Abstract
Cancer patients can harbor significant numbers of CD8 and CD4 T cells with specificities to tumor antigens (Ags). Yet, in most cases, such T cells fail to eradicate the tumor in vivo. Here, we investigated the interference of Ag-specific CD4+CD25+ regulatory T cells (Treg) with the tumor-specific CD8 T cell immune response in vivo, by monitoring the homing, expansion, and effector function of both subsets in draining and nondraining lymph nodes. The results show that CD8 cells expand to the same extent and produce similar levels of IFN-γ in the presence or absence of Ag-specific Treg. Nevertheless, these Treg abrogate CD8 T cell-mediated tumor rejection by specifically suppressing the cytotoxicity of expanded CD8 cells. The molecular mechanism of suppression involves TGF-β because expression of a dominant-negative TGF-β receptor by tumor-specific CD8 cells renders them resistant to suppression and is associated with tumor rejection and unimpaired cytotoxicity.
CD4+CD25+ regulatory T cells (Treg) are negative regulators of T cell immune responses in vitro and in vivo (1, 2). Upon TCR ligation, Treg can suppress the proliferation of both CD4 and CD8 cells in coculture experiments (3). Recently, however, it has been shown that the regulation of CD4 T cell responses in vivo can have different kinetics (reviewed in ref. 4). When coinjected into normal mice, both Treg and naïve CD4 cells initially proliferate upon recognition of cognate antigen (Ag). Furthermore, Treg do not influence the commitment of Ag-experienced CD4 cells to produce IFN-γ and IL-2. However, Treg do suppress cytokine secretion and interfere with the proliferation and possibly survival of CD4 cells later during the immune response (5-7). Treg also can control the magnitude of recall CD8 T cell responses in different settings that include viral (8, 9) and bacterial (10) infections as well as allograft transplantation in vivo (11, 12). A role in Treg function has been attributed to IL-2, which stimulates Treg and in turn may inhibit division of memory CD8 T cells (13, 14). However, these studies did not trace Ag-specific Treg in draining lymph nodes (LN), and it is not clear to what extent the different outcomes reflect accumulation of Treg by specific homing and local expansion. In fact, most studies were conducted with polyclonal Treg of unknown specificity where such questions cannot be addressed, and, therefore, the mode of inhibition cannot be correlated with specific Treg accumulation. Analysis of tumor-bearing patients suggests that suppression of CD8 T cell cytotoxicity by Treg may be causally related to tumor progression, because tumor-specific CD8 cells and tumor-specific CD4 Treg frequently accumulate in tumors from melanoma patients, and tumor-specific CD8 cells fail to exert cytotoxic T lymphocyte effector function (15-17).
This study investigates how Treg suppress primary CD8 T cell immune responses directed against tumor cells expressing influenza hemagglutinin (HA) as a surrogate tumor-specific Ag. Naïve CD8 and regulatory CD4 T cells with transgenic receptors specific for distinct peptides of HA were used to allow us to follow the fate of these cells in tumor draining LN. The results show that Treg interfere with CD8 T cell-mediated tumor rejection relatively early during the immune response, and the mechanism by which Treg suppress naïve CD8 cells differs from the ones that have been reported for memory CD8 cells (10-12, 14). Treg influenced neither the kinetics of proliferation nor the commitment of recently activated CD8 cells to produce inflammatory cytokines. Nevertheless, CD8 cells failed to undergo normal functional maturation in the presence of Treg as evidenced by the fact that their cytotoxic potential to destroy specific targets in vivo was abolished. Thus, these experiments reveal a previously unreported fine-tuning by Treg on different effector functions of CD8 cells.
There is considerable controversy over a putative role of TGF-β in the Treg-dependent regulation of immune responses (18). Although two recent studies suggested that TGF-β has a crucial role in the suppression of CD8 T cell-mediated diabetes, perhaps by mechanisms that allow more potent expansion of Treg (19) and mechanisms that may affect unknown functions of CD8 cells (20), the essential role of TGF-β has been debated by others (18). Here, we show that Treg-dependent inhibition of tumor-specific CD8 T cell-mediated cytotoxicity requires expression of the TGF-β receptor by CD8 cells. These data suggest a specific role of TGF-β signaling in the inhibition of cytotoxicity independent of cellular proliferation.
Materials and Methods
Mice. T cell receptor (TCR)-HA recombination activating gene-deficient (RAG-/-) mice expressing a TCR specific for H2-IEd/HA107-119, TCR-CL4 RAG-/- mice expressing a TCR specific for H2-Kd/HA512-520, and pgk-HA × TCR-HA mice were generated as described in refs. 21, 22, and 23, respectively. BALB/c mice were purchased from Taconic Farms. BALB/c Thy1.1 mice were obtained from Paul Allen (Washington University School of Medicine, St. Louis). Dominant-negative TGFβR type II (dnTGFβRII) B6 mice (24) were backcrossed to TCR-CL4 RAG-/- BALB/c mice for five or more generations.
T Cells. HA-specific CD8 cells from TCR-CL4 RAG-/- mice, HA-specific CD4 cells from TCR-HA RAG-/- mice, and HA-specific CD4 Treg from pgk-HA × TCR-HA mice were purified as described in ref. 23. Where indicated, cells were labeled with 10 μM carboxyfluorescein diacetate-succinimidyl ester (CFSE; Molecular Probes) prior to adoptive transfer. Dendritic cells (DCs) were magnetically purified (>98% CD11c+) from spleens of BALB/c mice that had been implanted with a Flt-3L secreting melanoma cell line (25).
Tumors. The CT26 tumor cell line was derived from a chemically induced murine colon carcinoma. The tumor cell line CT44 was generated by transfecting CT26 cells with a fusion protein of influenza HA and EGFP (23). We injected 106 CT44 and CT26 cells s.c. into the upper side of the left and right hind paws of anesthetized animals, respectively (23).
Flow Cytometry. All mAbs were obtained from Becton Dickinson, except anti-HA (clone 37.38), which was obtained from Southern Biotechnology Associates. Anti-TCR-HA (clone 6.5) was purified in our laboratory. Surface and intracellular staining and flow-cytometric analysis were performed as described in ref. 26.
In Vivo Cytotoxic T Lymphocyte Assays. Splenocytes from BALB/c mice were labeled with either 1 or 10 μM CFSE (namely, CFSE+ or CFSE2+), then incubated for 1 h at 37°C with either no peptide or 1 μg/ml HA107-119 peptide, respectively. Recipient mice were injected i.v. with a 1:1 ratio of the two populations (2 × 107 cells). Tumor-draining LN cells were obtained 16 h later and stained with appropriate mAbs, including anti-B220 mAb. Percentage specific lysis was calculated as follows: % specific lysis = [1 - (B220+ CFSE+ events/B220+ CFSE2+ events)] × 100.
Results
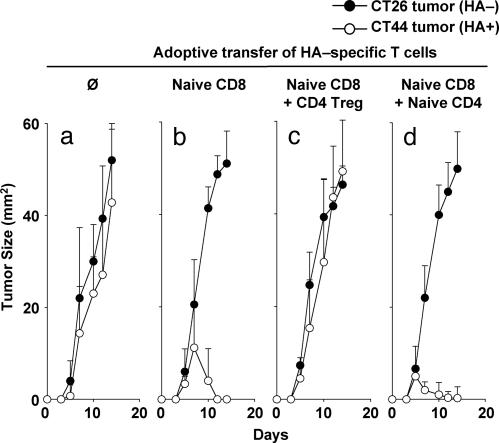
Treg Abrogate CD8 Tumor Immunity. The specificity of CD8 T cell-mediated tumor responses was analyzed in normal healthy BALB/c mice that were injected s.c. with both 106 HA+ tumor cells (CT44, right foot) and 106 HA- tumor cells (CT26, left foot) on day 0. Both tumors grew equally well in the transplanted mice, indicating the lack of an efficient tumor-specific immune response in normal mice (Fig. 1a). However, adoptive transfer of 105 naïve CD8 cells bearing a transgenic TCR specific for the Kd-restricted HA512-520 peptide (hereafter termed HA-specific CD8 cells) 1 day before tumor challenge resulted in specific rejection of CT44 tumors (Fig. 1b). CD8 T cell-mediated rejection of CT44 tumors was apparent between days 7 and 10 and was complete on day 14 (Fig. 1b). In contrast, CT26 tumors within the same individuals were not rejected (Fig. 1b).
Fig. 1.
HA-specific CD8 T cells selectively reject HA-expressing tumors in the absence of HA-specific CD4 Treg. On day -1, BALB/c mice were not treated (a) or were adoptively transferred with different combinations of HA-specific T cells as follows: 105 naïve CD8 T cells (b), 105 naïve CD8 T cells and 105 CD4 Treg (c), or 105 naïve CD8 and 105 naïve CD4 T cells (d). On day 0, two colon carcinoma cell lines, namely CT26 (HA-) and CT44 (HA+), were s.c. inoculated in the right and left footpads, respectively. Tumor size was measured over a period of 2 wk. Results show mean and SD values of nine or more mice from at least three independent experiments.
We then investigated whether CD4 Treg with specificity for a class II-restricted HA epitope may influence primary CD8 T cell immune responses directed against the class I restricted HA peptide (bystander suppression). HA-specific CD4 Treg were obtained from mice expressing influenza-HA under the control of the ubiquitous pgk promoter (pgk-HA) and a transgenic TCR (TCR-HA) specific for the I-Ed-restricted HA107-119 peptide (26). BALB/c mice were adoptively transferred with a mixture of 105 naïve HA-specific CD8 cells and 105 HA-specific CD4 Treg and subsequently challenged with CT26 and CT44 tumors. The results showed that Treg completely abrogated CD8 T cell-mediated rejection of CT44 tumors (Fig. 1c), in that the kinetic of CT44 tumor growth was as fast as that displayed by the CT26 tumor within the same individual (Fig. 1c), and comparable with the kinetic of CT44 or CT26 tumor growth in mice that did not receive HA-specific CD8 cells (Fig. 1a).
In contrast to the Treg-dependent abrogation of CD8 T cell-mediated tumor rejection, the cotransfer of 105 nonregulatory CD4 cells with the same specificity as the Treg did not abrogate but rather enhanced tumor rejection by CD8 cells (Fig. 1d), in line with the reported role of CD4 cells in augmenting immune responses by CD8 cells (21, 27). As reported in ref. 23, adoptive transfer of naïve HA-specific CD4 cells alone did not mediate rejection of CT44 tumors. The steady-state levels of adoptively transferred T cells in these experiments in the absence of Ag challenge were comparable for both CD4 and CD8 cells, in the range of 0.01% Ag-specific T cells among total CD8 or CD4 cells, respectively. These experiments, therefore, indicate that Treg were able to interfere effectively at early stages with the CD8 immune response.
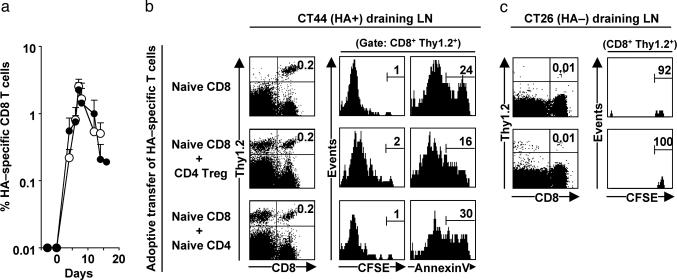
CD8 T Cell Homing and Expansion. Because CD4 Treg were reported to impair CD8 T cell proliferation in vitro (28), we investigated whether a similar mechanism contributed to the Treg-dependent impairment of the CD8 immune response in vivo. In initial experiments, CFSE-labeled HA-specific Thy1.2 CD8 cells were adoptively transferred into Thy1.1 BALB/c mice that subsequently were challenged with CT44 and CT26 tumors simultaneously. The number of HA-specific CD8 cells (defined as Thy1.2+ CD8+) rapidly increased 200-fold in CT44 draining LN (Fig. 2 a and b), whereas HA-specific CD8 cells did not accumulate in CT26 draining LN (Fig. 2c). As shown in Fig. 2b, the accumulation of CD8 cells in CT44 draining LN was accompanied by extensive cell division. Similar accumulation and expansion of HA-specific CD8 cells was observed when cotransferred with HA-specific naïve CD4 cells (Fig. 2b). The CD4 T cell population (defined as Thy1.2+ CD4+) also rapidly expanded in CT44 draining LN, reaching 2.9 ± 0.4% of CD4 cells on day 7, which is equivalent to 3.3 ± 2.0 HA-specific CD4 cells per HA-specific CD8 cell. The Ag-specific proliferation of both HA-specific CD8 and CD4 cells in CT44 draining LN indicated that HA512-520 and HA107-119 peptides were effectively presented in this environment. Because CT44 tumor cells do not express class II MHC molecules, the results indicate that HA Ags were released from tumor cells and presented by class II MHC positive cells, most likely DCs, in the draining LN.
Fig. 2.
HA-specific CD4 Treg neither impair proliferation nor accelerate apoptosis of HA-specific CD8 T cells. Thy1.1 BALB/c mice were adoptively transferred on day -1 with different combinations of CFSE-labeled Thy1.2 HA-specific T cells and challenged with CT26 (HA-) and CT44 (HA+) tumors on day 0 as described in Fig. 1. (a) CT44 draining LN from mice that received either naïve HA-specific CD8 T cells (○) or naïve HA-specific CD8 T cells and HA-specific CD4 Treg (•) were collected at different time points, and frequency of the transferred CD8 T cells (defined as CD8+Thy1.2+) was quantified by flow cytometry. (b) CD8 T cells in CT44 draining LN were assessed on day 6 for cell division (CFSE distribution) and apoptosis (binding to annexin V). (c) Similar analysis was performed with cells retrieved in CT26 tumor draining LN at the same time point. Similar results were obtained on days 4 and 8 (data not shown). Results show mean and SD values of six or more mice from at least three independent experiments.
In further experiments, the fate of HA-specific CD8 cells that were cotransferred with HA-specific CD4 Treg was studied. The presence of Treg did not alter the expansion of HA-specific CD8 cells because the kinetic of expansion of CD8 cells in the CT44 draining LN remained unchanged or only slightly reduced after cotransfer with Treg. This notion was further supported by the observation that the dilution of CFSE label was not significantly different in CD8 cells expanding in the absence or presence of Treg (Fig. 2b). HA-specific CD4 Treg also accumulated within the same LN, starting from ≈0.01% of CD4 cells immediately after adoptive transfer (data not shown) and reaching 3.4 ± 0.8% of all LN CD4 cells on day 7 (Fig. 2b). HA-specific CD4 Treg accumulated slightly faster than HA-specific CD8 cells in CT44 draining LNs, because we found 2.8 ± 1.2 HA-specific Treg per HA-specific CD8 cell on day 7. Neither CD8 cells nor Treg accumulated in the LN draining the HA-negative CT26 tumor (Fig. 2c). HA-specific CD4 Treg did not accelerate apoptosis of HA-specific CD8 cells, because the vast majority of cells remained annexin V-negative in the presence or absence of Treg (Fig. 2b). Collectively, these data indicate that Treg did not interfere with the accumulation and division of tumor-specific CD8 cells in CT44 draining LN.
CD8 T Cell Differentiation Markers. In additional assays it was analyzed whether Treg interfered with the expression of differentiation cell surface molecules on HA-specific CD8 cells. To this end naïve HA-specific CD8 cells were adoptively transferred into BALB/c mice subsequently challenged with CT44. A significant fraction of Ag-primed HA-specific CD8 cells acquired an activated CD25+CD127-CD69+CD44highCD62Llow phenotype, as demonstrated on day 7 in CT44 draining LN (see Fig. 6, which is published as supporting information on the PNAS web site). Similar phenotypic changes were observed when HA-specific CD8 cells were cotransferred with either naïve HA-specific CD4 cells or HA-specific CD4 Treg (Fig. 6). Because HA-specific CD8 cells acquired an activated phenotype irrespective of the absence or presence of Treg, expression of these markers could not be used as a diagnostic tool to predict survival of tumors.
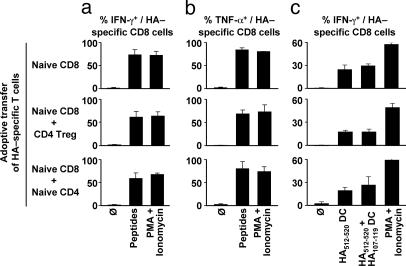
CD8 T Cell Cytokines. Ag-primed effector CD8 cells release cytokines and lytic molecules that mediate a local inflammatory response and effect target cell apoptosis (29, 30). The influence of HA-specific Treg on the functional activity of CD8 cells was investigated by analyzing cytokine production in HA-specific CD8 cells on d 7 after tumor challenge. The majority of HA-specific CD8 cells produced IFN-γ and TNF-α upon restimulation with exogenous Kd-restricted HA512-520 peptide or phorbol 12-myristate 13-acetate (PMA)/ionomycin (Fig. 3 a-c and Fig. 7, which is published as supporting information on the PNAS web site). Cotransfer of HA-specific CD4 Treg with HA-specific CD8 cells did not inhibit cytokine production. This result occurred whether the cells were restimulated only with class I MHC-restricted peptide, with both class I and class II MHC-restricted peptides, or with PMA and ionomycin, indicating that the reactivation of Treg in vitro did not suppress cytokine production by activated CD8 cells (Fig. 3 a and b).
Fig. 3.
HA-specific CD4 Treg do not control the production of inflammatory cytokines by HA-specific CD8 T cells. Thy1.1 BALB/c mice were adoptively transferred on day -1 with different combinations of Thy1.2 HA-specific T cells and challenged with CT44 (HA+) tumors on day 0 as described in Fig. 1, from which CT44 draining LN cells were collected on day 6. HA-specific CD8 T cells were analyzed for IFN-γ (a) and TNF-α (b) production after 4-h restimulation with exogenously added HA512-520 and HA107-119 peptides or PMA/ionomycin. (c) HA-specific CD8 T cells were analyzed for IFN-γ production after 4-h restimulation with DCs previously pulsed with only HA512-520 or both HA512-520 and HA107-119 peptides, or PMA/ionomycin. Results show mean and SD values of nine or more mice from at least three independent experiments.
We further investigated whether peptide presentation by Kd+ I-Ed+ antigen-presenting cells only (i.e., favoring close proximity of Treg and CD8 cells) could selectively impair CD8 T cell activity. To this end, purified splenic DCs (>97% CD11c+ Kd+ I-Ed+) were pulsed with only the class I MHC-restricted peptide or with both class I and class II MHC-restricted peptides and used as antigen-presenting cells. Stimulation by PMA/ionomycin was used in controls. In the absence of HA-specific CD4 Treg, a significant fraction of HA-specific CD8 cells produced IFN-γ upon restimulation with peptide-pulsed DCs (Fig. 3c). This fraction was lower than the number of IFN-γ-producing HA-specific CD8 cells upon restimulation with PMA/ionomycin or with exogenously added HA peptide(s) (Fig. 3a). The difference is likely due to reduced Ag presentation when peptide-pulsed DCs were used (Fig. 3c) instead of supplying cultures with peptides without subsequent removal of nonbound peptide (Fig. 3 a and b). Importantly, the presence of HA-specific CD4 Treg did not interfere with cytokine production by CD8 cells even when the CD4 cells were restimulated with the class II MHC-restricted HA peptide (Fig. 3c). Collectively these experiments indicate that the Treg did not interfere with the ability of CD8 cells to produce IFN-γ at early stages of the immune response.
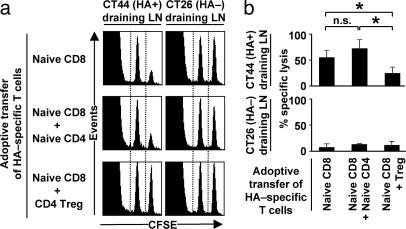
Suppression of CD8 T Cell Cytotoxicity. The cytotoxicity of HA-specific CD8 cells was addressed by in vivo readouts on day 6 after tumor challenge. In the absence of HA-specific CD4 Treg, HA-specific CD8 T cells specifically killed HA512-520 peptide-pulsed targets in CT44 draining LNs but not in CT26 draining LNs. The presence of HA-specific CD4 Treg abolished the specific cytotoxic activity of HA-specific CD8 cells (P < 0.0001; Fig. 4). No in vivo killing of HA+ targets was observed in popliteal LNs of nonchallenged mice or in CT44 draining LNs from CT44-bearing mice that did not receive HA-specific CD8 cells (data not shown). Importantly, because Treg did not interfere with the proliferation of naïve CD8 cells, the observed reduced cytotoxic activity in the presence of Treg in CT44 draining LNs indicates direct suppression of cytolytic activity rather than indirect suppression through impairment of proliferation.
Fig. 4.
HA-specific CD4 Treg suppress the cytotoxic activity of HA-specific CD8 T cells in vivo. BALB/c mice were adoptively transferred on day -1 with different combinations of HA-specific T cells and challenged with CT44 (HA+) tumors on day 0 as described in Fig. 1. Mice were injected intravenously with a 1:1 mixture of syngeneic splenocytes previously labeled with 10 μM CFSE and pulsed with HA512-520 peptide and of cells previously labeled with 1 μM CFSE but not pulsed with peptide. CT26 and CT44 draining LN were collected 16 h later, and Ag-specific killing of HA512-520 positive targets was measured by flow cytometry. (a) Representative examples for in vivo killing of HA+ targets. (b) Summary of in vivo killing of HA+ targets. n.s., not significant; *, P < 0.0001. Results show mean and SD values of nine or more mice from at least three independent experiments.
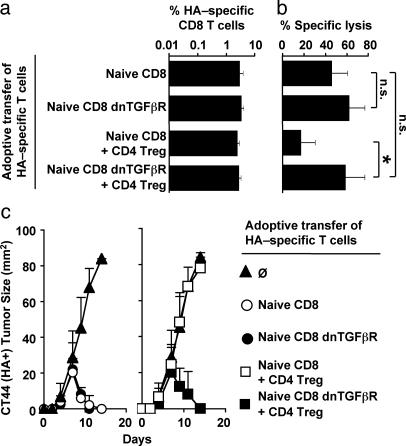
TGF-β-Dependent Suppression. A potential role of TGF-β in the suppression of tumor rejection by CD8 cells was analyzed by introducing a dominant-negative TGF-β receptor into the tumor-specific CD8 cells, by crossing mice with the transgenic TCR specific for Kd/HA512-520 with mice harboring a dnTGFβR (24). Expression of dnTGFβR by HA-specific CD8 cells did not alter their naïve phenotypic status at least in 4- to 6-wk-old animals, because the vast majority of both dnTGFβR and WT HA-specific CD8 cells were CD25-CD127+CD45RBhighCD69-CD44lowCD62Lhigh (see Fig. 8, which is published as supporting information on the PNAS web site), whereas some dnTGFβR HA-specific CD8 cells in older animals (12-16 wk old) did exhibit the CD44 activation marker (data not shown). Therefore, CD8 cells used in further experiments were from 4- to 6-wk-old mice. When 105 naïve dnTGFβR or WT HA-specific CD8 cells were transferred into BALB/c mice, both populations of cells displayed similar kinetics of expansion in response to challenge with CT44 tumors (Fig. 5a) and rejected CT44 tumors (Fig. 5c). Thus, expression of the dnTGFβR in CD8 cells did not alter their expansion and the kinetic of tumor rejection. When coinjected with Treg, dnTGFβR and WT HA-specific CD8 cells also similarly expanded in response to challenge with CT44 tumors (Fig. 5a). However, expression of dnTGFβR by HA-specific CD8 cells interfered with the suppression of tumor immunity by Treg because the kinetic of tumor rejection mediated by dnTGFβR HA-specific CD8 cells was comparable with the one mediated by WT HA-specific CD8 cells in the absence of Treg (Fig. 5c). To address whether this reaction resulted from a failure of Treg to suppress cytotoxicity, the effector function of dnTGFβR and WT CD8 cells in CT44 draining LN on day 7 was compared. Whereas the HA-specific CD8 cells efficiently lysed HA-peptide pulsed syngeneic target cells in vivo only in the absence of Treg, the cytotoxicity displayed by dnTGFβR HA-specific CD8 cells in the presence or absence of Treg was indistinguishable (Fig. 5b). Thus, expression of dnTGFβR by HA-specific CD8 cells was sufficient to render CD8 cells resistant to regulation by Treg and allowed efficient tumor rejection by cytolytic activity.
Fig. 5.
Suppression of HA-specific CD8 T cell cytotoxic activity by HA-specific CD4 Treg requires TGF-β receptor signaling. On day -1, 105 naïve HA-specific CD8 T cells expressing or not expressing dnTGFβR were adoptively transferred into BALB/c mice either alone or with 105 HA-specific CD4 Treg. Mice were challenged with CT44 (HA+) tumors on day 0. (a) HA-specific CD8 T cells in CT44 draining LN were quantified on day 6. (b) In vivo cytotoxic activity in CT44 draining LN was measured on day 6, as described in Fig. 4. n.s., not significant; *, P < 0.0001. (c) Tumor size was measured over a period of 2 wk in mice not receiving (Left) or receiving (Right) Treg. Results show mean and SD values of nine or more mice from at least three independent experiments.
Discussion
This report describes the influence of HA-specific CD4+CD25+ Treg on the primary response of naïve HA-specific CD8 cells against HA-expressing tumors in vivo. The results show the following: (i) HA-specific CD8 cells were capable of rejecting HA-expressing tumors, whereas HA-negative tumors were not affected; (ii) HA-specific Treg effectively interfered with tumor rejection; (iii) suppression affected neither proliferation of CD8 cells during the first 10 days of the response nor the acquisition of a fully activated surface phenotype; (iv) suppression affected only the cytolytic activity of CD8 cells and had no impact on IFN-γ or TNF-α secretion; and (v) the molecular mechanism of CD8 suppression essentially involved TGF-β signaling because CD8 cells incapable of TGF-β signaling were resistant to suppression. Thus, Treg can effectively suppress the early tumor-specific immune response by CD8 cells by inhibiting their cytolytic activity in a TGF-β-dependent manner.
It has been shown that Treg can suppress the proliferation of CD4 and CD8 cells in vitro (3, 28, 31) and that in vivo Treg are capable of interfering with the proliferation of as well as production of IFN-γ by CD8 cells (8, 10, 12, 14). Indeed, secretion of IFN-γ by CD8 cells has been suggested to play a critical role in tumor rejection (32, 33). However, most of these studies have used polyclonal Treg where specific homing and expansion cannot be studied and no correlation between Ag-specific Treg and inhibition of CD8 cells can be made. Here, we have analyzed the impact of Treg on a primary CD8 immune response under conditions where CD8 cells and Treg have specificity for different peptides of the same protein expressed by tumor cells and where initially both naïve CD8 cells and Treg are present at a low frequency, as might be expected under physiological conditions. This procedure permits one to follow the Ag-specific responses of both cell types in the tumor draining LN and to correlate the mode of inhibition with local expansion. The data show that during the first week both the Ag-specific CD8 cells as well as the Ag-specific Treg home to and expand in the tumor draining LN and that the expanding Treg do not affect the proliferation of CD8 cells. Of interest is the observation that despite this finding, Treg interfere with tumor rejection by CD8 cells, which otherwise is complete by day 10 or shortly thereafter. Because suppression of proliferation could not explain these results, we analyzed whether Treg interfered with the effector functions of CD8 cells. Although no significant impairment of IFN-γ production was observed by day 7, there was an almost complete suppression of CD8-mediated cytolytic activity at this point in time. This suppression was essentially dependent on TGF-β signaling because CD8 cells with a dominant negative TGF-β receptor were resistant to suppression. This study also illustrates the importance of the Ag specificity of Treg on the control of CD8 cells, a feature of the immune system that is still poorly defined in vivo. It is noteworthy that the HA-specific CD8 cells were not controlled by the abundant CD4+CD25+ T cell repertoire endogenously present in the recipient mice, but were impaired selectively by lower numbers of HA-specific Treg, at least upon extensive Ag-specific proliferation.
We show that suppression of immune responses in vivo can occur by affecting distinct effector mechanisms at different stages of an immune response. In interpreting the results, one can envisage a scenario in which Treg and CD8 cells initially do not effectively interact with each other because of the low frequency of each population in the draining LN and, hence, physical separation of the two subsets. Thus, both populations expand significantly and thereby reach a frequency allowing a more effective interaction either directly or through Ag-presenting cell intermediates. It appears that at this stage, the suppression of cytolytic activity is more effective than the suppression of cytokine production or proliferation, i.e., cytotoxicity appears most sensitive to suppression, a notion consistent with observations by others that these effector functions can be regulated independently (27, 34-37), perhaps because they require different intensity of TCR signaling (38). Also, the cytokine requirement for proliferation of primary and memory CD8 cells may differ, and, hence, Treg may be more efficient in suppressing the proliferation of memory than of primary CD8 T cells. It is tempting to speculate that in vivo Treg-mediated suppression inhibits TCR signaling, the intensity of which controls different effector functions. This hypothesis could explain the reported suppression of CD8 T cell proliferation (8, 10) and/or IFN-γ secretion (9) in cases of viral or bacterial infection, under conditions in which the ratio of Ag-specific Treg and CD8 cells is unknown. Our results also show that in this model of tumor rejection, loss of cytolytic activity is sufficient to allow tumor outgrowth even when tumor-specific T cells still produce high levels of IFN-γ.
Independent evidence suggests that the suppression of CD8 T cell cytotoxicity by Treg may be common in cancer. It is of interest that tumor-specific CD8 cells frequently accumulate in tumors of melanoma patients but fail to exert cytolytic effector function (15), especially under conditions where tumor-specific CD4 Treg also could be identified within the tumor stroma (16, 17). These observations are consistent with results described in this study showing that tumor-specific CD4 Treg did not perturb the proliferation of HA-specific CD8 cells but suppressed their cytolytic activity in vivo. Such suppression may not only occur in draining LN but also among extravasated CD4 and CD8 cells within the tumor. In this context, it is of considerable interest that the pathway of immune suppression of cytolytic activity essentially depends on signaling by the TGF-β receptor on CD8 cells. Although previous studies on TGF-β-mediated inhibition have not addressed specific effector functions (20), our observations provide a rational explanation for earlier findings indicating that T-cell-specific blockade of TGF-β signaling renders mice resistant to tumors that otherwise would have been lethal (39). TGF-β-mediated suppression of CD8 T cell cytolytic activity in vitro has been studied by several investigators, and it has been reported by some that TGF-β interferes at early phases of the cytotoxic response but is rather ineffective once cells have developed cytolytic activity (40). However, there are also studies describing a more direct inhibition of cytolytic activity by TGF-β by interfering with the production of pore-forming protein independently of proliferation (41) that are consistent with our observations. Our results, thus, show that under conditions where the homing and accumulation of Ag-specific T cells and their CD8 targets can be tightly monitored, it is possible to identify a specific effector CD8 T cell function, namely cytolytic activity, that is most susceptible to TGF-β-mediated inhibition of regulatory T cells.
Supplementary Material
Acknowledgments
We thank Ludger Klein for critical suggestions, Silke Paust for discussions, Thorsten Mempel (CBR Institute, Harvard Medical School) and Glenn Dranoff (Dana-Farber Cancer Institute, Harvard Medical School) for providing us Flt3L-expressing tumor cells and advice, and Dina Lazik for excellent technical assistance. M.L.C. was supported bya Cancer Research Institute Investigator Award in Cancer Immunology; M.P. was supported by Swiss National Foundation Grant PBLAB-100856 and Human Frontier Science Program Organization Grant LT00369/2003; and K.K. was supported by Ruth L. Kirschstein National Research Service Award R33 CA97728-02 and Idea Award DAMD17-02-1-0361.
Author contributions: H.v.B. and K.K. designed research; M.-L.C., M.J.P., and K.K. performed research; M.-L.C., M.J.P., and K.K. analyzed data; L.G., R.A.F., and R.W. contributed new reagents/analytic tools; and K.K. and M.J.P. wrote the paper.
Abbreviations: Ag, antigen; CFSE, carboxyfluorescein diacetate-succinimidyl ester; DC, dendritic cell; dnTGFβR, dominant-negative TGFβR type II; HA, influenza hemagglutinin; LN, lymph node; PMA, phorbol 12-myristate 13-acetate; RAG-/-, recombination activating gene-deficient; TCR, T cell receptor; Treg, CD4+CD25+ regulatory T cells.
References
- 1.Shevach, E. M. (2004) Semin. Immunol. 16, 69-71. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi, S. (2004) Annu. Rev. Immunol. 22, 531-562. [DOI] [PubMed] [Google Scholar]
- 3.Suri-Payer, E., Amar, A. Z., Thornton, A. M. & Shevach, E. M. (1998) J. Immunol. 160, 1212-1218. [PubMed] [Google Scholar]
- 4.von Boehmer, H. (2003) J. Exp. Med. 198, 845-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker, L. S., Chodos, A., Eggena, M., Dooms, H. & Abbas, A. K. (2003) J. Exp. Med. 198, 249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisson, S., Darrasse-Jeze, G., Litvinova, E., Septier, F., Klatzmann, D., Liblau, R. & Salomon, B. L. (2003) J. Exp. Med. 198, 737-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamazaki, S., Iyoda, T., Tarbell, K., Olson, K., Velinzon, K., Inaba, K. & Steinman, R. M. (2003) J. Exp. Med. 198, 235-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suvas, S., Kumaraguru, U., Pack, C. D., Lee, S. & Rouse, B. T. (2003) J. Exp. Med. 198, 889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dittmer, U., He, H., Messer, R. J., Schimmer, S., Olbrich, A. R., Ohlen, C., Greenberg, P. D., Stromnes, I. M., Iwashiro, M., Sakaguchi, S., et al. (2004) Immunity 20, 293-303. [DOI] [PubMed] [Google Scholar]
- 10.Kursar, M., Bonhagen, K., Fensterle, J., Kohler, A., Hurwitz, R., Kamradt, T., Kaufmann, S. H. & Mittrucker, H. W. (2002) J. Exp. Med. 196, 1585-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin, C. Y., Graca, L., Cobbold, S. P. & Waldmann, H. (2002) Nat. Immunol. 3, 1208-1213. [DOI] [PubMed] [Google Scholar]
- 12.Dai, Z., Li, Q., Wang, Y., Gao, G., Diggs, L. S., Tellides, G. & Lakkis, F. G. (2004) J. Clin. Invest. 113, 310-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malek, T. R., Yu, A., Vincek, V., Scibelli, P. & Kong, L. (2002) Immunity 17, 167-178. [DOI] [PubMed] [Google Scholar]
- 14.Murakami, M., Sakamoto, A., Bender, J., Kappler, J. & Marrack, P. (2002) Proc. Natl. Acad. Sci. USA 99, 8832-8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zippelius, A., Batard, P., Rubio-Godoy, V., Bioley, G., Lienard, D., Lejeune, F., Rimoldi, D., Guillaume, P., Meidenbauer, N., Mackensen, A., et al. (2004) Cancer Res. 64, 2865-2873. [DOI] [PubMed] [Google Scholar]
- 16.Wang, H. Y., Lee, D. A., Peng, G., Guo, Z., Li, Y., Kiniwa, Y., Shevach, E. M. & Wang, R. F. (2004) Immunity 20, 107-118. [DOI] [PubMed] [Google Scholar]
- 17.Curiel, T. J., Coukos, G., Zou, L., Alvarez, X., Cheng, P., Mottram, P., Evdemon-Hogan, M., Conejo-Garcia, J. R., Zhang, L., Burow, M., et al. (2004) Nat. Med. 10, 942-949. [DOI] [PubMed] [Google Scholar]
- 18.Piccirillo, C. A., Letterio, J. J., Thornton, A. M., McHugh, R. S., Mamura, M., Mizuhara, H. & Shevach, E. M. (2002) J. Exp. Med. 196, 237-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng, Y., Laouar, Y., Li, M. O., Green, E. A. & Flavell, R. A. (2004) Proc. Natl. Acad. Sci. USA 101, 4572-4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green, E. A., Gorelik, L., McGregor, C. M., Tran, E. H. & Flavell, R. A. (2003) Proc. Natl. Acad. Sci. USA 100, 10878-10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirberg, J., Baron, A., Jakob, S., Rolink, A., Karjalainen, K. & von Boehmer, H. (1994) J. Exp. Med. 180, 25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan, D. J., Liblau, R., Scott, B., Fleck, S., McDevitt, H. O., Sarvetnick, N., Lo, D. & Sherman, L. A. (1996) J. Immunol. 157, 978-983. [PubMed] [Google Scholar]
- 23.Klein, L., Trautman, L., Psarras, S., Schnell, S., Siermann, A., Liblau, R., von Boehmer, H. & Khazaie, K. (2003) Eur. J. Immunol. 33, 806-814. [DOI] [PubMed] [Google Scholar]
- 24.Gorelik, L. & Flavell, R. A. (2000) Immunity 12, 171-181. [DOI] [PubMed] [Google Scholar]
- 25.Mora, J. R., Bono, M. R., Manjunath, N., Weninger, W., Cavanagh, L. L., Rosemblatt, M. & Von Andrian, U. H. (2003) Nature 424, 88-93. [DOI] [PubMed] [Google Scholar]
- 26.Klein, L., Khazaie, K. & von Boehmer, H. (2003) Proc. Natl. Acad. Sci. USA 100, 8886-8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyman, M. A., Aung, S., Biggs, J. A. & Sherman, L. A. (2004) J. Immunol. 172, 6558-6567. [DOI] [PubMed] [Google Scholar]
- 28.Piccirillo, C. A. & Shevach, E. M. (2001) J. Immunol. 167, 1137-1140. [DOI] [PubMed] [Google Scholar]
- 29.Trapani, J. A. & Smyth, M. J. (2002) Nat. Rev. Immunol. 2, 735-747. [DOI] [PubMed] [Google Scholar]
- 30.Shankaran, V., Ikeda, H., Bruce, A. T., White, J. M., Swanson, P. E., Old, L. J. & Schreiber, R. D. (2001) Nature 410, 1107-1111. [DOI] [PubMed] [Google Scholar]
- 31.Somasundaram, R., Jacob, L., Swoboda, R., Caputo, L., Song, H., Basak, S., Monos, D., Peritt, D., Marincola, F., Cai, D., et al. (2002) Cancer Res. 62, 5267-5272. [PubMed] [Google Scholar]
- 32.Qin, Z., Schwartzkopff, J., Pradera, F., Kammertoens, T., Seliger, B., Pircher, H. & Blankenstein, T. (2003) Cancer Res. 63, 4095-4100. [PubMed] [Google Scholar]
- 33.Blankenstein, T. & Qin, Z. (2003) Curr. Opin. Immunol. 15, 148-154. [DOI] [PubMed] [Google Scholar]
- 34.Veiga-Fernandes, H., Walter, U., Bourgeois, C., McLean, A. & Rocha, B. (2000) Nat. Immunol. 1, 47-53. [DOI] [PubMed] [Google Scholar]
- 35.Snyder, J. E., Bowers, W. J., Livingstone, A. M., Lee, F. E., Federoff, H. J. & Mosmann, T. R. (2003) Nat. Med. 9, 231-235. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez, J., Aung, S., Marquardt, K. & Sherman, L. A. (2002) J. Exp. Med. 196, 323-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apostolou, I., Sarukhan, A., Klein, L. & von Boehmer, H. (2002) Nat. Immunol. 3, 756-763. [DOI] [PubMed] [Google Scholar]
- 38.Valitutti, S., Muller, S., Dessing, M. & Lanzavecchia, A. (1996) J. Exp. Med. 183, 1917-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorelik, L. & Flavell, R. A. (2001) Nat. Med. 7, 1118-1122. [DOI] [PubMed] [Google Scholar]
- 40.Erard, F., Garcia-Sanz, J. A., Moriggl, R. & Wild, M. T. (1999) J. Immunol. 162, 209-214. [PubMed] [Google Scholar]
- 41.Smyth, M. J., Strobl, S. L., Young, H. A., Ortaldo, J. R. & Ochoa, A. C. (1991) J. Immunol. 146, 3289-3297. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.