Abstract
Clinical trials have shown that AS03-adjuvanted H5N1 and A(H1N1)pdm09 vaccines are highly immunogenic, although with an increased reactogenicity profile relative to non-adjuvanted vaccines in terms of the incidence of common injection site and systemic adverse events (AEs). We evaluated pooled safety data from 22,521 adults who had received an AS03-adjuvanted H5N1 or A(H1N1)pdm09 influenza or control vaccine with the purpose to identify medically-attended AEs (MAEs), including subsets of serious AEs (SAEs), potentially immune-mediated diseases (pIMDs), and AEs of special interest (AESI), and to explore a potential association of these AEs with the administration of an AS03-adjuvanted influenza vaccine. For participants who had received an AS03-adjuvanted vaccine, the relative risks (RRs) for experiencing a MAE or a SAE compared to control group (participants who had received a non-adjuvanted vaccine or saline placebo) were 1.0 (95% confidence interval [CI]: 0.9; 1.1) and 1.1 (95% CI: 0.9; 1.4), respectively. The overall RRs for experiencing an AESI or a pIMD (AS03-adjuvanted vaccine/control) were 1.2 (95% CI: 0.9; 1.6) and 1.7 (95% CI: 0.8; 3.8), respectively. Thirty-8 participants in the AS03-adjuvanted vaccine group had a pIMD reported after vaccine administration, yielding an incidence rate (IR) of 351.9 (95% CI: 249.1; 483.1) per 100,000 person-years. The estimated IRs in the AS03-adjuvanted vaccine group were greater than the literature reported rates for: facial paresis/VIIth nerve paralysis, celiac disease, thrombocytopenia and ulcerative colitis. These results do not support an association between AS03-adjuvanted H5N1 and A(H1N1)pdm09 vaccines and the AEs collected in the trials included in the analysis.
Keywords: safety, potential immune-mediated disease, A(H1N1)pdm09 vaccine, H5N1 vaccine, influenza A(H5N1), pandemic influenza A(H1N1), pooled analysis
Abbreviations
- AE
adverse event
- pIMD
potential immune-mediated disease
- AESI
adverse event of special interest
- MAE
medically-attended adverse event
- SAE
serious adverse event
- NOCD
new onset chronic disease
- CI
confidence interval
- RR
relative risk
- SD
standard deviation
- IR
incidence rate
- GCP
Good Clinical Practice
- CBER
Center for Biologics Evaluation and Research
- CHMP
Committee for Medicinal Products for Human Use
- MedDRA
Medical Dictionary for Regulatory Activities
- PT
preferred term
Introduction
Modeling exercises have shown that vaccines, if available in sufficient quantity, could markedly attenuate the impact of an advancing influenza pandemic.1,2 Two major challenges include the limitation of influenza antigen manufacturing capacity in the face of a target for immunization comprising essentially 100% of the population, and the exacerbating factor of intrinsic poor immunogenicity of at least some potential pandemic vaccine antigens, notably avian strains. Partially in response to this problem, GlaxoSmithKline (GSK) Vaccines developed the Adjuvant System AS03, an oil-in-water emulsion containing α-tocopherol, squalene, and the surfactant polysorbate-80. Influenza vaccine trials showed that AS03 enhances antibody and T-cell immune responses and has marked antigen-sparing effects.3-9 Due to this antigen-sparing effect, AS03-adjuvanted influenza vaccine regimens using 3.75 μg of hemagglutinin content per dose fulfilled the immunogenicity criteria based on hemagglutination-inhibiting antibody titers, as set forth by both the US Food and Drug Administration Center for Biologics Evaluation and Research (CBER) and the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency,3,4,7-9 increasing the potential for vaccine supplies in the event of a pandemic.
A highly pathogenic avian influenza A (H5N1) virus emerged in 1997 and was associated with high mortality rates in infected persons;10 subsequently, the World Health Organization designated a number of H5N1 influenza virus strains as suitable for inclusion in vaccines. GSK Vaccines initiated an H5N1 vaccine development program and licensed 2 H5N1vaccines: one manufactured in Dresden, Germany (licensed in Europe) containing 3.75μg A/Vietnam/1194/2004 hemagglutinin and AS03A adjuvant and one manufactured in Quebec, Canada containing 3.75μg A/ Indonesia/5/2005 hemagglutinin and AS03A adjuvant (licensed in Europe, Canada and the United States). During the 2009/2010 A(H1N1)pdm09 pandemic, AS03A-adjuvanted split-virion vaccines against the A/California/7/2009 H1N1 strain were developed and licensed based on the experience acquired with H5N1 vaccines.4,11 Data from the GSK Vaccines’ clinical development program for immunization against influenza caused by the H5N1 subtype and A(H1N1)pdm09 have shown that AS03-adjuvanted H5N1 and A(H1N1)pdm09 vaccines are highly immunogenic.3-9,12 In addition, a variety of observational studies conducted during the 2009/2010 pandemic, as well as one controlled efficacy trial, have demonstrated that AS03-adjuvanted vaccines with reduced antigen content are effective in controlling pandemic influenza disease.13-16 Despite an increased reactogenicity profile relative to non-adjuvanted vaccines, in terms of the incidences of common and transient injection site and systemic adverse events,4,7-9,12 these vaccines are anticipated to have an acceptable safety profile for addressing pandemic threats.17,18
In early 2009, in preparation of marketing applications for AS03-adjuvanted inactivated split virion H5N1 vaccine using antigen manufactured in Quebec, Canada, GSK undertook an integrated safety analysis of data from 12,281 adults who received one of the AS03-adjuvanted H5N1 vaccines in completed clinical trials. Subsequent to GSK's clinical development of AS03-adjuvanted H1N1 vaccines in response to the 2009/2010 swine-origin A(H1N1)pdm09 pandemic, a second integrated safety analysis was generated, incorporating both the H5N1 and A(H1N1)pdm09 experience. This second analysis aggregated data from 22,521 adult recipients of GSK's AS03-adjuvanted H5N1 and A(H1N1)pdm09 vaccines. Because the AS03 Adjuvant System may be used in broad populations to address future influenza pandemic threats, it was considered important to characterize the safety profile of this class of AS03-adjuvanted inactivated split virion influenza vaccines in the setting of prospective clinical trials and to search for potential associations with low-frequency adverse events that may be associated with their administration to adults.
The aim of the present analysis was to summarize all available safety data from completed clinical trials in adults who received GSK's AS03-adjuvanted H5N1 and A(H1N1)pdm09 vaccines and to examine the occurrence of medically-attended adverse events (MAEs), including the subsets of serious adverse events (SAEs), potentially immune-mediated diseases (pIMDs) and adverse events (AEs) that the CHMP considered as worthy of closer safety monitoring following administration of pandemic influenza vaccine (AEs of special interest [AESIs]).19 By analyzing a large pooled database, our aim was to assess uncommon events in these AE classes, which might have occurred in 0.1% or less recipients of the adjuvanted vaccines. These are discussed in the context of background disease rates and post-marketing safety data available in the literature.
Results
Study population
Data from a total of 22,521 adults enrolled in clinical trials fullfiling the eligibility criteria for this analysis and who had received at least one dose of either adjuvanted study vaccine or control were included (Table 1); 16,160 received an AS03-adjuvanted influenza vaccine (either H5N1 [11,376 participants] or A(H1N1)pdm09 [4784 participants] with any dose of AS03 [either AS03A or AS03B, containing half of the volume of adjuvant emulsion contained in the AS03A/dose]) in controlled or uncontrolled clinical trials. Of these, 13,325 had been enrolled in controlled trials and followed for approximately 10,783 person-years. The corresponding control group included 6,361 participants who had received non-adjuvanted H5N1 or A(H1N1)pdm09 vaccine, non-adjuvanted seasonal influenza vaccine or placebo; the total follow-up for control participants was 5,161 person-years. The mean age (± standard deviation [SD]) of the participants was 44.0 ± 18 .1 y (median: 42 years) and 56% were women. The demographic characteristics of the participants included in the analysis are shown in Table 2.
Table 1.
Overview of clinical trials performed in adults and included in the pooled analysis of safety
| Clinical trial code(s) (NCT number) | Age range in years | Majority race | Blinding | Influenza Vaccine(s)* Dose (schedule), N vaccinated | Control (schedule), N vaccinated |
|---|---|---|---|---|---|
| Controlled trials | |||||
| Q-H5N1–001 (NCT00510874)12 | 18–64 | White/ Caucasian | Observer-blind | Q H5N1 3.75 AS03B 2Do (21d) = 151 D H5N1 3.75 AS03B 2Do (21d) = 148 Q H5N1 3.75 AS03A 2Do (21d) = 152 D H5N1 3.75 AS03A 2Do (21d) = 151 Q H5N1 1.9 AS03B 2Do (21d) = 50 Q H5N1 1.9 AS03A 2Do (21d) = 50 |
Q H5N1 3.75 2Do (21d) = 78 |
| Q-H5N1–002 | |||||
| (NCT00616928)66 | ≥18 | White/ Caucasian | Observer-blind | Q H5N1 3.75 AS03A 2Do (21d) = 3422 | Placebo = 1139 |
| Q-H5N1–005 | |||||
| (NCT00510874)69 | ≥18 | White/ Caucasian | Observer-blind | Q H5N1 3.75 AS03B 1Do = 239 Q H5N1 3.75 AS03A 1Do = 119 Q H5N1 7.5 AS03B 1Do = 241 Q H5N1 7.5 AS03A 1Do = 122 |
Placebo = 120 |
| Q-H5N1–010 | |||||
| (NCT00771615)70 | 19–65 | White/ Caucasian | Observer-blind | Q H5N1 3.75 AS03A 1D = 420 (booster extension of Q-Pan H5N1–001) |
Q H5N1 3.75 1D = 230 |
| D-H5N1–002 (NCT00449670)71 | 18–60 | Asian | Observer-blind | D H5N1 3.75 AS03A 2Do (21d) = 961 | D H5N1 3.75 2Do (21d) = 245 |
| D-H5N1–007 (NCT00309634)3 | 18–60 | White/ Caucasian | Observer-blind | D H5N1 30 AS03A 2Do (21d) = 49 D H5N1 15 AS03A 2Do (21d) = 50 D H5N1 7.5 AS03A 2Do (21d) = 50 D H5N1 3.75 AS03A 2Do (21d) = 51 |
D H5N1 30 2Do (21d) = 50 D H5N1 15 2Do (21d) = 50 D H5N1 7.5 2Do (21d)= 50 D H5N1 3.75 2Do (21d) = 50 |
| D-H5N1–008/011 (NCT00319098)11 | ≥18 | White/ Caucasian | Observer-blind | D H5N1 15 AS03A 2Do (21d) = 3801 |
Fluarix™/placebo = 1269 |
| D-H5N1–010/021 (NCT00397215)72 | >60 | White/ Caucasian | Open | D H5N1 7.5 AS03A 2Do (21d) = 159 D H5N1 3.75 AS03A 2Do (21d) = 165 |
D H5N1 7.5 2Do (21d)= 52 D H5N1 3.75 2Do (21d) = 61 |
| Q-H1N1–001 (NCT00985088)9 | >18 | White/ Caucasian | Observer-blind | Q H1N1 3.75 AS03A 2Do (21d) = 222 Q H1N1 3.75 AS03A 1Do = 221 Q H1N1 1.9 AS03B 2Do (21d) = 114 Q H1N1 1.9 AS03B 1Do = 112 |
Q H1N1 15 1Do = 223 Q H1N1 7.5 2Do (21d) = 115 Q H1N1 7.5 1Do = 111 Q H1N13.75 2Do (21d) = 222 |
| Q-H1N1–002 (NCT00979602)7 | >18 | White/ Caucasian | Observer-blind | Q H1N1 3.75 AS03A 1Do = 2025 | Q H1N1 15 1Do = 2023 |
| Q-H1N1–019 (NCT00985673)73 | 19–40 | White/ Caucasian | Observer-blind |
FluLaval™ followed by 2Do of Q H1N1 3.75 AS03A (21d) = 104 FluLaval™ + Q H1N1 3.75 AS03A followed by 1Do of Q H1N1 3.75 AS03A (21d) = 100 2Do of Q H1N1 3.75 AS03A (21d) followed by FluLaval™ = 102 |
2Do of Q H1N1 15 (21d) followed by FluLaval™ = 101 FluLaval™ + Q H1N1 15 followed by 1Do of Q H1N1 15 (21d) = 102 FluLaval™ followed by 2Do of Q H1N1 15 (21d) = 102 |
| D-H1N1–007 (NCT00989287)6 | 18–60 | White/ Caucasian | Open | D H1N1 3.75 AS03A 2Do (21d) = 64 | D H1N1 15 2Do (21d) =66 |
| D-H1N1–021 (NCT00951041)4 | 18–60 | White/ Caucasian | Observer-blind | D H1N1 3.75 AS03A 2Do (21d) = 64 | D H1N1 15 2Do (21d) =66 |
| D-H1N1–033 (NCT00989287)6 | 18–60 | White/ Caucasian | Observer-blind | D H1N1 3.75 AS03A 2Do (21d) = 65 | D H1N1 3.75 2Do (21d) =66 |
| Uncontrolled trials | |||||
| Q-H5N1–009 (NCT00695669)74 | 18–64 | White/ Caucasian | Open | Q H5N1 3.75 AS03A 2Do (21d) = 78 Q H5N1 3.75 AS03A 2Do (14d) = 78 Q H5N1 3.75 AS03A 2Do (7d) = 78 Q H5N1 3.75 AS03A 2Do (0d) = 78 |
None |
| Q-H5N1–011 (NCT00742885)75 | 20–64 | Asian | Open | Q H5N1 3.75 AS03A 2Do (21d) = 100 | None |
| D-H5N1–012 (NCT00430521)76 | 18–60 | White/ Caucasian | 0pen | D H5N1 3.75 AS03A 2Do (21d) = 512 | None |
| D-H5N1–015 (NCT00506350)77 | 19–61 | White/ Caucasian | Open | D H5N1 3.75 AS03A 1D for subjects primed with AS =151 D H5N1 3.75 AS03A 2D for subjects primed without AS = 149** D H5N1 3.75 AS03A 2D for unprimed subjects = 50 | None |
| D-H5N1–030 (NCT00449670)78 | 18–60 | Asian | Observer-blind | D H5N1 3.75 AS03A 2Do (21d) = 501 (Booster extension of D-Pan H5N1–002 | None |
| D-H5N1–038 (NCT00652743)78 | 18–60 | Asian | Open | D H5N1 3.75 AS03A 1D = 623 | None |
| D-H5N1–041 (NCT00812981)79 | 18–60 | White/ Caucasian | Observer-blind | D H5N1 3.75 AS03A 2Do (21d) = 320 | None |
| Q-H1N1–016 (NCT00989612)80 | 20–64 | Asian | Open | Q H1N1 3.75 AS03A 2Do (21d) = 100 | None |
| D-H1N1–008 (NCT00975884)81 | >18 | White/ Caucasian | Open | D H1N1 3.75 AS03A 2Do (21d) = 138 D H1N1 3.75 AS03A 1Do = 102 | None |
| D-H1N1–017 (NCT00979407)82 | 18–60 | White/ Caucasian | Double blind | Q H1N1 3.75 AS03A 2Do (21d) = 167 D H1N1 3.75 AS03A 2Do (21d) = 167 | None |
| D-H1N1–018 (NCT00968890)83 | >60 | White/ Caucasian | Open for H1N1 vaccine, observer-blind for Fluarix™/placebo | D H1N1 3.75 AS03A 2Do (21d) + Fluarix™ at dose 1/placebo at dose 2 = 84 D H1N1 3.75 AS03A 2Do (21d) + placebo at dose 1/Fluarix™ at dose 2 = 84 | None |
| D-H1N1–020 (NCT00971425)83 | >60 | White/ Caucasian | Single blind | D H1N1 3.75 AS03A 2Do (21d) then Fluarix™ = 72 Fluarix™ then D H1N1 3.75 AS03A 2Do (21d) = 73 | None |
| D-H1N1–022 (NCT00975884) | >18 | White/ Caucasian | Open | D H1N1 1.9 AS03A 2Do (21d) = 184 D H1N1 1.9 AS03A 2Do (6m) = 122 | None |
| D-H1N1–024 (NCT00992511)84 | >18 | White/ Caucasian | Double blind (with respect to D H1N1 lots) | D H1N1 3.75 AS03A 2Do (21d) = 148 D H1N1 3.75 AS03A 1Do = 152 | None |
Q, antigen produced in GSK facilities in St. Foy, Quebec; D antigen produced in GSK facilities in Dresden, Germany; Do, dose; d, day (interval between vaccine doses); N, number of participants enrolled and vaccinated.
* Vaccine formulations with quantity of HA (in micrograms) administered and presence of AS03.
**Data not included.
Table 2.
Demographic parameters of participants in the trials included in analysis
| Controlled trials |
Controlled and uncontrolled trials |
||
|---|---|---|---|
| H5N1/ A(H1N1)pdm09 AS03 | Control | H5N1/ A(H1N1)pdm09 AS03 | |
| Number of participants | 13,325 | 6,361 | 16,160 |
| Age, mean (SD) | 44.1 (18.3) | 44.8 (18.6) | 44.0 (18.1) |
| Women, n (%) | 7,621 (57.2) | 3,626 (57.0) | 9,103 (56.3) |
| Race | |||
| White/Caucasian | 11,204 (84.1) | 5,510 (86.6) | 13,441 (83.2) |
| African heritage / African American | 636 (4.8) | 320 (5.0) | 662 (4.1) |
| Asian - east Asian heritage | 623 (4.7) | 165 (2.6) | 953 (5.9) |
| Asian - south east Asian heritage | 404 (3.0) | 117 (1.8) | 407 (2.5) |
| White - Arabic / north African heritage | 145 (1.1) | 70 (1.1) | 162 (1.0) |
| Other | 313 (3.3) | 179 (5.8) | 535 (3.3) |
SD, standard deviation; n (%), number (percentage of sparticipant) in a given category.
Assessment of medically-attended adverse events
In the analysis including controlled trials, at least one MAE (see definition in the Methods section) was reported for 3,208 participants in the adjuvanted vaccine group and for 1,931 participants in the control group (Table 3). The corresponding relative risk (RR) for any MAE was 1.0 (95% confidence interval [CI]: 0.9; 1.1) (Fig. 1A). The RR for MAEs of Grade 3 severity (see definition in the Methods section) was also 1.0 (95% CI: 0.8; 1.1). RR computed at the level of individual preferred terms (PTs) showed that 3 PTs had been reported more frequently (based on non-overlapping 95% CIs) in the adjuvanted vaccine group: diarrhea, seasonal allergy and vulvovaginal candidiasis (Table 4). When only AS03A-containing vaccines were considered, one additional PT was identified: cystitis. This event was observed with an RR of 1.9 (95% CI: 1.0; 3.8). Conversely, 4 PTs were more frequently reported as MAEs in the control group: allergic rhinitis, gastroesophageal reflux disease, injection site swelling and musculoskeletal chest pain. In the analyses performed separately for the H5N1 and A(H1N1)pdm09 trials, 2 additional PTs were identified for H5N1 trials associated with control vaccination for fall with an RR of 0 (95% CI: 0; 0.8) and carpal tunnel syndrome with an RR of 0 (95% CI: 0; 0.7). Considering individual PTs for Grade 3 MAEs, only myocardial infarction had an RR with the 95% CI excluding 1 (RR = 0, 95% CI: 0; 0.8).
Table 3.
Incidence rate (per 1,000 person-years) of medically-attended adverse events, serious adverse events, potential immune-mediated diseases and adverse events of special interest in controlled and uncontrolled trials (total vaccinated cohorts)
| Controlled trials |
Controlled and uncontrolled trials |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| H5N1/ A(H1N1)pdm09 AS03 N = 13,325 |
Control N = 6,361 |
H5N1/ A(H1N1)pdm09 AS03 N = 16,160 |
|||||||
| T | n | IR (95% CI) | T | n | IR (95% CI) | T | n | IR (95% CI) | |
| At least one MAE | 7448.8 | 3208 | 430.7 (415.9; 445.8) | 4389 | 1931 | 439.9 (420.5; 459.9) | 8112.2 | 3742 | 461.3 (446.6; 476.3) |
| At least one Grade 3 MAE | 7448.8 | 643 | 86.3 (79.7; 93.2) | 4389 | 367 | 83.7 (75.3; 92.6) | 8112.2 | 765 | 94.3 (87.7; 101.2) |
| At least one SAE | 10782.8 | 388 | 35.9 (32.5; 39.7) | 5161 | 189 | 36.6 (31.6; 42.2) | 12939.4 | 507 | 39.2 (35.8; 42.7) |
| At least one pIMD | 8846.4 | 31 | 3.5 (2.4; 4.9) | 4963.6 | 11 | 2.3 (1.2; 3.9) | 10797.1 | 38 | 3.5 (2.5; 4.8) |
| At least one AESI | 8846.4 | 203 | 22.9 (19.9; 26.3) | 4963.6 | 86 | 17.4 (13.9; 21.4) | 10797.1 | 245 | 22.7 (19.9; 25.7) |
| At least one AESI (identified by SMQ) | 8846.4 | 76 | 8.6 (6.8; 10.8) | 4963.6 | 24 | 4.9 (3.1;7.2) | 10797.1 | 86 | 7.9 (6.4; 9.9) |
N, total number of participants; T, total follow-up time in years calculated as sum of (Ns*Ts); n, number of participants reporting at least one symptom; IR, incidence rate (per 1,000 person-years) of participants reporting at least one event; CI, confidence interval; MAE, medically-attended adverse event; SAE, serious adverse event; pIMD, potential immune mediated disease; AESI, adverse events of special interest; SMQ, Standardised Medical Dictionary for Regulatory Activities (MedDRA) queries
Figure 1.
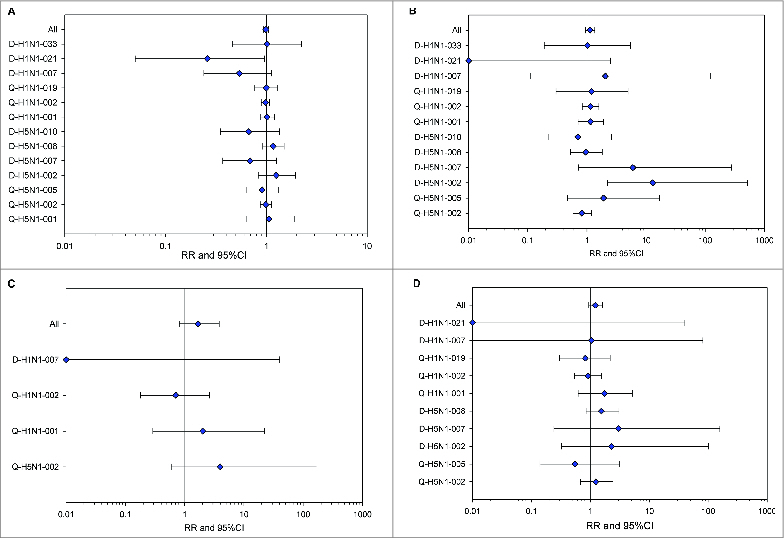
Forest plots showing relative risks of medically-attended adverse events (A), serious adverse events (B), potential immune-mediated diseases (C) and adverse events of special interest (D), in participants who received A(H1N1)pdm09 or H5N1 AS03-adjuvanted vaccines in controlled trials (total vaccinated cohorts). Trials in which no endpoint of interest was reported are not included in the figure. RR, relative risk; CI, confidence interval.
Table 4.
Incidence rate (per 100,000 person-years) and estimated relative risk in controlled trials for medically-attended events by preferred terms with the 95% CI of the relative risk excluding 1.0 (total vaccinated cohorts)
| H5N1/ A(H1N1)pdm09 AS03 N = 13,325 T = 7448.8 |
Control N = 6,361 T = 4,389 |
RR (AS03 over Control) |
||||
|---|---|---|---|---|---|---|
| MedDRA Preferred Term | n | IR (95% CI) | n | IR (95% CI) | RR (95% CI) | p-value interact |
| At least one MAE | 3208 | 43067.1 (41586.4; 44580.6) | 1931 | 43995.9 (42049.9; 45997.5) | 0.99 (0.93; 1.05) | 0.259 |
| Diarrhea | 38 | 510.1 (361.0; 700.2) | 10 | 227.8 (109.3; 419.0) | 2.91 (1.40; 6.63) | 0.399 |
| Gastroesophageal reflux disease | 36 | 483.3 (338.5; 669.1) | 38 | 865.8 (612.7; 1188.4) | 0.51 (0.31; 0.86) | 0.463 |
| Injection site swelling* | 0 | 0 (0; 49.5) | 4 | 91.1 (24.8; 233.3) | 0 (0; 0.63) | 1.000 |
| Seasonal allergy | 14 | 187.9 (102.8; 315.3) | 2 | 45.6 (5.5; 164.6) | 4.94 (1.09; 45.80) | 0.761 |
| Vulvovaginal candidiasis | 10 | 134.2 (64.4; 246.9) | 1 | 22.8 (0.6; 126.9) | 7.96 (1.09; 350.95) | 0.543 |
| Musculoskeletal chest pain | 9 | 120.8 (55.2; 229.4) | 18 | 410.1 (243.1; 648.2) | 0.34 (0.13; 0.83) | 0.639 |
| Allergic rhinitis | 4 | 53.7 (14.6; 137.5) | 9 | 205.1 (93.8; 389.3) | 0.22 (0.05; 0.85) | 0.855 |
| Cystitis** | 42 | 615.8 (443.8; 832.3) | 15 | 341.8 (191.3; 563.7) | 1.93 (1.03; 3.83) | 0.185 |
| Carpal tunel syndrome*** | 0 | 0 (0; 83.5) | 3 | 222.5 (45.9; 650.2) | 0 (0; 0.74) | 1.000 |
| Fall*** | 0 | 0 (0; 83.5) | 3 | 222.5 (45.9; 650.2) | 0 (0; 0.81) | not calculated |
N, total number of participants; T, total follow-up time in year calculated as sum of (Ns*Ts); n, number of participants reporting at least one event; 95% CI, exact 95% Confidence Interval; IR, incidence rate (per 100,000 person-years) of participants reporting at least one event; RR, Relative Risk (AS03-adjuvanted vaccine over Control group) adjusted by study effect; p-value interact, 2-sided Exact Breslow and Day Test for heterogeneity
*Solicited AE with a duration >4 days.
** For cystitis the incidence rate (per 100,000 person-years) and estimated RR was calculated in AS03A-adjuvanted vaccines and control groups based on controlled trials only.
*** For carpal tunel syndrome and fall the incidence rates (per 100,000 person-years) and estimated RRs were calculated in AS03-adjuvanted H5N1 vaccines and control groups based on controlled trials only. For ‘fall’, the three events were reported in the same trial while for ‘carpal tunnel syndrome’, the events were reported in two different trials. Therefore, the p-values could be calculated for ‘carpal tunnel syndrome’ but not for ‘fall’.
When both controlled and uncontrolled trials were analyzed, the IR of MAEs and MAEs of Grade 3 severity in the adjuvanted vaccine group was 461.3 (95% CI: 446.6; 476.3) per 1,000 person-years and 94.3 (95% CI: 87.7; 101.2) per 1,000 person-years, respectively (Table 3).
Assessment of serious adverse events
In the analysis including controlled trials, at least one SAE (definition is provided in the Methods section) was reported for 388 participants in the adjuvanted vaccine group and 189 participants in the control group, yielding an RR of 1.1 (95% CI: 0.9; 1.4) (Table 3, Fig. 1B). Similar RRs and 95% CIs were observed when only the controlled trials with AS03A-adjuvanted vaccine were analyzed (results not shown). The 95% CI of the RRs for all individual SAE PTs included 1.0. As the p-value for heterogeneity using the 2-sided exact Breslow and Day test was <0 .05, a review of aggregate data was performed for each of the controlled trials. The RRs for each of the controlled trials are presented in Fig. 1B.
In the analysis including controlled and uncontrolled trials, 507 participants who had received an adjuvanted vaccine reported at least one SAE during the follow-up, accounting for an incidence rate of 39.2 (95% CI: 35.8; 42.7) per 1,000 person-years (Table 3). When only AS03A-adjuvanted vaccine trials were assessed, the incidence rate was 38.7 (95% CI: 35.3; 42.4) per 1,000 person-years.
Fourteen events were considered by the reporting investigators to have a causal relationship with the study vaccine: 11 reported after an adjuvanted vaccine administration and 3 reported after the receipt of placebo control. None of these events were fatal. Four of these events were pIMDs: autoimmune hepatitis, thrombocytopenia and polymyalgia rheumatica reported for participants in the adjuvanted vaccine group and multiple sclerosis reported for a participant in the control group.
Fatal SAEs were reported for a total of 37 participants (25 in the adjuvanted vaccines and 12 in the control groups) and none were considered by the reporting investigators or GSK to be related to the study vaccine. Considering only controlled trials, the RR for experiencing a fatal SAE was 1.0 (95% CI: 0.4; 2.7).
Assessment of potential immune-mediated disorders and adverse events of special interest
Forty-3 pIMDs were identified for 42 participants enrolled in controlled trials: 31 participants in the adjuvanted vaccine group (21 had received an H5N1 and 10 an A(H1N1)pdm09 vaccine) and 11 participants in the control group (1 in the H5N1 trials and 10 in the A(H1N1)pdm09 trials) (Table 3). The RR for experiencing a pIMD (adjuvanted vaccine/control) was 1.7 (95% CI: 0.8; 3.8) for any AS03 formulation (Fig. 1C) and 1.8 (95% CI: 0.9; 4.1) for the AS03A formulation. When analyzed separately, the RR was 6.9 (95% CI: 1.1; 283.4) and 1.0 (95% CI: 0.4; 2.7) for H5N1 and A(H1N1)pdm09 adjuvanted vaccine groups, respectively.
There were 38 participants enrolled in controlled and uncontrolled trials (23 who had received an H5N1 adjuvanted vaccine and 15 who had received an A(H1N1)pdm09 adjuvanted vaccine) who experienced a pIMD after the primary vaccination. The incidence rate of pIMDs was 3.5 (95% CI: 2.5; 4.8) per 1,000 person-years, 3.8 (95% CI: 2.4; 5.7) per 1,000 person-years and 3.2 (95% CI: 1.8; 5.2) per 1,000 person-years in H5N1/ A(H1N1)pdm09, H5N1 and A(H1N1)pdm09 adjuvanted vaccine groups, respectively.
Analyses performed at the level of individual PTs did not suggest an increased risk for any individual diagnosis in participants who had received an adjuvanted vaccine (Table 5). The pIMDs reported in controlled and uncontrolled trials included: facial paresis or VIIth nerve paralysis (7 cases), polymyalgia rheumatica (7 cases), psoriasis (4 cases), autoimmune thyroiditis or Basedow's disease (3 cases), celiac disease (3 cases), thrombocytopenia (3 cases), radiculitis and/or radiculopathy (3 cases), multiple sclerosis (2 cases), uveitis (2 cases), ulcerative colitis (2 cases), rheumatoid arthritis (2 cases), neuritis (1 case) and optic neuritis (1 case).
Table 5.
Incidence rate and relative risk of potential immune mediated disease preferred terms reported in multiple trial participants in AS03-adjuvanted vaccines and control groups and background rates reported in the literature
| Participants considered | Total N | N Rate per 100,000 person-years ( 95% CI) for adjuvanted vaccine recipients* | N Rate per 100,000 person-years (95% CI) for control recipients** | Relative Risk*** (95% CI) | Background rates reported in the literature |
|---|---|---|---|---|---|
| Facial paresis and/or VIIth nerve paralysis | |||||
| Controlled and uncontrolled trials | 7 | 6 55.6 (20.4; 121.0) |
1 20.1 (0.5; 112.3) |
Not calculated | 13.1 – 46.725–31 |
| Controlled trials | 5 | 4 45.2 (12.3; 115.8) |
1 20.1 (0.5, 112.3) |
2.3 (0.2; 118.1) |
|
| Polymyalgia rheumatica or temporal arteritis | |||||
| Controlled and uncontrolled trials | 7 | 6 55.6 (20.4; 121.0) |
1 20.1 (0.5; 112.3) |
Not calculated | 12.7 – 112.6 32–34 |
| Controlled trials | 6 | 5 56.5 (18.4; 131.9) |
1 20.1 (0.5; 112.3) |
2.6 (0.3; 131.4) |
|
| Psoriasis | |||||
| Controlled and uncontrolled trials | 4 | 3 27.8 (5.7; 81.2) |
1 20.1 (0.5; 112.3) |
Not calculated | 38.5 – 140.0 35,36 |
| Controlled trials | 3 | 2 22.6 (2.7; 81.7) |
1 20.1 (0.5; 112.3) |
0.7 (0.0; 39.3) |
|
| Autoimmune thyroiditis or Basedow's disease | |||||
| Controlled and uncontrolled trials | 3 | 3 33.9 (7.0; 99.1) |
0 0.0 (0.0; 74.3) |
Not calculated | 2.2 – 41.9 37–40 |
| Controlled trials | 3 | 3 33.9 (7.0; 99.1) |
0 0.0 (0.0; 74.3) |
Infinity (0.3; Infinity) |
|
| Celiac disease | |||||
| Controlled and uncontrolled trials | 3 | 2 22.6 (2.7; 81.6) |
1 20.1 (0.5; 112.3) |
Not calculated | 1.4 – 12.9 43,44 |
| Controlled trials | 3 | 2 22.6 (2.7; 81.6) |
1 20.1 (0.5; 112.3) |
1.5 (0.1; 91.6) |
|
| Multiple sclerosis | |||||
| Controlled and uncontrolled trials | 2 | 1 9.3 (0.2; 51.6) |
1 20.1 ( 0.5; 112.3) |
Not calculated | 4.6 – 25.0 25,30 41,42 |
| Controlled trials | 1 | 0 0 (0.0; 41.7) |
1 20.1 (0.5; 112.3) |
0.0 (0.0; 39.0) |
|
| Thrombocytopenia | |||||
| Controlled and uncontrolled trials | 3 | 3 33.9 (7.0; 99.1) |
0 0 (0.0; 74.3) |
Not calculated | 0.0 – 7.1 25,41,85 |
| Controlled trials | 3 | 3 33.9 (7.0; 99.1) |
0 0 (0.0; 74.3) |
Infinity (0.4; Infinity) |
|
| Radiculitis or radiculopathy | |||||
| Controlled and uncontrolled trials | 3 | 1 11.3 (0.3; 63.0) |
2 40.3 (4.9; 145.6) |
Not calculated | 83.2 45 |
| Controlled trials | 3 | 1 11.3 (0.3; 63.0) |
2 40.3 (4.9; 145.6) |
0.3 (0.0; 7.1) |
|
| Uveitis | |||||
| Controlled and uncontrolled trials | 2 | 1 11.3 (0.3; 63.0) |
1 20.1 (0.5; 112.3) |
Not calculated | 10.5 – 52.4 41,50 |
| Controlled trials | 2 | 1 11.3 (0.3; 63.0) |
1 20.1 (0.5; 112.3) |
0.6 (0.0; 53.5) |
|
| Ulcerative colitis | |||||
| Controlled and uncontrolled trials | 2 | 2 22.6 (2.7; 81.7) |
0 0.0 (0.0; 74.3) |
Not calculated | 0.3 – 17.0 46,47 |
| Controlled trials | 2 | 2 22.6 (2.7; 81.7) |
0 0.0 (0.0; 74.3) |
Infinity (0.1; Infinity) |
|
| Rheumatoid arthritis | |||||
| Controlled and uncontrolled trials | 2 | 1 11.3 (0.3, 63.0) |
1 20.1 (0.5; 112.3) |
Not calculated | 8.3 – 36.0 41,48,49 |
| Controlled trials | 2 | 1 11.3 (0.3; 63.0) |
1 20.1 (0.5; 112.3) |
0.6 (0.0; 52.2) |
*Total person-years = 10,797 for controlled and uncontrolled H5N1 and A(H1N1)pdm09 trials and 8,846 for controlled H5N1 and A(H1N1)pdm09 trials.
**Total person-years = 4,964.
***RR was computed for the controlled trials only.
N, number of participants; CI, confidence interval; Infinity explicit value cannot be defined due to the absence of cases in the control group.
The RR of experiencing a CHMP-defined adverse event of special interest (AESI)15 (see Methods for definition) was 1.2 (95% CI: 0.9; 1.6) for the combined (H5N1/ A(H1N1)pdm09) adjuvanted vaccine group (Fig. 1D). The RR for experiencing an AESI was 2.7 (95% CI: 1.2; 7.0) and 1.1 (95% CI: 0.5; 2.2) for the H5N1 adjuvanted vaccine and the A(H1N1)pdm09 adjuvanted vaccine groups, respectively. Analyses for each adjuvanted vaccine cohort performed at the level of the individual PTs suggested no increased risk for any individual diagnosis in the adjuvanted vaccine group (results not shown). For 2 AESIs identified by narrow Standardised Medical Dictionary for Regulatory Activities (MedDRA) queries (SMQs), anaphylaxis and convulsion, that are not considered pIMDs, the calculated RRs were 2.1 (95% CI: 0.2; 105.2) and 1.5 (95% CI: 0.3; 8.8). For controlled and uncontrolled trials analyzed together, 245 participants who had received an adjuvanted vaccine experienced an event identified as an AESI, giving an incidence rate of 22.7 (95% CI: 19.9; 25.7) per 1,000 person-years of follow-up (Table 3).
Discussion
In this analysis, pooled safety data obtained from clinical trials in adults who had received H5N1 or A(H1N1)pdm09 vaccine antigens together with the α-tocopherol and squalene-based oil-in-water emulsion adjuvant system AS03 were analyzed to identify MAEs, including subsets of SAEs, pIMDs, and AESI, and to explore a potential association of these relatively low frequency events with the administration of an AS03-adjuvanted influenza vaccine.
In controlled trials, 1,288 individual PTs were reported as MAEs in both adjuvanted and control groups. Of the large number of individual PTs reported as MAEs, 4 events were more frequently reported in the adjuvanted vaccines groups (cystitis [only in the AS03A group], diarrhea, vulvovaginal candidiasis and seasonal allergy), and 6 events were more frequently reported in the control group (allergic rhinitis, gastroesophageal reflux disease, injection site swelling, musculoskeletal chest pain, fall and carpal tunnel syndrome). A mechanistic basis for the association of the MAEs which occurred more commonly in AS03 recipients is not obvious. Vulvovaginal candidiasis and cystitis, as well as diarrhea, might imply an alteration in the gastro-intestinal or urogenital microflora, but are also common medical events in the general population.20,21 There has been no evidence for alterations of mucosal microflora in repeated dose animal toxicity studies of AS03-adjuvanted influenza vaccines, where exposures are much higher on a weight adjusted basis.22,23 Cystitis occurred almost exclusively in women, thus a direct irritant effect in the bladder present due to an AS03 component or degradant would be unexpected, as males typically have longer bladder retention times for urine. Considering seasonal allergy, it is of interest to note that a prior history of atopy or asthma, antedating vaccine exposure, was present in 10 of 16 participants reporting this MAE (including 9 adjuvanted vaccine recipients and one control). Additionally, a related diagnosis, allergic rhinitis, appeared with increased frequency in the control group. Mechanistic data from animal models suggest that AS03 elicits balanced Th1 and Th2 responses, rather than the Th2-dominated response often associated with allergy.24 Future studies of AS03 vaccines should monitor these classes of events, but it is reassuring that the analysis did not suggest an increase in the overall incidence of MAEs (either Grade 3 or of any severity) in the adjuvanted influenza vaccines group (RR 1.0; 95% CI: 0.9 - 1.1) compared with the control group.
The small number of SAEs, and their disparate nature, made it difficult to draw conclusions on the association of individual PTs with treatments. When analyzed as an aggregate across all trials, no substantial imbalance between adjuvanted vaccine and control recipients was observed. Similar results were observed in all trials with the exception of the D-H5N1–002 trial. In study D-H5N1–002, potential sources of bias contributing to the overall SAE imbalance (RR 13.0; 95% CI: 2.2; 523.5) may have included the 4:1 randomization ratio for adjuvanted vaccines versus placebo and, more importantly, the much longer follow-up period for adjuvanted vaccine recipients (up to 36 months after the primary course as compared to only 6 months for control recipients). In this regard, it is notable that 34 of the 51 SAEs reported in the D-H5N1–002 adjuvanted vaccine group occurred after the 6-month initial follow-up period, and during a time interval when there was no safety follow-up of controls.
The analyses presented here consistently yielded point estimates for the RR of the aggregate groups of pIMDs and AESIs which were greater than 1, although the 95% CIs for these RRs consistently included 1.0. For the composite endpoint of all pIMDs and AESIs analyzed in aggregate in both A(H1N1)pdm09 and H5N1 trials, the RRs were 1.7 (95% CI: 0.8; 3.8) and 1.2 (95% CI: 0.9; 1.6), respectively. In the H5N1 sub-analysis the RRs for pIMDs and AESIs were 6.9 (95% CI: 1.1; 283.4) and 1.4 (95% CI: 1.0; 2.2), whereas the A(H1N1)pdm09 sub-analysis did not generate an RR with a 95% CI that excluded 1.0. While the observation periods for A(H1N1)pdm09 trials were evenly balanced (3174.7 y for adjuvanted vaccine recipients vs. 3192.4 y for control participants in controlled trials, or a ratio of 1:1), there was a marked imbalance of total safety follow-up of adjuvanted vaccine recipients in H5N1 trials (5671.7 person-years versus 1771.2 person-years in controls), due to both unbalanced treatment allocation and differing lengths of safety follow-up for adjuvant recipients and control subjects. In the same manner noted for the SAE contrast in study D-H5N1–002 above, the randomization ratio of 3:1 for H5N1 trials overall, compared to approximately 1:1 in A(H1N1)pdm09 trials, may have contributed to the differences observed in RRs for very low frequency events such as pIMDs in H5N1 vs. A(H1N1)pdm09 trials. A post-hoc analysis of pIMDs was performed in which the pIMD diagnoses that had been withdrawn or changed by investigators, or had been considered a pre-existing disease and/or with clear alternative causes, were removed. This analysis showed that the RR for pIMDs increased from 1.7 to 4.2, although with a substantially wider CI (95% CI: 1.0; 38.8). There was an overlap between the CHMP-specified AESIs and the pre-defined list of pIMDs (except for anaphylaxis and convulsion; for all other AESIs, the list of PTs used to define AESIs includes the PTs from the pIMD list). Therefore subcategories of pIMDs were used to calculate RR for AESI and thus the discussion of the latter applies to AESIs.
When all pIMD cases were analyzed, the point estimates of the incidence rates in the adjuvanted vaccine group were greater than the literature reported rates for facial paresis/VIIth nerve paralysis, celiac disease, thrombocytopenia and ulcerative colitis.25-50 The incidence rate for celiac disease was also higher than those in the literature for the control group. For facial paresis, an alternative cause had been considered more likely by the investigator in one case (secondary to a viral illness rather than to vaccination); and for 5 of 7 cases, the time from the most recent vaccine administration to onset had been <1 or ≥ 78 days, and thus outside the generally accepted risk window of 7 to 30 d postulated for seasonal influenza vaccines,51 although within the one-year risk window recently proposed for adjuvated vaccines.52 Two subjects with celiac disease reported were found to have symptom onsets and/or diagnostic findings which had preceded the administration of the investigational test articles. In one case of thrombocytopenia, the diagnosis as an immune-mediated disorder was unlikely based on the presence of another, more probable, cause: chemotherapy for Stage IV small cell lung cancer. In another subject, decreased platelet count had occurred as part of a symptom complex which was attributed to an allergic reaction to sulfonamide following treatment with trimethoprim-sulfamethoxazole. Among the 2 cases of ulcerative colitis reported, the temporal relationship to vaccination for one case was questionable considering that the participant had a 6-month complex gastrointestinal medical history prior to vaccine administration. An immune-mediated etiology related to vaccination can be questioned for other diagnoses. Two cases of neuritis were reported, both in the adjuvanted vaccine group and both with time from the most recent vaccine administration to onset that may not be consistent with a vaccine-induced auto-immune phenomenon. One case of optic neuritis had an onset more than 3 y following the second vaccine dose; while another report of “neuritis” had onset within hours after the first vaccine dose, clinical features suggestive of radial nerve injury due to improper injection technique, and resolution 83 d post-vaccination. One participant with autoimmune thyroiditis and one with multiple sclerosis were found to have symptom onsets and/or diagnostic findings which had preceded the administration of the investigational test articles. Based on the clinical data included in this pooled analysis, there was no consistent pathogenic mechanism implicated across the spectrum of pIMD diagnoses observed; some were antibody-mediated diseases, whereas others are believed to be T-cell mediated or even primarily non-immunologic.
For convulsion, an AESI not considered a pIMD, 9 cases were reported in the adjuvanted vaccine group and 3 cases in the control group, with an RR of 1.5 (95% CI: 0.3; 8.8).
Our findings are similar to those reported in post-marketing studies. A GSK-sponsored prospective cohort post-authorization safety study (PASS) in the United Kingdom, which enrolled 9,142 participants, reported only 2 AEs which are part of the CHMP AESI list, with the observed number higher than expected: neuritis (1 case within 31 days) and convulsions (8 cases with onset within 181 days).53 A retrospective cohort study that examined the risk of neurological and autoimmune diagnoses following vaccination with adjuvanted A(H1N1)pdm09 vaccine in >1 million persons reported an increased risk for Bell's palsy (hazard ratio [HR] 1.3), paresthesia (HR 1.1) and inflammatory bowel disease (HR 1.1).54 A slightly increased risk for a broad range of neurological and immune-related diagnoses in persons who received an adjuvanted A(H1N1)pdm09 vaccine (Pandemrix™) was reported in another population-based prospective cohort study conducted in Sweden.55
Several retrospective studies suggest an association between vaccination with the A(H1N1)pdm09 vaccine Pandemrix™ during 2009/2010 pandemic and the subsequent onset of narcolepsy in persons less than 21 y of age as well as in adults.54,56-65 These retrospective observational studies alone will remain insufficient to determine whether the observed increased risk of narcolepsy was solely related to the vaccine, or whether a complex interplay of other factors, including the A(H1N1)pdm09 virus itself, may have had an etiologic role. Further research is needed to elucidate the chain of events that resulted in narcolepsy and the potential roles of genetic and environmental factors. In the clinical trials included in our analysis, only one case of somnolence was reported in a participant who had received an adjuvanted vaccine in a controlled trial. The onset was on Day 33 following vaccination, and the condition was reported as persisting after 352 days, but with no additional diagnostic procedures carried out by the investigator to rule out or substantiate the possible diagnosis of narcolepsy. Following the reports of potential association between Pandemrix™ and narcolepsy, the database was screened but no other cases of narcolepsy or similar suspect terms were identified. The current analysis did not include participants in the adolescent age group which is most commonly affected by new onset of narcolepsy and, given the rarity of this diagnosis would not be expected to be informative unless the risk of narcolepsy or other rare disease states such as Guillain-Barré syndrome was markedly greater than that suggested by the epidemiologic data. 54,56-65
Although the analysis presented here represents a large dataset in which a wide range of vaccine safety data were collected, a variety of limitations must be recognized. Because of the low incidence rates of some of the diagnoses of interest here, even this pooled analysis has low power to exclude the possibility that a modest increase in risk may exist in adjuvanted vaccine recipients. This consideration is counterbalanced, to some extent, by the recognition that many of the intrinsic limitations and biases of this pooled analysis, including unbalanced randomization, unbalanced periods of safety follow-up, and the increased acute reactogenicity of adjuvanted vaccines 8,9,11,66 may all operate to exaggerate an apparent association of uncommon events with the adjuvanted products or stimulate enhanced reporting by adjuvanted vaccine recipients. Conversely, the inclusion of some trials with only 6 month follow-up periods may have reduced the likelihood of recognition of diseases with insidious onset, such as rheumatoid arthritis and polymyalgia rheumatica.
The use of literature-reported rates as a reference for the incidence of safety outcomes also has important limitations, such as age, geographical and seasonal differences in disease, diagnostic procedures or case definitions that may be associated with false signaling or failure to identify a true signal,67 and the limited or heterogeneous available data for some diseases. Differences in methodologies and populations may account for the differences observed between literature reported rates of AEs and those observed in our clinical trials. Stimulated reporting of AEs in the controlled trials included in the analysis ensured a more accurately estimation of the IR than the methods commonly used in epidemiologic studies, but could also induce exaggerated background rates relative to purely observational data sets.
Conclusions
The results of this analysis do not suggest a statistically significant association between the use AS03-adjuvanted H5N1 and A(H1N1)pdm09 influenza vaccines and the reporting of MAEs, SAEs, AESIs, or pIMDs, although small increases in the risk of such events cannot be ruled out. In the setting of an advancing pandemic with attendant morbidity, mortality, and severe economic and social disruption, the benefit/risk ratio resulting from a small increased risk, if real, associated with vaccines which could both reduce mortality and limit the spread of pandemic disease may well be deemed acceptable. Further clinical trials evaluating AS03-adjuvanted influenza vaccines will likely be performed to ensure that the utility of the adjuvant is confirmed for new evolving pandemic threat strains such as A(H7N9) or A(H10N8). These trials must continue to closely monitor safety overall, including pIMDs, so that the best possible information can be available to guide use of these important medical counter measures.
Methods
Objectives
The objective of this analysis was to increase the likelihood of detecting MAEs, including subsets of SAEs, pIMDs, and AESI after the primary vaccination series, based on the maximum sample size attainable in participants with generally comparable data, and to explore a potential association of these AEs with the receipt of an AS03-adjuvanted influenza vaccine.
Eligibility criteria and design of trials included in the analysis
Data from 28 multicenter, open-label, observer- or double-blind clinical trials conducted in adults and sponsored by GSK were included in the analysis. All completed clinical trials that had included at least one adjuvanted monovalent A(H1N1)pdm09 or H5N1 vaccine formulation and had had a locked safety dataset covering at least 6 months after first vaccine exposure were included in the analysis (Table 1). The adjuvant system was either AS03A or AS03B. Of all trials, 26 had been randomized and 14 had been controlled with a plain unadjuvanted antigen vaccine or placebo. The data lock point for the analysis of the trials was 16 March 2011. All participants from the trials who had received at least one dose of either adjuvanted study vaccine or control were included in the analysis. Participants were included into the appropriate analysis group according to the vaccine received. We pooled recipients of H5N1 and A(H1N1)pdm09 antigens, regardless of site of manufacture (Dresden, Germany or St-Foy, Quebec, Canada), and regardless of antigen or adjuvant dose.
H5N1 vaccine recipients had received one of the 4 antigen dose levels (3.75μg, 7.5μg, 15μg or 30μg) from the A/Indonesia/05/2005/PR8-IBCDC-RG2, A/Vietnam/1194/04-like NIBRG-14 or A/turkey/Turkey/1/2005-like NIBRG-23 strains. Similar antigen dose levels of A/California/7/2009/NYMC X-179A strain had been administered to the A(H1N1)pdm09 vaccine recipients. The AS03-adjuvanted influenza vaccine doses had been prepared by mixing the antigen and AS03 prior to administration. AS03 is an oil-in-water emulsion-based adjuvant system containing squalene (10.69mg per dose for AS03A and 5.345mg per dose for AS03B), α-tocopherol (11.86mg per dose for AS03A and 5.93mg per dose for AS03B) and polysorbate 80 (4.86mg per dose for AS03A and 2.43mg per dose for AS03B). The control group included participants who had received saline placebo alone or split virion unadjuvanted pandemic H5N1 or A(H1N1)pdm09 antigen alone or commercially-available seasonal influenza vaccines (Fluarix™ or Fluviral™/FluLaval™ [GSK Vaccines]).
Collection of safety data
To obtain information on pIMDs (a subset of AEs that included both clear autoimmune diseases and other inflammatory and/or neurologic disorders which may or may not have autoimmune etiologies) for all trials included, GSK had initially requested investigators to report any AE considered as a potentially “new onset chronic disease” (NOCD) with onset up to 6 months after administration of the first dose of the study vaccine. Reports were subsequently screened using a pre-defined list of potential chronic diseases or signs/symptoms of NOCD. This list included chronic diseases such as autoimmune disorders, allergies, asthma and signs/symptoms of these diseases. For trials initiated after December 2008, investigators had been requested to identify AEs with potential underlying immunologic mechanisms using a pre-defined list of terms capturing major pIMD categories: neuro-inflammatory, musculoskeletal, skin, gastrointestinal, hepatobiliary, hematologic disorders and autoimmune metabolic diseases.
Additionally, the database was searched for AESIs. The AESIs were those AEs that the CHMP considered as worthy of closer safety monitoring following administration of pandemic influenza vaccine and included: anaphylaxis, Bell's palsy, convulsion, demyelination, encephalitis, Guillain-Barré syndrome, neuritis and vasculitis.19 There was an overlap between the CHMP-specified AESIs and the pre-defined list of pIMDs. The AESIs anaphylaxis and convulsion are not considered pIMDS; however, for all other AESIs, the list of PTs and SMQs used to define AESIs includes the PTs from the pIMD list.
For each of the AEs experienced, the participant had been asked if he/she had received medical attention, defined as hospitalization, an emergency room visit or an otherwise unscheduled visit to or from medical personnel for any reason. AEs characterized by such unscheduled medical care were designated MAEs .The severity of all MAEs had been graded by investigators on a scale of 1–3, with Grade 1 for those that had not interfered with everyday activities and Grade 3 for those that had prevented everyday activities. A SAE had been defined as an event that had resulted in death, had been life-threatening, had required hospitalization or prolongation of hospitalization, had resulted in disability/incapacity, had been a congenital anomaly/birth defect in the offspring of a trial participant or had been considered by medical judgment as medically significant.
The duration of follow-up for the events of interest had varied between the trials and was ≥85 days for MAEs and ≥182 days for SAEs, pIMDs and AESIs.
Ethics
All trials had been conducted in accordance with Good Clinical Practice (GCP), the principles of the Declaration of Helsinki, and all applicable regulatory requirements within each local or national jurisdiction. The trial protocols and informed consent forms had been reviewed and approved by Independent Ethics Committees prior to study initiation, and all participants gave written informed consent. The summaries of the trial protocols are available at www.gsk-clinicalstudyregister.com.
Statistical analyses
All analyses were performed on the total vaccinated cohort, which included all participants who had received at least one dose of adjuvanted vaccine/control. Verbatim descriptions of pIMDs, MAEs and SAEs were coded for analysis using MedDRA PTs. MedDRA coding was performed before unblinding, at the time of finalization of the individual clinical trial data sets.
The following analyses were performed: (i) comparative safety analysis of AS03-adjuvanted vaccines vs. control considering controlled trials only and including either all participants or (ii) analysis including only AS03A-adjuvanted vaccines (excluding participants who received a half dose of the adjuvant, i.e. AS03B); (iii) safety characterization of AS03-adjuvanted vaccines based on both controlled and uncontrolled trials including either all participants or (iv) analysis including only AS03A-adjuvanted vaccines (excluding participants who received a half dose of the adjuvant).
Person-year incidence rates (per 1,000 or per 100,000 person-years) were computed for all endpoints, with exact 95% CIs for both adjuvanted vaccine and control groups, in both controlled and uncontrolled trials. Computation of person-year incidence was based on individual participant follow-up. The duration of follow-up (in days) was defined as the time between the date of the first vaccination and the date of the last data collection of the endpoints.
For the controlled trials, the RR of an event in participants who had received an adjuvanted vaccine relative to that in control recipients was estimated, with an exact 95% CI, using the exact conditional logistic regression adjusted for the trial effect.68 Homogeneity of the common RR across the different trials was assessed using a 2-sided exact Breslow and Day test. The p-value for homogeneity was calculated across all trials when there had been at least one event in each treatment group and when the events had been reported in more than one study. A p-value below 0.05 was considered as indicative of heterogeneity. When no cases had been identified in control recipients, a lower bound of the 95% CI was estimated. Multiplicity was not accounted for in the analysis of disproportionality. Each RR for which the 95% CI excluded 1.0 was accepted as suggestive of a potential treatment effect without controlling for the risk for false positive signals.
Data from the literature were used to evaluate the probability of observing pIMDs and to assess how the incidence rates before and after case review match those reported in a non-exposed population. For each pIMD, the annual incidences reported from different sources were used to calculate a median incidence rate per 100,000 person-years.
Disclosure of Potential Conflict of Interest
At the time of the study, all authors, except Anne Hepburn, were employees of the GlaxoSmithKline group of companies. Bruce L. Innis, Walthere Dewe, Harry Seifert, David W. Vaughn, Lou F. Fries, Ping Li and Catherine Cohet report ownership of stock options and/or restricted shares. Lou F. Fries is the employee of another biopharmaceutical company (Novavax) developing a different adjuvanted influenza vaccine. Anne Hepburn has nothing to disclose.
Acknowledgments
The authors wish to thank all investigators, study participants and nurses who contributed to the clinical trials included in the present analyses. We are grateful to all teams of GSK Biologicals for their contribution to this study, especially Dorothy Slavin, Anne Schuind, Rosalia Calamera, Nathalie Garcon, Anu Madan, Olivier Godeaux, Patricia Izurieta, and Miguel Madariaga. The authors would also like to thank Adriana Rusu (XPE Pharma & Science on behalf of GlaxoSmithKline Vaccines) for writing support and Bruno Dumont (Business & Decision Life Sciences on behalf of GlaxoSmithKline Vaccines) for editorial assistance and manuscript coordination.
Trademark Statement
Fluarix™, Fluviral™, FluLaval™ and Pandemrix™ are trademarks of the GlaxoSmithKline group of companies.
Funding
Studies Q-H5N1–001, Q-H5N1–002, Q-H5N1–005, Q-H5N1–010, Q-H1N1–001, Q-H1N1–002, and Q-H1N1–019 were funded by the US Department of Health and Human Services (HHS), Assistant Secretary of Preparedness and Response (ASPR), Biomedical Advanced Research and Development Authority (BARDA). Study Q-Pan-009 was funded by the Public Health Agency of Canada. GlaxoSmithKline Biologicals SA provided funding for all studies and was involved in all stages of the clinical trials conduct and analysis. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data.
References
- 1.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature 2006; 442:448-52; PMID:16642006; http://dx.doi.org/ 10.1038/nature04795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Germann TC, Kadau K, Longini IM. Jr, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci USA 2006; 103:5935-40; PMID:16585506; http://dx.doi.org/ 10.1073/pnas.0601266103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, Devaster JM, Leroux-Roels G. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 2007; 370:580-9; PMID:17707753; http://dx.doi.org/ 10.1016/S0140-6736(07)61297-5 [DOI] [PubMed] [Google Scholar]
- 4.Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine 2010; 28:1740-5; PMID:20034605; http://dx.doi.org/ 10.1016/j.vaccine.2009.12.014 [DOI] [PubMed] [Google Scholar]
- 5.Leroux-Roels I, Bernhard R, Gerard P, Drame M, Hanon E, Leroux-Roels G. Broad Clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One 2008; 3:e1665; PMID:18301743; http://dx.doi.org/ 10.1371/journal.pone.0001665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roman F, Clement F, Dewe W, Walravens K, Maes C, Willekens J, De Boever F, Hanon E, Leroux-Roels G. Effect on cellular and humoral immune responses of the AS03 adjuvant system in an A/H1N1/2009 influenza virus vaccine administered to adults during two randomized controlled trials. Clin Vaccine Immunol 2011; 18:835-43; PMID:21450978; http://dx.doi.org/ 10.1128/CVI.00480-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang WH, Dionne M, Kyle M, Aggarwal N, Li P, Madariaga M, Godeaux O, Vaughn DW. Long-term immunogenicity of an AS03-adjuvanted influenza A(H1N1)pdm09 vaccine in young and elderly adults: an observer-blind, randomized trial. Vaccine 2013; 31:4389-97; PMID:23856331; http://dx.doi.org/ 10.1016/j.vaccine.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poder A, Simurka P, Li P, Roy-Ghanta S, Vaughn D. An observer-blind, randomized, multi-center trial assessing long-term safety and immunogenicity of AS03-adjuvanted or unadjuvanted H1N1/2009 influenza vaccines in children 10-17 years of age. Vaccine 2014; 32:1121-9; PMID:24252703; http://dx.doi.org/ 10.1016/j.vaccine.2013.11.031 [DOI] [PubMed] [Google Scholar]
- 9.Ferguson M, Risi G, Davis M, Sheldon E, Baron M, Li P, Madariaga M, Fries L, Godeaux O, Vaughn D. Safety and Long-term Humoral Immune Response in Adults After Vaccination With an H1N1 2009 Pandemic Influenza Vaccine With or Without AS03 Adjuvant. J Infect Dis 2012; 205:733-44; PMID:22315336; http://dx.doi.org/ 10.1093/infdis/jir641 [DOI] [PubMed] [Google Scholar]
- 10.Fedson DS. Preparing for pandemic vaccination: an international policy agenda for vaccine development. J Public Health Policy 2005; 26:4-29; PMID:15906873; http://dx.doi.org/ 10.1057/palgrave.jphp.3200008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rumke HC, Bayas JM, de Juanes JR, Caso C, Richardus JH, Campins M, Rombo L, Duval X, Romanenko V, Schwarz TF, et al. . Safety and reactogenicity profile of an adjuvanted H5N1 pandemic candidate vaccine in adults within a phase III safety trial. Vaccine 2008; 26:2378-88; PMID:18407382; http://dx.doi.org/ 10.1016/j.vaccine.2008.02.068 [DOI] [PubMed] [Google Scholar]
- 12.Langley JM, Frenette L, Ferguson L, Riff D, Sheldon E, Risi G, Johnson C, Li P, Kenney R, Innis B, et al. . Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis 2010; 201:1644-53; PMID:20423222; http://dx.doi.org/ 10.1086/652701 [DOI] [PubMed] [Google Scholar]
- 13.Skowronski DM, Janjua NZ, De Serres G, Hottes TS, Dickinson JA, Crowcroft N, Kwindt TL, Tang P, Charest H, Fonseca K, et al. . Effectiveness of AS03 adjuvanted pandemic H1N1 vaccine: case-control evaluation based on sentinel surveillance system in Canada, autumn 2009. BMJ 2011; 342:c7297; PMID:21292718; http://dx.doi.org/ 10.1136/bmj.c7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Buynder PG, Dhaliwal JK, Van Buynder JL, Couturier C, Minville-LeBlanc M, Garceau R, Tremblay F. Protective effect of single-dose adjuvanted pandemic influenza vaccine in children. Influenza Other Respir Viruses 2010; 4:171-178; PMID:20629771; http://dx.doi.org/ 10.1111/j.1750-2659.2010.00146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wichmann O, Stöcker P, Poggensee G, Altmann D, Walter D, Hellenbrand W, Krause G, Eckmanns T. Pandemic influenza A(H1N1) 2009 breakthrough infections and estimates of vaccine effectiveness in Germany 2009-2010. Euro Surveill 2010; 15:pii=19561; PMID:20460094 [PubMed] [Google Scholar]
- 16.Nolan T, Roy-Ghanta S, Montellano M, Weckx L, Ulloa-Gutierrez R, Lazcano-Ponce E, Kerdpanich P, Safadi M, Cruz-Valdez A, Litao S, et al. . Relative efficacy of AS03-adjuvanted pandemic H1N1 influenza vaccine in children: results of a controlled, randomized efficacy trial. J Infect Dis 2014. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Medicines Agency Assessment report for Pandemic influenza vaccine (H5N1) (split virion, inactivated, adjuvanted) GlaxoSmithKline Biologicals. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001206/WC500049547.pdf. Accessed 14 Feb 2014. [Google Scholar]
- 18. Product information leaflet AREPANRIX™ H1N1 AS03-Adjuvanted H1N1 Pandemic Influenza Vaccine. Available at: http://www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/prodpharma/legislation/interimorders-arretesurgence/prodinfo-vaccin-eng.pdf. Accessed 14 Feb 2014. [Google Scholar]
- 19.European Medicines Agency CHMP Recommendations for the Pharmacovigilance Plan as part of the Risk Management Plan to be submitted with the Marketing Authorisation Application for a Pandemic Influenza Vaccine. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2010/01/WC500051739.pdf. Accessed 14 Feb 2014 [Google Scholar]
- 20.Sobel JD. Vulvovaginal candidosis. Lancet 2007; 369:1961-71; PMID:17560449; http://dx.doi.org/ 10.1016/S0140-6736(07)60917-9 [DOI] [PubMed] [Google Scholar]
- 21.Stamm WE, Norrby SR. Urinary tract infections: disease panorama and challenges. J Infect Dis 2001; 183(Suppl 1):S1-4; PMID:11171002; http://dx.doi.org/ 10.1086/318850 [DOI] [PubMed] [Google Scholar]
- 22. Toxicology Review of Influenza A (H5N1) virus monovalent vaccine, adjuvanted. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM379021.pdf. Accessed 10 Mar 2014 [Google Scholar]
- 23.European Medicines Agency CHMP assessment report for Pandemrix. London, 24 September 2009 Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000832/WC500095422.pdf. Accessed 10 Mar 2014 [Google Scholar]
- 24.Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H, et al. . Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011; 29:2461-73; PMID:21256188; http://dx.doi.org/ 10.1016/j.vaccine.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 25.Black S, Eskola J, Siegrist CA, Halsey N, Macdonald N, Law B, Miller E, Andrews N, Stowe J, Salmon D, et al. . Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet 2009; 374:2115-22; PMID:19880172; http://dx.doi.org/ 10.1016/S0140-6736(09)61877-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandenburg NA, Annegers JF. Incidence and risk factors for Bell's palsy in Laredo, Texas: 1974-1982. Neuroepidemiology 1993; 12:313-25; PMID:8309506; http://dx.doi.org/ 10.1159/000110333 [DOI] [PubMed] [Google Scholar]
- 27.Campbell KE, Brundage JF. Effects of climate, latitude, and season on the incidence of Bell's palsy in the US Armed Forces, October 1997 to September 1999. Am J Epidemiol 2002; 156:32-9; PMID:12076886; http://dx.doi.org/ 10.1093/aje/kwf009 [DOI] [PubMed] [Google Scholar]
- 28. deDiego Prim JI. MP, Madero R, Gavilan J. Seasonal patterns of idiopathioc facial paralysis: a 16-year study. Otolaryngol Head Neck Surg 1999; 120:269-71; PMID:9949364; http://dx.doi.org/ 10.1016/S0194-5998(99)70418-3 [DOI] [PubMed] [Google Scholar]
- 29.Katusic SK, Beard CM, Wiederholt WC, Bergstralh EJ, Kurland LT. Incidence, clinical features, and prognosis in Bell's palsy, Rochester, Minnesota, 1968-1982. Ann Neurol 1986; 20:622-7; PMID:3789675; http://dx.doi.org/ 10.1002/ana.410200511 [DOI] [PubMed] [Google Scholar]
- 30.Kurz X, Domergue F, Slattery J, Segec A, Szmigiel A, Hidalgo-Simon A. Safety monitoring of Influenza A/H1N1 pandemic vaccines in EudraVigilance. Vaccine 2011; 29:4378-87; PMID:21501644; http://dx.doi.org/ 10.1016/j.vaccine.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 31.Morris AM, Deeks SL, Hill MD, Midroni G, Goldstein WC, Mazzulli T, Davidson R, Squires SG, Marrie T, McGeer A, et al. . Annualized incidence and spectrum of illness from an outbreak investigation of Bell's palsy. Neuroepidemiology 2002; 21:255-61; PMID:12207155; http://dx.doi.org/ 10.1159/000065645 [DOI] [PubMed] [Google Scholar]
- 32.Cimmino MA, Zaccaria A. Epidemiology of polymyalgia rheumatica. Clin Exp Rheumatol 2000; 18:S9-11; PMID:10948749 [PubMed] [Google Scholar]
- 33.Doran MF, Crowson CS, O'Fallon WM, Hunder GG, Gabriel SE. Trends in the incidence of polymyalgia rheumatica over a 30 year period in Olmsted County, Minnesota, USA. J Rheumatol 2002; 29:1694-7; PMID:12180732 [PubMed] [Google Scholar]
- 34.Smeeth L, Cook C, Hall AJ. Incidence of diagnosed polymyalgia rheumatica and temporal arteritis in the United Kingdom, 1990-2001. Ann Rheum Dis 2006; 65:1093-8; PMID:16414971; http://dx.doi.org/ 10.1136/ard.2005.046912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huerta C, Rivero E, Rodriguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol 2007; 143:1559-65; PMID:18087008 [DOI] [PubMed] [Google Scholar]
- 36.Icen M, Crowson CS, McEvoy MT, Dann FJ, Gabriel SE, Maradit Kremers H. Trends in incidence of adult-onset psoriasis over three decades: a population-based study. J Am Acad Dermatol 2009; 60:394-401; PMID:19231638; http://dx.doi.org/ 10.1016/j.jaad.2008.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abraham-Nordling M, Torring O, Lantz M, Hallengren B, Ohrling H, Lundell G, Calissendorff J, Jorneskog G, Wallin G. Incidence of hyperthyroidism in Stockholm, Sweden, 2003-2005. Eur J Endocrinol 2008; 158:823-7; PMID:18505903; http://dx.doi.org/ 10.1530/EJE-07-0877 [DOI] [PubMed] [Google Scholar]
- 38.Berglund J, Ericsson UB, Hallengren B. Increased incidence of thyrotoxicosis in Malmo during the years 1988-1990 as compared to the years 1970-1974. J Intern Med 1996; 239:57-62; PMID:8551201; http://dx.doi.org/ 10.1046/j.1365-2796.1996.415757000.x [DOI] [PubMed] [Google Scholar]
- 39.Brownlie BE, Wells JE. The epidemiology of thyrotoxicosis in New Zealand: incidence and geographical distribution in north Canterbury, 1983-1985. Clin Endocrinol (Oxf) 1990; 33:249-59; PMID:2225482; http://dx.doi.org/ 10.1111/j.1365-2265.1990.tb00489.x [DOI] [PubMed] [Google Scholar]
- 40.Galofre JC, Garcia-Mayor RV, Fluiters E, Fernandez-Calvet L, Rego A, Paramo C, Andrade MA. Incidence of different forms of thyroid dysfunction and its degrees in an iodine sufficient area. Thyroidology 1994; 6:49-54; PMID:7536450 [PubMed] [Google Scholar]
- 41.Klein NP, Ray P, Carpenter D, Hansen J, Lewis E, Fireman B, Black S, Galindo C, Schmidt J, Baxter R. Rates of autoimmune diseases in Kaiser Permanente for use in vaccine adverse event safety studies. Vaccine 2010; 28:1062-8; PMID:19896453; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.115 [DOI] [PubMed] [Google Scholar]
- 42.Modrego PJ, Pina MA. Trends in prevalence and incidence of multiple sclerosis in Bajo Aragon, Spain. J Neurol Sci 2003; 216:89-93; PMID:14607307; http://dx.doi.org/ 10.1016/j.jns.2003.07.007 [DOI] [PubMed] [Google Scholar]
- 43.Cook B, Oxner R, Chapman B, Whitehead M, Burt M. A thirty-year (1970-1999) study of coeliac disease in the Canterbury region of New Zealand. N Z Med J 2004; 117:U772; PMID:15014561 [PubMed] [Google Scholar]
- 44.Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ., 3rd Trends in the identification and clinical features of celiac disease in a North American community, 1950-2001. Clin Gastroenterol Hepatol 2003; 1:19-27; PMID:15017513; http://dx.doi.org/ 10.1053/jcgh.2003.50004 [DOI] [PubMed] [Google Scholar]
- 45.Radhakrishan K, Litchy WJ, O’Fallon WM, Kurland LT. Epidemiology of cervical radiculopathy: a population-based study from Rochester, Minnesota, 1976-1990. Brain 1994; 117:325-35; PMID:8186959; http://dx.doi.org/ 10.1093/brain/117.2.325 [DOI] [PubMed] [Google Scholar]
- 46.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011; 140:1785-94; PMID:21530745; http://dx.doi.org/ 10.1053/j.gastro.2011.01.055 [DOI] [PubMed] [Google Scholar]
- 47.Loftus EV. Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am 2002; 31:1-20; PMID:12122726; http://dx.doi.org/ 10.1016/S0889-8553(01)00002-4 [DOI] [PubMed] [Google Scholar]
- 48.Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L, SERAP Study Group. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology (Oxford) 2008; 47:1088-92; PMID:18511475; http://dx.doi.org/ 10.1093/rheumatology/ken205 [DOI] [PubMed] [Google Scholar]
- 49.Pedersen JK, Kjaer NK, Svendsen AJ, Horslev-Petersen K. Incidence of rheumatoid arthritis from 1995 to 2001: impact of ascertainment from multiple sources. Rheumatol Int 2009; 29:411-5; PMID:18853167; http://dx.doi.org/ 10.1007/s00296-008-0713-6 [DOI] [PubMed] [Google Scholar]
- 50.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology 2004; 111:491-500; PMID:15019324; http://dx.doi.org/ 10.1016/j.ophtha.2003.06.014 [DOI] [PubMed] [Google Scholar]
- 51.Zhou W, Pool V, DeStefano F, Iskander JK, Haber P, Chen RT; VAERS Working Group. A potential signal of Bell's palsy after parenteral influenza vaccines: reports to the Vaccine Adverse Event Reporting System (VAERS) – United States, 1991-2001. Pharmacoepidemiol Drug Saf 2004; 13:505-10; PMID:15317028; http://dx.doi.org/ 10.1002/pds.998 [DOI] [PubMed] [Google Scholar]
- 52.Tavares Da Silva F, De Keyser F, Lambert PH, Robinson WH, Westhovens R, Sindic C. Optimal approaches to data collection and analysis of potential immune mediated disorders in clinical trials of new vaccines. Vaccine. 2013; 31:1870-6; PMID:23391600; http://dx.doi.org/ 10.1016/j.vaccine.2013.01.042 [DOI] [PubMed] [Google Scholar]
- 53.Nazareth I, Tavares F, Rosillon D, Haguinet F, Bauchau V. Safety of AS03-adjuvanted split-virion H1N1 (2009) pandemic influenza vaccine: a prospective cohort study. BMJ Open 2013; 3:e001912; PMID:23388195; http://dx.doi.org/ 10.1136/bmjopen-2012-001912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bardage C, Persson I, Ortqvist A, Bergman U, Ludvigsson JF, Granath F. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm, Sweden. BMJ 2011; 343:d5956; PMID:21994316; http://dx.doi.org/ 10.1136/bmj.d5956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Persson I, Granath F, Askling J, Ludvigsson JF, Olsson T, Feltelius N. Risks of neurological and immune-related diseases, including narcolepsy, after vaccination with Pandemrix: a population- and registry-based cohort study with over 2 years of follow-up. J Intern Med 2014; 275:172-90; PMID:24134219; http://dx.doi.org/ 10.1111/joim.12150 [DOI] [PubMed] [Google Scholar]
- 56.Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, Himanen SL, Hublin C, Julkunen I, Olsen P, et al. . AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One 2012; 7:e33536; PMID:22470453; http://dx.doi.org/ 10.1371/journal.pone.0033536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jokinen J, Nohynek H, Honkanen J, Vaarala O, Partinen M, Hublin C, Kilpi T. Association between the pandemic vaccine and narcolepsy in adults. Cohort study based on confirmed register data. THL 2013. Available at: http://www.julkari.fi/bitstream/handle/10024/104482/URN_ISBN_978-952-245-921-3.pdf?sequence=1 . Accessed 14 Feb 2014 [Google Scholar]
- 58.Medical Products Agency (MPA) – Sweden Occurrence of narcolepsy with cataplexy among children and adolescents in relation to the H1N1 pandemic and Pandemrix vaccinations - Results of a case inventory study by the MPA in Sweden during 2009-2010. Thirty June 2011 Available at: http://www.lakemedelsverket.se/upload/nyheter/2011/Fallinventeringsrapport_pandermrix_110630.pdf. Accessed 14 Feb 2014. [Google Scholar]
- 59.Persson I, Granath F, Askling J, Ludvigsson JF, Olsson T, Feltelius N. Risks of neurological and immune-related diseases, including narcolepsy, after vaccination with Pandemrix: a population- and registry-based cohort study with over 2 years of follow-up. J Intern Med 2014;275:172-90; PMID:24134219; http://dx.doi.org/ 10.1111/joim.12150 [DOI] [PubMed] [Google Scholar]
- 60.Szakacs A, Darin N, Hallbook T. Increased childhood incidence of narcolepsy in western Sweden after H1N1 influenza vaccination. Neurology 2013;80:1315-21; PMID:23486871; http://dx.doi.org/ 10.1212/WNL.0b013e31828ab26f [DOI] [PubMed] [Google Scholar]
- 61.Department of Health (Ireland) Investigation of an increase in the incidence of narcolepsy in children and adolescents in 2009 and 2010. Final Report of National Narcolepsy Study Steering Committee (2012). Available at: http://www.dohc.ie/publications/pdf/Final_Report_of_National_Narcolepsy_Study_Steering_Committee.pdf?direct=1. Accessed 14 Feb 2014 [Google Scholar]
- 62.Dauvilliers Y, Arnulf I, Lecendreux M, Monaca Charley C, Franco P, Drouot X, d'Ortho MP, Launois S, Lignot S, Bourgin P, et al. . Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain 2013; 136:2486-96; PMID:23884811; http://dx.doi.org/ 10.1093/brain/awt187 [DOI] [PubMed] [Google Scholar]
- 63. European Center for Disease Prevention and Control Infections, Vaccinations and Narcolepsy Background rates and Case-control Study, Final Study Report VAESCO-Narcolepsy v1.9.7 ECDC Technical report, Oct. 2012. Narcolepsy in association with pandemic influenza vaccination (a multi-country European epidemiological investigation). Stockholm: ECDC; September 2012 Available at: http://ecdc.europa.eu/en/publications/Publications/Vaesco%20report%20FINAL%20with%20cover.pdf. Accessed 14 Feb 2014 [Google Scholar]
- 64.Miller E, Andrews N, Stellitano L, Stowe J, Winstone AM, Shneerson J, Verity C. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. BMJ 2013; 346:f794; PMID:23444425; http://dx.doi.org/ 10.1136/bmj.f794 [DOI] [PubMed] [Google Scholar]
- 65.Heier MS, Gautvik KM, Wannag E, Bronder KH, Midtlyng E, Kamaleri Y, Storsaeter J. Incidence of narcolepsy in Norwegian children and adolescents after vaccination against H1N1 influenza A. Sleep Med 2013; 14:867-71; PMID:23773727; http://dx.doi.org/ 10.1016/j.sleep.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 66.Langley JM, Risi G, Caldwell M, Gilderman L, Berwald B, Fogarty C, Poling T, Riff D, Baron M, Frenette L, et al. . Dose-sparing H5N1 A/Indonesia/05/2005 pre-pandemic influenza vaccine in adults and elderly adults: a phase III, placebo-controlled, randomized study. J Infect Dis 2011; 203:1729-38; PMID:21606531; http://dx.doi.org/ 10.1093/infdis/jir172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lieu TA, Kulldorff M, Davis RL, Lewis EM, Weintraub E, Yih K, Yin R, Brown JS, Platt R; Vaccine Safety Datalink Rapid Cycle Analysis Team. Real-time vaccine safety surveillance for the early detection of adverse events. Med Care 2007; 45:S89-95; PMID:17909389; http://dx.doi.org/ 10.1097/MLR.0b013e3180616c0a [DOI] [PubMed] [Google Scholar]
- 68.Breslow NE, Day W. Statistical Methods in Cancer research: the analysis of case-control studies. Oxford: Oxford University Press; 1980.; [PubMed] [Google Scholar]
- 69.Langley J, Frenette L, Jeanfreau R, Chu L, McNeil S, Halperin S, Dramé M, Fries L, Vaughn D. Immunogenicity of a Heterologous H5N1 Booster Vaccine 6 and 18 Months after Primary Vaccination in Adults. 49th Annual Meeting of the Infectious Diseases Society of America -; 2011 October 20-23, Boston. [DOI] [PubMed] [Google Scholar]
- 70.Risi G, Frenette L, Langley JM, Li P, Riff D, Sheldon E, Vaughn DW, Fries L. Immunological priming induced by a two-dose series of H5N1 influenza antigen, administered alone or in combination with two different formulations of AS03 adjuvant in adults: results of a randomised single heterologous booster dose study at 15 months. Vaccine 2011; 29:6408-18; PMID:21554915; http://dx.doi.org/ 10.1016/j.vaccine.2011.04.072 [DOI] [PubMed] [Google Scholar]
- 71.Chu DW, Hwang SJ, Lim FS, Oh HM, Thongcharoen P, Yang PC, Bock HL, Dramé M, Gillard P, Hutagalung Y, et al. . Immunogenicity and tolerability of an AS03(A)-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine 2009; 27:7428-35; PMID:19683087; http://dx.doi.org/ 10.1016/j.vaccine.2009.07.102 [DOI] [PubMed] [Google Scholar]
- 72.Heijmans S, De Meulemeester M, Reynders P, Giet D, Demanet E, Devresse PY, Icardi G, Dramé M, Roman F, Gillard P. Immunogenicity profile of a 3.75-μg hemagglutinin pandemic rH5N1 split virion AS03A-adjuvanted vaccine in elderly persons: a randomized trial. J Infect Dis 2011; 203:1054-62; PMID:21450995; http://dx.doi.org/ 10.1093/infdis/jiq174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langley JM, Frenette L, Chu L, McNeil S, Halperin S, Li P, Vaughn D. A randomized, controlled non-inferiority trial comparing A(H1N1)pmd09 vaccine antigen, with and without AS03 adjuvant system, co-administered or sequentially administered with an inactivated trivalent seasonal influenza vaccine. BMC Infect Dis. 2012; 12:279; PMID:23110320; http://dx.doi.org/ 10.1186/1471-2334-12-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lasko B, Reich D, Madan A, Roman F, Li P, Vaughn D. Rapid immunization against H5N1: a randomized trial evaluating homologous and cross-reactive immune responses to AS03(A)-adjuvanted vaccination in adults. J Infect Dis 2011; 204:574-81; PMID:21791660; http://dx.doi.org/ 10.1093/infdis/jir328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagai H, Ikematsu H, Tenjinbaru K, Maeda A, Dramé M, Roman FP. A phase II, open-label, multicentre study to evaluate the immunogenicity and safety of an adjuvanted prepandemic (H5N1) influenza vaccine in healthy Japanese adults. BMC Infect Dis 2010; 10:338; PMID:21108818; http://dx.doi.org/ 10.1186/1471-2334-10-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwarz TF, Horacek T, Knuf M, Damman HG, Roman F, Dramé M, Gillard P, Jilg W. Single dose vaccination with AS03-adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine 2009; 27:6284-90; PMID:19856521; http://dx.doi.org/ 10.1016/j.vaccine.2009.01.040 [DOI] [PubMed] [Google Scholar]
- 77.Leroux-Roels I, Roman F, Forgus S, Maes C, De Boever F, Dramé M, Gillard P, van der Most R, Van Mechelen M, Hanon E, et al. . Priming with AS03 A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine 2010; 28:849-57; PMID:19835828; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.017 [DOI] [PubMed] [Google Scholar]
- 78.Gillard P, Chu DW, Hwang SJ, Yang PC, Thongcharoen P, Lim FS, Dramé M, Walravens K, Roman F. Long-term booster schedules with AS03A-adjuvanted heterologous H5N1 vaccines induces rapid and broad immune responses in Asian adults. BMC Infect Dis 2014; 14:142; PMID:24628789; http://dx.doi.org/ 10.1186/1471-2334-14-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang PC, Yu CJ, Chang SC, Hsieh SM, Drame M, Walravens K, Roman F, Gillard P. Safety and immunogenicity of a split-virion AS03A-adjuvanted A/Indonesia/05/2005 (H5N1) vaccine in Taiwanese adults. J Formos Med Assoc 2012; 111:333-9; PMID:22748624; http://dx.doi.org/ 10.1016/j.jfma.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 80.Ikematsu H, Nagai H, Kawashima M, Kawakami Y, Tenjinbaru K, Maeda A, Li P, Gillard P, Roman F. Immunogenicity and safety of a novel AS03(A)-adjuvanted H1N1 2009 pandemic influenza vaccine in adults in Japan. Hum Vaccin 2010; 6:888-93; PMID:20980795; http://dx.doi.org/ 10.4161/hv.6.11.12851 [DOI] [PubMed] [Google Scholar]
- 81.Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)-Adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis 2010; 51:668-77; PMID:20687838; http://dx.doi.org/ 10.1086/655830 [DOI] [PubMed] [Google Scholar]
- 82.Launay O, Duval X, Fitoussi S, Jilg W, Kerdpanich A, Montellano M, Schwarz TF, Watanveerade V, Wenzel JJ, Zalcman G, et al. . Extended antigen sparing potential of AS03-adjuvanted pandemic H1N1 vaccines in children, and immunological equivalence of two formulations of AS03-adjuvanted H1N1 vaccines: results from two randomised trials. BMC Infect Dis 2013; 13:1-11; PMID:23280237; http://dx.doi.org/ 10.1186/1471-2334-13-435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peeters M, Regner S, Vaman T, Devaster JM, Rombo L. Safety and immunogenicity of an AS03-adjuvanted A(H1N1)pmd09 vaccine administered simultaneously or sequentially with a seasonal trivalent vaccine in adults 61 years or older: data from two multicentre randomised trials. Vaccine 2012; 30:6483-91; PMID:22885014; http://dx.doi.org/ 10.1016/j.vaccine.2012.07.081 [DOI] [PubMed] [Google Scholar]
- 84.Duval X, Caplanusi A, Laurichesse H, Deplanque D, Loulergue P, Vaman T, Launay O, Gillard P. Flexibility of interval between vaccinations with AS03A-adjuvanted influenza A (H1N1) 2009 vaccine in adults aged 18-60 and >60 ears: a randomized trial. BMC Infect Dis 2012; 12:162; PMID:22824474; http://dx.doi.org/ 10.1186/1471-2334-12-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yong M, Schoonen WM, Li L, Kanas G, Coalson J, Mowat F, Fryzek J, Kaye JA. Epidemiology of paediatric immune thrombocytopenia in the General Practice Research Database. Br J Haematol 2010; 149:855-64; PMID:20377590; http://dx.doi.org/ 10.1111/j.1365-2141.2010.08176.x [DOI] [PubMed] [Google Scholar]


