Abstract
Many drugs of abuse exert their addictive effects by increasing extracellular dopamine in the nucleus accumbens, where they likely alter the plasticity of corticostriatal glutamatergic transmission. This mechanism implies key molecular alterations in neurons in which both dopamine and glutamate inputs are activated. Extracellular signal-regulated kinase (ERK), an enzyme important for long-term synaptic plasticity, is a good candidate for playing such a role. Here, we show in mouse that d-amphetamine activates ERK in a subset of medium-size spiny neurons of the dorsal striatum and nucleus accumbens, through the combined action of glutamate NMDA and D1-dopamine receptors. Activation of ERK by d-amphetamine or by widely abused drugs, including cocaine, nicotine, morphine, and Δ9-tetrahydrocannabinol was absent in mice lacking dopamine- and cAMP-regulated phosphoprotein of Mr 32,000 (DARPP-32). The effects of d-amphetamine or cocaine on ERK activation in the striatum, but not in the prefrontal cortex, were prevented by point mutation of Thr-34, a DARPP-32 residue specifically involved in protein phosphatase-1 inhibition. Regulation by DARPP-32 occurred both upstream of ERK and at the level of striatal-enriched tyrosine phosphatase (STEP). Blockade of the ERK pathway or mutation of DARPP-32 altered locomotor sensitization induced by a single injection of psychostimulants, demonstrating the functional relevance of this regulation. Thus, activation of ERK, by a multilevel protein phosphatase-controlled mechanism, functions as a detector of coincidence of dopamine and glutamate signals converging on medium-size striatal neurons and is critical for long-lasting effects of drugs of abuse.
Keywords: dopamine D1 receptor, drug addiction, NMDA receptor, nucleus accumbens, protein kinase
Many drugs of abuse share the ability to stimulate dopamine transmission in the nucleus accumbens (1). They are thought to mimic the effects of naturally reinforcing stimuli and divert the normal role of dopamine neurons in coding reward prediction errors (2). Dopamine modulates long-term depression and potentiation at glutamatergic corticostriatal synapses, and current models of striatal circuits suggest that the regulation of the plasticity of corticostriatal transmission is a central mechanism of dopamine-controlled-learning (3, 4). One prediction of these models is that key biochemical events should occur specifically in neurons in which both dopamine and glutamate inputs are activated. Activation of extracellular signal-regulated kinase (ERK) is a candidate for such a role because it depends on both dopamine and glutamate receptors (5). In addition, ERK activity is known to be important for long-term synaptic plasticity (6), and its pharmacological blockade prevents the transcriptional and rewarding effects of cocaine and Δ9-tetrahydrocannabinol (THC) (5, 7). To determine the role of ERK in the long-term action of dopamine in the striatum, it is critical to identify in which neurons it is activated and the mechanism of this activation. In the present study, using a variety of pharmacological tools and mutant mice, we show that activation of ERK occurs in a specific subset of striatal neurons and requires the stimulation of D1 dopamine receptors (D1Rs) and NMDA glutamate receptors (NMDARs). We found that this coincidence detection results from the striatal-specific regulation of both serine/threonine and tyrosine protein phosphatases and is initiated by phosphorylation of the protein phosphatase-1 (PP-1) inhibitor dopamine- and cAMP-regulated phosphoprotein of Mr 32,000 (DARPP-32) on a single threonine residue (Thr-34). Our results provide evidence that this mechanism has important consequences for the long-term effects of psychostimulants and may have a general role in the action of many drugs of abuse.
Methods
Experiments were carried out in accordance with the guidelines of the French Agriculture and Forestry Ministry for handling animals (Decree 87849, License 01499) in either male 8-week old C57BL/6J mice or male and female mutant mice and matched controls from previously described lines (8-10). Mice were habituated to daily saline i.p. injections during the 3 days preceding the experiments. For immunoblotting, microdisks were prepared from sections of rapidly frozen brains and lysed in a 1% (vol/vol) SDS solution at 100°C. For immunofluorescence, mice were perfused transcardially with 4% paraformaldehyde in 0.1 M Na2HPO4/NaH2PO4 buffer (pH 7.5). Cells with fluorescence above background were counted by using a computerized image analyzer, in two to four brain sections per animal, bilaterally for each region of interest. Locomotor activity was measured by consecutive interruption of two adjacent beams (¼ tour) in a circular corridor with four infrared beams placed at every 90° (Imetronic, Pessac, France) in a low-luminosity environment. Statistical analyses were done by using one-way ANOVA followed by Bonferonni's test or Dunnett's test. Detailed procedures and reagents are described in Supporting Experimental Procedures, which is published as supporting information on the PNAS web site.
Results
Psychostimulants Activate ERK in a Subset of Striatal Medium-Size Spiny Neurons (MSNs). To investigate the molecular basis for ERK regulation, we examined its activation by phosphorylation in mouse striatum in response to acute injections of d-amphetamine (d-amph) by using antibodies specific for the active form of the kinase. In agreement with previous results obtained with cocaine (5, 11), we found a robust increase in phospho-ERK (P-ERK) immunoreactivity in the striatum of mice treated with d-amph. P-ERK was detected in neuronal nuclei and perikarya, as well as in the surrounding neuropil, in the dorsal striatum, and in the shell (Fig. 7, which is published as supporting information on the PNAS web site) and core (not shown) of the nucleus accumbens. P-ERK immunoreactivity was found in MSNs, the principal type of striatal neurons, because it colocalized with several markers of these neurons: DARPP-32 (12), striatal-enriched tyrosine phosphatase (STEP) (13) (Fig. 1a and Table 1, which is published as supporting information on the PNAS web site), and Gαolf (14) (data not shown). No P-ERK immunoreactivity was found in cholinergic interneurons identified with antibodies against choline acetyltransferase (Fig. 1a).
Fig. 1.
d-amph-induced ERK activation in MSNs. (a) Double immunolabeling of P-ERK1/2 (red) and the indicated proteins (green) in the shell of the nucleus accumbens, 15 min after i.p. injection of d-amph (10 mg/kg) (see also Table 1). ChAT, choline acetyltransferase; Dyn, dynorphin; Enk, enkephalin. (b) Double immunolabeling of P-ERK (red) and phospho-Thr-34-DARPP-32 (P-DARPP-32, green) in vehicle-treated (Upper) or d-ampf-treated (Lower) mice. (c) Fifteen minutes before d-amph (+) or saline (-) injection, mice were treated with saline or MK801 (0.1 mg/kg). Neurons immunoreactive for P-DARPP-32 were counted, and the proportion of neurons immunoreactive for both P-DARPP-32 and P-ERK is indicated as filled bars. Virtually all P-ERK-positive neurons were P-DARPP-32-positive (not shown). Data are means ± SEM (neurons per shell field, five mice per group, d-amph-treated vs. control: *, P < 0.05; antagonist vs. saline pretreatment: º, P < 0.05). Photographs are single confocal sections. (Scale bars: 10 μm.)
MSNs are chemically heterogeneous: some express dynorphin and high levels of D1Rs, whereas others express enkephalin and high levels of D2 receptors (15). After d-amph injection, P-ERK was found in ≈60% of dynorphin-positive neurons, but in only ≈3% of enkephalin-positive neurons (Fig. 1a and Table 1). Localization of P-ERK in D1R-expressing cells was also supported by their double labeling with D1R antibodies (Fig. 1a). Because D1R acts mainly by raising cAMP levels and activating cAMP-dependent protein kinase (PKA), we compared the distribution of P-ERK and phospho-Thr-34-DARPP-32, a major PKA substrate in MSNs (12), by using phosphorylation state-specific antibodies (Fig. 8, which is published as supporting information on the PNAS web site). P-ERK was detected in a subpopulation of phospho-Thr-34-DARPP-32-positive neurons (≈65%), and virtually never in other striatal neurons (Fig. 1 b and c and Table 1). Thus, in response to d-amph, ERK phosphorylation is more restricted than PKA activation, within a subpopulation of dynorphin- and D1R-positive MSNs, indicating that it requires a stimulus in addition to dopamine.
ERK Activation Selectively Requires a Combined Action of Glutamate NMDARs and Dopamine D1Rs. Using immunoblotting of brain samples obtained after rapid freezing, we compared the phosphorylation of ERK to that of the glutamate receptor 1 (GluR1) subunit of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, a well characterized PKA substrate, phosphorylated on Ser-845 in response to D1R stimulation (16). A robust increase in phosphorylation of GluR1 and both ERK isoforms (predominantly ERK2) was observed in response to d-amph (Figs. 2a and 7b). ERK and GluR1 phosphorylation was rapid (maximal at 10 min), transient (return to baseline at 30 min), and dose-dependent (Fig. 7 c and d). Phosphorylation of both ERK and GluR1 was blocked by prior administration of the D1R antagonist SCH23390 (Fig. 2a). These results were confirmed by using D1R-knockout mice (Fig. 2b).
Fig. 2.
Regulation of ERK and GluR1 phosphorylation in the mouse striatum in vivo by d-amph. Fifteen minutes after injection of saline (-)or d-amph (10 mg/kg) (+), phosphorylation of ERK2 and GluR1 at Ser-845 were quantified in the striatum by immunoblotting in mice pretreated with saline or the D1R antagonist SCH23390 (0.25 mg/kg, i.p.) 30 min before saline or d-amph (a), in wild-type (wt) or D1R knockout (D1R-KO) mice (b), or in mice pretreated with saline or the NMDAR antagonist MK801 (0.1 mg/kg) 30 min before saline or d-amph (c). Results are expressed as percentages of controls. Data are means ± SEM (five mice per group, d-amph-treated vs. control: *, P < 0.05; knockout vs. wild type or antagonist vs. saline pretreatment: º, P < 0.05).
The requirement for glutamate stimulation was then examined. Pretreatment of mice with an NMDA receptor antagonist, MK801, prevented ERK phosphorylation, whereas it did not alter GluR1 phosphorylation (Fig. 2c). Similarly, as judged by quantification of the immunofluorescence data, MK801 prevented phosphorylation of ERK, but not of DARPP-32 (Fig. 1c). Taken together, these results indicate that phosphorylation of GluR1 and DARPP-32 through the PKA pathway requires activity of D1R but not of NMDAR. In contrast, ERK phosphorylation requires stimulation of both D1R and NMDAR, showing its dependence on a coincident activity of dopamine and glutamate transmission in a subset of D1R-containing MSNs.
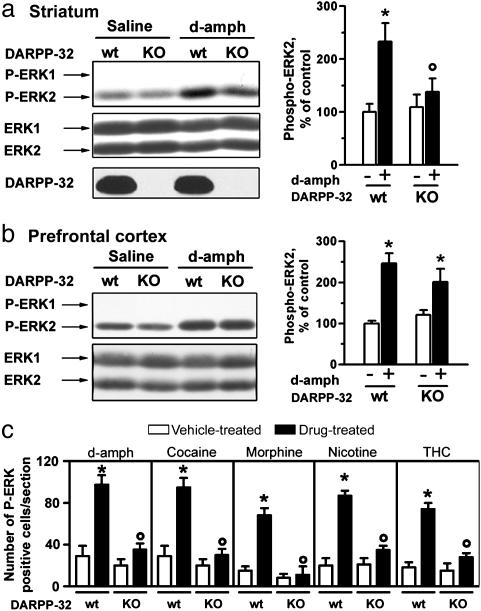
DARPP-32 Is Required for Activation of the ERK Pathway in the Striatum by Drugs of Abuse. MSNs express high levels of DARPP-32, a protein that regulates several phosphorylation cascades (17). In DARPP-32-knockout mice, ERK activation in response to d-amph was dramatically reduced (Fig. 3a). Psychostimulants activate ERK in several other brain regions (11) such as the prefrontal cortex, in which DARPP-32 expression is much lower than in the striatum (12). As illustrated in Fig. 3b, activation of ERK by d-amph was similar in the prefrontal cortex of wild-type and DARPP-32-knockout mice, suggesting that DARPP-32 plays a specific role in ERK activation in the striatum. DARPP-32 has a high degree of sequence similarity with inhibitor-1, another PKA-regulated inhibitor of PP-1, also expressed in MSNs (18). However, ERK activation was unchanged in inhibitor-1 knockout mice (data not shown), supporting a selective role of DARPP-32 in this response.
Fig. 3.
Requirement of DARPP-32 in the activation of ERK in the striatum by drugs of abuse. (a) Immunoblot analysis (quantification in Right) of striatal extracts from wild-type (wt) and DARPP-32-knockout (KO) mice 15 min after i.p. injection of saline or d-amph (10 mg/kg). (b) Immunoblot analysis of prefrontal cortex extracts as in a. (c) Quantification of P-ERK-positive cells in sections of the nucleus accumbens from wt and DARPP-32-KO mice 10 min after injection of d-amph (10 mg/kg, i.p.) or cocaine (20 mg/kg, i.p.), or 20 min after injection of morphine (5 mg/kg, s.c.), nicotine (0.4 mg/kg, s.c.), or THC (1 mg/kg, i.p.), and their respective vehicle-treated controls. Data are means ± SEM (five mice per group, drug-treated vs. control: *, P < 0.05; KO vs. wt: º, P < 0.05).
We have recently shown that activation of ERK in the nucleus accumbens shell is a shared property of diverse drugs of abuse (11). We compared the role of DARPP-32 in ERK phosphorylation in response to d-amph, cocaine, morphine, nicotine, and THC in the nucleus accumbens shell (Fig. 3c). ERK activation was blocked for all these drugs in DARPP-32-knockout mice. These results demonstrate that DARPP-32-dependent activation of ERK in the striatum is a general mechanism common to a variety of drugs of abuse.
DARPP-32 Phosphorylation on Residue Thr-34 Is Specifically Required for Activation of the ERK Pathway by Cocaine in the Striatum. DARPP-32 has several regulatory functions that depend on the phosphorylation of distinct residues by different kinases (17), and the absence of DARPP-32 could alter the activation of ERK in the striatum by several mechanisms. Phosphorylation of DARPP-32 on Thr-34 is necessary and sufficient for high-affinity inhibition of PP-1 (19, 20). To investigate the precise role of PP-1 inhibition in ERK activation by psychostimulants, we used mutant mice in which Thr-34 of DARPP-32 was replaced by alanine (10). Cocaine-induced phosphorylation of ERK was prevented in the dorsal striatum and the nucleus accumbens core and shell of Thr-34 → Ala-DARPP-32 mutant mice (Fig. 4a and Fig. 9a, which is published as supporting information on the PNAS web site). In contrast, the mutation had no effect on ERK phosphorylation in the prefrontal cortex. Similar results were observed when the mice were treated with d-amph (data not shown). On the other hand, in mice bearing a point mutation of Thr-75, a residue phosphorylated by CDK5 and responsible for inhibition of PKA (21), ERK activation in the dorsal striatum, the nucleus accumbens core and shell, and the prefrontal cortex was similar to that in wild-type littermates (Figs. 4b and 9b). When we examined the levels of several proteins involved in striatal signal transduction we found that levels of ERK1 and ERK2 were selectively increased in the striatum of Thr-34 → Ala, but not Thr-75 → Ala mutant mice (Fig. 10, which is published as supporting information on the PNAS web site). This change suggested a compensatory reaction to the alteration of the ERK pathway in the striatum.
Fig. 4.
DARPP-32 phosphorylation on residue Thr-34 is required for activation of ERK in the striatum by cocaine. (a) The number of P-ERK-positive neurons was quantified in dorsal striatum (DStr), nucleus accumbens (shell and core), and prefrontal cortex (Pfx) in wild-type (wt) and Thr-34 → Ala DARPP-32 (T34A) mutant mice 10 min after i.p. injection of saline or cocaine (20 mg/kg). (b) Same experiment in wild-type and Thr-75 → Ala DARPP-32 (T75A) mutant mice. (c) Phosphorylation of transcription factor Elk-1 Ser-383 was examined in wild-type and Thr-34 → Ala DARPP-32 (T34A) mutant mice 10 min after i.p. injection of saline or cocaine (20 mg/kg). The number of immunofluorescent neuronal nuclei labeled with phosphorylation state-specific antibodies was quantified in dorsal striatum (DStr), nucleus accumbens (shell and core), and prefrontal cortex (Pfx). Data are means ± SEM; six mice per group; treated vs. control: *, P < 0.01; wild type vs. mutant: º, P < 0.05.
The above results were obtained by measuring the level of ERK phosphorylation on its activation loop. Although this phosphorylation is highly correlated with ERK activity, it was important to determine whether increased ERK phosphorylation actually resulted in its functional activation. To test this possibility, we examined the in vivo phosphorylation of a major substrate of ERK, the transcription factor Elk-1 (22). As previously reported (7, 23), d-amph and cocaine induced a strong phosphorylation of Elk-1 on Ser-383 in the dorsal striatum, the nucleus accumbens shell and core, and the prefrontal cortex (Fig. 4c). Phosphorylation of Elk-1 was still detected at 30 min (data not shown). We verified that the observed Elk-1 phosphorylation resulted from ERK activation by pretreating mice with SL327, an inhibitor of mitogen-activated protein (MAP)-kinase/ERK kinase (MEK) that crosses the blood-brain barrier (24). SL327 pretreatment completely prevented Elk-1 phosphorylation (data not shown). DARPP-32 was necessary for the regulation of Elk-1, because drug-induced phosphorylation of Elk-1 was prevented in the dorsal striatum and nucleus accumbens of DARPP-32 Thr-34 → Ala mutant mice (Fig. 4c). In contrast, DARPP-32 mutation had no effect within the prefrontal cortex (Fig. 4c). These results show that DARPP-32 phosphorylation on Thr-34 is a critical element in the regulation of the ERK pathway in vivo in response to drugs of abuse.
Role of DARPP-32 and ERK Activation in Behavioral Sensitization. Psychostimulants have been reported to alter behavioral responses in rats weeks after a single injection (25). To explore the contribution of DARPP-32-mediated ERK activation in lasting behavioral effects of these drugs, we used an experimental protocol allowing analysis of the delayed consequences of a single acute administration of psychostimulant (25). Mice received a first injection of drug and were challenged with a test injection either 2 or 7 days later (Fig. 5 and Fig. 11, which is published as supporting information on the PNAS web site). Wild-type mice displayed increased locomotion in response to the second injection of cocaine, and this sensitization was more pronounced in the group of mice that received the challenge injection at the later time point (Figs. 5 and 11). To evaluate the role of ERK in this response, mice were treated with SL327 before the first injection of cocaine. We selected a dose of SL327 (30 mg/kg) that decreased ERK activation by >80% in the striatum but had no major effect on spontaneous locomotion (data not shown) or acute response to cocaine (Fig. 5a). The increased responsiveness to the challenge injection of cocaine (Fig. 5a) or d-amph (Fig. 11a) was dramatically reduced in SL327-pretreated mice, indicating a role for ERK in the induction of sensitization. In contrast, SL327 had no effect on the expression of sensitization, i.e., it did not decrease the enhanced locomotor response when it was administered just before the challenge injection (data not shown). Locomotor sensitization to cocaine was blocked in DARPP-32-knockout (Fig. 11 b and c) and Thr-34 → Ala mutant mice (Figs. 5b and 11d). In contrast sensitization was not altered in Thr-75 → Ala mutant mice (data not shown). These experiments indicate that the ERK pathway, as well as DARPP-32-mediated PP-1 inhibition, is critical for the establishment of long-term alterations of behavioral responses to a second exposure to cocaine.
Fig. 5.
Role of DARPP-32 and ERK activation in behavioral sensitization. (a) Locomotor activity was measured in response to a first injection (1st inj) of cocaine (20 mg/kg, i.p.) in mice pretreated with vehicle (open bars) or SL327 (30 mg/kg i.p., filled bars), 30 min before cocaine injection. Locomotor activity in response to vehicle injection did not differ between saline-pretreated (145 ± 68¼ turns per 60 min) and SL327-pretreated (119 ± 27¼ turns per 60 min) mice. The response to a test injection of cocaine (Test inj) was measured either 2 days (2d) or 7 days (7d) later. (b) The locomotor effects of the first and test injections of cocaine were examined by using the same protocol in wild-type (wt) and Thr-34 → Ala DARPP-32 (T34A) mutant mice (11 mice per group). *, P < 0.01 test vs. first injection; º, P < 0.01 SL327 vs. saline pretreatment, or mutant vs. wild type.
DARPP-32 Regulates ERK by Preventing Its Dephosphorylation and Increasing Its Phosphorylation. We next examined the mechanism by which DARPP-32 alters the ERK pathway, taking into account the fact that ERK is likely not a substrate for PP-1 (26). The observed increase in psychostimulant-induced ERK phosphorylation could be the result of either a decrease in phosphatase activity or an increase in kinase activity. Both of these possibilities were examined. ERK activation occurred in MSNs that express high levels of STEP (Fig. 1a), a protein tyrosine phosphatase that inactivates ERK by dephosphorylating the tyrosine residue of its activation loop (27, 28). Phosphatases of the STEP family lose their ability to dephosphorylate ERK when they are phosphorylated by PKA on a regulatory serine residue within their ERK-binding domain, termed the kinase interaction motif (28). Dephosphorylation of this site, a reaction catalyzed by several phosphatases, including PP-1 (29), permits STEP to bind to and dephosphorylate ERK. d-amph stimulated the phosphorylation of the 46-kDa isoform of STEP in the striatum, as shown by an increase in its apparent molecular weight (Fig. 6a). This effect was prevented in DARPP-32-knockout mice (Fig. 6a), supporting the regulation of STEP by PP-1 in vivo.
Fig. 6.
Requirement of DARPP-32 in d-amph-induced phosphorylation of STEP and MEK. (a) Regulation of STEP phosphorylation in striatum from wild-type (wt) and DARPP-32-knockout (KO) mice injected with saline or d-amph (10 mg/kg, i.p.). Phosphorylation of STEP (P-STEP) is indicated by the upward shift of the 46-kDa isoform (STEP46). STEP46 phosphorylation was expressed as the ratio P-STEP46/total STEP46. (b) Regulation of MEK phosphorylation in striatum from wt and KO mice injected with saline or d-amph (10 mg/kg, i.p.). MEK phosphorylation was expressed as percentage of controls. Data are means ± SEM (six mice per group; treated vs. control: *, P < 0.01; treated KO mice vs. wt: º, P < 0.05). (c) Schematic representation of the role of phosphatase regulation in the stimulation of ERK after activation of D1R and NMDAR by acute psychostimulant administration.
We also investigated the regulation of the signaling cascade that leads to ERK activation. ERK is phosphorylated on both threonine and tyrosine by the dual-specificity protein kinase MEK. MEK is itself activated by phosphorylation. d-amph increased the phosphorylation of MEK in the striatum of wild-type mice, but not of DARPP-32-knockout mice (Fig. 6b). These data indicate that DARPP-32 controls the activation of ERK through at least two mechanisms (Fig. 6c): (i) by preventing its dephosphorylation by STEP, and (ii) by increasing its phosphorylation by MEK.
Discussion
Our results show that drugs of abuse activate ERK in a subpopulation of D1R-containing medium-sized striatal neurons. This activation requires stimulation of both D1R and NMDAR because it is prevented by blocking either type of receptor. In contrast, in vivo activation of PKA in these neurons, as monitored by the phosphorylation of two of its substrates, GluR1 and DARPP-32, necessitates activation of D1R, but not of NMDAR. The regulation by the two types of receptors accounts for the selective phosphorylation of ERK in a subpopulation of the neurons in which DARPP-32 is phosphorylated, as a consequence of the more widespread activation of PKA. Activation of ERK in MSNs thus requires the concomitant activity of two types of receptors and behaves as a functional coincidence detector.
We have identified the molecular mechanisms of this functional interaction between the D1R- and the NMDAR-controlled signaling pathways necessary to activate ERK. DARPP-32, the PP-1 inhibitor that is highly enriched in MSNs, is necessary for this cross-talk, because the activation of ERK was blocked in DARPP-32-knockout mice. The use of a knockin point mutation demonstrated that a single residue of DARPP-32, Thr-34, was responsible for its ability to control ERK phosphorylation in response to psychostimulants, providing evidence for the role of PKA-regulated inhibition of PP-1. Inhibition of PP-1 appeared to be important at several levels for activating the ERK cascade. On the one hand, it prevented ERK dephosphorylation by STEP, by maintaining this tyrosine phosphatase in a phosphorylated, inactive, state. On the other hand, it was also critical upstream of ERK, because MEK phosphorylation in response to psychostimulants was dramatically reduced in DARPP-32-null mice. This latter effect might result from the inhibition by phospho-Thr-34-DARPP-32 of a negative regulation exerted by PP-1 at the level of MEK or upstream.
Electrical stimulation of corticostriatal neurons can activate ERK in the striatum (30), presumably through NMDAR and a Ca2+/calmodulin-dependent pathway (31, 32). Several synergistic mechanisms coupling NMDAR and the ERK pathway have been reported, including stimulation of Ras-GRF (33), a guanine-nucleotide-exchange factor that activates Ras, and inhibition of SynGAP (34, 35), a GTPase-activating protein that inactivates Ras. Our results suggest that after administration of psychostimulants, stimulation of D1R and the resulting DARPP-32-mediated inhibition of PP-1 potentiate the activation of the ERK pathway depending on NMDAR, insufficient by itself to give rise to a detectable phosphorylation of ERK in these experimental conditions. This mechanism appears selective for striatal neurons because DARPP-32 was not required for ERK activation in the prefrontal cortex. Importantly, the control of the ERK pathway by DARPP-32 in the nucleus accumbens shell was common to all drugs of abuse tested, indicating that it may play a critical role in long-term effects of these drugs.
Abundant evidence indicates that the ERK pathway is required for long-term synaptic plasticity at both transcriptional and posttranscriptional levels in many brain regions (6, 36). Regulation of transcription is also known to play a central role in the long-term effects of drugs of abuse (37). We found that activation of Elk-1, a substrate of ERK, and an essential component of the ternary complex factor involved in the regulation of many immediate early genes (38), was altered after either pharmacological blockade of the ERK pathway or mutation of DARPP-32 Thr-34. This finding supports a role of this pathway for gene regulation in vivo. Moreover, the long-lasting behavioral consequences of a single exposure to drugs, as revealed by locomotor sensitization, were dependent on both DARPP-32 Thr-34 and ERK activation, further attesting to the functional importance of this pathway. The molecular events regulated by ERK and necessary for the establishment of sensitization are not known, but they are likely to involve ERK-dependent regulation of transcription. It should be noted that ERK activation in response to cocaine injection in sensitized mice was similar to that observed in naïve animals (data not shown).
Our results have interesting implications for understanding striatal function. The flow of information through the striatum is largely based on the highly convergent glutamatergic inputs coming from the cerebral cortex. Dopamine controls corticostriatal transmission at multiple levels and modulates the plasticity of corticostriatal synapses (3). The activation of ERK provides a mechanism by which long-term transcription-dependent alterations can be selectively implemented in neurons exposed to both increased extracellular dopamine and a high degree of activity of glutamatergic terminals from the cortex or other striatal afferent pathways. Thus, the control of the ERK pathway by DARPP-32 in the striatum could allow the integration of the context-dependent activity of glutamatergic neurons and the error-in-reward-prediction signal provided by dopamine neurons to alter the plasticity of corticostriatal synapses. Previous evidence suggests that such regulatory mechanisms may be altered after chronic dopamine depletion (39). On the other hand, this mechanism is likely to be exaggerated after exposure to drugs of abuse that induce a marked and diffuse increase in extracellular dopamine. ERK activation may facilitate long-lasting alterations in a subset of striatal neurons activated by converging corticostriatal inputs triggered by environmental and internal stimuli concomitant with or preceding drug intake. We suggest that this mechanism may play a role in the development of addiction to drugs of abuse, as well as in physiological reward-controlled learning.
Supplementary Material
Acknowledgments
We thank Dilja Krueger and Hilary North for characterization of the phospho-DARPP-32 antibody; Drs. R. Luedtke and J. M. Trzaskos for their gift of D1R antibodies and SL327, respectively; and Philippe Bernard for his assistance in breeding mice. Marina Picciotto and Marc Flajolet are gratefully acknowledged for critical reading of the manuscript. This work was supported in part by grants from the Mission Interministérielle de Lutte contre la Drogue et la Toxicomanie (to J.-A.G. and J.C.), the Fondation Schlumberger pour l'Enseignement et la Recherche, and the Fondation Liliane Bettencourt (to J.-A.G.); the Action Concertée Incitative Physiologie Développement (to J.C. and J.-A.G.); the National Institutes of Health (Grants KO2 MH01527 and RO1 MH52711 to P.J.L.); The Peter Jay Sharp Foundation (to P.G.), and the National Institutes of Health (Grants MH40899 and DA10044 to P.G. and A.C.N.).
Author contributions: E.V., V.P., A.C.N., D.H., and J.-A.G. designed research; E.V., V.P., H.E., J.-C.C., A.C.N., and A.S. performed research; P.S., S.P., J.C., P.J.L., A.C.N., and P.G. contributed new reagents/analytic tools; E.V., D.H., and J.-A.G. analyzed data; and E.V., A.C.N., P.G., D.H., and J.-A.G. wrote the paper.
Abbreviations: d-amph, d-amphetamine; DARPP-32, dopamine- and cAMP-regulated phosphoprotein of Mr 32,000; D1R, dopamine D1 receptor; ERK, extracellular signal-regulated kinase; GluR1, glutamate receptor 1; MEK, mitogen-activated protein kinase/ERK kinase; MSN, medium-size spiny neuron; NMDAR, NMDA receptor; P-ERK, phospho-ERK; PKA, cAMP-dependent protein kinase; PP-1, protein phosphatase-1; STEP, striatal-enriched tyrosine phosphatase; THC, Δ9-tetrahydrocannabinol.
See Commentary on page 253.
References
- 1.Di Chiara, G. (1999) Eur. J. Pharmacol. 375, 13-30. [DOI] [PubMed] [Google Scholar]
- 2.Schultz, W. (2002) Neuron 36, 241-263. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds, J. N. J. & Wickens, J. R. (2002) Neural Netw. 15, 507-521. [DOI] [PubMed] [Google Scholar]
- 4.Hyman, S. E. & Malenka, R. C. (2001) Nat. Rev. Neurosci. 2, 695-703. [DOI] [PubMed] [Google Scholar]
- 5.Valjent, E., Corvol, J. C., Pages, C., Besson, M. J., Maldonado, R. & Caboche, J. (2000) J. Neurosci. 20, 8701-8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas, G. M. & Huganir, R. L. (2004) Nat. Rev. Neurosci. 5, 173-183. [DOI] [PubMed] [Google Scholar]
- 7.Valjent, E., Pages, C., Rogard, M., Besson, M. J., Maldonado, R. & Caboche, J. (2001) Eur. J. Neurosci. 14, 342-352. [DOI] [PubMed] [Google Scholar]
- 8.Drago, J., Gerfen, C. R., Lachowicz, J. E., Steiner, H., Hollon, T. R., Love, P. E., Ooi, G. T., Grinberg, A., Lee, E. J., Huang, S. P., et al. (1994) Proc. Natl. Acad. Sci. USA 91, 12564-12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fienberg, A. A., Hiroi, N., Mermelstein, P. G., Song, W., Snyder, G. L., Nishi, A., Cheramy, A., O'Callaghan, J. P., Miller, D. B., Cole, D. G., et al. (1998) Science 281, 838-842. [DOI] [PubMed] [Google Scholar]
- 10.Svenningsson, P., Tzavara, E. T., Carruthers, R., Rachleff, I., Wattler, S., Nehls, M., McKinzie, D. L., Fienberg, A. A., Nomikos, G. G. & Greengard, P. (2003) Science 302, 1412-1415. [DOI] [PubMed] [Google Scholar]
- 11.Valjent, E., Pages, C., Hervé, D., Girault, J. A. & Caboche, J. (2004) Eur. J. Neurosci. 19, 1826-1836. [DOI] [PubMed] [Google Scholar]
- 12.Ouimet, C. C., Miller, P. E., Hemmings, H. C., Jr., Walaas, S. I. & Greengard, P. (1984) J. Neurosci. 4, 111-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lombroso, P. J., Naegele, J. R., Sharma, E. & Lerner, M. (1993) J. Neurosci. 13, 3064-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hervé, D., Lévi-Strauss, M., Marey-Semper, I., Verney, C., Tassin, J.-P., Glowinski, J. & Girault, J. A. (1993) J. Neurosci. 13, 2237-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaguchi, Y. (1997) Neurosci. Res. 27, 1-8. [DOI] [PubMed] [Google Scholar]
- 16.Snyder, G. L., Allen, P. B., Fienberg, A. A., Valle, C. G., Huganir, R. L., Nairn, A. C. & Greengard, P. (2000) J. Neurosci. 20, 4480-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svenningsson, P., Nishi, A., Fisone, G., Girault, J. A., Nairn, A. C. & Greengard, P. (2004) Annu. Rev. Pharmacol. Toxicol. 44, 269-296. [DOI] [PubMed] [Google Scholar]
- 18.Gustafson, E. L., Girault, J. A., Hemmings, H. C., Jr., Nairn, A. C. & Greengard, P. (1991) J. Comp. Neurol. 310, 170-188. [DOI] [PubMed] [Google Scholar]
- 19.Desdouits, F., Cheetham, J. J., Huang, H. B., Kwon, Y. G., da Cruz e Silva, E. F., Denefle, P., Ehrlich, M. E., Nairn, A. C., Greengard, P. & Girault, J. A. (1995) Biochem. Biophys. Res. Commun. 206, 652-658. [DOI] [PubMed] [Google Scholar]
- 20.Hemmings, H. C., Jr., Nairn, A. C., Elliott, J. I. & Greengard, P. (1990) J. Biol. Chem. 265, 20369-20376. [PubMed] [Google Scholar]
- 21.Bibb, J. A., Snyder, G. L., Nishi, A., Yan, Z., Meijer, L., Fienberg, A. A., Tsai, L. H., Kwon, Y. T., Girault, J. A., Czernik, A. J., et al. (1999) Nature 402, 669-671. [DOI] [PubMed] [Google Scholar]
- 22.Sweatt, J. D. (2001) J. Neurochem. 76, 1-10. [DOI] [PubMed] [Google Scholar]
- 23.Konradi, C., Cole, R. L., Heckers, S. & Hyman, S. E. (1994) J. Neurosci. 14, 5623-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atkins, C. M., Selcher, J. C., Petraitis, J. J., Trzaskos, J. M. & Sweatt, J. D. (1998) Nat. Neurosci. 1, 602-609. [DOI] [PubMed] [Google Scholar]
- 25.Vanderschuren, L. J., Schmidt, E. D., De Vries, T. J., Van Moorsel, C. A., Tilders, F. J. & Schoffelmeer, A. N. (1999) J. Neurosci. 19, 9579-9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou, B., Wang, Z. X., Zhao, Y., Brautigan, D. L. & Zhang, Z. Y. (2002) J. Biol. Chem. 277, 31818-31825. [DOI] [PubMed] [Google Scholar]
- 27.Paul, S., Nairn, A. C., Wang, P. & Lombroso, P. J. (2003) Nat. Neurosci. 6, 34-42. [DOI] [PubMed] [Google Scholar]
- 28.Pulido, R., Zuniga, A. & Ullrich, A. (1998) EMBO J. 17, 7337-7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nika, K., Hyunh, H., Williams, S., Paul, S., Bottini, N., Tasken, K., Lombroso, P. J. & Mustelin, T. (2004) Biochem. J. 378, 335-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sgambato, V., Pagès, C., Rogard, M., Besson, M. J. & Caboche, J. (1998) J. Neurosci. 18, 8814-8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanhoutte, P., Barnier, J. V., Guibert, B., Pagès, C., Besson, M. J., Hipskind, R. A. & Caboche, J. (1999) Mol. Cell. Biol. 19, 136-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarzschild, M. A., Cole, R. L., Meyers, M. A. & Hyman, S. E. (1999) J. Neurochem. 72, 2248-2255. [DOI] [PubMed] [Google Scholar]
- 33.Krapivinsky, G., Krapivinsky, L., Manasian, Y., Ivanov, A., Tyzio, R., Pellegrino, C., Ben-Ari, Y., Clapham, D. E. & Medina, I. (2003) Neuron 40, 775-784. [DOI] [PubMed] [Google Scholar]
- 34.Kim, J. H., Liao, D., Lau, L. F. & Huganir, R. L. (1998) Neuron 20, 683-691. [DOI] [PubMed] [Google Scholar]
- 35.Chen, H. J., Rojas-Soto, M., Oguni, A. & Kennedy, M. B. (1998) Neuron 20, 895-904. [DOI] [PubMed] [Google Scholar]
- 36.Valjent, E., Hervé, D., Caboche, J. & Girault, J. A. (2003) Curr. Neuropharmacol. 1, 165-174. [Google Scholar]
- 37.Nestler, E. J. (2001) Nat. Rev. Neurosci. 2, 119-128. [DOI] [PubMed] [Google Scholar]
- 38.Buchwalter, G., Gross, C. & Wasylyk, B. (2004) Gene 324, 1-14. [DOI] [PubMed] [Google Scholar]
- 39.Gerfen, C. R., Miyachi, S., Paletzki, R. & Brown, P. (2002) J. Neurosci. 22, 5042-5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.