24 (77%) 95%CI: 58.9;90.4 |
22 (79%) 95%CI: 59.0;91.7 |
18 (64%) 95%CI: 44.1;81.4 |
17 (61%) 95%CI: 40.6;78.5 |
| Fever | 9 (29%) | 6 (21%) | 5 (18%) | 4 (14%) |
| Headache | 15 (48%) | 12 (43%) | 12 (43%) | 14 (50%) |
| Malaise | 8 (26%) | 10 (36%) | 3 (11%) | 7 (25%) |
| Myalgia | 13 (42%) | 9 (32%) | 8 (29%) | 13 (46%) |
| Asthenia | 10 (32%) | 8 (29%) | 7 (25%) | 11 (39%) |
Injection site pain was the most frequently reported injection site reaction in each group after both the first (range: 18–26%) and second vaccinations with any dengue vaccine formulation (range: 25–32%). Most injection site reactions were mild. In all there were 3 subjects vaccinated with dengue vaccine who reported Grade 3 injection site reactions. Almost all injection site reactions appeared within 3 d of injection and lasted for 1–3 d (data not shown).
Headache was the most common solicited systemic reaction after the first (range: 50–68%) and second injections (range: 43–48%) among subjects vaccinated with any dengue vaccine formulation, while fever was the least common after any dose (range: 13–29%). Most systemic reactions were mild to moderate. In all there were 7 subjects vaccinated with dengue vaccine who reported Grade 3 reactions. Solicited systemic reactions appeared throughout the 3 week reporting period, but most appeared within the first 3 d and lasted between 1–3 d (data not shown).
Unsolicited adverse events occurring within 28 d of injection were reported by 64%–66% of participants per group after the first injection of any dengue vaccine formulation and by 52%–58% after the second. Of these events, most were considered unrelated to vaccination. Vaccine-related unsolicited adverse events (i.e., unsolicited adverse reactions) were reported for up to 5 participants per group after each injection and were mainly injection site reactions including rash, pruritus and hematoma. Two unsolicited adverse reactions occurred within 30 minutes after the second injection: nausea in the blended CYD/VDV group and dizziness in the CYD-TDV group. All reactions resolved spontaneously within one day.
No SAEs occurred during the 28 d after any vaccination. Two non-fatal SAEs occurred between Day 28 after the first vaccination and the second vaccination periods, one after bivalent vaccination, and the other one after the third dose of JE vaccine. None of them were considered vaccine related.
Most participants in all groups had biological values within the normal range after both the first and second injections. There was a trend toward a modest elevation in aspartate aminotransferase (AST), alanine aminotransferase (ALT) and creatine phosphokinase (CPK) levels after the first injection in the blended CYD/VDV group. Mean AST values rose from 26.2 ± 9.8 U/L at day 0 and peaked at 30.6 ± 24.0 U/L after 14 days; mean ALT values rose from 30.9 ± 18.3 U/L at day 0 and peaked at 35.2 ± 30.3 U/L after 28 days; and mean CPK values rose from 116 ± 70.1 U/L at day 0 and peaked at 380 ± 1394 after 14 d (Fig. 2).
Figure 2.
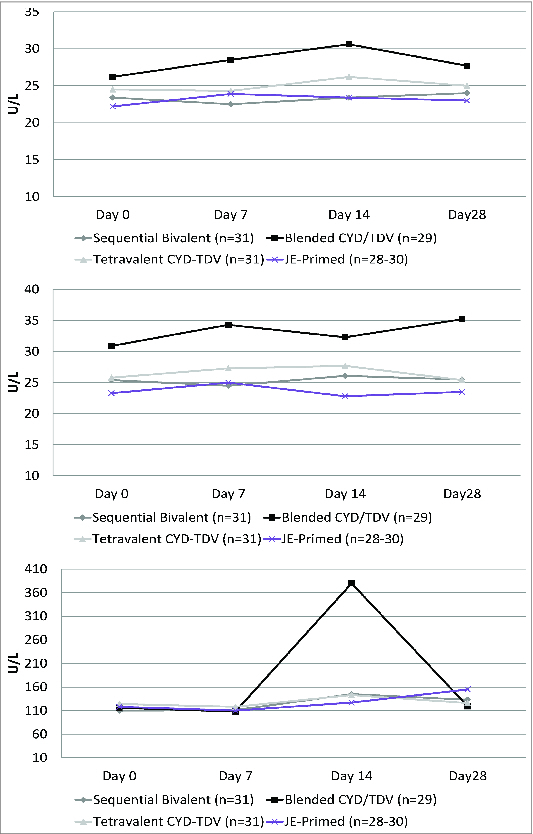
Mean aspartate aminotransferase –AST- (top), alanine aminotransferase –ALT- (middle) and creatine phosphokinase –CPK- (bottom) measurements on days 0, 7, 14, and 28 after the first vaccination.
Viraemia
The proportion of participants with detectable viraemia according to the non-serotype specific RT-PCR test was comparable (81%–93%) after the first tetravalent vaccination with either the tetravalent CYD vaccine, or the blended formulation, and lower (65%) after bivalent vaccination (Table 3). Viraemia was below the lower limit of quantitation (LLOQ) in the majority of cases, and the highest value (5.78 log10 GEq/ml) observed in the tetravalent CYD group. When analyzed with the serotype-specific assays, CYD-4 then CYD-3 were most commonly detected after tetravalent vaccination. CYD-4 viremia was quantified in 3 subjects in the CYD-TDV group (5.3, 5.4, and 5.7 log10 GEq/ml). After the first bivalent vaccination CYD-3 viraemia was detected more frequently than in the other groups and quantified in 2 subjects (5.1, and 5.2 log10 GEq/ml), CYD-1 viremia was quantified in 2 subjects in the CYD-TDV group (4.9 and 5.3 log10 GEq/ml) and serotype 2 viremia in 3 subjects in the blended CYD/VDV group (5.4, 5.4, and 5.7 log10 GEq/ml). No dengue viraemia was detected after JE vaccination.
Table 3.
Vaccine virus viraemia 7, 14, or 21 d after first and second injections (n (%) with detectable and quantifiable viraemia)
| First injection |
Second injection |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sequential Bivalent | Blended CYD/VDV | Tetravalent CYD-TDV | JE-primed | Sequential Bivalent | Blended CYD/VDV | Tetravalent CYD-TDV | JE-primed | |
| Vaccine(s) injected | CYD-1;3 | CYD-1;3;4/VDV-2 | CYD-TDV | JE | CYD-2;4 | CYD-1;3;4/VDV-2 | CYD-TDV | CYD-TDV |
| Non-serotype specific | ||||||||
| N | 31 | 29 | 31 | 29 | 31 | 28 | 29 | 27 |
| Detectable viraemia | 20 (65%) | 27 (93%) | 25 (81%) | 0 | 5 (16%) | 1 (4%) | 1 (3%) | 18 (67%) |
| Quantifiable viraemia | 2 (6%) | 1 (3%) | 2 (67%) | 0 | 1 (3%) | 0 | 0 | 0 |
| DENV-1 | ||||||||
| Detectable viraemia | 2 (6%) | 1 (3%) | 4 (13%) | 0 | 0 | 0 | 0 | |
| Quantifiable viraemia | 0 | 0 | 2 (7%) | 0 | 0 | 0 | 0 | |
| DENV-2 | ||||||||
| Detectable viraemia | 0 | 3 (10%) | 2 (6%) | 0 | 5 (18%) | 0 | 0 | |
| Quantifiable viraemia | 0 | 3 (10%) | 0 | 0 | 2 (7%) | 0 | 0 | |
| DENV-3 | ||||||||
| Detectable viraemia | 16 (52%) | 8 (28%) | 7 (23%) | 0 | 1 (4%) | 0 | 3 (11%) | |
| Quantifiable viraemia | 2 (10%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| DENV-4 | ||||||||
| Detectable viraemia | 0 | 24 (83%) | 21 (68%) | 4 (13%) | 0 | 0 | 15 (56%) | |
| Quantifiable viraemia | 0 | 0 | 3 (1%) | 1 (3%) | 0 | 0 | 1 (4%) | |
After the second injection of either tetravalent vaccine, viremia (non-serotype-specific assay) was detected in one participant per group (Table 3). After injection of the complementary bivalent CYD-2;4 vaccine, overall viremia was detected only in 5 participants (i.e., less frequently than after the previous CYD-1;3 vaccination, or after the first tetravalent vaccination in the other groups). CYD-4 viremia was detected in only 4 participants after CYD-2;4 vaccination, compared with 21 and 24 in the 2 tetravalent vaccine groups respectively, and quantified in only one subject (5.4 log10 GEq/ml) (Table 3). Serotype 2 viremia was quantified in 2 subjects in the blended CYD/VDV group (5.8 and 6.7 log10 GEq/ml). After CYD-TDV vaccination in the JE-primed group, the proportion with detectable viremia was 67%, and CYD-4 was the serotype most commonly detected but only quantified in one subject (5.4 log10 GEq/ml).
Of the 3 sets of samples, collected on days 7, 14 and 21, viraemia peaked on day 7 after each injection (data not shown).
Immunogenicity
Humoral immune response
Baseline dengue seropositivity was observed in up to 5 participants per group. On day 28 after the first injection of either tetravalent vaccine formulation, the serotype-specific seropositivity rates (Fig. 3) and GMTs (Table 4) were comparable between groups and were highest against serotype 4. After bivalent vaccination, the immune response to serotype 1 was similar to that seen with either tetravalent vaccine, while the immune response against serotype 3 (GMT: 297; seropositivity rate: 84%) was higher than in the other groups.
Figure 3.
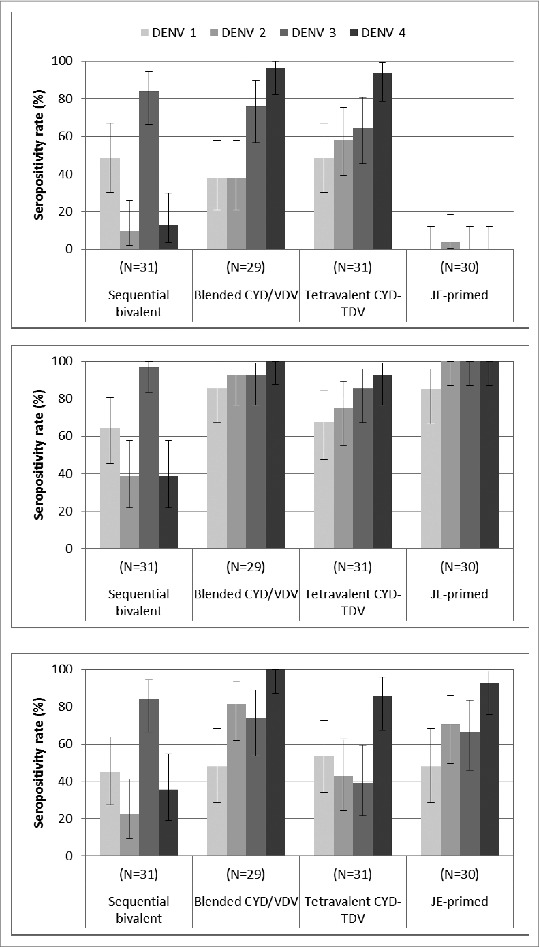
Serotype-specific seropositivity rate (percentage of subjects with PRNT50 titres ≥10 ) 28 d after the first (top) and second (middle) injections, and 1 y (bottom) after the first injection, assessed with PRNT assay using the dengue vaccine candidates’ parental wild-type strains as the challenge virus.
Table 4.
Geometric mean titres (95% confidence interval) of dengue antibodies 28 d after the first and second injections and 365 d after the first injection
| Bivalent | Blended CYD/VDV | Tetravalent CYD-TDV | JE-primed | |
|---|---|---|---|---|
| First injection | ||||
| Serotype 1 | 18 (10; 32) | 15 (9;28) | 17 (10;31) | 5 (5;5) |
| Serotype 2 | 7 (5;9) | 17 (8;33) | 32 (16;65) | 6 (4;7) |
| Serotype 3 | 297 (124;715) | 64 (31;133) | 23 (13;39) | 5 (5;5) |
| Serotype 4 | 7 (5;10) | 552 (299;1019) | 468 (226;968) | 5 (5;5) |
| Second injection | ||||
| Serotype 1 | 23 (15;38) | 54 (30;96) | 28 (15;50) | 33 (21;51) |
| Serotype 2 | 10 (7;15) | 152 (79;293) | 43 (23;79) | 101 (78;130) |
| Serotype 3 | 159 (97;260) | 127 (71;229) | 46 (29;73) | 81 (63;103) |
| Serotype 4 | 21 (10;44) | 246 (159;382) | 173 (97;307) | 430 (267;694) |
| 365 d post-dose 1 | ||||
| Serotype 1 | 14 (9;22) | 14 (9;22) | 18 (10;30) | 11 (8;16) |
| Serotype 2 | 8 (6;11) | 55 (32;94) | 16 (9;29) | 23 (15;37) |
| Serotype 3 | 61 (36;104) | 36 (20;64) | 11 (7;16) | 18 (12;27) |
| Serotype 4 | 13 (8;24) | 103 (69;155) | 72 (44;117) | 67 (45;99) |
Three months after the first vaccination with either tetravalent vaccine, GMTs were similar to those observed on day 28 against serotypes 1, serotype 2, and serotype 3; however, GMTs were lower for serotype 4 (range 147–214)(data not shown).
The second injection of either tetravalent vaccine increased the seropositivity rate to all serotypes. In these 2 groups, GMTs against serotypes 1, 2 and 3 were higher after the second injection than after the first one, whereas the GMT against serotype 4 and measured 28 d after vaccination was lower after the second injection than after the first dose. GMTs against each serotype appeared higher after the second injection of the blended CYD/VDV tetravalent vaccine than after the second injection of CYD-TDV.
In the bivalent vaccination group, injection of the CYD-2;4 vaccine bivalent group elicited a low immune response to these 2 serotypes, particularly for serotype 4, lower than seen after primary tetravalent vaccination, and a slight booster response to serotype 1.
Immune responses after the first injection of CYD-TDV in JE-primed participants were in general higher than after the first injection in the other groups. Furthermore, the immune responses elicited by one CYD-TDV vaccination in JE-primed participants appeared to be higher than after second injection in the other groups (Fig. 3, Table 4). However, this effect appeared time limited and by day 365 the level of antibodies measured after one injection of CYD-TDV in JE-primed participants was comparable with the one after 2 injections in those receiving 2 doses of CYD-TDV. On day 365, the most persistent response was seen in the blended CYD/VDV formulation group.
Cellular immune response
Due to poor viability and yield of the PBMCs prepared at the investigator sites many samples could not be analyzed or had to be rejected. Therefore, results were not obtained for the same set of participants at each visit.
In response to in vitro restimulation with CYD viruses, cytokine profiles in all groups were dominated by IFN-γ (Fig. 4). These responses were mostly serotype-specific: subjects vaccinated with CYD-1,-3 showed a T cell response dominated by these serotypes, with lower responses against serotypes 2 and 4. In this group, serotypes 1 and 3 were still dominant after the second, heterologous, vaccination with CYD-2,-4. In contrast, after CYD-TDV vaccination, serotypes 2 and 4 were dominant after the first vaccination. After vaccination with the blended CYD/VDV tetravalent vaccine, responses to in vitro re-stimulation with CYD2 were comparable with those in the CYD-TDV Group. Supernatant IFN-γ cytokine results after 2 doses showed a predominance of serotype 4 when the 4 serotypes were injected concomitantly.
Figure 4.
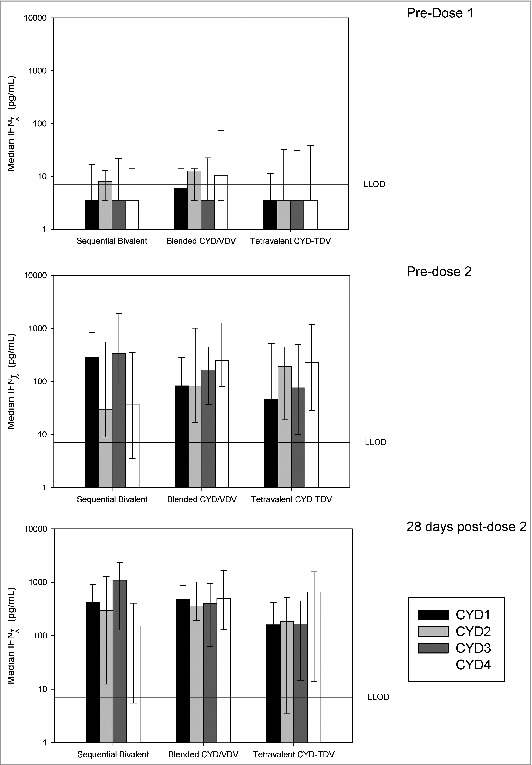
Dengue serotype-specific cellular IFN-γ responses measured before and after vaccination upon stimulation by CYD-1 to 4. Median IFN-γ secretion measured by CBA in supernatants of PBMCs stimulated in vitro for 4 d with monovalent CYD1–4 collected from vaccinees before primary vaccination and before and 28-days after secondary vaccination. Vertical bars represent the median. The error bars represent Q1 and Q3. The reference line represents the lower limit of detection (LLOD) of 7.1 pg/mL. The values obtained for unstimulated condition (medium) were substracted from values obtained upon stimulation for each subject and visit.
Anti-NS3 responses measured after stimulation with dengue or YF-17D peptide pools showed that the injection of the different CYD formulations induced the generation of IFNg-dominated, YF-17D-specific CD8+ cells. Responses against DEN backbone were almost exclusively detected in the blended CYD/VDV tetravalent vaccine (not shown).
Discussion
This is the first and only clinical trial that reports safety and immunogenicity results of bivalent or blended CYD/VDV formulations. The results of this study provide important information on the nature and potential role of the different components of the CYD vaccine on both humoral and cellular immunological response. In addition, this is the first study to assess the priming effect of JE vaccination, which reflects the situation in countries where this vaccination is recommended. Earlier clinical studies showed that the CYD tetravalent dengue vaccine was well tolerated and a 3-dose regimen elicited balanced antibody responses against the 4 dengue serotypes, as measured by the Vero-cell based PRNT assay.10-12 After the first vaccination however, responses were seen to be directly predominantly against serotype 4. Parallel investigations in a monkey model suggested that interference between the CYD vaccine viruses, due to differential in vivo replication of the 4 viruses and epitope dominance, could be modulated with alternative vaccination strategies.9 This phase II clinical study was therefore designed to investigate these alternative vaccination strategies in humans. It confirmed that tetravalent CYD vaccination was appropriate for continued development in Phase III studies.
The sequential bivalent regimen was successful in increasing the proportion of responders and titres against serotype 3, but failed to increase responses to serotype 1. Furthermore, the immunity induced by vaccination with CYD-1;3 appeared to limit the replication of CYD-4 after the subsequent CYD-2;4 vaccination and diminished immune responses to CYD-4. Indeed the rate of detectable CYD-4 viraemia and CYD-4-specific seropositivity after the CYD-2;4 vaccination was lower than that observed after CYD-TDV vaccination in either flavivirus naïve or JE-primed participants. In other words, antibodies elicited by bivalent CYD-1;3 vaccination did not enhance replication of the CYD-2 and CYD-4 viruses when injected more than 3 months later, and indeed appeared to have blunted it.
It is hypothesized that antibody-dependent enhancement (ADE) explains the increased likelihood of severe dengue disease occurring in the case of a secondary infection with a heterotypic dengue virus, as well as in infants when maternally-derived, anti-dengue antibodies titres have waned to sub-neutralizing levels.13 Pre-existing, non-neutralizing, heterotypic antibodies resulting from a prior dengue infection would enhance infection via the binding of virus-antibody complexes to the surface of cells expressing receptors for the Fc fragment of IgG antibodies (FcγR). Extrapolating this hypothesis to vaccination, it has been suggested that incomplete seroprotection against all 4 serotypes, such as that which occurs before the completion of a multiple-dose vaccination regimen, may enhance viremia and reactogenicity upon subsequent vaccinations or enhance disease severity if the case of wild-type dengue infection. Results from sequential bivalent vaccination show that partial immunization with the CYD viruses do not have enhancing effects, at least in the short term (<3.5 months), and instead present cross-neutralizing activity toward heterologous vaccine serotypes. The strongest evidence so far in support of the safety of a multiple dose vaccination CYD-TDV regimen, is provided by the phase IIb efficacy study in Ratchaburi, Thailand where no increased risk for severe disease was seen during the year between the first and third vaccination of more than 2600 children.14 A similar lack of enhanced reactogenicity and replication after sequential, heterotypic dengue vaccinations was recently reported by Durbin et al. who investigated live, attenuated, monovalent dengue vaccines based on the DEN4Δ30 vaccine virus.15
While sequential complementary bivalent vaccination, administered 8 weeks apart, induced a balanced response against all 4 serotypes in monkeys, it did not in humans. It is possible that the interval of time during which serotype cross-neutralization takes place is longer in humans than in monkeys, making this sequential bivalent approach inefficient in humans within a short interval of time. Such questions regarding optimal time intervals between 2 administrations of live vaccines have also been raised by other authors.16
The blended CYD-1;3;4/VDV-2 vaccine appeared to increase the immune response particularly after the second vaccination and this response appeared to be longer lasting. Despite the limitation of the cellular immunity analyses due to poor sample viability, significant cellular responses were seen in samples collected from this group against both structural and DENV NS3 antigens. CD8+ responses against DENV NS3 were almost exclusively detected in this group, in agreement with previous analyses comparing in naive volunteers anti-NS3 T cell responses induced by VDVs and CYDs.17 In addition, analyses of cellular responses in the group immunized by sequential bivalent vaccines further demonstrated the DEN serotype specificity of the induced T cell responses.
Pre-existing immunity to yellow fever has been observed to enhance the immune response to CYD vaccination in humans: 2 CYD-TDV vaccinations given to YF-primed individuals were seen to be similarly immunogenic to 3 CYD-TDV vaccinations in flavivirus-naïve individuals, one month after the last vaccination.18 Here JE-vaccination of humans also appeared to provide some priming effect. Indeed, the highest seropositivity rates and GMTs to the 4 serotypes in our study were seen after one CYD-TDV vaccination in JE-primed participants. However, the persistence of this priming effect is unclear, as titres observed at the 1 y timepoint were comparable between the CYD-TDV group and the JE-primed group. It has been suggested that the initial effect is due in a significant part to the induction of a cross-reactive response of short duration, maybe involving IgM antibodies. Such shorter duration of low-affinity responses compared to high-avidity ones has also been observed for T cell responses.19 Importantly, JE priming had no visible effect on either the safety profile or viraemia, allowing the CYD dengue vaccine to be evaluated in trials in populations who are routinely vaccinated against JE.
This study was not designed to document the immunogenicity of a full dengue vaccination schedule, which is now being evaluated in phase III trials with a 0–6–12 month vaccination regimen. However, overall, immune responses were consistent with previously reported observations after the first 2 dengue vaccinations.10-12,18 CYD-4 was immunodominant in all groups that received all 4 serotypes simultaneously, particularly after the first vaccination in flavivirus-naïve individuals. Antibody responses increased (higher GMTs and seropositivity rates) and broadened (better balance between serotypes) after the second dengue vaccination. After a third dose of tetravalent CYD vaccination, which was not tested here, immune responses have been shown to consistently result in a balanced antibody response against all 4 serotypes, as measured with a PRNT50 assay on Vero cells.11,12,18,20-22 A microneutralization assay was originally planned to determine antibody titers to each of the 4 dengue virus serotypes in this study. However, the PRNT50 assay on Vero cells was later adopted in the CYD project. Hence, the report of results from this study was delayed to ensure consistency of immunogenicity data throughout the project.
All of the tested vaccination regimens were well tolerated and had an acceptable safety profile, consistent with previous studies.10-12,18,22 Injection site pain was consistently the most common injection site reaction and headache the most common systemic reaction. Levels of viraemia were low and generally below the level of quantitation, again consistent with results from previous clinical studies, as well as in studies conducted in a monkey model.9
There are number of limitations to this exploratory study that should be acknowledged, including the relatively small sample size and the fact that only descriptive analyses were performed. In addition, results of 4 of the original 5 arms are presented in this report due to intellectual property reasons; however, the conclusions of the study still remain valid. Vaccination with the 4 CYD vaccines twice in the blended CYD/VDV and tetravalent CYD-TDV groups, but only once in the bivalent and JE-primed groups, also means that the results must be interpreted with caution. JE-VAX®, a vaccine manufactured by Sanofi Pasteur and commercially available at the time of the study, was selected for JE priming. Although this vaccine has been discontinued and other cell culture JE vaccines are available, we believe this was a valid option for JE priming purposes, as it induces JE-specific neutralizing antibody responses like the currently available commercial vaccines. The CYD vaccination schedule used here differs from the 3-dose schedule in flavivirus naïve individuals that is currently under investigation and development.
In conclusion, this exploratory study showed that all of the tested dengue vaccination regimens were well tolerated and no safety issues were observed. A blended formulation containing VDV2 combined with CYD1, 3 and 4 appeared to show some benefit in terms of both antibody and cellular immune responses. The bivalent vaccination approach showed no advantage in terms of immunogenicity or safety, but the observation that a first bivalent vaccination against dengue serotypes 1 and 3 did not enhance the replication, reactogenicity, and immunogenicity of a second bivalent vaccination against serotypes 2 and 4, is of particular importance as it implies that partial immunity elicited by incomplete tetravalent CYD vaccination (e.g., after the first vaccination) will not enhance disease in the case of infection with dengue before the completion of the full vaccination regimen.
Materials and Methods
Trial design and participants
In an open, randomized, controlled, phase IIa trial conducted in 2008–2009, 150 healthy adults aged 18–45 y were enrolled at 2 centers in Mexico City, a dengue non-endemic area. The main exclusion criteria were: pregnancy or breast-feeding, human immunodeficiency virus, hepatitis B or C seropositivity, immunodeficiency or any other chronic illness that could interfere with the results, previous residence in or travel of >2 weeks to areas with high dengue endemicity, a history of flavivirus infection or previous vaccination against flavivirus disease. Women who were capable of conceiving were required to use an effective method of contraception or abstinence for at least 4 weeks before the first injection until at least 4 weeks after the last injection.
Participants were randomized into 5 groups and vaccinations were performed on day [D] 0 and D105 (±15 days) (Table 5). In order not to delay the publication of the present results, which bring important information on the nature and potential role of the humoral and cellular response induced by the CYD vaccine, one of the groups (i.e., the concomitant bivalent group that received CYD-1;3 and CYD-2;4 concomitantly) had to be excluded from this report for intellectual property reasons. Therefore, the following 4 groups are presented: 1. the sequential bivalent group received CYD-1;3 on day 0 and CYD-2;4 on day 105 (±15 days), 2. the blended CYD/VDV received a tetravalent combination of CYD-1;3;4 and a Vero cell adapted attenuated dengue serotype 2 vaccine (VDV-2), 3.the tetravalent CYD-TDV group received CYD-TDV, and 4. the JE-primed group received JE vaccine on days -14 and -7 and 0 followed by CYD-TDV on day 105.
Table 5.
Vaccination schedule
| Day 0 | Day 105 | |
|---|---|---|
| Sequential bivalent | Bivalent CYD-1;3 | Bivalent CYD-2;4 |
| Blended CYD/CYD | Tetravalent blend of CYD-1;3;4 + VDV-2 | Tetravalent blend CYD-1;3;4 + VDV-2 |
| Tetravalent CYD-TDV | Tetravalent CYD | Tetravalent CYD |
| JE-primed | JE vaccine | Tetravalent CYD |
*CYD = ChimeriVax™ dengue / VDV = Vero dengue vaccine.
Clinical assessments were performed: at screening; on days -14 and -7 (JE-primed group only); on days 0, 7, 14, 21, 28 and 60 after each of the 2 injections; and on day 365 after the first injection
The trial was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practices, as well as national and local ethical requirements. All participants gave their written informed consent prior to entering the trial.
Vaccines
All vaccines were supplied by Sanofi Pasteur and were provided as single doses, in the form of a powder and solvent for reconstitution immediately prior to injection. All injections were administered subcutaneously to the deltoid region of the upper arm.
The bivalent and tetravalent dengue vaccines contained approximately 5 log10 tissue culture infectious dose (TCID50) of each serotype of CYD contained in the vaccine. The blended tetravalent CYD-1;3;4/VDV-2 vaccine contained 4 log10 TCID50 of VDV-2 per 0.5 ml dose. The CYD-1–4 viruses have been described previously.7 VDV-2 was derived from the live, attenuated 16681 PDK 53 strain and was plaque purified and adapted to production in Vero cells,23 as it has been done for VDV3.24.
Japanese encephalitis virus vaccine was a commercially available inactivated vaccine presented as a lyophilized powder for reconstitution in 1 ml of solvent (JE-VAX®).
Safety and reactogenicity
The following were recorded: unsolicited systemic adverse events (any untoward medical occurrence) or reactions occurring within 30 minutes after each injection, solicited injection site reactions (pain, erythema, edema) occurring within 7 d after injection, solicited systemic adverse reactions (fever, headache, malaise, myalgia, asthenia) occurring within 21 d after injection, unsolicited adverse events (AEs) or reactions—AEs judged to be related to the vaccine by the investigator—occurring within 28 d after injection, serious adverse events occurring at any time, and out-of-normal range laboratory results (biochemistry, haematology) on days 0, 7, 14 and 28 after each injection. Grade 3 non-measurable adverse reactions were those incapacitating and preventing daily activities. Grade 3 measurable adverse reactions were defined as: a fever >39 °C, erythema or edema ≥5 cm.
Participants were provided with a safety diary card, a thermometer and a ruler to record adverse events and reactions and their intensity.
Viraemia
As part of the safety evaluation, the presence of CYD-1–4 or VDV-2 was assessed in serum collected 7, 14 and 21 d after each injection. Analyses were performed by sponsor's Global Clinical Immunology laboratory (Sanofi Pasteur, Swiftwater, PA, USA).
Analyses for CYD-1–4 viraemia were performed in 2 steps, as previously described.10 Briefly, a first, non-serotype-specific, reverse transcriptase-polymerase chain reaction (RT-PCR) was used to detect the presence of any of the 4 CYD viruses. Samples that were positive in this first test were then analyzed using 4 CYD serotype-specific quantitative RT-PCRs. In the non-serotype-specific RT-PCR, RNA was extracted from the serum using a commercial kit and a RT-PCR was carried out with primers from the yellow fever core gene sequence. In the serotype-specific RT-PCRs, RNA was again extracted from the serum using a commercial kit and a RT-PCR was carried out with serotype-specific primers from the envelope non-structural protein 1 junction gene sequence for each serotype. A dengue RT-PCR for serotype 2 was performed in the blending CYD/VDV group as the tetravalent blending formulation administered to this group contained the VDV-2 virus.
Immunogenicity
Humoral immune response
Antibody levels to each of the 4 dengue virus serotypes were determined by 50% plaque reduction neutralisation test on serum collected on the day of each injection and 28 d later, as well as on day 365 after the first injection.25 Briefly, serial fold2- dilutions dilutions of serum to be tested (previously heat-inactivated) were mixed with a constant challenge dose of each dengue serotype DENV-1, -2, -2, or -4 (expressed as plaque forming unit [PFU]/mL). The mixtures were inoculated into wells of a 24-well plate of confluent Vero cell monolayers. After incubation for several days, dengue virus infection is indicated by formation of plaques. The neutralizing antibody titer is calculated as the highest reciprocal dilution (1/dil) of serum at which ≥50 % reduction in viral plaque count is observed (PRNT50). The lower limit of quantitation of the dengue PRNT50 is 10 and samples with titres ≥10 were considered seropositive. Similarly to 2 other clinical studies performed at the same time as this one, the PRNT assay replaced the initially planned microneutralization test.18,26 Analyses were performed by the sponsor's Global Clinical Immunology laboratory (Sanofi Pasteur, Swiftwater, PA, USA).
Cellular immune response
Specific immunity was evaluated as previously at the Sponsor's Research Department by cytometric bead array (CBA) and intracellular cytokine staining (ICS) after antigenic stimulation of cells collected in 3 groups of subjects (sequential bivalent, blended CYD/VDV and tetravalent CYD-TDV groups) before and 28 d after each immunization.17 Briefly, PBMCs were adjusted to 2 × 106 cells/mL in RPMI+GSP+5%SAB and 2.5 × 105 cells (125μL) were added to each well of a 96-well round bottom plate. PBMCs were stimulated in simplicate in vitro for 4 d at 37°C ± 1°C with 0.5 multiplicity of infection (MOI) monovalent vaccines CYD-1, -2,-3, -4. CY110, vaccine stabilizer, was used as negative control and a combination of 1μg/mL Phyto-hemagglutinin (Remel) and 10ng/mL phorbol-myristate-acetate (PMA) (Sigma) was used as positive control Secreted Th1/Th2 cytokines were measured by CBA (Human Th1/Th2 Cytokine Kit, Becton Dickinson, San Jose, USA) following the manufacturer's instructions. Data were acquired with the BD FACSArray Bioanalyzer (Becton Dickinson, San Jose, USA) and analyzed with BD FCAP software. Values were calculated as Δ(D28-D0) stimulation-Δ(D28-D0) medium.
Statistical methods
The study was descriptive, with no hypothesis testing. The planned sample size of 30 participants per group provided a 95% probability of observing an event with a true incidence of 9.5%.
All analyses were descriptive and presented by group with 95% confidence intervals (CIs) for the main parameters. Unsolicited adverse events were coded according to System Organ Class (SOC) and Preferred Term using the Medical Dictionary for Regulatory Activities (MedDRA). For neutralizing antibody data, geometric mean titres (GMTs) were calculated. Participants with titres ≥10 were considered seropositive for the corresponding serotype. Statistical analyses were performed using SAS® software (version 8.2 or later).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Grenville Marsh at Sanofi Pasteur for for assisting with preparation of manuscript drafts. The authors would like to thank all the volunteers who participated in the trial, and the study-site personnel for their contributions to the study. In addition, thanks are due to the following people within Sanofi Pasteur: Mark Boaz, Dany de Grave and Branda Hu, for their contributions in the development and improvement of immunological assays, and the Global Clinical Immunology Department for conducting the immunological assays; Martin Sanchez-Ruiz for study management and logistics; and Fernando Noriega, Melanie Saville, and Nadia Tornieporth for their contributions to the study design and development.
Funding
This study was funded by Sanofi Pasteur. GD, RF, BZ, AB, AH, BG and JL are employed by Sanofi Pasteur. JFGH has no conflict of interest.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature 2013; 496:504-7; PMID:23563266; http://dx.doi.org/ 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brathwaite Dick O, San Martin JL, Montoya RH, del Diego J, Zambrano B, Dayan GH. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg 2012; 87:584-93; PMID:23042846; http://dx.doi.org/ 10.4269/ajtmh.2012.11-0770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmons CP, Farrar JJ, Nguyen vV, Wills B. Dengue N Engl J Med 2012; 366:1423-32; PMID:22494122; http://dx.doi.org/ 10.1056/NEJMra1110265 [DOI] [PubMed] [Google Scholar]
- 4.Kurane I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp Immunol Microbiol Infect Dis 2007; 30:329-40; PMID:17645944; http://dx.doi.org/ 10.1016/j.cimid.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 5.Murrell S, Wu SC, Butler M. Review of dengue virus and the development of a vaccine. Biotechnol Adv 2011; 29:239-47; PMID:21146601; http://dx.doi.org/ 10.1016/j.biotechadv.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 6.Sabchareon A, Wallace D, Lang J, Bouckenooghe A, Moureau A. Efficacy of tetravalent dengue vaccine in Thai schoolchildren - Authors' reply. Lancet 2013; 381:1094-5; PMID:23540847; http://dx.doi.org/ 10.1016/S0140-6736(13)60755-2 [DOI] [PubMed] [Google Scholar]
- 7.Guirakhoo F, Arroyo J, Pugachev KV, Miller C, Zhang ZX, Weltzin R, Georgakopoulos K, Catalan J, Ocran S, Soike K, et al. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J Virol 2001; 75:7290-304; PMID:11462001; http://dx.doi.org/ 10.1128/JVI.75.16.7290-7304.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson KB, Gibbons RV, Edelman R, Eckels KH, Putnak RJ, Innis BL, Sun W. Interference and facilitation between dengue serotypes in a tetravalent live dengue virus vaccine candidate. J Infect Dis 2011; 204:442-50; PMID:21742844; http://dx.doi.org/ 10.1093/infdis/jir279 [DOI] [PubMed] [Google Scholar]
- 9.Guy B, Barban V, Mantel N, Aguirre M, Gulia S, Pontvianne J, Jourdier TM, Ramirez L, Gregoire V, Charnay C, et al. Evaluation of interferences between dengue vaccine serotypes in a monkey model. Am J Trop Med Hyg 2009; 80:302-11; PMID:19190230 [PubMed] [Google Scholar]
- 10.Poo J, Galan F, Forrat R, Zambrano B, Lang J, Dayan GH. Live-attenuated Tetravalent Dengue Vaccine in Dengue-naive Children, Adolescents, and Adults in Mexico City:Randomized Controlled Phase 1 Trial of Safety and Immunogenicity. Pediatr Infect Dis J 2011; 30:e9-17; http://dx.doi.org/ 10.1097/INF.0b013e3181fe05af [DOI] [PubMed] [Google Scholar]
- 11.Morrison D, Legg TJ, Billings CW, Forrat R, Yoksan S, Lang J. A novel tetravalent dengue vaccine is well tolerated and immunogenic against all 4 serotypes in flavivirus-naive adults. J Infect Dis 2010; 201:370-7; PMID:20059357; http://dx.doi.org/ 10.1086/649916 [DOI] [PubMed] [Google Scholar]
- 12.Capeding RZ, Luna IA, Bomasang E, Lupisan S, Lang J, Forrat R, Wartel A, Crevat D. Live-attenuated, tetravalent dengue vaccine in children, adolescents and adults in a dengue endemic country: randomized controlled phase I trial in the Philippines. Vaccine 2011; 29:3863-72; PMID:21477675; http://dx.doi.org/ 10.1016/j.vaccine.2011.03.057 [DOI] [PubMed] [Google Scholar]
- 13.Halstead SB. Dengue. Lancet 2007; 370:1644-52; PMID:17993365; http://dx.doi.org/ 10.1016/S0140-6736(07)61687-0 [DOI] [PubMed] [Google Scholar]
- 14.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 2012; 380:1559-67; PMID:22975340; http://dx.doi.org/ 10.1016/S0140-6736(12)61428-7 [DOI] [PubMed] [Google Scholar]
- 15.Durbin AP, Schmidt A, Elwood D, Wanionek KA, Lovchik J, Thumar B, Murphy BR, Whitehead SS. Heterotypic dengue infection with live attenuated monotypic dengue virus vaccines: implications for vaccination of populations in areas where dengue is endemic. J Infect Dis 2011; 203:327-34; PMID:21208923; http://dx.doi.org/ 10.1093/infdis/jiq059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons M, Burgess T, Lynch J, Putnak R. Protection against dengue virus by non-replicating and live attenuated vaccines used together in a prime boost vaccination strategy. Virology 2010; 396:280-8; PMID:19913867; http://dx.doi.org/ 10.1016/j.virol.2009.10.023 [DOI] [PubMed] [Google Scholar]
- 17.Guy B, Nougarede N, Begue S, Sanchez V, Souag N, Carre M, Chambonneau L, Morrisson DN, Shaw D, Qiao M, et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 2008; 26:5712-21; PMID:18762226; http://dx.doi.org/ 10.1016/j.vaccine.2008.08.019 [DOI] [PubMed] [Google Scholar]
- 18.Lanata CF, Andrade T, Gil AI, Terrones C, Valladolid O, Zambrano B, Saville M, Crevat D. Immunogenicity and safety of tetravalent dengue vaccine in 2-11 year-olds previously vaccinated against yellow fever: randomized, controlled, phase II study in Piura, Peru. Vaccine 2012; 30:5935-41; PMID:22863660; http://dx.doi.org/ 10.1016/j.vaccine.2012.07.043 [DOI] [PubMed] [Google Scholar]
- 19.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature 2009; 458:211-4; PMID:19182777; http://dx.doi.org/ 10.1038/nature07657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran NH, Luong CQ, Vu TQH, Forrat R, Lang J, Quoc DV, Bouckenooghe , Wartel TA. Safety and Immunogenicity of Recombinant, Live Attenuated Tetravalent Dengue Vaccine (CYD- TDV) in Healthy Vietnamese Adults and Children. J Vaccines Vaccin 2012; 3:7. [Google Scholar]
- 21.Leo YS, Wilder-Smith A, Archuleta S, Shek LP, Chong CY, Leong HN, Low CY, Oh ML, Bouckenooghe A, Wartel TA, et al. Immunogenicity and safety of recombinant tetravalent dengue vaccine (CYD-TDV) in individuals aged 2-45 y: Phase II randomized controlled trial in Singapore. Hum Vaccin Immunother 2012; 8:1259-71; PMID:22894958; http://dx.doi.org/ 10.4161/hv.21224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabchareon A, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Margolis HS, Letson GW. Dengue infection in children in Ratchaburi, Thailand: a cohort study. I. Epidemiology of symptomatic acute dengue infection in children, 2006-2009. PLoS Negl Trop Dis 2012; 6:e1732; PMID:22860141; http://dx.doi.org/ 10.1371/journal.pntd.0001732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabchareon A, Lang J, Chanthavanich P, Yoksan S, Forrat R, Attanath P, Sirivichayakul C, Pengsaa K, Pojjaroen-Anant C, Chokejindachai W, et al. Safety and immunogenicity of tetravalent live-attenuated dengue vaccines in Thai adult volunteers: role of serotype concentration, ratio, and multiple doses. Am J Trop Med Hyg 2002; 66:264-72; PMID:12139219 [DOI] [PubMed] [Google Scholar]
- 24.Sanchez V, Gimenez S, Tomlinson B, Chan P, Thomas N, Forrat R, Chambonneau L, Lang J, Guy B. Innate and adaptive cellular immunity in flavivirus-naive human recipients of a live-attenuated dengue serotype 3 vaccine produced in Vero cells (VDV3). Vaccine 2006; 24:4914-26; PMID:16632108; http://dx.doi.org/ 10.1016/j.vaccine.2006.03.066 [DOI] [PubMed] [Google Scholar]
- 25.Timiryasova TM, Bonaparte MI, Luo P, Zedar R, Hu BT, Hildreth SW. Optimization and validation of a plaque reduction neutralization test for the detection of neutralizing antibodies to four serotypes of dengue virus used in support of dengue vaccine development. Am J Trop Med Hyg 2013; 88:962-70; PMID:23458954; http://dx.doi.org/ 10.4269/ajtmh.12-0461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dayan GH, Thakur M, Boaz M, Johnson C. Safety and immunogenicity of three tetravalent dengue vaccine formulations in healthy adults in the USA. Vaccine 2013; 31:5047-54; PMID:24021313; http://dx.doi.org/ 10.1016/j.vaccine.2013.08.088 [DOI] [PubMed] [Google Scholar]


