Abstract
Placental mesenchymal dysplasia (PMD) is a rare placental disorder characterized by placental enlargement and areas of abnormal, enlarged, grape-like villi. This condition may resemble a partial hydatidiform mole and may occur associated with Beckwith–Wiedemann syndrome (BWS) or in phenotypically normal fetuses. There were 110 cases reported so far. We describe one case with typical gross and microscopic placental lesions.
Keywords: Placenta, Placenta Diseases, Hydatidiform mole
INTRODUCTION
Placental mesenchymal dysplasia (PMD) is a rare placental disorder characterized by placental enlargement and areas of abnormal, enlarged, grape-like villi.1,2 This condition may resemble a partial hydatidiform mole, but lacks trophoblastic proliferation. It may be found in cases associated with Beckwith–Wiedemann syndrome (BWS), intrauterine growth restriction (IUGR) and in phenotypically normal fetuses.3,4
We report the gross and microscopic findings of a placenta with mesenchymal dysplasia.
CASE REPORT
A previously healthy 26-year-old woman, gravida 5 para 2 (the remaining past obstetric history was not available), had vaginal delivery of a 2230g live born female infant at the 36th week of pregnancy (-0,98 z score, 16 percentile, adequate for gestational age).5
The newborn had no malformations at birth and the Apgar score was of 9 at 1 minute and 9 at 5 minutes.
Pre natal records were not available, but post partum serology for toxoplasmosis, hepatitis B and C were negative and IgG was positive for rubella and cytomegalovirus.
The infant presented jaundice and underwent phototherapy. Echocardiogram, cranial ultrasound and urinary tract ultrasound were normal. The infant was discharged at the fourth day of life.
GROSS FINDINGS
The trimmed placenta weighted 1415 g and measured 28.0 × 25.0 x 7,0 cm, all dimensions over the 99 percentile for gestational age.6,7 The umbilical cord was sent with a length of 7.0 cm and 0.8 cm diameter, with only two vessels. The chorionic vessels were tortuous and cirsoid (with g aneurysmal dilatation). Their cut surface showed extensive thrombosis in the lumen and increased thickness of the walls.
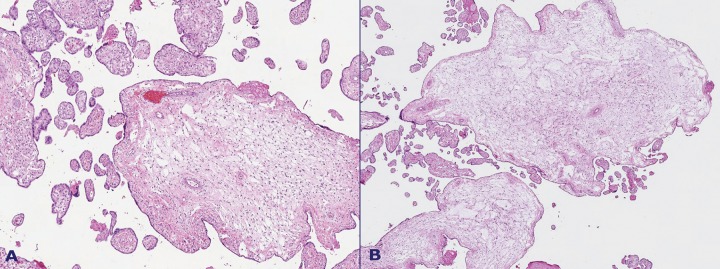
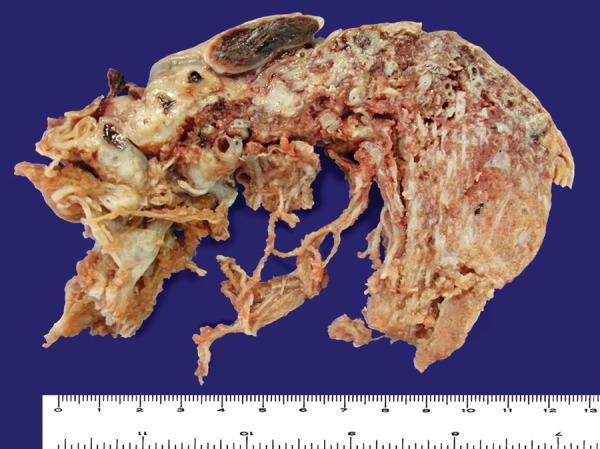
The cut surfaces of the placenta showed an admixture of normal-looking areas and numerous clusters of grape-like fluid-filled vesicles measuring up to 2.0 cm in diameter and noodle-shaped villi (Figures 1-2). Both subamniotic and intraplacental hematoma were seen.
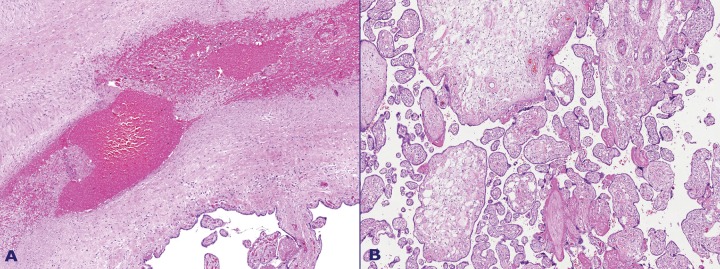
Figure 1. A – The cut surface of the placenta showing varicose chorionic vessels on the top. Myxoid areas on the cut surface of stem villi are visible B – A normal area of villi (arrow head) interfacing with noodle-like enlarged villi (arrow). A varicose chorionic vessel with obstructive thrombosis is seen on the right.
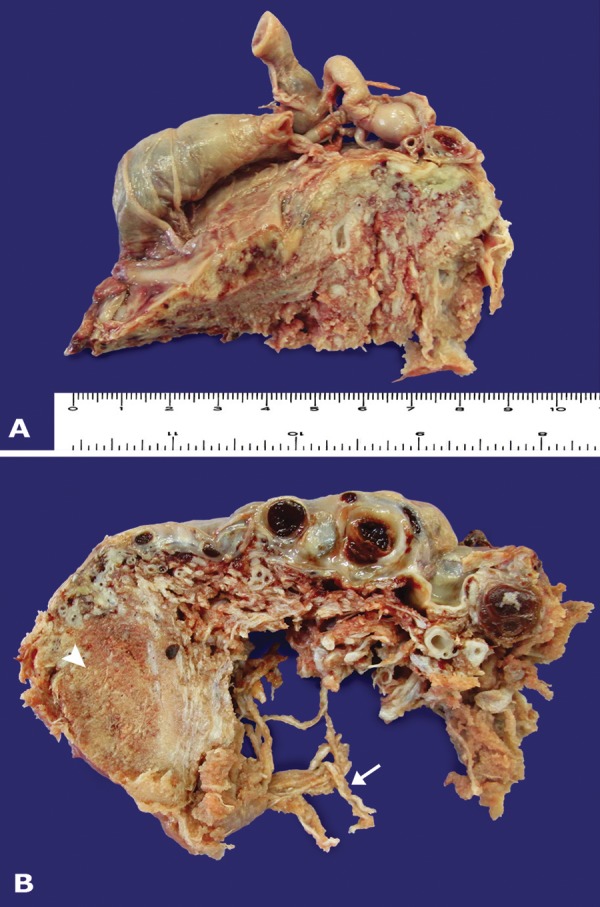
Figure 2. Another area showing typical PMD findings. Dilated chorionic vessels presenting with thrombosis, and a cut surface area of the placenta showing normal areas adjacent to areas with cystic myxoid appearance.
HISTOLOGIC FINDINGS
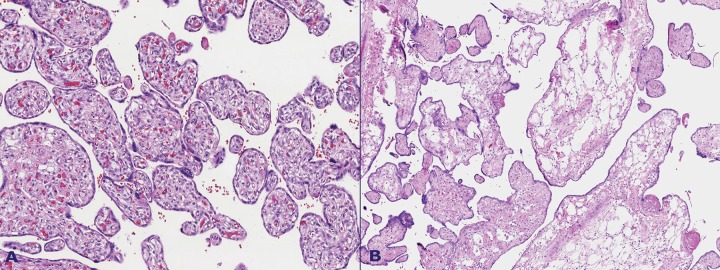
Microscopic examination revealed an admixture of normal terminal and stem villi and enlarged cystic villi. These villi were characterized by a loose, edematous, and myxoid stroma with fewer and smaller vessels than their normal counterparts; they were sometimes absent, and their location was peripheral in the villous structure. The stroma cells were mostly spindle-like or stellate in shape (Figures 3, 4, 5 and 6).
Figure 3. A – Mixed population of villi. Mature third trimester villi and enlarged villi with a myxoid or hydropic appearance. The vessels in the latter are decreased in number, are located on the villous periphery, and have thickened walls; B – Enlarged villi showing marked stromal edema. The stroma is composed of loose connective tissue, mainly spindle cells.
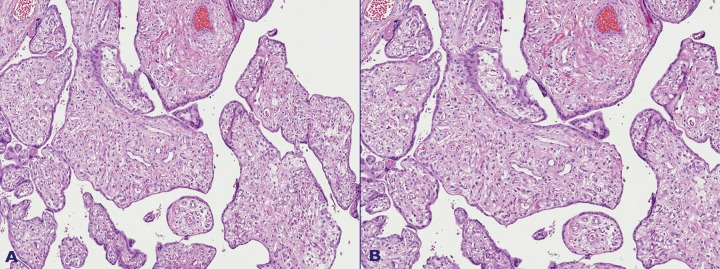
Figure 4. A – Enlarged, dysplastic villi among mature third trimester villi showing chorangiosis; B – Boxed area of Figure 4A. The stroma of the dysplastic villi is composed of loose connective tissue, with spindle cells; cisternae may form as a result of fluid accumulation. The villous vessels are decreased in number and located in the periphery. There are no throphoblastic hyperplasia or inclusions.
Figure 5. A – Immature third trimester villi showing chorangiosis with numerous capillary vessels, more than 10 per villus, in numerous villi; B – Enlarged villi showing marked stromal edema. The stroma is composed of loose connective tissue.
Figure 6. A – Chorangiomatosis with enlarged villi with a loose stroma, proliferative capillary vascular channels, and intervening stromal cells; some vessels have enlarged walls and others have dilated lumen; B – Villi with hyalinized stroma were found adjacent to dysplastic villi. No trophoblastic hyperplasia is seen.
There were no proliferations or inclusions on the lining trophoblast. These enlarged villi were accompanied by multifocal groups of immature enlarged villi, with 10 or more vessels, that is, chorangiosis (Figure 5A). Another lesion found was multifocal diffuse chorangiomatosis (Figure 6A) with numerous enlarged villi showing a proliferation of vascular channels.
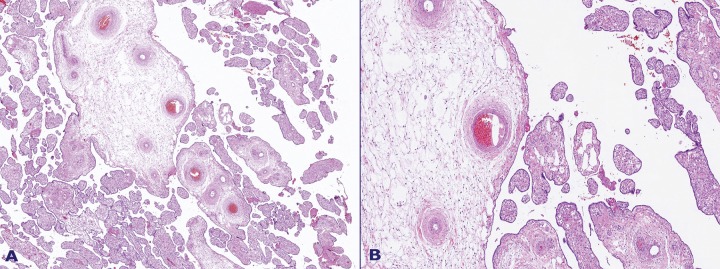
The grossly visible thrombosis on dilated vessels at the chorionic plate showed an occlusive pattern (Figure 7). The umbilical artery showed mural thrombosis. There were also groups of avascular terminal villi and hemorrhagic endovasculitis.
Figure 7. A – Occlusive vascular thrombosis and hemorrhagic endovasculitis; B – Enlarged stem villi adjacent to mature third trimester villi.
DISCUSSION
PMD is a rare disorder that was first described by Moscoso and colleagues in 1991.8 It is found in approximately 0.02% of pregnancies,1,9 with 110 cases described so far. There is a female preponderance, with female:male ratio of 3.6-4:1.4,9,10 This condition has been associated with Beckwith-Wiedmann syndrome (BWS) but it is also related to phenotypically normal fetuses,2,4 and is compatible with fetal life.1,11 BWS is characterized by omphalocele, macroglossia, macrosomia, visceromegaly, hemihypertrophy, and histologic adrenal cytomegaly,2,11 however none of these lesions were apparent at birth in this case. Cases without BWS present a high rate of IUGR4,9, (50%), and 40% result in fetal demise or neonatal death. In our case the birth weight was in the lower limit of normality (10-50 percentiles).5
Gross findings include an enlarged placenta presenting areas of cystic villous change: enlarged villous structures with a myxoid appearance. Enlarged chorionic vessels with a varicose appearance and thrombosis of the lumen are also found.2-4
Microscopically, there are two populations of villi: (1) mostly normal for the gestational age; and (2) enlarged villi, sometimes described as hydropic. The latter present a myxoid stroma harboring spindle cells without atypia or prominent mitotic activity4 and loose connective tissue sometimes forming cisternae. The trophoblast shows no hyperplasia or inclusions.1,2,4,12
The stromal matrix is composed mainly of hyaluronic acid, as has been demonstrated by special staining techniques such as Alcian blue followed by enzymatic digestion.3,4
Chorionic vessels may present aneurysmatic and varicose dilatation, while those in the affected villi tend to be smaller in size and have thicker walls than their normal counterparts, being sometimes completely obliterated. These vessels are peripheral in the villous structure. Avascular villi may also be found.2,3
It has been proposed that the dilatation of chorionic vessels may be a result of circulatory imbalance due to poor vascularization of the dysplastic cotyledons. Ultrasonographic studies in some cases of placentae diagnosed after birth with PMD showed no vascular abnormalities of chorionic vessels until mid-gestation.2 Other findings have been described in association with PMD, such as single-artery umbilical cord, tortuous umbilical cord, umbilical cord with furcate insertion, and chorangioma.
Vascular malformations associated with PMD might represent a form of congenital malformation of the mesoderm1,4, and there may be a relation to simultaneous mesenchymal disorders of the newborn, such as liver and skin hemangiomas.3,11 In our case, we found chorangiomatosis, chorangiosis, and a single umbilical artery associated with PMD.
Chorangiosis is defined as more than 10 capillaries per terminal villus in 10 villi in several regions of the placenta.13 Chorangiomatosis is the presence of proliferative capillary vascular channels and intervening stromal cells surrounded by trophoblast. It is similar to chorangioma, but involves several villi throughout the placenta, without nodule formation.13
The most common karyotype associated with PMD is 46,XX.2 Other instances were associated with androgenic/biparental mosaicism,4 and there were also cases that presented with aneuploidy. BWS has been related to imprinting alterations on 11p15.5, which may affect the expression of IGF-2, thus influencing growth of the fetus and placenta.11,14
Another gene implied in the pathogenesis of this condition is Xp22.31, which is expressed as VEGF-D, related to angiogenesis4 and thus potentially to the mesenchymal abnormalities found in PMD and eventually in the fetus.
Since its similarity to the partial hydatidiform mole on its ultrasonographic and histologic presentation, PMD was first described as a “pseudo partial mole”.2,8,11 The differential diagnosis can be achieved by different methods. Ultrasonographic assessment and maternal serum beta-human chorionic gonadotrophin can be obtained prenatally, while histologic examination will show the absence of trophoblastic hyperplasia in PMD.2,4 Elevated maternal serum alpha-fetoprotein levels have been associated with PMD.1,15 The karyotype can also separate these diseases, since a partial hydatidiform mole will present as triploid, while most cases of PMD will be diploid.
The gross and histologic findings in this case were consistent with PMD.
CONCLUSION
PMD is a rare condition, more frequently involving the karyotype 46,XX and has been associated with other conditions, such as BWS and mesenchymal defects. Although its cause remains unknown, a number of studies associate PMD with possible genetic mutations and related diseases.
The similarity in presentation between PMD and partial hydatidiform mole makes the differential diagnosis necessary since the fetus in PMD is generally viable. When diagnosed, PMD should raise the suspicion for BWS.
Further studies should aim at defining any genetic mutations related to PMD, and whether they are related to fetal mesenchymal disorders.
Footnotes
Toscano MP, Schultz R. Placental mesenchymal dysplasia: case report with gross and histological findings. Autopsy Case Rep [Internet]. 2014;4(4):51-6. http://dx.doi.org/10.4322/acr.2014.039
REFERENCES
- 1.Gibson BR, Muir-Padilla J, Champeaux A, Suarez ES. Mesenchymal dysplasia of the placenta. Placenta. 2004;25(7):671-2. http://dx.doi.org/10.1016/j.placenta.2003.12.008. PMid: [DOI] [PubMed] [Google Scholar]
- 2.Jauniaux E, Nicolaides KH, Hustin J. Perinatal features associated with placental mesenchymal dysplasia. Placenta. 1997;18(8):701-6. http://dx.doi.org/10.1016/S0143-4004(97)90012-6. PMid: [DOI] [PubMed] [Google Scholar]
- 3.Ohyama M, Kojyo T, Gotoda H, Sato T, Ijiri R, Tanaka Y. Mesenchymal dysplasia of the placenta. Pathol Int. 2000;50(9):759-64. http://dx.doi.org/10.1046/j.1440-1827.2000.01100.x. PMid: [DOI] [PubMed] [Google Scholar]
- 4.Pham T, Steele J, Stayboldt C, Chan L, Benirschke K. Placental mesenchymal dysplasia is associated with high rates of intrauterine growth restriction and fetal demise: A report of 11 new cases and a review of the literature. Am J Clin Pathol. 2006;126(1):67-78. http://dx.doi.org/10.1309/RV45HRD53YQ2YFTP. PMid: [DOI] [PubMed] [Google Scholar]
- 5.Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857-68. http://dx.doi.org/10.1016/S0140-6736(14)60932-6. PMid: [DOI] [PubMed] [Google Scholar]
- 6.Thompson JMD, Irgens LM, Skjaerven R, Rasmussen S. Placenta weight percentile curves for singleton deliveries. BJOG. 2007;114(6):715-20. http://dx.doi.org/10.1111/j.1471-0528.2007.01327.x. PMid: [DOI] [PubMed] [Google Scholar]
- 7.Benirschke K, Kaufmann P, Baergen RN, editors. Pathology of the human placenta. New York: Springer; 2006. cap. 28; p. 1019-26. Normative values and tables. [Google Scholar]
- 8.Moscoso G, Jauniaux E, Hustin J. Placental vascular anomaly with diffuse mesenchymal stem villous hyperplasia. A new clinico-pathological entity? Pathol Res Pract. 1991;187(2-3):324-8. http://dx.doi.org/10.1016/S0344-0338(11)80791-0. PMid: [DOI] [PubMed] [Google Scholar]
- 9.Li H, Li L, Tang X, Yang F, Yang KX. Placental mesenchymal dysplasia: a case of a normal-appearing fetus with intrauterine growth restriction. Int J Clin Exp Pathol. 2014;7(8):5302-7. PMid:. [PMC free article] [PubMed] [Google Scholar]
- 10.Ohira S, Ookubo N, Tanaka K, et al. Placental mesenchymal dysplasia: chronological observation of placental images during gestation and review of the literature. Gynecol Obstet Invest. 2013;75(4):217-23. http://dx.doi.org/10.1159/000350661. PMid: [DOI] [PubMed] [Google Scholar]
- 11.Paradinas FJ, Sebire NJ, Fisher RA, et al. Pseudo-partial moles: placental stem vessel hydrops and the association with Beckwith-Wiedemann syndrome and complete moles. Histopathology. 2001;39(5):447-54. http://dx.doi.org/10.1046/j.1365-2559.2001.01256.x. PMid: [DOI] [PubMed] [Google Scholar]
- 12.Matsui H, Iitsuka Y, Yamazawa K, et al. Placental mesenchymal dysplasia initially diagnosed as partial mole. Pathol Int. 2003;53(11):810-3. http://dx.doi.org/10.1046/j.1440-1827.2003.01550.x. PMid: [DOI] [PubMed] [Google Scholar]
- 13.Ogino S, Redline RW. Villous capillary lesions of the placenta: distinctions between chorangioma, chorangiomatosis, and chorangiosis. Hum Pathol. 2000;31(8):945-54. http://dx.doi.org/10.1053/hupa.2000.9036. PMid: [DOI] [PubMed] [Google Scholar]
- 14.Milani D, Pezzani L, Tabano S, Miozzo M. Beckwith-Wiedemann and IMAGe syndromes: two very different diseases caused by mutations on the same gene. Appl Clin Genet. 2014;7:169-75. PMid:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lage JM. Placentomegaly with massive hydrops of placental stem villi, diploid DNA content, and fetal omphaloceles: possible association with Beckwith-Wiedemann syndrome. Hum Pathol. 1991;22(6):591-7. http://dx.doi.org/10.1016/0046-8177(91)90237-J. PMid: [DOI] [PubMed] [Google Scholar]