Abstract
Background
Cervical and ocular Vestibular Evoked Myogenic Potentials (VEMPs) have become common clinical vestibular assessments. However, VEMP testing requires high intensity stimuli, raising concerns regarding safety with children, where sound pressure levels may be higher due to their smaller ear canal volumes.
Purpose
The purpose of this study was to estimate the range of peak-to-peak equivalent sound pressure levels (peSPLs) in child and adult ears in response to high intensity stimuli (i.e., 100 dB normal hearing level (nHL)) commonly used for VEMP testing and make a determination of whether acoustic stimuli levels with VEMP testing are safe for use in children.
Research Design
Prospective Experimental.
Study Sample
Ten children (4–6 years) and ten young adults (24 – 35 years) with normal hearing sensitivity and middle ear function participated in the study.
Data Collection and Analysis
Probe microphone peSPL measurements of clicks and 500 Hz tonebursts (TBs) were recorded in tubes of small, medium, and large diameter, and in a Brüel & Kjær Ear Simulator Type 4157 to assess for linearity of the stimulus at high levels. The different diameter tubes were used to approximate the range of cross-sectional areas in infant, child, and adult ears, respectively. Equivalent ear canal volume and peSPL measurements were then recorded in child and adult ears. Lower intensity levels were used in the participant’s ears to limit exposure to high intensity sound. The peSPL measurements in participant ears were extrapolated using predictions from linear mixed models to determine if equivalent ear canal volume significantly contributed to overall peSPL and to estimate the mean and 95% confidence intervals of peSPLs in child and adult ears when high intensity stimulus levels (100 dB nHL) are used for VEMP testing without exposing subjects to high-intensity stimuli.
Results
Measurements from the coupler and tubes suggested: 1) each stimuli was linear, 2) there were no distortions or non-linearities at high levels, and 3) peSPL increased with decreased tube diameter. Measurements in participant ears suggested: 1) peSPL was approximately 3 dB larger in child compared to adult ears, and 2) peSPL was larger in response to clicks compared to 500 Hz TBs. The model predicted the following 95% confidence interval for a 100 dB nHL click: 127–136.5 dB peSPL in adult ears and 128.7–138.2 dB peSPL in child ears. The model predicted the following 95% confidence interval for a 100 dB nHL 500 Hz TB stimulus: 122.2 – 128.2 dB peSPL in adult ears and 124.8–130.8 dB peSPL in child ears.
Conclusions
Our findings suggest that 1) when completing VEMP testing, the stimulus is approximately 3 dB higher in a child’s ear, 2) a 500 Hz TB is recommended over a click as it has lower peSPL compared to the click, and 3) both duration and intensity should be considered when choosing VEMP stimuli. Calculating the total sound energy exposure for your chosen stimuli is recommended as it accounts for both duration and intensity. When using this calculation for children, consider adding 3 dB to the stimulus level.
Keywords: Vestibular, Vestibular Evoked Myogenic Potentials, Children
INTRODUCTION
The purpose of this study was to estimate the range of peak-to-peak equivalent sound pressure levels (peSPLs) in child and adult ears in response to high intensity stimuli (i.e., 100 dB normal hearing level (nHL)) commonly used for Vestibular Evoked Myogenic Potential (VEMP) testing and make a determination of whether acoustic stimuli levels with VEMP testing are safe for use in children. VEMPs are used to assess vestibular end organ and nerve function. The VEMP is recorded by stimulating the vestibular system with a high intensity air or bone conducted acoustic signal and then measuring subsequent muscle responses. The cervical VEMP (cVEMP) is measured from the sternocleidomastoid muscle (SCM) and is used to assess inferior vestibular nerve and saccular function (Colebatch et al, 1994). The ocular VEMP (oVEMP) is measured from the inferior oblique (extraocular) muscle and is speculated to assess primarily superior nerve and utricular function; however, it may receive some contributions from the saccule (Todd, 2007; Weber et al, 2012; Todd, 2014).
Children with hearing loss are at increased risk for vestibular loss due to the close anatomical relationship and similar developmental time course during embryology between the vestibular system and cochlea, thus making them susceptible to the same genetic and environmental factors (Kaga et al, 1981; Inoue et al, 2013). The risk of vestibular loss increases depending on both the etiology and degree of hearing loss. An estimated 30–70% of children with profound hearing loss will have some degree of vestibular dysfunction (Arnivg, 1955; Buchman et al, 2004; Jafari and Asad, 2011). Vestibular dysfunction has also been noted in varying degrees in children with Connexin 26 mutations, cytomegalovirus (CMV), Usher (Type 1 and 3), and Pendred, among others (Cushing et el, 2008; O’Reilly et al, 2010).
When vestibular testing is necessary for children, VEMPs are an ideal part of the pediatric vestibular battery as VEMPs provide ear-specific information and do not elicit dizziness like other vestibular tests (i.e., rotary chair and caloric testing). However, despite the benefits of VEMP testing in children, high-intensity stimuli (100 dB nHL) are required to elicit the response. Sudden sensorineural hearing loss in response to VEMP testing has been reported in only 1 subject (Mattingly et al, 2015). However, in spite of the low incidence of adverse effects following VEMP testing, the use of high-intensity stimuli raise concerns regarding safety, particularly with children, where sound levels may be higher due to smaller ear canal volumes than adults. In adults, VEMP stimulus levels (133 dB SPL) have been shown to affect cochlear function, as 27% (n = 8/30) of subjects reported muffled hearing after VEMP testing (Krause et al, 2013). While no measureable changes were documented with respect to pure tone audiometry, significant decreases in Distortion Product Otoacoustic Emission levels post VEMP testing were reported, indicating the high intensity stimulus level used in VEMP testing may have a negative effect on cochlear function in some individuals (Krause et al, 2013).
Similar studies with VEMP have not been completed in children, however, comparisons of peSPL between adults and children have been completed with regard to auditory brainstem response (ABR) testing. Sininger et al (1997) investigated differences in peSPL between infant and adult ear canals using click and tone burst (TB) stimuli during ABR. They reported higher average peSPLs in infant, compared to adult, ears for each stimulus type, noting average differences as large as 24 dB for 4000 Hz, 17 dB for clicks and 3 dB for 500 Hz TB. Given the significant differences in peSPLs in smaller ear canals, there are concerns that VEMP stimuli, which are typically presented at a higher level than stimuli used for threshold ABRs, may result in unsafe levels in the ear, particularly when testing children.
Recommendations for safe maximum presentation levels in VEMP are difficult to develop because current noise exposure standards and units are not specific to VEMP stimuli. These standards are targeted toward adults and evaluate the excess risk of hearing impairment for a 40 year working lifetime (NIOSH, 1998). These standards were not developed to be applied to single noise exposures. Therefore, when making recommendations about safe exposure levels, clinicians should keep in mind that specific noise exposure recommendations do not exist for VEMP stimuli, and do not exist for children.
The purpose of this study was to: 1) determine if equivalent ear canal volume significantly contributes to the peSPLs in the ear canals of adults and children in response to 100 dBnHL click and 500 Hz TB stimuli, which are commonly used for VEMP testing, 2) predict the range of peSPLs generated in ear canals in response to these stimuli, and 3) compare the peSPL ranges to current noise standards for safety determination. We hypothesized that peSPLs would be significantly higher in children’s ears due to their smaller ear canal volumes. To minimize the potential for noise-induced hearing loss, we chose not to subject the children or adults to 100 dB nHL VEMP stimuli in the event that it generated unsafe peSPLs in subjects’ ears. Instead, we measured peSPLs in response to lower intensity stimuli (75, 80, and 85 dB nHL) and used linear mixed modeling to determine if equivalent ear canal volume significantly contributed to overall peSPL, and to estimate the mean and 95% confidence intervals of peSPLs in child and adult ears in response to a 100 dBnHL click and 500 Hz TB stimulus. This information was used to help make a determination of whether acoustic stimuli levels with VEMP testing are safe for use in children based on noise standards.
METHODS AND MATERIALS
Participants
Ten children (mean age: 5 years, range 4–6 years, 5 males) and ten young adults (mean age: 28.7 years, range 24–35 years, 4 males) with normal hearing sensitivity and normal middle ear function participated in the study. Hearing screening and tympanometry (226 Hz) were completed bilaterally for each subject. Hearing was screened at 20 dB HL at all octave frequencies from 250 through 4000 Hz. Tympanometry was considered normal if peak pressure was between −100 and 30 daPa and peak admittance was greater than or equal to 0.3 mmhos. Participants not meeting the above criteria were excluded from the study. Ears with tympanic membrane perforations or pressure equalization tubes were also excluded. Informed consent was obtained from all subjects for testing approved by the Institutional Review Board at Boys Town National Research Hospital.
Procedures
Probe microphone peSPL measurements were recorded in tubes of small, medium, and large diameter, and in a Brüel & Kjær Ear Simulator Type 4157 to assess the linearity of the stimulus at high levels. The different diameter tubes were used to approximate the range of cross-sectional areas in infant, child, and adult ears, respectively.
Equivalent ear canal volume and peSPLs were measured from one ear selected at random for each of the participants yielding data from 6 left ears and 4 right ears for the child participants and 7 left ears and 3 right ears for the adult participants. Equivalent ear canal volumes were measured during tympanometry using a 226 Hz probe tone (Otoflex, Otometrics, Schaumberg, IL or GSI Tympstar, Grason-Stadler, Eden Prairie, MN). The term equivalent ear canal volume is used because the ear canal volume is not measured directly during tympanometry. Only an estimate of the volume of air between the probe tip and the tympanic membrane” is measured, which can overestimate ear canal volume (Wiley et al, 2002).
Probe microphone measurements
The pressure response levels of individual transients were quantified in terms of the peSPL. The properties of transient levels assessed using peSPL and peak sound pressure level (pSPL) are described in Burkard (2006). For any given transient sound, the peSPL may be 3 to 9 dB lower than the pSPL. This difference is related to the fact that the peSPL is defined using both positive and negative polarities of the pressure waveform, and relating SPL of a continuous sine wave with the same peak-to-peak value, whereas the pSPL is defined based on the maximum absolute value of the pressure waveform. The peSPL was used in this study because it included information on the pressure transient from both positive and negative polarities.
Click and 500 Hz TB were presented using the Interacoustics Otoaccess Eclipse software through ABR-3A transducers. The click stimulus was presented with a rarefaction onset with a 0.5 ms duration at a rate of 5.1 Hz. The 500 Hz TB stimulus was presented with a rarefaction onset with a 2 cycle rise/fall time and 1 cycle plateau (10 ms) at a rate of 5.1 Hz. These stimuli durations were chosen as they have been reported to be optimal in VEMP acquisition (Welgampola et al, 2001; Huang et al, 2005). The peSPL of these stimuli were measured by a calibrated ER-7C microphone. As shown in Figure 1, the ER-7C microphone with Etymotic preamplifier (figure 1A) was coupled to an ER7-14C probe tube, which was threaded through the foam portion of the insert earphone (E-A-RLINK 3A [adult] and 3B [child]) along the center tube, which was coupled to the ABR-3A transducers, and then placed in the tubes, artificial ear simulator and subject’s ear (figure 1B). Insertion of the insert earphone was considered optimal if the outer edge of the insert was flush with tube, artificial ear simulator or external ear canal opening. The peak to peak mean voltages of the stimuli were measured with a Tektronix TDS 2002 digital oscilloscope. Audacity (version 2.0.4) software was used to save the measured waveforms with a CardDeluxe sound card (Digital Audio Labs). The manufacturer specification for microphone sensitivity of the ER-7 microphone was used to convert the peak to peak voltages to peSPL. The means of the peSPLs were computed in MATLAB (version 2013b). The mean levels in the coupler were calculated over several measurements and found to have negligible variability. In addition to verifying the measurements made with the oscilloscope, Audacity was also used to check for evidence of distortion in the waveforms, which was not found with the levels reported here.
Figure 1.
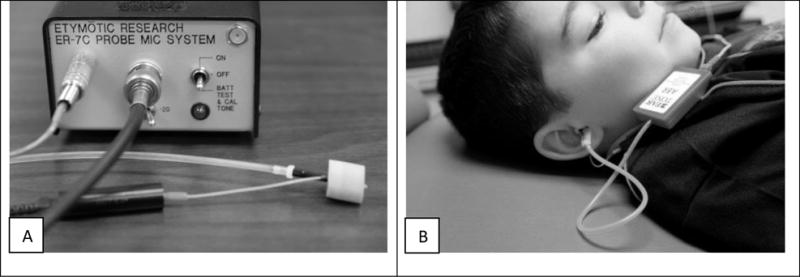
(A) Depiction of the ER-7C microphone coupled to an ER7-14C probe tube and threaded through the foam portion of the insert earphone along the center tube, which was coupled to the ABR-3A transducers, and then (B) placed in the subject’s ear.
Probe microphone peSPL measurements of a click and 500 Hz TB were recorded in 3 dB steps from 85 to 100 dB nHL in tubes of small (0.48 cm diameter, 30.48 m length, and 551.6 cm3 volume), medium (0.64 cm diameter, 152.4 m length, and 4902.7 cm3 volume), and large diameter (0.79 cm diameter, 30.48 m length, and 1494 cm3 volume), and in a Brüel & Kjær Artificial Ear Simulator Type 4157. These intervals were measured to verify the stimulus levels remained linear at high intensity levels. The tubes were sufficiently long that any reflected signal from the opposite end was delayed so far in time and small in amplitude that the peSPL measurement was not influenced by any reflections. Probe microphone peSPL measurements in the tubes were used to determine the relative increase in peSPL associated with changes in ear canal diameter. The artificial ear simulator was used as it represents all ear canals when calibrating insert earphones.
Probe microphone peSPL measurements were recorded in child and adult ears at the following intensity levels: 75, 80, and 85 dB nHL. Probe microphone peSPL measurements were recorded for approximately 10 seconds for each stimulus and level. These intensity levels were used for the participant ears to reduce exposure to high intensity stimuli. Participants lay quietly while recordings were obtained. Children were allowed to watch a short video or cartoon at reduced volume.
The peSPL measurements in participant ears at these low intensity levels (75, 80, and 85 dB nHL) were extrapolated using predictions from linear mixed models to determine if equivalent ear canal volume significantly contributed to overall peSPL and to estimate the mean and 95% confidence intervals of peSPLs in child and adult ears when high intensity stimulus levels (100 dB nHL) are used for VEMP testing without exposing subjects to high-intensity stimuli.
RESULTS
Probe Microphone peSPLs in the Artificial Ear Simulator and Tubes
Recorded peSPL values from the tubes and artificial ear simulator are shown in Table 1. Regression analysis was completed to verify that the peSPL for both stimulus types (click and 500 Hz TB) across all conditions (small, medium, and large tubes and an artificial ear simulator) were linear. Shown in Figure 2, peSPLs were linear across all conditions (i.e., small, medium, and large tubes and artificial ear simulator); r = 0.976 – 1.0, p < 0.001) for both stimulus types (Click (Figure 2A, left) and 500 Hz TB (Figure 2B, right)), indicating that both stimuli maintained linear growth at high levels.
Table 1.
Recorded peSPL values from the tubes and artificial ear simulator
| Stimuli | Small Diameter Tube | Medium Diameter Tube | Large Diameter Tube | Ear Simulator | |
|---|---|---|---|---|---|
|
| |||||
| dBnHL | peSPL | peSPL | peSPL | peSPL | |
| CLICK | 85 | 118.2 | 117.0 | 114.1 | 116.5 |
| 88 | 121.1 | 120.0 | 117.0 | 119.4 | |
| 91 | 124.1 | 122.9 | 120.0 | 122.3 | |
| 94 | 126.9 | 125.8 | 122.8 | 125.1 | |
| 97 | 129.8 | 128.5 | 125.6 | 127.8 | |
| 100 | 132.2 | 131.0 | 128.1 | 130.2 | |
|
| |||||
| 500 Hz TB | 85 | 102.8 | 101.1 | 97.4 | 108.4 |
| 88 | 105.9 | 103.8 | 100.5 | 111.5 | |
| 91 | 109.0 | 106.8 | 103.2 | 114.7 | |
| 94 | 112.3 | 110.2 | 106.5 | 118.0 | |
| 97 | 115.5 | 113.3 | 109.7 | 120.9 | |
| 100 | 118.5 | 116.3 | 112.0 | 123.8 | |
Figure 2.
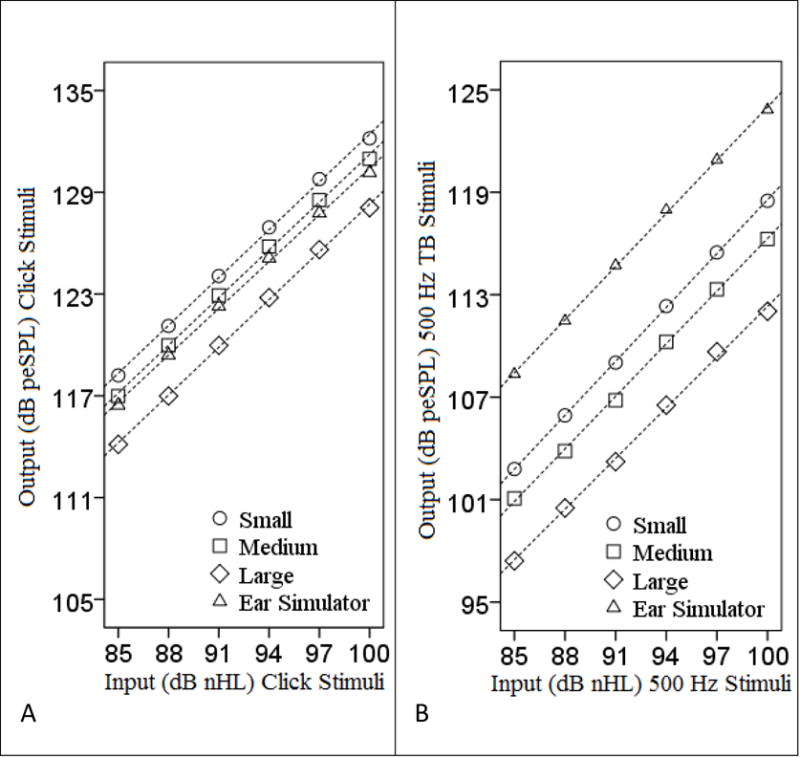
Input (dB nHL) and output (dB peSPL) of the (A) click and (B) 500 Hz TB stimuli up to maximum stimulus level (100 dB nHL) in accord with the manufacturer specification. Dashed lines represent the linear regression lines fit to the data.
For both stimuli, the peSPL tended to decrease as the tube diameter increased. A one-way ANOVA was completed to determine if these peSPL differences were statistically significant. There was no significant difference in mean peSPL between tube size for either 500 Hz TB stimuli (F (2, 17) = 1.559, p = 0.243) or for click stimuli (F (2, 17) = 0.97, p = 0.402), suggesting that tube diameter did not have a significant effect on overall peSPL in a reflection-less tube.
Responses in Participant Ears
Probe microphone peSPL measurements were obtained in child and adult ears at lower intensity levels (75, 80, and 85 dB nHL). Regression analysis suggested that peSPLs were linear for both stimulus types (click and 500 Hz TB,) across child (click: r = 0.744, p < 0.001; 500 Hz TB: r = 0.94, p < 0.001) and adult ears (click: r = 0.729, p < 0.001; 500 Hz TB: r = 0.806, p < 0.001). The r-values are smaller in the subject ears, compared to the tubes and artificial ear simulator, which we attribute to the variability in ear canal size across subject ears compared to the fixed size of the tubes and artificial ear simulator.
In response to 75, 80, and 85 dB nHL click and 500 Hz TB stimuli, corresponding mean peSPLs in adult and child ears can be found in Table 2. A 3-way between groups ANOVA was performed to examine the main effects and interactions of input level, age group, and stimulus type as they relate to peSPL, with peSPL as the dependent variable and input level (75, 80, and 85 dB nHL stimuli), age group (adult versus child), and stimulus type (click versus 500 Hz TB) as the fixed factors. As expected, there were significant main effects for input level (F (2,114) = 99.7, p < 0.001), age group (F (1,114) = 12.6, p = 0.001), and stimulus type (F (1,114) = 120.3, p < 0.001), with peSPL increasing as the input level increased, peSPL being larger in child compared to adult ears, and peSPL being larger in response to clicks compared to 500 Hz TB. There were no 2-way or 3-way interactions otherwise.
Table 2.
Mean [SD] and range of peSPLs for children and adults in response to 75, 80, and 85 dB nHL click and 500 Hz TB stimuli.
| Stimuli | Child | Adult | ||
|---|---|---|---|---|
|
| ||||
| mean [SD] | Range | mean [SD] | Range | |
| Click 75 dB nHL | 107.7 [3.6] | 99.2 – 112.2 | 106.0 [3.8] | 100.1 – 111.3 |
| Click 80 dB nHL | 113.1 [4.3] | 102.5 – 117.8 | 111.2 [4.0] | 104.5 – 116.3 |
| Click 85 dB nHL | 118.3 [4.3] | 107.7 – 123.3 | 116.4 [4.2] | 109.5 – 121.5 |
| 500 Hz TB 75 dB nHL | 101.6 [1.2] | 99.5 – 103 | 99.0 [3.2] | 93.1 – 102.5 |
| 500 Hz TB 80 dB nHL | 106.9 [1.1] | 104.8 – 108.5 | 104.3 [3.2] | 98.3 – 107.7 |
| 500 Hz TB 85 dB nHL | 111.8 [2.2] | 106 – 113.7 | 109.6 [3.2] | 103.6 – 112.9 |
We hypothesized the difference in peSPLs in children’s ears to be due to their smaller equivalent ear canal volumes, as measured during tympanometry. Mean (SD) equivalent ear canal volumes in the child group (0.735 ml (0.04)) were significantly smaller than mean equivalent ear canal volumes in the adult group (1.024 ml (0.08), t = 3.185, p = 0.005).
Predicting peSPL at high intensity stimulus levels
A click stimulus with a presentation level of 100 dB nHL, in accord with the manufacturer specification of stimulus level (Interacoustics Otoaccess Eclipse), resulted in a measured level of 130.2 dB peSPL in an artificial ear simulator. Similarly, a 500 Hz TB stimulus with a presentation level of 100 dB nHL had a measured level of 123.8 dB peSPL. One drawback of assuming the stimulus level in the artificial ear simulator matches the stimulus level in a patient’s ear is the lack of variability (i.e., small standard deviation) in the artificial ear simulator. Across patients’ ears, peSPL variability can be higher, as noted in the raw data (Table 2), due to individual differences in insertion depth, ear canal size, and other factors. This is particularly important with VEMPs, which are recorded in response to high levels where individuals on the upper end of variability may be subjected to high intensity sound levels which may result in temporary or permanent hearing loss. While not significant, measurements done in tubes and in adult vs child ears (shown above) demonstrated that peSPL increases with smaller diameter size. Therefore, with linear mixed modeling, we created a statistical model using the peSPLs from the subjects’ ears in response to low levels (to 75, 80, and 85 dB nHL click and 500 Hz TB stimuli) and equivalent ear canal volumes to: 1) determine if equivalent ear canal volume significantly contributes to peSPL and 2) predict the range of peSPLs generated in equivalent ear canals in response to high intensity (100 dB nHL) stimuli. To generate the model, the fixed effect was group (child = 0 and adult = 1), with equivalent ear canal volume (ml) as a covariate, input level (click or 500 Hz TB in dB nHL) as a repeating within-subjects factor and compound symmetry as the assumed pattern of covariance. Compound symmetry was used given the small sample size and single covariate. For either stimulus type, the model was significant: click stimuli [F = 66.831, p < 0.001]; 500 Hz TB stimuli, [F = 78.987, p < 0.001]. Shown in Table 3, as expected, the measured peSPL level in the ear increased as the input level from the transducer increased. However, equivalent ear canal volume did not significantly contribute to the model for either stimulus type.
Table 3.
Summary Parameters from Mixed Modeling
| Parameter | Click | 500 Hz Tone burst | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Estimate | Standard Error | Significance | Estimate | Standard Error | Significance | |
|
|
||||||
| Intercept | 34.85 | 3.78 | < 0.001 | 27.08 | 2.69 | < 0.001 |
| Adult | 0.4 | 2.2 | 0.857 | −1.3 | 1.38 | 0.357 |
| Equivalent Ear Canal Volume | −7.36 | 4.57 | 0.126 | −4.5 | 2.86 | 0.134 |
| Input Level | 1.04 | 0.02 | < 0.001 | 1.04 | 0.02 | <0.001 |
The model generated above yielded the following equations for estimating peSPL for adults and children:
Using average equivalent ear canal volumes from the adult (1.024 ml) and child (0.735 ml) participants, we used the model to estimate the peSPL generated in adult and child ear canals if a high intensity stimulus (100 dB nHL) was used. The predicted peSPL for a 100 dB nHL click in adults was 131.7 dB peSPL and 133.4 dB peSPL for children, shown in Table 4 (compared to 130.2 dB peSPL in an artificial ear simulator). The model predicted the following 95% confidence interval for a 100 dB nHL click: 127–136.5 dB peSPL in adult ears and 128.7–138.2 dB peSPL in child ears.
Table 4.
Mean and 95% Confidence Intervals (CI) of predicted peSPLs (and estimated pSPLs and dBA in italics) for children and adults for a 100 dB nHL click and 500 Hz TB stimuli. To convert from peSPL to pSPL, 4.5 dB was added to the click stimuli and 3.1 dB was added to 500 Hz TB stimuli. To convert from peSPL to dBA, 3 dB was subtracted.
| Stimuli | Predicted Child | Predicted Adult | Artificial Ear Simulator | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean | 95% CI | Mean | 95% CI | |||
| Click 100 dB nHL | peSPL | 133.4 | 128.7 – 138.2 | 131.7 | 127 – 136.5 | 130.2 |
| pSPL | (137.9) | (133.2 – 142.7) | (136.2) | (131.5 – 141) | (134.7) | |
| dBA* | – | – | – | – | – | |
|
| ||||||
| 500 Hz 100 dB nHL | peSPL | 127.8 | 124.8 – 130.8 | 125.2 | 122.2 – 128.2 | 123.8 |
| pSPL | (130.9) | (127.9 – 133.9) | (128.3) | (125.3 – 131.3) | (126.9) | |
| dBA | (124.8) | (121.8–127.8) | (122.2) | (122.2 – 125.2) | (120.8) | |
Clicks cannot be reliably converted to dBA.
Similarly for a 500 Hz TB stimulus, using average equivalent ear canal volumes from the adult (1.024 ml) and child (0.735 ml) participants, the predicted peSPL for a 100 dBnHL 500 Hz TB in adults was 125.2 dB peSPL and 127.8 dB peSPL for children, shown in Table 4 (compared to 123.8 dB peSPL in an artificial ear simulator). The model predicted the following 95% confidence interval for a 100 dB nHL 500 Hz TB stimulus: 122.2 – 128.2 dB peSPL in adult ears and 124.8–130.8 dB peSPL in child ears.
DISCUSSION
The purpose of this study was to: 1) determine if equivalent ear canal volume significantly contributes to the peSPLs in ear canals of adults and children in response to stimuli commonly used for VEMP testing, 2) predict the range of peSPLs generated in ear canals in response to these stimuli, and 3) compare these peSPL ranges to current noise standards for safety determination. First we wanted to insure that the stimuli used were linear. When assessing the stimulus linearity in the tubes, artificial ear simulator, and participants’ ears, each stimulus (click and 500 Hz TB) was linear. There were no distortions or non-linearities at high levels.
We hypothesized that peSPLs would be significantly higher in the child ears compared to adult ears and that this difference would be attributed to equivalent ear canal volume. In the tube conditions, which estimated cross-sectional ear canal areas in infant, child, and adults ears, respectively, peSPL decreased as tube diameter increased; however, the difference was not significant. In these conditions, the tube lengths were sufficiently long that the effect of volume was negligible. Consistent with this hypothesis, regardless of the stimulus type, peSPLs were significantly greater in the child ears, and the children exhibited significantly smaller equivalent ear canal volumes. However, when input into a linear mixed model, equivalent ear canal volume did not significantly contribute to the model predicting peSPL for either stimulus. There are several factors that could account for equivalent ear canal volume not contributing to the model. It could be that insertion depth of the insert earphone when measuring the peSPL and insertion depth of the tympanometry probe when measuring equivalent ear canal volume were different. Equivalent ear canal volume obtained from tympanometry is an estimate of the volume measured between the probe tip and tympanic membrane, and could be overestimating ear canal volume. It could also be that the sample size was not large enough for equivalent ear canal volume to reach significance in the statistical model, however, linear mixed modeling was the best statistical choice for making predictions about high level inputs. Nonetheless, because peSPLs were significantly greater in the child ears suggests that greater care should be taken to limiting exposure in children’s ears.
Next, we predicted the range of peSPLs generated in participant ears in response to a 100 dB nHL click and 500 Hz TB. Prediction ranges are shown in Table 4. While the artificial ear simulator accurately estimated mean peSPL measured in adult and child ears, it does not account for the variability found in participant ears. As shown in Table 2, the difference in the means, at most, between adult and child ears was 2.6 dB peSPL, suggesting that when completing VEMP testing, the stimulus is approximately 3 dB higher in a child’s ear.
Lastly, we compared the peSPL ranges to current noise standards for safety determination. This is difficult for several reasons. First, in relevant noise-exposure standards, noise is defined as either impulse or continuous, and VEMP stimuli do not fall neatly into either category. Second, these noise standards were developed for adults consistently exposed to noise over the course of a 40 year work life, not for one time exposures in-the-ear as is the case during VEMP testing. However, although these standards are not meant to prevent all risk to noise induced hearing loss, they do establish a damage-risk criteria and excess risk that may be considered when evaluating the safety of VEMP stimuli.
For impulse noise, the Occupational Safety and Health Administration (OSHA) recommends no exposures greater than 140 dB pSPL (OSHA, n.d.), while the National Institute of Occupational Safety and Health (NIOSH) recommends no exposures greater than 140 dBA (NIOSH, 1998), where dBA refers to the decibel measured using the A frequency-weighting scale, which is typically used to measure noise exposure for hearing protection (Earshen, 2003). In the present study, the SPL of transients was reported in peSPL, which can be 3 – 9 dB lower than its pSPL (Burkard, 2006). For the current data, pSPL was calculated in the ear simulator condition. For a 100 dB nHL input, the pSPL was 3.1 dB greater than peSPL for the 500 Hz TB and 4.5 dB greater for the click. Therefore, adding 3.1 dB to the measured peSPLs for the 500 Hz TB and 4.5 dB to the click provide estimates of the pSPLs in subject ears (Table 4). For a click stimulus (Table 4), the estimated pSPL suggests that OSHAs permissible range for impulse noise would be exceeded (> 140 dB pSPL) for some individuals in both the adult and child groups. For a 500 Hz TB stimulus (Table 4), the estimated pSPL suggests that levels are within OSHAs permissible range for impulse noise (< 140 dB pSPL) for both adults and children. Because these are estimations, this is not to say that presentation levels for clicks are unsafe for all persons, however, it does provide a rationale for choosing a 500 Hz TB over the click stimulus. Additionally, the 500 Hz TB produces larger responses due to the frequency tuning characteristics of the otolith organs (Welgampola and Colebatch, 2001; Janky and Shepard, 2009; Park et al, 2010).
When comparing to OSHA and NIOSH’s recommendations for continuous noise both recommendations are contingent on the intensity and duration of the noise. OSHA recommends no overall sound exposures greater than 90 dBA for longer than 8 hours. As the duration is halved, the permissible exposure level is increased by 5 dBA (i.e., a140 dBA noise can be heard for 0.4688 minutes or 28.13 seconds) (Suter, 2003). NIOSH’s approach is more conservative. As the duration is halved the permissible exposure level is increased by 3 dBA (Suter, 2003), therefore, we use OSHA’s recommendations for comparison. First, we convert peSPL to dBA, The 500 Hz TB is attenuated by 3 – 4 dB in the A weighted scale (Suter, 2003). Subtracting 3 dB from our measured peSPLs can provide estimates of the dBA SPL of the transients (Table 4). For a 500 Hz TB stimulus, the estimated dBA suggests that levels are within OSHA’s permissible range for both adults and children as long as the stimulus is not presented for more than 225 seconds for adults and 112 seconds for children. Again, these are estimations. Therefore, this is not to say that presentation levels are unsafe for all persons, however, it does provide a basis for accounting for both duration and intensity.
Alternatively, Colebatch and Rosengren (2014) suggest calculating the total sound energy exposed to the ear, which is contingent on the sound intensity and duration of the VEMP stimulus. Total sound energy exposure is calculated using the following formula: Intensity (SPL) + 10 × log10 (stimulus duration in seconds). For the stimuli used in this study, the total sound energy exposure for two trials (200 stimuli) of a 500 Hz TB (10 ms duration) at 100 dB nHL (135 dB SPL; this intensity is measured by the manufacture as root mean square) would be:
According to Colebatch and Rosengren (2014), this value exceeds the recommended exposure level of 132 dB. Similar to OSHA and NIOSH standards, this equation was formulated from industry standards which were developed to protect adults working in noisy conditions and were not meant to be applied to clinical tests or noise exposures for children. Children may be more susceptible to complications due to noise exposure. Excessive noise exposure resulting in hearing loss, temporary or permanent, may lead to both short term and lifelong negative effects. Therefore, when using this calculation for children, consider adding 3 dB to the stimulus level.
While reducing the stimulus intensity may not be an option, given the otolith organ’s high threshold (i.e., 110–115 dB SPL) (Colebatch et al, 1994; Welagampola et al, 2001; Janky and Shepard, 2009), reducing stimulus duration or using a bone conducted stimulus can decrease overall noise exposure. Singh and Apeksha (2014) noted that a 1 ms rise/fall time with 0 ms plateau resulted in larger cVEMP amplitudes and reduced test-retest variability in the wave forms. Reducing rise/fall times has the added benefit of decreasing the stimulus duration which would lessen the sound energy exposure (Singh and Apeksha, 2014). This is useful when needing to complete several trials without over exposing the patient/subject to excessive amounts of sound. Another possible option for decreasing sound exposure during VEMP testing is using bone conduction with head taps or a reflex hammer; however, further research regarding the safety of bone conduction stimulation is still needed (Papathanasiou, 2014). Bone conduction VEMP is also affective when conductive hearing losses are present (Halmagyi, 1995), tends to be faster to complete, and may be more reliable then with air conducted stimuli (Wackym, 2012).
It should be noted that our child data reflect children ages 4 – 6 years, and the ear canal reaches adult size at age 7–8 (Feigin et al, 1989). Therefore, particular care should be taken when performing VEMP testing in infants and children under the age of 4 years.
CONCLUSIONS
While industry standards were not developed for VEMP testing, and few studies have documented adverse side effects when completing VEMP testing, care should be taken, particularly when testing children. Our findings suggest that 1) when completing VEMP testing, the stimulus is approximately 3 dB higher in a child’s ear, 2) a 500 Hz TB is recommended over a click as it has lower peSPL compared to the click and has a greater response amplitude due to the tuning characteristics of the otolith organs (Janky and Shepard, 2009; Park et al, 2010), 3) both duration and intensity should be considered when choosing VEMP stimuli. Calculating the total sound energy exposure for your chosen stimuli is recommended as it accounts for both duration and intensity. When using this calculation for children, consider adding 3 dB to the stimulus level. Additional care can be taken by reducing stimulus rise and fall times (Shall, 2009; Singh and Apeksha, 2014), starting at lower stimulus intensities (i.e., 115 dB SPL) for children and then increasing the stimulus intensity only if necessary.
Acknowledgments
Source of Funding: Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number P20GM109023 and by the National Institute on Deafness and Other Communication Disorders under award number R03DC015318.
Thanks to Kendra Schmid, Ph.D., of the College of Public Health, University of Nebraska Medical Center, for her assistance with statistical analysis.
Abbreviations
- cVEMP
Cervical Vestibular Evoked Myogenic Potentials
- oVEMP
Ocular Vestibular Evoked Myogenic Potentials
- NIOSH
National Institute of Occupations Safety and Health
- OSHA
Occupational Safety and Health Administrations
- peSPL
Peak to peak sound pressure level
- pSPL
Peak sound pressure level
- nHL
Normal hearing level
- dBA
Decibels A-filter weighting
Footnotes
Podium presentation at American Balance Society meeting in Scottsdale, AZ on March 5th, 2014
Conflicts of Interest: No conflicts of interest
Reference List
- Arnivg J. Vestibular function in deafness and severe hardness of hearing. Acta Otolaryngol. 1955;45:283–288. doi: 10.3109/00016485509124281. [DOI] [PubMed] [Google Scholar]
- Buchman CA, Joy J, Hodges A, Telischi FF, Balkany TJ. Vestibular effects of cochlear implantation. Laryngoscope. 2004;114:1–22. doi: 10.1097/00005537-200410001-00001. [DOI] [PubMed] [Google Scholar]
- Burkard R. Calibration of Acoustic Transients. Brain Res. 2006;1091:27–31. doi: 10.1016/j.brainres.2006.02.132. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994;57:190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, Rosengren SM. Safe levels of acoustic stimulation: comment on “effects of acoustic stimuli used for vestibular evoked myogenic potential studies on the cochlear function”. Otol Neurotol. 2014;35:932–933. doi: 10.1097/MAO.0000000000000289. [DOI] [PubMed] [Google Scholar]
- Cushing SL, Papsin BC, Rutka JA, James AL, Gordon KA. Evidence of vestibular and balance dysfunction in children with profound sensorineural hearing loss using cochlear implants. Laryngoscope. 2008;118:1814–1823. doi: 10.1097/MLG.0b013e31817fadfa. [DOI] [PubMed] [Google Scholar]
- Earshen JJ. Sound Measurements: Instrumentation and Noise Descriptors. In: Berger EH, Royster LH, Royster JD, Driscoll DP, Layne M, editors. The Noise Manual. Fairfax: American Industrial Hygiene Association; 2003. p. 54. [Google Scholar]
- Feigin JA, Kopun JG, Stelmachowicz PG, Gorga MP. Probe-tube microphone measures of ear-canal sound pressure levels in infants and children. Ear Hear. 1989;10:254–258. doi: 10.1097/00003446-198908000-00008. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Yavor RA, Colebatch JG. Tapping the head activates the vestibular system: a new use for the clinical reflex hammer. Neurology. 1995;45:1927–1929. doi: 10.1212/wnl.45.10.1927. [DOI] [PubMed] [Google Scholar]
- Huang T, Su H, Cheng P. Effect of click duration on vestibular-evoked myogenic potentials. Acta Oto-Largyngologica. 2005;125:141–144. doi: 10.1080/00016480410016900. [DOI] [PubMed] [Google Scholar]
- Inoue A, Iwasaki S, Ushio M, Chihara Y, Fujimoto C, Egami N, Yamasoba T. Effect of vestibular dysfunction on the development of gross motor function in children with profound hearing loss. Audiol Neurootol. 2013;18:143–151. doi: 10.1159/000346344. [DOI] [PubMed] [Google Scholar]
- Jafari Z, Asad MS. The effect of saccular function on static balance ability of profound hearing-impaired children. Int J Pediatr Otorhinolaryngol. 2011;75:919–924. doi: 10.1016/j.ijporl.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Janky KL, Shepard N. Vestibular evoked myogenic potential (VEMP) testing: normative threshold response curves and effects of age. J Am Acad Audiol. 2009;20:514–522. doi: 10.3766/jaaa.20.8.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga K, Suzuki JI, Marsh RR, Tanaka Y. Influence of labyrinthine hypoactivity on gross motor development of infants. Ann N Y Acad Sci. 1981;374:412–420. doi: 10.1111/j.1749-6632.1981.tb30887.x. [DOI] [PubMed] [Google Scholar]
- Krause E, Mayerhofer A, Gurkov R, Drexl M, Braun T, Olzowy B, Boetzel K. Effects of acoustic stimuli used for vestibular evoked myogenic potential studies on the cochlear function. Otol Neurotol. 2013;34:1186–1192. doi: 10.1097/MAO.0b013e31829ce7b4. [DOI] [PubMed] [Google Scholar]
- Mattingly JK, Portnuff CD, Hondorp BM, Cass SP. Sudden Bilateral Hearing Loss After Cervical and Ocular Vestibular Evoked Myogenic Potentials. Otol Neurotol. 2015;36:961–964. doi: 10.1097/MAO.0000000000000764. [DOI] [PubMed] [Google Scholar]
- National Institute of Occupational Safety and Health (NIOSH) Criteria for a recommended standard: occupational noise exposure. Cincinnati, OH: U.S. Government Printing Office; 1998. ((DHHS)(NIOSH) Publication No. 98-126). [Google Scholar]
- O’Reilly RC, Morlet T, Nicholas BD, Josephson G, Horlbeck D, Lundy L, Mercado A. Prevalence of vestibular and balance disorders in children. Otol Neurotol. 2010;31:1441–1444. doi: 10.1097/MAO.0b013e3181f20673. [DOI] [PubMed] [Google Scholar]
- Occupational Safety and Health Administration (OSHA) Regulations (Standards-29 CFR 1910.95) (n.d.) Retrieved from https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=9735 Retrieved on 2/3/2015.
- Papathanasiou ES, Murofushi T, Akin FW, Colebatch JG. International guidelines for the clinical application of cervial vestibular evoked myogenic potentials: An expert consensus report. Clin Neurophysiol. 2014;125:658–666. doi: 10.1016/j.clinph.2013.11.042. [DOI] [PubMed] [Google Scholar]
- Park H, Lee IS, Shin JE, Lee YJ, Park MS. Frequency-tuning characteristics of cervical and ocular vestibular evoked myogenic potentials induced by air-conducted tone bursts. Clin Neurophysiol. 2010;121:85–89. doi: 10.1016/j.clinph.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Roberts L, Cacace AT. Jendrassik maneuver facilitates cVEMP amplitude: some preliminary observations. J Am Acad Audiol. 2014;25:237–243. doi: 10.3766/jaaa.25.3.2. [DOI] [PubMed] [Google Scholar]
- Singh N, Apeksha K. The effect of rise/fall time of 500 Hz short tone bursts on cervical vestibular evoked myogenic potential. J Vestib Res. 2014;24:25–31. doi: 10.3233/VES-130503. [DOI] [PubMed] [Google Scholar]
- Sininger Y, Abdala C, Cone-Wesson B. Auditory threshold sensitivity of the human neonate as measured by the auditory brainstem response. Hear Res. 1997;104:27–38. doi: 10.1016/s0378-5955(96)00178-5. [DOI] [PubMed] [Google Scholar]
- Shall MS. The importance of saccular function to motor development in children with hearing impairments. Int J Otolaryngol. 2009 doi: 10.1155/2009/972565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter AH. Standards and Regulations. In: Berger EH, Royster LH, Royster JD, Driscoll DP, Layne M, editors. The Noise Manual. Fairfax: American Industrial Hygiene Association; 2003. pp. 639–668. [Google Scholar]
- Todd N, Rosengren S, Aw S, Colebatch J. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol. 2007;118:381–390. doi: 10.1016/j.clinph.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Todd N. The ocular vestibular evoked myogenic potential (OVEMP), ten years old. Clin Neurophysiol. 2014;125:439–441. doi: 10.1016/j.clinph.2013.09.034. [DOI] [PubMed] [Google Scholar]
- Wackym PA, Ratigan JA, Birck JD, Johnson SH, Doornink J, Bottlang M, Gardiner SK, Black FO. Rapid cVEMP and oVEMP responses elicited by a novel head striker and recording device. Otol Neurotol. 2012;33:1392–1400. doi: 10.1097/MAO.0b013e318268d234. [DOI] [PubMed] [Google Scholar]
- Weber KP, Rosengren SM, Michels R, Strum V, Straumann D, Landau K. Single motor unit activity in human extraocular muscles during the vestibulo-ocular reflex. J Physiol. 2012;590:3091–3101. doi: 10.1113/jphysiol.2011.226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgampola MS, Colebatch JG. Characteristics of tone burst-evoked myogenic potentials in the sternocleidomastoid muscles. Otol Neurotol. 2001;22:796–802. doi: 10.1097/00129492-200111000-00014. [DOI] [PubMed] [Google Scholar]
- Wiley T, Stoppenback DT. Basic Principles of Acoustic Immittance Measures. In: Katz J, Burkard RF, Medwetsky L, editors. Handbook of Clinical Audiology. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 169–170. [Google Scholar]


