Abstract
Traditionally, vaccines have been composed of live attenuated or killed micro-organisms. Alternatively, individual protein subunits or other molecular components of the micro-organism can serve as the antigen and trigger an antibody response by the immune system. The immune system is a coordinated molecular and cellular response that works in concert to check the spread of infection. In the past decade, there has been much progress on DNA vaccines. DNA vaccination includes using the coding segments of a viral or bacterial genome to generate an immune response. However, the potential advantage of combining an RNA molecule with inorganic nanoparticle delivery should be considered, with the goal to achieve immuno-synergy between the two and to overcome some of the current limitations of DNA vaccines and traditional vaccines.
INTRODUCTION
Genetic medicines hold tremendous potential and there is a large literature already devoted to the topic of DNA vaccines. However considerably less work has been done on RNA-based vaccines despite several potential advantages, such as avoiding insertion, replication and transcription steps. Today there are literally thousands of papers in the literature also involving RNA nanoparticles. However few, if any, have considered whether the immunological profile of inorganic nanoparticles could synergize with the RNA to potentiate immune response. Here we summarize progress to date on RNA vaccines and address the molecular correlates and immunological responses desirable for potential RNA vaccine nanoparticles and their continued progression.
RNA VACCINES
While DNA vaccines have long been heralded, more recently an interest in RNA vaccines has emerged. RNA vaccines are being evaluated against a variety of diseases as summarized in Table 1.
Table 1.
Diseases with potential RNA vaccines
| Disease | RNA type | Delivery | Model | Reference |
|---|---|---|---|---|
| Cancer | mRNA | Naked | N/A | 1 |
| HIV | RNAi | Lipid Nanoparticles; Subcutaneous | Mouse | 2 |
| Melanoma Cancer | mRNA | Gene Gun | Mouse | 3 |
| Melanoma Cancer | mRNA | Naked; Intradermal injection | Human | 4 |
| Type I Allergy | mRNA | Naked | Mouse | 5 |
| Avian influenza virus | Self-amplifying RNA | Naked; intramuscular | Chicken | 6 |
| Cytomegalovirus | Self-amplifying RNA | Naked; intramuscular or subcutaneous | Human | 7 |
| Flavivirus | Self-replicating viral RNA | Gene gun | Mouse | 8 |
| H7N9 Influenza | Self-amplifying RNA | Lipid Nanoparticles | Mouse | 9 |
| Measles | Self-amplifying RNA | Naked; intradermal | Infant Macaques | 10 |
As shown in Table 1, diseases for which RNA vaccines have been tested include; viruses such as Avian influenza virus, Cytomegalovirus (CMV), Flavivirus, Hepatitis C, HIV, influenza, and some cancers (e.g.- melanoma) and measles. Delivery of the RNA vaccines has been accomplished via physical means such as electroporation or particle based methods such as gene gun and lipid nanoparticles. Routes of RNA vaccine administration include; subcutaneous, intradermal and intramuscular. Except for one case where RNAi was used, the overwhelming majority of RNA vaccines are constructed from either mRNA or self-amplifying RNA (saRNA), and these are discussed in the next section.
RNA VACCINE CONSTRUCTION
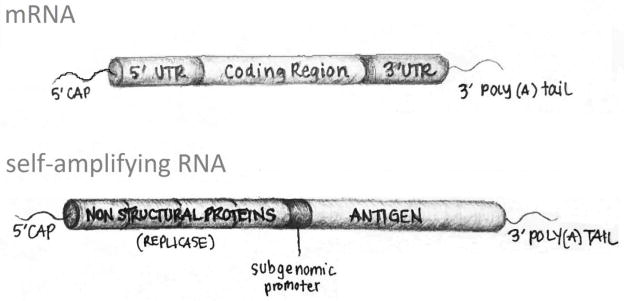
For RNA vaccines the delivery of a translatable RNA sequence into the cytoplasm circumvents many of the complications of DNA delivery, in that nuclear localization is not required and there are no integration, replication or transcription steps necessary. The structural elements an RNA vector must contain in order to be directly translated to protein once present in the cytosol is illustrated below in Figure 1.
FIGURE 1.
Illustration of the functional sequences in an mRNA or saRNA construct
Figure 1 illustrates basic structural features of RNA vaccine expression vectors. For example mRNA constructs contain a 5′-G cap, a 5′-untranslated region (UTR), the coding sequence for the antigenic protein, a 3′-UTR, and a 3′-poly(A) tail. The vaccine antigen to be expressed is encoded in the coding region. Within the 5′-UTR are sequences that promote the assembly of and stabilization of the ribosome on the mRNA. The 3′-UTR is generally thought to mediate mRNA decay rate. Self-amplifying RNA (saRNA) is a replicon-based vaccine that is longer than other RNA-based vaccines with typical molecular weights of approximately 10 kb.11 The most studied saRNA vaccines have been derived from alphavirus genomes. Alphaviruses consist of a single copy of positive, single-stranded RNA that contains genomic RNA encoding its own replication machinery and sub genomic RNA that encodes viral proteins, such as caspid and glycoproteins.12 When creating the vaccine, the sub genomic RNA encoding the viral structural proteins is replaced with RNA that encodes the antigen(s) of interest. First, the replicase complex is translated in the cytoplasm. The replicase then synthesizes a genomic anti-sense RNA that is used as a template for the genomic positive-strand RNA synthesis as well as for the sub genomic RNA encoding the structural antigen.13 No viral particles are produced because their genetic sequences have been replaced by those encoding the desired antigens and there is no cell-to-cell transmission. saRNA vaccines have the potential to be more potent than mRNA vaccines because of their ability to replicate and induce a stronger, broader, and more effective humoral and cellular immune response.14 Their delivery has advanced from being packaged into virus-like particles into synthetic formulations (i.e., lipid nanoparticles15,16), which doesn’t require a cell culture step. This provides simplicity and safety benefits that using a virus would impose.
DOUBLE-STRANDED RNA (dsRNA)
Double-stranded RNA (dsRNA) is an alternative approach to an encoding RNA strand, but which has also been shown to elicit an immune response. Because dsRNA is often associated with and produced by viruses, it is therefore recognized by the host’s immune cells as an indicator of viral infection. In viral infections, host cells recognize viral components, such as dsRNA, via Toll-like receptors (TLRs), a family of innate immune receptors that recognize pathogen-associated molecular patterns (PAMPs).17,18,19 One type of dsRNA, for which there has been a lot of recent interest, is polyinosinic:polycytidylic acid, poly I:C. Poly I:C (pIC) is a synthetic analog of viral dsRNA with one strand composed entirely of cytidine and the other strand of inosine. It has long been suggested as an adjuvant to potentiate vaccine immunogenic activity, but is more recently being considered for combination chemotherapy20 or even as a stand-alone treatment.21 Diseases being tested for potential pIC treatment are summarized in Table 2.
Table 2.
Diseases With Potential Poly I:C dsRNA Treatment
| Disease | Delivery Method | Model | Reference |
|---|---|---|---|
| Brain Tumors | Poly-ICLC; intramuscular | Human | 22 |
| Cancer | Vaccine containing poly I:C | Human | 23 |
| Cancers | Poly ICLC + IL-2 injections | Human | 24 |
| Cerebral Ischaemia/reperfusion injury | Naked; Intraperitoneal and intravascular injection | Mouse | 25 |
| Gastric Adenocarcinoma | Liposomes; Subcutaneous | Mouse | 26 |
| Glioblastoma | Poly-ICLC; intramuscular | Human | 27 |
| Hepatitis B | Naked; hydrodynamic injection | Mouse | 28 |
| Herpes Zoster in children with cancer | Topical Poly I:C | Human | 29 |
| Influenza A Virus | Naked; Intranasal | Mouse | 30 |
| Influenza A/Puerto Rico/8/34 virus | Naked; intramuscular and intranasal | Mouse | 31 |
| Laser induced Skin Wounds | Topical Poly I:C | Human and mouse | 32 |
| Leukemia (solid tumors) | Poly ICLC injection | Human | 33 |
| Severe Acute Respiratory Syndrome | Naked; Intranasal | Mouse | 34 |
As shown in Table 2, a variety of cancer and infectious diseases are being treated with pIC. In some cases, the pIC is used more as an adjuvant in conjunction with vaccine or in combination with cytokine (IL-2). However, in many cases the pIC or the related compound poly ICLC (polyinosinic-polycytidilic acid-polylysine-carboxymethylcellulose) is used as the active immunotherapeutic agent itself. Similar to RNA vaccines, in some cases, naked pIC is delivered locally such as subcutaneous or intramuscular injection or administered via intranasal, intraperitoneal or intravascular injection. Many of these have progressed beyond mouse studies into humans.
IMMUNOLOGICAL CORRELATES
One of the key factors for immunological activity of any potential RNA nanoparticle is the activation of innate and adaptive systems. A robust molecular response needs to be triggered for the cells of the immune system to communicate with each other and perform their critical functions. Table 3 presents an overview of these signaling systems.
Table 3.
| Immunity | Cytokine | Producer Cells | Receiver Cell | Response |
|---|---|---|---|---|
| Innate | Tumor necrosis factor-alpha (TNF-α) | Monocytes, macrophages dendritic cells, TH1 cells and others | Endothelial, Macrophages Neutrophils, Hepatocytes, Fibroblasts, Muscle and Fat Cells, Hypothalamus | Production of: chemokine (to induce diapedesis, chemotaxis, and leukocyte recruitment), acute phase protein, interleukin-1 (IL-1), extracellular killing, stimulation of catabolism for energy conversion, fever and sleep |
| /*Innate | Type I/Interferons | Any virus infected cell IFN-α is produced by T-lymphocytes, B-lymphocytes, NK cells, monocytes, macrophages. IFN-β is produced by virus-infected cells, fibroblasts, macrophages, epithelial cells, and endothelial cells | All uninfected cells | Production of enzymes capable of degrading mRNA. Induction of MHC-I antigen expression needed for recognition of antigens by cytotoxic T-lymphocytes |
| Adaptive | Type II Interferons | Interferon-gamma (IFN-γ) is produced by activated TH1 cells, CD8+ cells and NK cells | Cytotoxic T-lymphocytes, NK cells, macrophages, monocytes, neutrophils, antigen-presenting cells | Induction of: proinflammatory responses, MHC-I and MHC-II and co-stimulatory molecules by APCs in order to promote cell-mediated immunity, promotes differentiation of T4-lymphocytes into TH1 cells and inhibits TH2 cell proliferation, activates macrophages and increases antimicrobial and tumoricidal activity |
| Adaptive | Interleukin-2 (IL-2) | T4 and T8-lymphocytes | NK, T and B cells | Acts as a growth factor, increases NK cell killing ability, stimulates antibody synthesis |
| Innate | Interleukin-6 (IL-6) | T-lymphocytes, macrophages, monocytes, endothelial cells, fibroblasts | Liver cells, B-lymphocytes, Neutrophils | Proliferation of neutrophils and acute phase protein production |
| Innate | Interleukin-10 (IL-10) | Macrophages and TH2 cells | Activated macrophages and dendritic cells | Inhibits production of IL-12, co-stimulator and MHC-II molecules, Promotes an anti-inflammatory response |
| Innate | Interleukin-12 (IL-12) | Macrophages and dendritic cells | T-lymphocytes and natural killer cells | Production of interferon-gamma and induction of killing activity |
| Innate | Chemokines: IL-8, CCL CXCL, MIP, MCP, GRO, and other molecules | Leukocytes, endothelial cells, epithelial cells, and fibroblasts | Leukocytes, white blood cells (WBCs), B and T-lymphocytes, dendritic cells | Leukocyte chemoattraction and regulation of cell movement through the lymph nodes and spleen. Release of killing agents for extracellular killing, ingestion of damaged tissue, increase leukocyte integrin affinity for vascular wall, ligands during diapedesis, regulation of actin polymerization |
| Adaptive | VCAM/ICAM, CD, MHC | Dendritic cell, antigen presenting cell | Effector T cell | T cell activation, cellular and humoral immunity, tolerance. ICAM/VCAM expressed by endothelial cells and promote leukocyte adhesion to vessel walls during extravasation. |
As shown in Table 3, the immune system has a number of specialized cells including; neutrophils, eosinophils, basophils, mast cells, monocytes, dendritic cells, and macrophages. Producer cells, such as macrophages and dendritic cells, signal the receiver cells through expression of various cytokines. Major cytokines include, but are not limited to: Tumor necrosis factors (TNFs) such as TNF-α and TNF-β, and a variety of interleukins, such as IL-1, IL-6, IL-10, IL-12 and IL-15 and IL-18. Each elicits a different response on a variety of specific receiver cells or even on organs, such as the hypothalamus. Responses are overt and robust, and range from acute inflammation, the recruitment of a variety of cells to the site of infection, the release of killing agents, the production of chemokines, chemolocation, and chemoattractant agents, as well as the signaling of a variety of other molecules involved. Receiver cells can be T cells, B lymphocytes, natural killer (NK) cells, neutrophils and other cells. The response of the receiver cell can be production of various classes of antibodies, MHC class I and II, cell differentiation and growth signals. Innate immune cells provide a quick, general response that typically leads to inflammation. These express genetically encoded receptors, called Toll-like receptors (TLRs), which recognize general danger- or microbe-associated patterns. These non-specific receptors cannot distinguish between strains of bacteria or viruses. 17,18,19 Despite their lack of specificity, innate immune cells are essential in that they also activate adaptive immunity. Upon recognition of a microbe, dendritic cells and macrophages consume the invader and become antigen-presenting cells that activate lymphocytes.37 Essentially, all cells involved in innate and adaptive immunity produce cytokines, but especially by T helper (TH) lymphocytes, which are a part of adaptive immunity.38,39 IL-2 is considered and early responder to adaptive immunity and activation of type II interferon is also considered indicative of adaptive immunity. In addition to antibody production other signaling molecules which get up-regulated in an adaptive immune response include VCAM, ICAM, CD and MHC molecules.
NANOPARTICLE IMMUNOLOGY
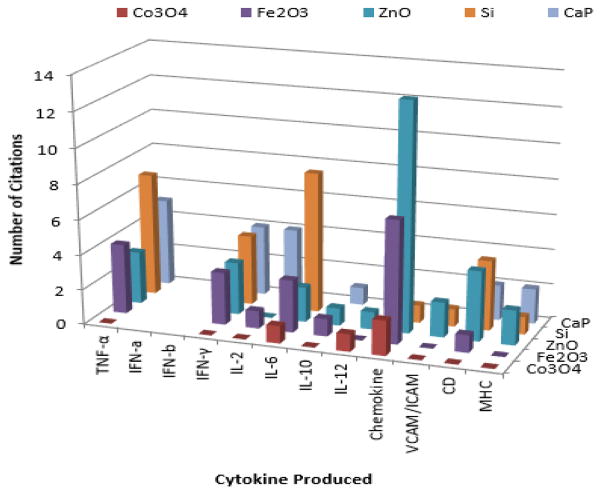
While a great deal of work has already been described for the immunology of organic nanoparticles, carbon nanotubes, liposomes, etc, there is a growing body of literature which suggests that inorganic nanoparticles are also immunologically active as highlighted in Figure 2.
FIGURE 2.
Figure 2 reflects the significant immuno-activity of nanoparticles. Here literature published the last 5–6 years for inorganic nanoparticles of general interest in biomedical nanotechnology including; silicon or mesoporous silicate nanoparticles (MSN), calcium phosphate (CaP), iron oxide (Fe2O3), zinc oxide (ZnO) and cobalt oxide (Co3O4) nanoparticles were compared. As can be clearly seen, all these types of nanoparticles have been reported to elicit some type of immunological response. Common molecular markers investigated include various interleukins, TNF-α, IFN-γ, chemokines and others. For example silicon oxide (SiO2), titanium dioxide (TiO2) and Fe3O4 have been shown to activate the transcription factor NF-kB leading to increased secretion of TNF-α, IL-1β, and interleukin-6 (IL-6) in cultured brain microglia and neuronal cells.54 In human vascular endothelial cells or human keratinocytes IL-6 induction has been reported to be mediated via potassium channel or P2Y11 receptor.55,56 While our work and others suggests IFN-γ, TNF-α, IL-1β may be indicative of nanoparticle pro-inflammatory activity,58–62 IL-6 is recently considered as the keystone cytokine in disease.62 Therefore the above results should be interpreted with some caution and may be cell type and route of administration dependent. These need to be balanced by modulation of CD, increases in serum IgG, IgM, chemokines, MHC-I, and MHC-II reported for MSN and other nanoparticles which could otherwise contribute a desirable immunological component to an RNA vaccine or double stranded RNA (dsRNA) as discussed next.66–68
ACHIEVING SYNERGY
Nanovaccines incorporating RNA may have several advantages. First the core nanoparticles themselves are capable of augmenting immunogenic activity as shown Fig. 2. Further more recent evidence for this is seen for example when MSN and CaP nanoparticles have been tested in conjunction with vaccine and have been shown to enhance and sustain T-cell responses and in the case of CaP to protect against influenza challenge.66–69 The RNA molecule does not have to encode an antigen to potentiate an immune response either, as shown previously for example with pIC where its signaling through Toll-like receptors (TLRs) and pathogen-associated molecular patterns (PAMP) are immuno-stimulatory. Thus another means of achieving synergy has been shown for example with pIC containing CaP nanoparticles which in addition to molecular signaling as above have the additional advantage of T cell activation and the induction of dendritic cell maturation.66,67
Lipid coated CaP co-loaded with pIC, STAT3 siRNA and ovalbumin (Ova) antigen have been referred to as nanovaccines and have been shown to induce an effective anti-tumor immunological response.70 Therefore similarly, another attractive approach for achieving synergy against cancer is to co-load the nanoparticle with pIC and another anti-tumor agent such as Zoledronic acid.71 As mentioned previously, pIC is a much needed double edged sword against cancer capable of stimulating an immunogenic and anticancer approach. Bob Langers group showed that surface functionalization is required for iron oxide to bind RNA,72 and this is true for pIC and consistent with our results for another magnetic nanocarrier, manganese oxide.73,74 There is however a great deal of current interest in targeted combination chemotherapy with pIC nanoparticles,75–77 especially as a novel approach for intratumoral immunotherapy against melanoma and potentially other cancers.78–80 For additional synergy it would be desirable to be able to load the RNA onto for example zinc oxide or cobalt oxide nanoparticles which have been shown to possess inherent anti-cancer activity themselves.81–84 A schematic of the synergistic advantage of such an approach is illustrated in Figure 3.
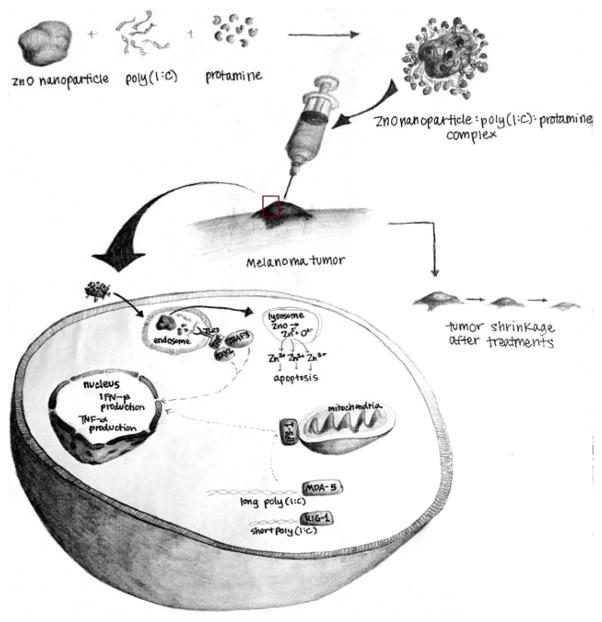
FIGURE 3.
Synergy expected between zinc oxide nanoparticle and pIC.
As shown in Figure 3, the delivery of pIC by zinc oxide nanoparticle would be expected to trigger an appropriate immune response as discussed above, and in addition activation of TLR3 causing translocation of interferon regulatory factor-3 (IRF-3) and nuclear factor (NF)-κB across the nuclear membrane into the nucleus where type I interferons and proinflammatory cytokines, including TNFα, IL-6 and IL-8, would be expected to induced. This would essentially activate the immune system against the tumor. Further through TLR3 signaling, as well as RIG-1 and MDA-5, poly IC would be expected to trigger apoptosis, and essentially cause a molecular chemotherapeutic effect. Further, mechanisms of apoptosis caused via nanoparticles, ROS induction, etc. are not expected to be the same as poly IC, and thus the molecular signaling pathways may be complementary leading to such immunochemotherapeutic potentiation.
CONCLUSION AND FUTURE DIRECTIONS
In summary, RNA vaccines are in development against a variety of cancers, viral infection and type I allergy. RNA vaccine vectors thus far have been either mRNA or self-amplifying RNA (saRNA) constructs. Double-stranded RNA (dsRNA) such as pIC is also immunogenic and in addition to anti-viral activity is being evaluated as an immunotherapy or combination immunochemotherapy against melanoma and several other solid tumors, as well as ischemia, severe acute respiratory syndrome, Hepatitis B, herpes zoster in children with cancer and others. While liposome delivery of either RNA vaccines or pIC has been evaluated, the immunogenicity reported for inorganic nanoparticles has gone surprisingly underexploited. As clearly in the literature the last few years ample evidence has now been reported, particularly for calcium phosphate (CaP) and mesoporous silicate nanoparticles (MSN), but also for iron oxide, zinc oxide and cobalt oxide, of pronounced immunogenic activity. In the last year CaP and MSN have shown profound application as nanovaccines accordingly.69, 70 RNA nanovaccines may therefore have promise and application against cancer, where in this case exploring the RNA nanobio interface of zinc or cobalt iron containing nanoparticles which could combine indigenous anti-cancer and immunogenic activity with direct RNA binding may therefore achieve the synergy long sought after. Particularly given recent reports of the antimetastatic activity of pIC85–87, it is imperative now to evaluate its activity in conjunction with nanomaterials.
Acknowledgments
This work was supported by National Cancer Institute Grant 7R15CA139390-03. The authors are also grateful for support from the Nanotechnology Innovation Center Kansas State (NICKS).
References
- 1.Diken M, Kreiter S, Selmi A, Türeci O, Sahin U. Antitumor vaccination with synthetic mRNA: strategies for in vitro and in vivo preclinical studies. Methods Mol Biol. 2013;969:235–246. doi: 10.1007/978-1-62703-260-5_15. [DOI] [PubMed] [Google Scholar]
- 2.Kim S-S, Peer D, Kumar P, et al. RNAi-mediated CCR5 Silencing by LFA-1-targeted Nanoparticles Prevents HIV Infection in BLT Mice. Molecular Therapy. 2010;18(2):370–376. doi: 10.1038/mt.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steitz J, Britten CM, Wölfel T, Tüting T. Effective induction of anti-melanoma immunity following genetic vaccination with synthetic mRNA coding for the fusion protein EGFP.TRP2. Cancer Immunol Immunother. 2006;55(3):246–253. doi: 10.1007/s00262-005-0042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weide B, Pascolo S, Scheel B, et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32(5):498–507. doi: 10.1097/CJI.0b013e3181a00068. [DOI] [PubMed] [Google Scholar]
- 5.Weiss R, Scheiblhofer S, Roesler E, Ferreira F, Thalhamer J. Prophylactic mRNA vaccination against allergy. Curr Opin Allergy Clin Immunol. 2010;10(6):567–574. doi: 10.1097/ACI.0b013e32833fd5b6. [DOI] [PubMed] [Google Scholar]
- 6.Kalhoro NH, Veits J, Rautenschlein S, Zimmer G. A recombinant vesicular stomatitis virus replicon vaccine protects chickens from highly pathogenic avian influenza virus (H7N1) Vaccine. 2009;27(8):1174–1183. doi: 10.1016/j.vaccine.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein DI, Reap EA, Katen K, et al. Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine. 2009;28(2):484–493. doi: 10.1016/j.vaccine.2009.09.135. [DOI] [PubMed] [Google Scholar]
- 8.Kofler RM, Aberle JH, Aberle SW, Allison SL, Heinz FX, Mandl CW. Mimicking live flavivirus immunization with a noninfectious RNA vaccine. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(7):1951–1956. doi: 10.1073/pnas.0307145101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hekele A, Bertholet S, Archer J, et al. Rapidly produced SAM® vaccine against H7N9 influenza is immunogenic in mice. Emerging Microbes and Infections. 2013;2:e52. doi: 10.1038/emi.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan C-H, Greer CE, Hauer D, et al. A Chimeric Alphavirus Replicon Particle Vaccine Expressing the Hemagglutinin and Fusion Proteins Protects Juvenile and Infant Rhesus Macaques from Measles. Journal of Virology. 2010;84(8):3798–3807. doi: 10.1128/JVI.01566-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geall AJ, Mandl CW, Ulmer JB. RNA: The new revolution in nucleic acid vaccines. Seminars in Immunology. 2013;25(2):152–159. doi: 10.1016/j.smim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Ulmer JB, Mason PW, Geall A, Mandl CW. RNA-based vaccines. Vaccine. 2012;30(30):4414–8. doi: 10.1016/j.vaccine.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 13.Leitner WW, Ying H, Restifo NP. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine. 1999;18(9–10):765–777. doi: 10.1016/s0264-410x(99)00271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akira S. Toll-like receptors and innate immunity. International Immunology. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 15.Geall AJ, Verma A, Otten GR, et al. Nonviral delivery of self-amplifying RNA vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(36):14604–14609. doi: 10.1073/pnas.1209367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Gascón A, del Pozo-Rodríguez A, Solinís MÁ. Development of nucleic acid vaccines: use of self-amplifying RNA in lipid nanoparticles. International Journal of Nanomedicine. 2014;9:1833–1843. doi: 10.2147/IJN.S39810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanaya K, Kondo K, Suzukawa K, Sakamoto T, Kikuta S, Okada K, Yamasoba T. Innate immune responses and neuroepithelial degeneration and regeneration in the mouse olfactory mucosa induced by intranasal administration of Poly(I:C) Cell and Tissue Research. 2014;357:279–299. doi: 10.1007/s00441-014-1848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaccines – Adjuvants. National Institute of Allergy and Infectious Diseases; Apr 3, 2012. [accessed 23 July 2015]. http://www.niaid.nih.gov/topics/vaccines/understanding/pages/adjuvants.aspx. [Google Scholar]
- 19.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 20.Khodadust R, Unsoy G, Gunduz U. Development of poly (I:C) modified doxorubicin loaded magnetic dendrimer nanoparticles for targeted combination therapy. Biomedicine and Pharmacotherapy. 2014;68(8):979–987. doi: 10.1016/j.biopha.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Salazar AM, Erlich RB, Mark A, Bhardwaj N, Herberman RB. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunology Research. 2014;2(8):720–4. doi: 10.1158/2326-6066.CIR-14-0024. [DOI] [PubMed] [Google Scholar]
- 22.Hartman LLR, Crawford JR, Makale MT, et al. Pediatric Phase II Trials of Poly-ICLC in the Management of Newly Diagnosed and Recurrent Brain Tumors. Journal of pediatric hematology/oncology. 2014;36(6):451–457. doi: 10.1097/MPH.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morse MA, Chapman R, Powderly J, et al. Phase I study utilizing a novel antigen-presenting cell-targeted vaccine with Toll-like receptor stimulation to induce immunity to self antigens in cancer patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(14):4844–4853. doi: 10.1158/1078-0432.CCR-11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ewel CH, Urba WJ, Kopp WC, Smith JW, 2nd, Steis RG, Rossio JL, Longo DL, Jones MJ, Alvord WG, Pinsky CM, et al. Polyinosinic-polycytidylic acid complexed with poly-L-lysine and carboxymethylcellulose in combination with interleukin 2 in patients with cancer: clinical and immunological effects. Cancer Research. 1992;52(11):3005–10. [PubMed] [Google Scholar]
- 25.Zhang X, Ha T, Lu C, et al. Poly (I:C) therapy decreases cerebral ischaemia/reperfusion injury viaTLR3-mediated prevention of Fas/FADD interaction. Journal of Cellular and Molecular Medicine. 2015;19(3):555–565. doi: 10.1111/jcmm.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu J, Hou Z, Han Q, Zhang C, Tian Z, Zhang J. Poly(I:C) Exhibits an Anti-Cancer Effect in Human Gastric Adenocarcinoma Cells Which is Dependent on RLRs. International Immunopharmacology. 2013 Nov;17(3):814–820. doi: 10.1016/j.intimp.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld MR, Chamberlain MC, Grossman SA, et al. A multi-institution phase II study of poly-ICLC and radiotherapy with concurrent and adjuvant temozolomide in adults with newly diagnosed glioblastoma. Neuro-Oncology. 2010;12(10):1071–1077. doi: 10.1093/neuonc/noq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J, Huang S, Zhao X, et al. Poly(I:C) Treatment Leads to Interferon-Dependent Clearance of Hepatitis B Virus in a Hydrodynamic Injection Mouse Model. In: Sandri-Goldin RM, editor. Journal of Virology. 18. Vol. 88. 2014. pp. 10421–10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman S, Hughes WT, Darlington RW, Kim HK. Evaluation of Topical Polyinosinic Acid-Polycytidylic Acid in Treatment of Localized Herpes Zoster in Children with Cancer: a Randomized, Double-Blind Controlled Study. Antimicrobial Agents and Chemotherapy. 1975;8(3):289–294. doi: 10.1128/aac.8.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao J, Wohlford-Lenane C, Zhao J, et al. Intranasal Treatment with Poly(I·C) Protects Aged Mice from Lethal Respiratory Virus Infections. Journal of Virology. 2012;86(21):11416–11424. doi: 10.1128/JVI.01410-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-Gil L, Goff PH, Hai R, García-Sastre A, Shaw ML, Palese P. A Sendai Virus-Derived RNA Agonist of RIG-I as a Virus Vaccine Adjuvant. Journal of Virology. 2013;87(3):1290–1300. doi: 10.1128/JVI.02338-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Q, Wang L, Lin Y, Ren X, Wen S, Du X, Lu T, Su SY, Yang X, Huang W, Zhou S, Wen F, Su SB. Toll-like Receptor 3 Ligand Polyinosinic:polycytidylic Acid Promotes Wound Healing in Human and Murine Skin. The Journal of Investigative Dermatology. 2012;132(8):2085–92. doi: 10.1038/jid.2012.120. [DOI] [PubMed] [Google Scholar]
- 33.Levine AS, Levy HB. Phase I-II Trials of Poly IC Stabilized with Poly-L-Lysine. Cancer Treatment Reports. 1978;62(11):1907–12. [PubMed] [Google Scholar]
- 34.Zhao J, Wohlford-Lenane C, Zhao J, et al. Intranasal Treatment with Poly(I·C) Protects Aged Mice from Lethal Respiratory Virus Infections. Journal of Virology. 2012;86(21):11416–11424. doi: 10.1128/JVI.01410-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janeway CA, Jr, Travers P, Walport M, et al. Principles of innate and adaptive immunity. 5. New York: Garland Science; 2001. Immunobiology: The Immune System in Health and Disease. Available from: http://www.ncbi.nlm.nih.gov/books/NBK27090/ [Google Scholar]
- 36.Keiser Gary. The Adaptive Immune System: Cell-Mediated Immunity: Cytokines. [accessed 13 July 2015];The Adaptive Immune System: Cell-Mediated Immunity: Cytokines. 2014 Sep 1; http://faculty.ccbcmd.edu/courses/bio141/lecguide/unit6/cellular/cmidefense/cytokines/cytokines.html.
- 37.Cavallo D, Ciervo A, Fresegna AM, Maiello R, Tassone P, Buresti G, Casciardi S, Iavicoli S, Ursini CL. Investigation on Cobalt Oxide Nanoparticles Cyto-genotoxicity and Inflammatory Response in Two Types of Respiratory Cells. Journal of Applied Toxicology. 2015;35(10):1102–13. doi: 10.1002/jat.3133. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Xia T, Meng H, Low-Kam C, Liu R, Pokhrel S, Lin S, Wang X, Liao YP, et al. Use of Metal Oxide Nanoparticle Band Gap to Develop a Predictive Paradigm for Oxidative Stress and Acute Pulmonary Inflammation. ACS Nano. 2012;6(5):4349–4368. doi: 10.1021/nn3010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandebriel RJ, De Jong WH. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnology, Science and Applications. 2012;5:61–71. doi: 10.2147/NSA.S23932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun J, Wang S, Zhao D, Hun FH, Weng L, Liu H. Cytotoxicity, Permeability, and Inflammation of Metal Oxide Nanoparticles in Human Cardiac Microvascular Endothelial Cells. Cell Biology and Toxicology. 2011;27(5):333–42. doi: 10.1007/s10565-011-9191-9. [DOI] [PubMed] [Google Scholar]
- 41.Hanley C, Thurber A, Hanna C, Punnoose A, Zhang J, Wingett DG. The Influences of Cell Type and ZnO Nanoparticle Size on Immune Cell Cytotoxicity and Cytokine Induction. Nanoscale Research Letters. 2009;4(12):1409–1420. doi: 10.1007/s11671-009-9413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palomäki J1, Karisola P, Pylkkänen L, Savolainen K, Alenius H. Engineered nanomaterials cause cytotoxicity and activation on mouse antigen presenting cells. Toxicology. 2010;267:15–131. doi: 10.1016/j.tox.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 43.Roy R, Parashar V, Chauhan LK, Shanker R, Das M, Tripathi A, Dwivedi PD. Mechanism of uptake of ZnO nanoparticles and inflammatory responses in macrophages require PI3K mediated MAPKs signaling. Toxicology in Vitro: An International Journal Published In Association With BIBRA. 2014;28(3):457–467. doi: 10.1016/j.tiv.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Cho W-S, Duffin R, Bradley M, et al. Predictive value of in vitro assays depends on the mechanism of toxicity of metal oxide nanoparticles. Particle and Fibre Toxicology. 2013;10:55. doi: 10.1186/1743-8977-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho W-S, Duffin R, Poland CA, et al. Metal Oxide Nanoparticles Induce Unique Inflammatory Footprints in the Lung: Important Implications for Nanoparticle Testing. Environmental Health Perspectives. 2010;118(12):1699–1706. doi: 10.1289/ehp.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Idikuda V, Jaiswal A, Wong YYW, Leung S, Daniels C, Lai J. Magnesium Oxide Nanoparticles Induce Cytotoxic and Proinflammatory Effects in Schwann cells and DRG Neurons. The FASEB Journal. 2012;26:1046.3. [Google Scholar]
- 47.Sun J, Wang S, Zhao D, Hun FH, Weng L, Liu H. Cytotoxicity, Permeability, and Inflammation of Metal Oxide Nanoparticles in Human Cardiac Microvascular Endothelial Cells. Cell Biology and Toxicology. 2011;27(5):333–42. doi: 10.1007/s10565-011-9191-9. [DOI] [PubMed] [Google Scholar]
- 48.Wu H-Y, Chung M-C, Wang C-C, Huang C-H, Liang H-J, Jan T-R. Iron oxide nanoparticles suppress the production of IL-1beta via the secretory lysosomal pathway in murine microglial cells. Particle and Fibre Toxicology. 2013;10:46. doi: 10.1186/1743-8977-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murray AR, Kisin E, Inman A, Young SH, Muhammed M, Burks T, Uheida A, Tkach A, Waltz M, Castranova V, Fadeel B, Kagan VE, Riviere JE, Monteiro-Riviere N, Shvedova AA. Oxidative stress and dermal toxicity of iron oxide nanoparticles in vitro. Cell Biochemistry and Biophysics. 2013;67(2):461–476. doi: 10.1007/s12013-012-9367-9. [DOI] [PubMed] [Google Scholar]
- 50.Vermeij EA, Koenders MI, Bennink MB, Crowe LA, Maurizi L, Vallée JP, Hofmann H, van den Berg WB, van Lent PLEM, van de Loo FAJ. The In-Vivo Use of Superparamagnetic Iron Oxide Nanoparticles to Detect Inflammation Elicits a Cytokine Response but Does Not Aggravate Experimental Arthritis. PLoS One. 2015;10(5):e0126687. doi: 10.1371/journal.pone.0126687. [DOI] [PMC free article] [PubMed] [Google Scholar]; Andujar P, Simon-Deckers A, Galateau-Sallé F, et al. Role of metal oxide nanoparticles in histopathological changes observed in the lung of welders. Particle and Fibre Toxicology. 2014;11:23. doi: 10.1186/1743-8977-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen B-A, Jin N, Wang J, et al. The effect of magnetic nanoparticles of Fe3O4 on immune function in normal ICR mice. International Journal of Nanomedicine. 2010;5:593–599. doi: 10.2147/ijn.s12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toki S, Omary RA, Wilson K, Gore JC, Peebles RS, Pham W. A comprehensive analysis of transfection-assisted delivery of iron oxide nanoparticles to dendritic cells. Nanomedicine: nanotechnology, biology, and medicine. 2013;9(8):1235–1244. doi: 10.1016/j.nano.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen CC, Liang HJ, Wang CC, Liao MH, Jan TR. Iron oxide nanoparticles suppressed T helper 1 cell-mediated immunity in a murine model of delayed-type hypersensitivity. International Journal of Nanomedicine. 2012;7:2729–2737. doi: 10.2147/IJN.S31054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xue Y, Wu J, Sun J. Four types of inorganic nanoparticles stimulate the inflammatory reaction in brain microglia and damage neurons in vitro. Toxicol Lett. 2012;214(2):91–8. doi: 10.1016/j.toxlet.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Yang L, Yan Q, Zhao J, Li J, Zong X, Yang L, Wang Z. The role of potassium channel in silica nanoparticle-induced inflammatory effect in human vascular endothelial cells in vitro. Toxicol Lett. 2013;223(1):16–24. doi: 10.1016/j.toxlet.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 56.Nagakura C, Negishi Y, Tsukimoto M, Itou S, Kondo T, Takeda K, Kojima S. Involvement of P2Y11 receptor in silica nanoparticles 30-induced IL-6 production by human keratinocytes. Toxicology. 2014;322:61–8. doi: 10.1016/j.tox.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Mansell A, Jenkins BJ. Dangerous liaisons between interleukin-6 cytokine and toll-like receptor families: a potent combination in inflammation and cancer. Cytokine Growth Factor Rev. 2013;24(3):249–56. doi: 10.1016/j.cytogfr.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 58.Medberry P, Dennis S, Van Hecke T, DeLong RK. pDNA bioparticles: comparative heterogeneity, surface, binding, and activity analyses. Biochem Biophys Res Commun. 2004;319(2):426–32. doi: 10.1016/j.bbrc.2004.04.188. [DOI] [PubMed] [Google Scholar]
- 59.Lee S, Kim MS, Lee D, Kwon TK, Khang D, Yun HS, Kim SH. The comparative immunotoxicity of mesoporous silica nanoparticles and colloidal silica nanoparticles in mice. Int J Nanomedicine. 2013;8:147–58. doi: 10.2147/IJN.S39534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Cristo L, Movia D, Bianchi MG, Allegri M, Mohamed BM, Bell AP, Moore C, Pinelli S, Rasmussen K, Riego-Sintes J, Prina-Mello A, Bussolati O, Bergamaschi E. Proinflammatory Effects of Pyrogenic and Precipitated Amorphous Silica Nanoparticles in Innate Immunity Cells. Toxicol Sci. 2015 Nov 25; doi: 10.1093/toxsci/kfv258. [DOI] [PubMed] [Google Scholar]
- 61.Winter M, Beer HD, Hornung V, Krämer U, Schins RP, Förster I. Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology. 2011;5(3):326–40. doi: 10.3109/17435390.2010.506957. [DOI] [PubMed] [Google Scholar]
- 62.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16(5):448–57. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 63.Park HJ, Sohn J-H, Kim Y-J, Park YH, Han H, Park KH, Lee K, Choi H, Um K, Choi I-H, Park J-Won, Lee J-H. Acute exposure to silica nanoparticles aggravate airway inflammation: different effects according to surface characteristics. Exp Mol Med. 2015;47(7) doi: 10.1038/emm.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiménez-Periáñez A, Abos Gracia B, López Relaño J, Diez-Rivero CM, Reche PA, Martínez-Naves E, Matveyeva E, Gómez del Moral M. Mesoporous silicon microparticles enhance MHC class I cross-antigen presentation by human dendritic cells. Clin Dev Immunol. 2013;2013:362163. doi: 10.1155/2013/362163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marzaioli V, Aguilar-Pimentel JA, Weichenmeier I, Luxenhofer G, Wiemann M, Landsiedel R, Wohlleben W, Eiden S, Mempel M, Behrendt H, Schmidt-Weber C, Gutermuth J, Alessandrini F. Surface modifications of silica nanoparticles are crucial for their inert versus proinflammatory and immunomodulatory properties. Int J Nanomedicine. 2014;9:2815–32. doi: 10.2147/IJN.S57396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sokolova V, Knuschke T, Kovtun A, Buer J, Epple M, Westendorf AM. The use of calcium phosphate nanoparticles encapsulating Toll-like receptor ligands and the antigen hemagglutinin to induce dendritic cell maturation and T cell activation. Biomaterials. 2010;31(21):5627–33. doi: 10.1016/j.biomaterials.2010.03.067. [DOI] [PubMed] [Google Scholar]
- 67.Zhou W, Moguche AO, Chiu D, Murali-Krishna K, Baneyx F. Just-in-time vaccines: Biomineralized calcium phosphate core-immunogen shell nanoparticles induce long-lasting CD8(+) T cell responses in mice. Nanomedicine. 2014;10(3):571–8. doi: 10.1016/j.nano.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kupferschmidt N, Qazi KR, Kemi C, Vallhov H, Garcia-Bennett AE, Gabrielsson S, Scheynius A. Mesoporous silica particles potentiate antigen-specific T-cell responses. Nanomedicine (Lond) 2014;9(12):1835–46. doi: 10.2217/nnm.13.170. [DOI] [PubMed] [Google Scholar]
- 69.Knuschke T, Sokolova V, Rotan O, Wadwa M, Tenbusch M, Hansen W, Staeheli P, Epple M, Buer J, Westendorf AM. Immunization with biodegradable nanoparticles efficiently induces cellular immunity and protects against influenza virus infection. J Immunol. 2013;190(12):6221–9. doi: 10.4049/jimmunol.1202654. [DOI] [PubMed] [Google Scholar]
- 70.Luo Z, Wang C, Yi H, Li P, Pan H, Liu L, Cai L, Ma Y. Nanovaccine loaded with poly I:C and STAT3 siRNA robustly elicits anti-tumor immune responses through modulating tumor-associated dendritic cells in vivo. Biomaterials. 2015;38:50–60. doi: 10.1016/j.biomaterials.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 71.Chen L, Ding Y, Wang Y, Liu X, Babu R, Ravis W, Yan W. Codelivery of zoledronic acid and double stranded RNA from core-shell nanoparticles. Int J Nanomedicine. 2013;8:137–45. doi: 10.2147/IJN.S38928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang S, Eltoukhy AA, Love KT, Langer R, Anderson DG. Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Letters. 2013;13(3):1059–64. doi: 10.1021/nl304287a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shukoor MI, Natalio F, Ksenofontov V, Tahir MN, Eberhardt M, Theato P, Schröder HC, Müller WE, Tremel W. Double-stranded RNA polyinosinic-polycytidylic acid immobilized onto gamma-Fe2O3 nanoparticles by using a multifunctional polymeric linker. Small. 2007;3(8):1374–8. doi: 10.1002/smll.200600664. [DOI] [PubMed] [Google Scholar]
- 74.Parker-Esquivel B, Flores KJ, Louiselle D, Craig M, Dong L, Garrad R, Ghosh K, Wanekaya A, Glaspell G, DeLong RK. Association of poly I:C RNA and plasmid DNA onto MnO nanorods mediated by PAMAM. Langmuir. 2012;28(8):3860–70. doi: 10.1021/la203998r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khodadust R, Unsoy G, Gunduz U. Development of poly (I:C) modified doxorubicin loaded magnetic dendrimer nanoparticles for targeted combination therapy. Biomed Pharmacotherapy. 2014;68(8):979–87. doi: 10.1016/j.biopha.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 76.Cobaleda-Siles M, Henriksen-Lacey M, Ruiz de Angulo A, Bernecker A, Gómez Vallejo V, Szczupak B, Llop J, Pastor G, Plaza-Garcia S, Jauregui-Osoro M, Meszaros LK, Mareque-Rivas JC. An iron oxide nanocarrier for dsRNA to target lymph nodes and strongly activate cells of the immune system. Small. 2014;10(24):5054–67. doi: 10.1002/smll.201401353. [DOI] [PubMed] [Google Scholar]
- 77.Hernández-Gil J, Cobaleda-Siles M, Zabaleta A, Salassa L, Calvo J, Mareque-Rivas JC. An Iron Oxide Nanocarrier Loaded with a Pt(IV) Prodrug and Immunostimulatory dsRNA for Combining Complementary Cancer Killing Effects. Adv Healthc Mater. 2015;4(7):1034–42. doi: 10.1002/adhm.201500080. [DOI] [PubMed] [Google Scholar]
- 78.Singh M, Overwijk WW. Intratumoral immunotherapy for melanoma. Cancer Immunol Immunother. 2015;64(7):911–21. doi: 10.1007/s00262-015-1727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salazar AM, Erlich RB, Mark A, Bhardwaj N, Herberman RB. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunol Res. 2014;2(8):720–4. doi: 10.1158/2326-6066.CIR-14-0024. [DOI] [PubMed] [Google Scholar]
- 80.Qu J, Hou Z, Han Q, Zhang C, Tian Z, Zhang J. Poly(I:C) exhibits an anti-cancer effect in human gastric adenocarcinoma cells which is dependent on RLRs. Int Immunopharmacol. 2013;17(3):814–20. doi: 10.1016/j.intimp.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 81.Chattopadhyay S, Dash SK, Ghosh T, Das D, Pramanik P, Roy S. Surface modification of cobalt oxide nanoparticles using phosphonomethyl iminodiacetic acid followed by folic acid: a biocompatible vehicle for targeted anticancer drug delivery. Cancer Nanotechnology. 2013;4(4–5):103–116. doi: 10.1007/s12645-013-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chattopadhyay S, Dash SK, Ghosh T, Das S, Tripathy S, Mandal D, Das D, Pramanik P, Roy S. Anticancer and immunostimulatory role of encapsulated tumor antigen containing cobalt oxide nanoparticles. Journal of Biological Inorganic Chemistry. 2013;18(8):957–73. doi: 10.1007/s00775-013-1044-y. [DOI] [PubMed] [Google Scholar]
- 83.Rasmussen JW, Martinez E, Louka P, Wingett DG. Zinc Oxide Nanoparticles for Selective Destruction of Tumor Cells and Potential for Drug Delivery Applications. Expert opinion on drug delivery. 2010;7(9):1063–1077. doi: 10.1517/17425247.2010.502560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liuba T, Vittoria R, Cristina R, Orazio V, Iorio MC, Vanacore R, Pietrabissa A, Cuschieri A. Zinc oxide nanoparticles as selective killers of proliferating cells. Int J Nanomedicine. 2011;6:1129–1140. doi: 10.2147/IJN.S16581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y, Sun R, Liu B, Deng M, Zhang W, Li Y, Zhou G, Xie P, Li G, Hu J. TLR3 activation inhibits nasopharyngeal carcinoma metastasis via downregulation of chemokine receptor CXCR4. Cancer Biol Ther. 2009;8:1826–30. doi: 10.4161/cbt.8.19.9437. [DOI] [PubMed] [Google Scholar]
- 86.Miller FR, Aslakson CJ, McEachern D. Modulation of host resistance to metastasis in the lungs of aged retired breeder mice. Invasion Metastasis. 1991;1:233–40. [PubMed] [Google Scholar]
- 87.Forte G, Rega A, Morello S, Luciano A, Arra C, Pinto A, Sorrentino R. Polyinosinic-polycytidylic acid limits tumor outgrowth in a mouse model of metastatic lung cancer. J Immunol. 2012;188:5357–64. doi: 10.4049/jimmunol.1103811. [DOI] [PubMed] [Google Scholar]