Abstract
Respiratory viruses cause significant morbidity and mortality in infants and young children worldwide. Current strategies to modulate the immune system and prevent or treat respiratory viral infections in this age group have shown limited success. Here, we demonstrate that a lysate derived from Gram-positive and Gram-negative organisms positively modulates protective antibody responses against both respiratory syncytial virus (RSV) and influenza virus in murine models of infection. Interestingly, despite the complex mixture of Toll-like receptor (TLR) agonists present in the bacterial lysate, the modulatory effects were mostly dependent on TLR4 signaling. Our results indicate that the use of simple formulations of TLR-agonists can significantly improve the immune response against critical pediatric respiratory pathogens.
Keywords: respiratory, virus, RSV, influenza, bacterial, lysates, extracts, adjuvants, immunomodulators
Introduction
Respiratory syncytial virus (RSV) and influenza virus cause significant morbidity and mortality in infants and young children.1-3 RSV is the main cause of hospitalization in this age group worldwide, whereas influenza is responsible for fatalities in young children and has been associated with asthma exacerbations in older children and adults.4-6 Approximately 95% of children are infected with RSV by the age of two, and 40% of preschool-aged children experience an influenza infection.7-10 Preventive and therapeutic interventions are urgently needed to reduce the burden of these childhood respiratory illnesses.
Many strategies have been proposed to modulate the immune system and prevent or treat respiratory viral infections.11,12 Use of type I interferons against influenza, corticosteroids to ameliorate the symptoms of bronchiolitis associated with RSV infections, and anti-inflammatory agents to modulate severity of RSV disease exemplify these approaches.13-16 Unfortunately, none has proven clinically successful.
On the other hand, vaccines to prevent viral respiratory diseases present important challenges.17 No pediatric vaccine is available today to protect infants younger than six months of age against any respiratory virus, and only influenza can be modulated through vaccination in older children and pregnant women.
Pattern recognition receptors (PRR) recognize highly conserved sets of molecular structures called pathogen-associated molecular patterns (PAMPs), which are foreign to the host, and specific for large groups of microbes.18-20 Toll-like receptors (TLRs) are a family of PRRs that recognize PAMPs and initiate signaling events leading to activation of innate host defenses. TLRs recognize conserved molecules derived from different classes of microorganisms, including Gram-positive and Gram-negative bacteria, RNA and DNA viruses, as well as fungi and protozoa.18-20 Signaling by TLRs in host cells induces expression of antimicrobial genes and production of inflammatory cytokines and chemokines.20,21 The production of pro-inflammatory cytokines upon sensing of microbial products is critical for innate and adaptive immune responses to infection.22 In the last decade, a role for TLRs in promoting B cell responses has been described.23
In recent years, bacterial lysates have been used successfully to modulate the risk of allergies and atopy.24 In this study, we tested a bacterial lysate containing different Gram-negative and Gram-positive microorganisms for its ability to activate TLRs and indirectly modulate antibody responses against RSV and influenza virus in murine models of infection.
Results
Bacterial lysate promotes inflammatory cytokine production and activates TLR2 and TLR4
First, we evaluated the immunomodulatory effects of the bacterial lysate in vitro, exploring pro-inflammatory cytokine production in THP-1 cells. The bacterial lysate promoted TNF-α and IL-1β production in THP-1 cells dose dependently (Fig. 1A & not shown). We then investigated the effect of bacterial lysates in human peripheral blood mononuclear cells (PBMC). Again, production of IL-6, IL-1β and TNF-α was dose-dependently increased after exposure of cells to bacterial products (Fig. 1B–D).
Figure 1.
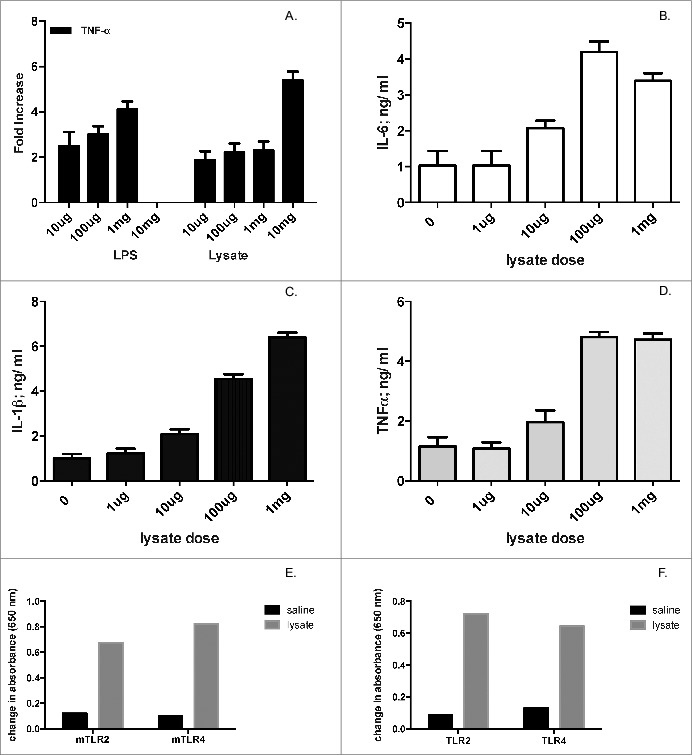
Bacterial lysate promotes inflammatory cytokine production and activates TLR2 and TLR4. (A) THP-1 cells (106 cells/well) were stimulated with various doses of LPS (for comparison) or bacterial lysate, and TNF-α production (in ng/mL) was subsequently measured in the supernatants 24hs post stimulation. Panel represents fold-increase in cytokine production. (B, C, D) Human PBMC (106 cells/well) were stimulated with different concentrations of bacterial lysate, and subsequently assayed for IL-6, IL-1β and TNF-α production 24 hs after stimulation. (E & F) Murine and human TLR activation by bacterial lysate in HEK293 cells expressing individual TLRs (see Materials and Methods). Ultrapure LPS from E. coli and peptidoglycan from E. coli K12 were used as positive controls for TLR4 and TLR2, respectively (data not shown). Two independent experiments were performed for each experimental paradigm; panels show one representative experiment of each pair.
Given the immunomodulatory effect of the bacterial lysate, we subsequently examined whether the lysate activated specific PRR. In particular, we were interested in exploring its effect on TLR2 and TLR4. Activation of TLR2 enhances germinal center formation and promotes secretion of IgM in B cells from tonsils,26 as well as IgA in mucosal B cells.27 TLR4 plays a critical role in B cell maturation and antibody production.23 TLR activation by lysate was evaluated on HEK293 cells expressing murine or human TLR2 or TLR4. The system relied on a secreted alkaline phosphatase reporter under the control of a promoter inducible by NFκB. The bacterial lysate was able to activate both human and murine TLR2 and TLR4 (Fig. 1E & F).
Bacterial lysate improves humoral immunity against respiratory viruses
We subsequently explored the role of bacterial lysate supplementation on antibody responses against respiratory viruses. For this purpose, BALB/c mice were treated intranasally (IN) with lysate for three consecutive days (days -1,0 and 1) before and during inoculation with influenza H1N1 virus. Influenza H1N1 virus titers in lungs of mice three days after inoculation with H1N1 plus lysate or placebo were not significantly different (mean H1N1 titers, with lysate: 1×104 pfu/ g vs. no lysate: 1.2×104 pfu/ g; p = 0.45). Levels of influenza-specific IgG antibodies were assayed in serum after infection. Virus-specific IgG levels against the H1 hemagglutinin were significantly enhanced by lysate supplementation when compared to those detected in mice inoculated only with influenza virus (Fig. 2A). Biological activity of these antibodies, assessed by characterizing their avidity for the H1 hemagglutinin, also revealed positive modulation by co-administration of lysate. Mice treated with lysate had anti-H1 IgG of greater avidity for influenza H1N1 compared to animals infected with H1N1 virus in the absence of bacterial lysate (Fig. 2B). Finally, we investigated whether the bacterial lysate enhanced protective responses against influenza. Protection against the virus was assessed by influenza hemagglutinin inhibition (HAI) assay, a response affected both by quantity and quality of the anti-H1 response. Indeed, HAI titers doubled in lysate-recipients infected with influenza A H1N1 when compared to recipients of virus alone (Fig. 2C).
Figure 2.
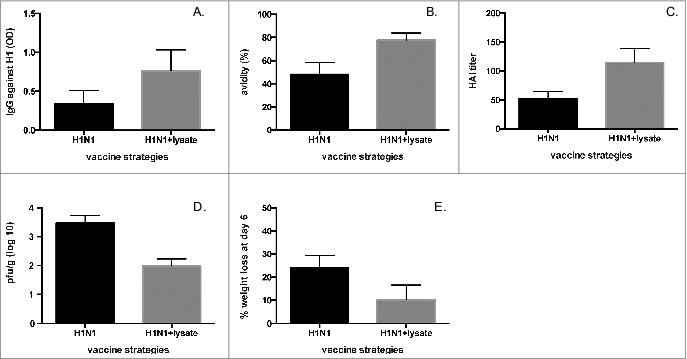
Bacterial lysate improves antibody response against influenza H1N1 virus. (A) Immunoassays for IgG against H1 (p for comparison = 0.028), (B) avidity of IgG for H1 (p for comparison = 0.042), and (C) hemagglutinin-inhibition of H1N1 influenza virus (p for comparison = 0.03) in serum from BALB/c mice obtained 60 days after intranasal infection with 106 PFU influenza virus A (A/new Caledonia 120/99-like (H1N1)) in the presence or absence of lysate as described in Materials and Methods. (D) BALB/c mice were inoculated with H1N1 (104.5 pfu) either alone or with bacterial lysate, challenged 28 days later with H1N1 (106 pfu), and lung viral titers were assessed 4 days after challenge. (E) Percentage of body weight loss was determined on the same experimental group described in (D) at day 6 after challenge. All experiments were performed in duplicates using 6-10 mice/group.
Intranasal co-inoculation of bacterial lysate with RSV also enhanced the anti-RSV antibody response (Fig. 3A). A positive effect of lysate co-administration was also observed for avidity of IgG against RSV F (Fig. 3B). Importantly, neutralization of RSV significantly improved in recipients of lysate compared to mice immunized with RSV alone (Fig. 3C). All these observations did not correlate with differences in RSV titers in the lungs of mice inoculated with RSV plus lysate vs. RSV alone (RSV lung titers four days post-inoculation, with lysate: 1×104 pfu/g; no lysate: 9×103 pfu/g; p = 0.86).
Figure 3.
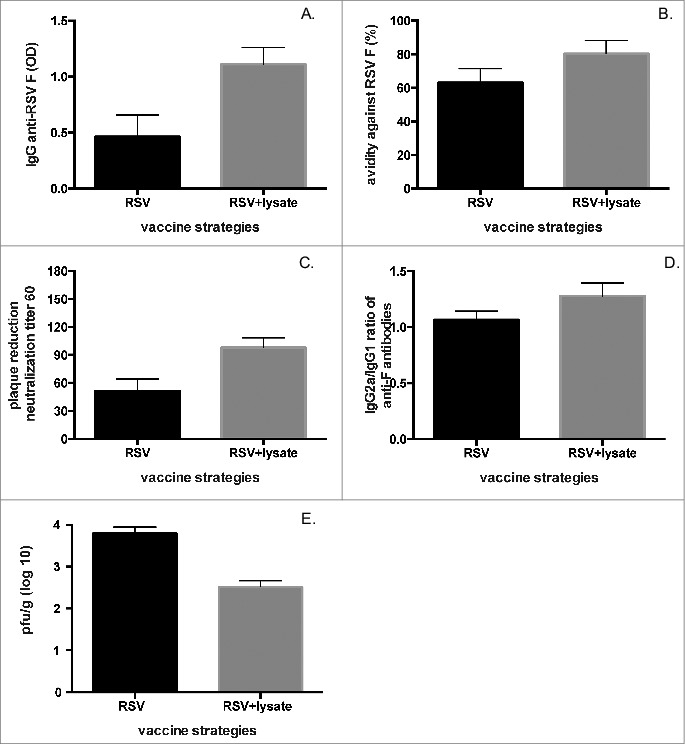
Bacterial lysate improves antibody response against RSV A2. (A) immunoassays for IgG against RSV F (p for comparison = 0.046), (B) avidity of IgG for RSV F (p for comparison = 0.038), (C) plaque reduction neutralization test (PRNT60) for RSV A2 (p for comparison<0.01), and (D) IgG2a/IgG1 ratio against RSV F (p = 0.04) in serum from BALB/c mice obtained 60 days after intranasal infection with RSV A2 (106 PFU) in the presence or absence of lysate as described in Materials and Methods. (E) BALB/c mice were inoculated with RSV A2 (104.5 pfu) either alone or with lysate, challenged 28 days later with the same virus (106 pfu), and viral titers in the lungs of the animals were assessed 4 days after challenge. All experiments were performed in duplicates using 6-8 mice/group.
Then, since RSV disease in children has been associated with a T helper type 2 (Th2) polarization of the immune response against the virus,28-31 we investigated whether the bacterial lysate could bias Th responses against RSV. Treatment with lysate decreased anti-RSV IgG1/IgG2a ratios post-infection, polarizing the anti-RSV response towards Th1 (Fig. 3D).
Finally, we inoculated 1×104.5 pfu of RSV alone or with lysate IN and challenged mice with 106 pfu of RSV IN 28 days after immunization (Fig. 3E). Interestingly, RSV lung titers after challenge were significantly lower in lysate recipients than in controls, showing that improved quantity and quality of protective responses translated into a better control of viral production, a parameter typically associated with milder illness (Fig. 3E). A similar protective effect upon secondary infection was observed for influenza H1N1, with decreased virus titer (Fig. 2D) and weight loss in lysate recipients (Fig. 2E).
The modulatory effect of the bacterial lysate is mainly elicited through TLR4
We then investigated whether the positive modulation of antibody production against respiratory viruses elicited by bacterial lysate was dependent on PRR activation. For this purpose, wild type C57BL/10, TLR2−/− and TLR4−/− mice were infected with RSV or influenza H1N1 in the presence or absence of bacterial lysate (Fig. 4).
Figure 4.
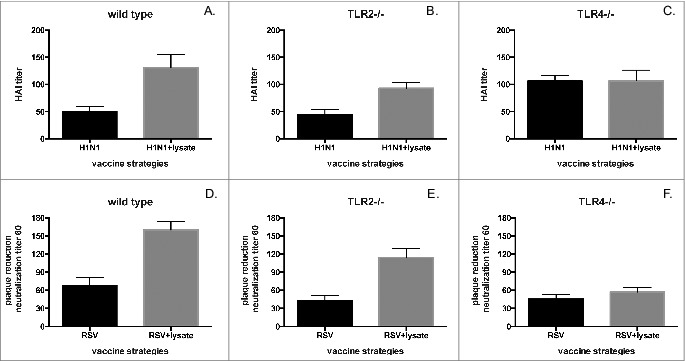
The modulatory effect of bacterial lysate is mainly elicited through TLR4. HAI titer of influenza A H1N1 virus in (A) wild type (p = 0.01), (B) TLR2−/− (p = 0.032) and (C) TLR4−/− (p = NS) mice 60 days after intranasal infection with 106 PFU of influenza A/new Caledonia 120/99-like (H1N1) in the presence or absence of lysate. PRNT60 for RSV in (D) wild type (p = 0.012), (E) TLR2−/− (p = 0.045) and (F) TLR4−/− (p = NS) mice 60 days after intranasal infection with RSV A2 (106 PFU) in the presence or absence of lysate.
As previously described for BALB/c mice, anti-influenza H1N1 protective HAI and anti-RSV neutralizing responses were greater in recipients of virus plus lysate than in mice inoculated only with either virus, both in WT and TLR2−/− mice, albeit at a somewhat reduced level in the knock-out animals (Fig 4 A, B, D, E). However, no differences in HAI or neutralizing anti-RSV responses were observed in TLR4−/− mice inoculated with viruses in the presence or absence of lysate (Fig. 4 C & F). These findings confirm the positive modulation elicited by bacterial lysate against respiratory pathogens, and identify TLR4 as a main mediator of these protective responses.
Discussion
In this manuscript, we describe a novel and simple intervention in mice that enhances the antibody response against respiratory viruses, the main pediatric agents of morbidity worldwide. We show that administration of a bacterial lysate containing various TLR agonists improves the humoral response against influenza and respiratory syncytial virus, enhancing the quantity, quality, and protective efficacy of antibodies in murine models of infection. Interestingly, despite the complex mixture of TLR agonists present in the bacterial lysate, the observed modulatory effects were mostly dependent on TLR4 signaling.
Several factors hamper vaccine development against respiratory viruses.16 Achieving protective antibody titers in young infants, for example, is challenging in the presence of transplacentally-acquired maternal antibodies that are sufficient to prevent vaccine take, but insufficient for protecting against wild type disease.32 Immune immaturity in infants affects protective responses against agents requiring protection early in life.33 Affinity maturation is critical to prevent severe immune imbalances resulting in disease enhancement when children are vaccinated against RSV or measles virus.17,34 Administration of bacterial lysates may modulate some of these responses, by enhancing IgG titers, promoting maturation of avidity against these agents, and improving protective responses. Similar observations have been reported using other PAMPs.23,35 Furthermore, TLR activation is critical to elicit affinity maturation against inactivated RSV vaccines, and prevent the enhanced bronchopneumonia resulting from RSV infection in infants immunized with non-replicating RSV vaccines.17 In influenza, TLR agonists have proven excellent adjuvants for non-replicating vaccines.36
The beneficial role of bacterial products in non-specifically modulating responses to infection is not surprising. Other adjuvants like complete Freund's, which contains heat-killed mycobacteria, have proven beneficial for immune responses when formulated as components of injectable vaccines.22 Similarly, administration of bacterial extracts by the oral route has been shown to stimulate protective mucosal immunity against viral and bacterial infections.37 In this report, we show that dried bacterial lysates delivered intranasally, following the natural route of inoculation by respiratory viruses, significantly boost specific immune responses against RSV and influenza. While our observations are in mice, co-habitation of humans with microbes for millennia suggests a strong evolutionary rationale for leveraging these interactions during routine bodily functions including protection against pathogens. In non-hygienic environments, the human immune system likely evolved to fight respiratory viral infections in the presence of a myriad of ubiquitous TLR agonists, and it is reasonable to assume that this multi-microbial context shaped and optimized the immune response against respiratory pathogens. The utilization of bacterial lysates to restore that context might have the potential to enhance anti-viral responses.
In summary, we show that intranasal delivery of cell lysates containing Gram-positive and Gram-negative bacterial products can activate the innate and adaptive immune response, resulting in positive modulation of protective antibody responses against critical pediatric respiratory pathogens.
Materials and Methods
Bacterial lysate
The bacterial lysate (final concentration 0.5 g/ml) contained 200 million organisms of each of the following bacteria: Streptococcus pneumoniae, Branhamella catarrhalis, Klebsiella pneumoniae, and Micrococcus spp. (respiratory micrococcus), and 100 million organisms of Haemophilus influenzae and Streptococcus spp. (i.e. S. anhemoliticus and S. viridans). Bacteria were sonicated to disrupt the bacterial cell wall, heated for an hour at 70°C, and then kept at 4°C for 24 hours before undergoing an additional cycle of 70°C treatment for an hour. After this treatment, the lysates were analyzed and shown not to contain any live organism.
Animals and treatment
BALB/c, C57BL/10, Tlr4−/− and Tlr2−/− mice were purchased from the Jackson Laboratory and kept in a controlled pathogen free animal facility. Antibody responses were assayed in groups of 6 to 10 female mice treated or not with the bacterial lysate (see Table 1 for composition; 10 mg/day) and infected intranasally (106 PFU) with either influenza virus A (A/New Caledonia 120/99-like (H1N1)) or respiratory syncytial virus, RSV (A2).
Table 1.
Lysate components and their interactions with TLRs
| TLR1 | TLR2 | TLR3 | TLR4 | TLR5 | TLR6 | TLR7 | TLR8 | TLR9 | |
|---|---|---|---|---|---|---|---|---|---|
| Streptococcus pneumoniae (Gram +) | − | + | − | + | + | + | − | − | + |
| Branhamella catarrhalis (Gram −) | − | UK | − | + | + | − | − | − | + |
| Haemophilus influenzae (Gram −) | − | + | UK | + | + | − | UK | − | + |
| Klebsiella pneumoniae (Gram −) | − | UK | − | + | + | − | − | − | + |
| Micrococcus (respiratory) (Gram +) | − | + | − | + | + | + | − | − | + |
UK: Unknown
Antibody assays
Sera were obtained from mice at 60 days post infection and tested for total anti-RSV F protein or total anti-H1 influenza antibodies using protein-specific immunoassays as described.25 For avidity determinations, a 10-minutes wash with a 7M urea solution (Sigma-Aldrich) was used before addition of secondary antibodies. T-helper cell bias studies analyzed the ratio between IgG1 and IgG2a by ELISA, using anti-IgG1 and anti-IgG2a HRP-conjugated antibodies (BD Biosciences) as previously described.17 Influenza hemagglutinin inhibition assay (HAI) and plaque reduction neutralization test (PRNT) were performed as described.17,25
In vitro assays
THP-1 cells grown on 96 well plates (106 cells/well) were incubated with various doses of bacterial lysate (10 μg, 1 mg, 10 mg) or LPS (10 μg, 100 μg, 1 mg). Supernatants were collected 24 h after stimulation, and TNF-α and IL-1β levels measured by ELISA (Biosource Europe). In addition, human peripheral blood mononuclear cells (PBMC) isolated from healthy volunteers using Ficoll-Hypaque gradients were grown at 106 cells/ml in RPMI cell culture media supplemented with 10% fetal bovine serum (FBS) and stimulated with different concentrations of bacterial lysate (1 μg, 10 μg, 100 μg, 1 mg). Levels of pro-inflammatory cytokines IL-6, TNF-α, and IL-1β present in supernatants were assayed by ELISA (Biosource Europe) 24 h after stimulation.
TLR ligand screening
TLR agonism was tested in HEK293 cells stably transfected with either TLR4 or TLR2 of human or murine origin (Invivogene). These cells express the soluble alkaline phosphatase (sAP) reporter gene under the control of the NF-κB promoter, which enables the quantification of cell activation by measuring sAP activity in medium containing specific enzyme substrates (OD at 655 nm). Ultrapure LPS from E. coli and peptidoglycan from E. coli K12 were used as positive controls for TLR4 and TLR2, respectively. No ligand (saline solution) was used as negative control.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The bacterial lysate used in these experiments was manufactured by Casasco, Argentina, who partly funded this study.
References
- 1.Simoes EA. Respiratory syncytial virus infection. Lancet 1999; 354: 847-52. [DOI] [PubMed] [Google Scholar]
- 2.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, and Anderson LJ. Bronchiolitis associated hospitalizations among US children, 1980-1996. JAMA 1999; 282:1440-6. [DOI] [PubMed] [Google Scholar]
- 3.Libster R, Bugna J, Coviello S, Hijano DR, Dunaiewsky M, Reynoso N, Cavalieri ML, Guglielmo MC, Areso MS, Gilligan T, et al. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Engl J Med 2010; 362: 45-55. [DOI] [PubMed] [Google Scholar]
- 4.Schanzer DL, Langley JM, and Tam TW. Hospitalization attributable to influenza and other viral respiratory illnesses in Canadian children. Pediatr Infect Dis J 2006; 25: 795-800. [DOI] [PubMed] [Google Scholar]
- 5.Iwane MK, Edwards KM, Szilagyi PG, Walker FJ, Griffin MR, Weinberg GA, Coulen C, Poehling KA, Shone LP, Balter S, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics 2004; 113:1758-64. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson KG, McNally T, Silverman M, Simons P, Stockton JD, and Zambon MC. Rates of hospitalization for influenza, respiratory syncytial virus and human metapneumovirus among infants and young children. Vaccine 2006; 24: 102-8. [DOI] [PubMed] [Google Scholar]
- 7.Monto AS, and Sullivan KM. Acute respiratory illness in the community: frequency of illness and the agents involved. Epidemiol Infect 1993; 110: 145-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, and Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179-86. [DOI] [PubMed] [Google Scholar]
- 9.Menec VH, Black C, MacWilliam L, and Aoki FY. The impact of influenza-associated respiratory illnesses on hospitalizations, physician visits, emergency room visits, and mortality. Can J Public Health 2003; 94: 59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr, and Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000; 342: 225-31. [DOI] [PubMed] [Google Scholar]
- 11.Hornef MW, Wick MJ, Rhen M. and Normark S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat Immunol 2002; 3: 1033-40. [DOI] [PubMed] [Google Scholar]
- 12.González PA, Carreño LJ, Bueno SM, Riedel CA, and Kalergis AM. Understanding respiratory syncytial virus infection to improve treatment and immunity. Curr Mol Med 2013; 13: 1122-39. [DOI] [PubMed] [Google Scholar]
- 13.Hall CB, Douglas RG, Jr, and Simons RL. Interferon production in adults with respiratory syncytial viral infection. Ann Intern Med 1981; 94: 53-5. [DOI] [PubMed] [Google Scholar]
- 14.Hayden FG, Fritz RS, Lobo MC, Alvord W, Strober W, and Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection: relation to symptom formation and host defense. J Clin Invest 1998; 101: 643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osterlund P, Pirhonen J, Ikonen N, Rönkkö E, Strengell M, Mäkelä SM, Broman M, Hamming OJ, Hartmann R, Ziegler T, et al. Pandemic H1N1 2009 influenza A virus induces weak cytokine responses in human macrophages and dendritic cells and is highly sensitive to the antiviral actions of interferon's. J Virol 2010; 84: 1414-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coviello S. and Polack FP. Prophylactic and therapeutic approaches against respiratory syncytial virus. Curr Med Chem Anti-Infective Agents 2005; 4: 67-73. [Google Scholar]
- 17.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med 2009; 15: 34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawai T. and Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010; 11: 373-84. [DOI] [PubMed] [Google Scholar]
- 19.Pasare C. and Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect 2004; 6:1382-7. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Kaisho T. and Akira S. Toll-like receptors. Annu Rev Immunol 2003; 21: 335-76. [DOI] [PubMed] [Google Scholar]
- 21.Janeway CA, Jr, and Medzhitov R. Innate immune recognition, Annu Rev Immunol 2002; 20: 197-216. [DOI] [PubMed] [Google Scholar]
- 22.Janeway CA, Travers P, Walport M. and Shlomchik M. Immunobiology. Fifth edition 2001; Garland Publishing. [Google Scholar]
- 23.Pasare C, and Medzhitov R. Control of B-cell responses by Toll-like receptor. Nature 2005; 438: 364-8. [DOI] [PubMed] [Google Scholar]
- 24.Lau S, Gerhold K, Zimmermann K, Ockeloen CW, Rossberg S, Wagner P, Sulser C, Bunikowski R, Witt I, Wauer J, et al. Oral application of bacterial lysate in infancy decreases the risk of atopic dermatitis in children with 1 atopic parent in a randomized, placebo-controlled trial. J Allergy Clin Immunol 2012; 129: 1040-7. [DOI] [PubMed] [Google Scholar]
- 25.Monsalvo AC, Batalle JP, Lopez MF, Krause JC, Klemenc J, Hernandez JZ, Maskin B, Bugna J, Rubinstein C, Aguilar L, et al. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med 2011; 17: 195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganley-Leal LM, Liu X, and Wetzler LM. Toll-like receptor 2-mediated human B cell differentiation. Clin Immunol 2006; 120: 272-84. [DOI] [PubMed] [Google Scholar]
- 27.Liang Y, Hasturk H, Elliot J, Noronha A, Liu X, Wetzler LM, Massari P, Kantarci A, Winter HS, Farraye FA, et al. Toll-like receptor 2 induces mucosal homing receptor expression and IgA production by human B cells. Clin Immunol 2011; 138: 33-40. [DOI] [PubMed] [Google Scholar]
- 28.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K. and Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422-34. [DOI] [PubMed] [Google Scholar]
- 29.Graham BS. Pathogenesis of respiratory syncytial virus vaccine-augmented pathology. Am J Respir Crit Care Med 1995; 152: S63-6. [DOI] [PubMed] [Google Scholar]
- 30.Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC., III and Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of FI RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J. Virol 1994; 68: 5321-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham BS, Henderson GS, Tang YW, Lu X, Neuzil KM, and Colley DG. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol 1993; 151: 2032-40. [PubMed] [Google Scholar]
- 32.Holt EA, Moulton LH, Siberry GK, and Halsey NA. Differential mortality by measles vaccine titer and sex. J Infect Dis 1993; 168:1087-96. [DOI] [PubMed] [Google Scholar]
- 33.Gans HA, Arvin AM, Galinus J, Logan L, DeHovitz R, and Maldonado Y. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA 1998; 280: 527-32. [DOI] [PubMed] [Google Scholar]
- 34.Polack FP, Hoffman SJ, Crujeiras G, and Griffin DE. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med 2003; 9: 1209-13. [DOI] [PubMed] [Google Scholar]
- 35.Shen P, Lampropoulou V, Stervbo U, Hilgenberg E, Ries S, Mecqinion A, and Fillatreau S. Intrinsic Toll-like receptor signalling drives regulatory function in B cells. Front Biosci (Elite Ed) 2013; 5: 78-86. [DOI] [PubMed] [Google Scholar]
- 36.Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS, Lu X, DeVos J, Hancock K, Katz JM, et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One 2010; 27: 5(10): e13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bessler WG, Vor dem Esche U, Masihi N. The bacterial extract OM-85 BV protects mice against influenza and Salmonella infection. Int Immunopharmacol 2010; 10:1086-90. [DOI] [PubMed] [Google Scholar]


