Substance-related disorders represent a major burden to afflicted individuals, their families, and society. Repeated drug use can result in addiction, manifested as an intense desire for the drug, along with an impaired ability to temper its use, even in the face of serious consequences. Many tens of millions of people are addicted to drugs of abuse, and with costs to society in the many hundreds of billions of dollars per annum, there is little doubt that understanding the consummate processes (followed by advances in treatments) represents a major goal for medicine (1, 2).
Although many transmitters have been implicated in the effects of the various types of drugs of abuse, dopamine and, more recently, glutamate have been most consistently associated with the reinforcing effects. Indeed, there is considerable evidence that the mesolimbic dopaminergic pathway plays a crucial role in the selection and orchestration of goal-directed behaviors, particularly those elicited by incentive stimuli. Drugs of abuse increase extracellular dopamine concentrations in limbic regions, including the nucleus accumbens (1, 2). However, a growing body of data suggests that increases in dopamine are not directly related to reward per se, but, rather, to the learned anticipation of reward and incentive salience (3). Notably, salience affects the motivation to seek the anticipated reward and facilitates conditioned learning (3).
The key role of conditioned learning has focused attention on neural systems that attribute incentive salience, most notably a circuit that includes glutamate projections from the prefrontal cortex to the nucleus accumbens and dorsal striatum. This finding has led to the hypothesis that key biochemical events underlying drug-induced changes in neural plasticity should occur specifically in neurons inervated by both dopamine and glutamate inputs.
In this issue of PNAS, Valjent et al. (4) provide an important link, implicating dopamine, cAMP-regulated phosphoprotein of 32,000 kDa (DARPP-32) glutamate as mediators of drug-related changes in the activity of the extracellular signal-regulated kinase (ERK) mitogen-activated protein (MAP) kinase pathway. Activation of the ERK cascade represents an exciting nexus for drug-induced changes in neural plasticity—it is known to be important for long-term synaptic plasticity, and its activation has been shown to depend on both dopamine and glutamate receptors.
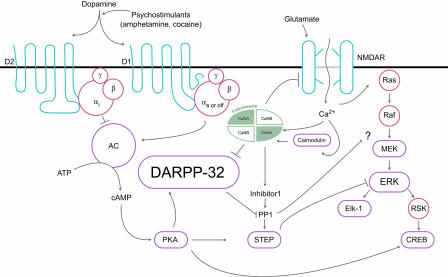
DARPP-32 is localized to neurons containing dopamine receptors and is a potent inhibitor of protein phosphatase 1, which plays a central role in dopaminergic and glutamatergic signaling, and in integrating the activity of these two pathways. Dopamine, acting through the D1 receptor and the adenylate cyclase-stimulatory G protein Gs/Golf, activates adenylate cyclase, thereby bringing about protein kinase A (PKA)-mediated phosphorylation of DARPP-32. By contrast, acting through the D2 receptor and the adenylate cyclase-inhibitory Gi proteins, dopamine reduces DARPP-32 phosphorylation (Fig. 1). Calcineurin acts as a principle mediator of dephosphorylation-dependent inactivation of DARPP-32 (see ref. 5 for a review). Consistent with these observations, knockout mice with disruption of the DARPP-32 gene have been previously shown to have alterations in their molecular, electrophysiological, and behavioral responses to dopamine, drugs of abuse, and antipsychotic medications (5–8). Furthermore, the calcineurin inhibitor FK506 modulates methamphetamine-induced behavioral changes in rats (9). Glutamate also promotes dephosphorylation of DARPP-32, presumably through calcium-dependent activation of calcineurin (5, 10). DARPP-32, as reported previously by Greengard et al. (5), occupies a unique position whereby it modulates, bidirectionally, both dopaminergic and glutamatergic signaling depending on which threonine (Thr) residue within the protein is phosphorylated. Phospho-Thr-34-DARPP-32 amplifies the D1/PKA pathway, whereas phosphor-Thr-75 DARPP-32 dampens it, thereby shifting the balance toward dephosphorylation of target substrates via PP-1 (5, 11). Thus, DARPP-32 has been postulated to represent a “molecular switch” that regulates and fine-tunes the phosphorylation state of PP-1 target proteins (Fig. 1).
Fig. 1.
Diagram of DARPP-32. STEP, striatal enriched phosphatase; NMDAR, NMDA glutamate receptors. Adapted with permission from ref. 11.
The new findings reported by Valjent et al. (4) strongly implicate the ERK signaling cascade as a putative downstream effector of DARPP-32-mediated changes in neural plasticity induced by drugs of abuse (4). In a broad sense, the ERK signaling cascade is a general mediator/marker of various forms of neural plasticity. The functions of ERK and related kinases in learning and memory are well documented, where both long-term potentiation and long-term depression rely on this pathway and both short-term and long-term effects are relevant (12). Furthermore, many of the cellular actions of neurotrophic factors are mediated through ERK activation after binding to a receptor tyrosine kinase (Trk). After Trk autophosphorylation and activation, the small G protein Ras activates the serine-threonine kinase Raf, which results in the subsequent phosphorylation of kinases MAP kinase/ERK kinase (MEK), ERK, and ribosomal S6 kinase (RSK). RSK, directly and indirectly, modulates a number proteins involved in neurotrophic and neuroplastic functions, including the transcription factor cAMP-response element-binding protein (CREB) (Fig. 1).
Valjent et al. (4) have provided further evidence for a nexus between dopaminergic and glutamatergic pathways, DARPP-32, and ERK, implying modalities of discourse among these pathways and proteins. They report that administration of d-amphetamine to mice increases phosphorylated (activated) ERK, specifically in medium spiny neurons of the dorsal striatum and nucleus accumbens. Furthermore, in the dorsal striatum, P-ERK was localized almost exclusively to a subset of neurons that express both the dopamine D1 receptor and phosphorylated DARPP-32. This effect was prevented when the drug was administered to DARPP-32 knockout mice. A similar effect of cocaine, nicotine, morphine, and Δ9-tetrahydrocannabinol on ERK phosphorylation in the nucleus accumbens was reduced in knockout mice.
Both dopamine D1R and NMDA receptor antagonists blocked the effects of d-amphetamine on ERK phosphorylation. Importantly, whereas the NMDA receptor antagonist blocked ERK activation, it had no effect on the phosphorylation of DARPP-32. The authors used DARRP-32 knock-in mice, where the PKA Thr-34 site is replaced by alanine to determine that both striatal and nucleus accumbens (but not prefrontal cortex) phosphorylation of ERK was prevented after administration of either cocaine or d-amphetamine, and they provided evidence for specificity at the PKA phosphorylation site. Furthermore, phosphorylation of the ERK substrate Elk-1 was increased by d-amphetamine and cocaine. Using a blood–brain barrier penetrant inhibitor of MEK (ERK kinase) or mice with the Thr-34 mutation prevented the phosphorylation of Elk-1. ERK activation and Elk-1 are responsible for many downstream transcriptional effects not yet fully studied vis-à-vis DARPP-32.
Finally, administration of the MEK inhibitor, similar to mutated DARPP-32 mice, prevented the behavioral sensitization effects of cocaine. However, the acute affects were not attenuated, and it is yet to be determined whether the altered behavioral response to secondary d-amphetamine exposure are due to blockage of development of behavioral sensitization to psychostimulants specifically or secondary to effects more generally attributable to learned behavior. It should be noted that ERK1 knockout mice are generally hyperactive and are hypersensitive to the rewarding properties of morphine (13). Administration of the MEK inhibitor to rats at higher doses increases activity both in an open-field test and in the forced-swim test model of antidepressant efficacy, suggesting that the ERK signaling cascade plays an important role in modulating a variety of complex behaviors (14).
Their findings indicate that two complementary mechanisms may be involved. First, striatal enriched phosphatase, a protein involved in dephosphorylation of ERK, appeared to be phosphorylated after d-amphetamine administration, as evidenced by a decrease in gel migration. Although not confirmed with phosphorylation site-specific antibodies, a likely explanation is an increase in phosphorylation of an inhibitory serine site. Consistent with the aforementioned effects of the MEK inhibitor, phosphorylation of MEK was also increased in the striatum of mice after administration of amphetamine. Both of these effects were prevented in DARPP-32 knockout mice.
In light of such convergence, we are reminded that signaling networks are finely tuned interconnected systems, with intricate positive and negative feedback loops.
An unresolved concern is whether a ubiquitous signaling pathway like the ERK cascade can play only a specific role, such as mediating the effects of drugs of abuse. Valjent et al. (4) correctly point out that there are different classifications of ERK-positive cells in the striatum that have different inputs, outputs, and functions. Furthermore, it is now clear that signaling systems are anchored and clustered, and, thus, subcellular localization of changes may also be critical in providing specificity. The ERK signaling network, nevertheless, is characterized by modules with well defined input and output, as well as by feedback loops and multistep regulatory controls (15). These features suggest that “learned behavior” of biological systems may well be stored within intracellular biochemical reactions that comprise signaling pathways like the ERK cascade (15). Future studies should address whether psychostimulants, antidepressants, and neuroleptics, which have been associated with ERK activation in the striatum, induce this effect in unique populations of neurons and, especially, those containing DARRP-32-positive cells (16, 17). Although few studies have directly contrasted the behavioral significance of ERK activation induced by these agents, it is reasonable that, in different neuronal circuits, ERK activation and duration alter subsets of behavior and that the roles of ERK cannot be simplified or generalized. In conclusion, Valjent et al. (4) have provided a clearer understanding of drug-induced alterations in a major regulatory hub of signaling networks; these findings have the potential to facilitate the development of novel therapeutics, agents that can modulate neural plasticity within the brain reward circuitry and break the vicious cycle of addiction.
Acknowledgments
We thank Irving I. Gottesman for valuable comments. This work was supported by the Intramural Research Program of the National Institute of Mental Heath, Foundation for the National Institutes of Health Neuroscience Research Fellowship (to T.D.G.), a National Association for Research on Schizophrenia and Depression Young Investigator Award (to T.D.G.), and the Stanley Research Foundation (to H.K.M.).
See companion article on page 491.
References
- 1.Hyman, S. E. & Malenka, R. C. (2001) Nat. Rev. Neurosci. 2, 695-703. [DOI] [PubMed] [Google Scholar]
- 2.Chao, J. & Nestler, E. J. (2004) Annu. Rev. Med. 55, 113-132. [DOI] [PubMed] [Google Scholar]
- 3.Volkow, N. D., Fowler, J. S., Wang, G. J. & Swanson, J. M. (2004) Mol. Psychiatry 9, 557-569. [DOI] [PubMed] [Google Scholar]
- 4.Valjent, E., Pascoli, V., Svenningsson, P., Paul, S., Enslen, H., Corvol, J.-C., Stipanovich, A., Caboche, J., Lombroso, P. J., Nairn, A. C., et al. (2005) Proc. Natl. Acad. Sci. USA 102, 491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greengard, P., Allen, P. B. & Nairn, A. C. (1999) Neuron 23, 435-447. [DOI] [PubMed] [Google Scholar]
- 6.Fienberg, A. A., Hiroi, N., Mermelstein, P. G., Song, W., Snyder, G. L., Nishi, A., Cheramy, A., O'Callaghan, J. P., Miller, D. B., Cole, D. G., et al. (1998) Science 281, 838-842. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi, P., Gubellini, P., Centonze, D., Picconi, B., Bernardi, G., Chergui, K., Svenningsson, P., Fienberg, A. A. & Greengard, P. (2000) J. Neurosci. 20, 8443-8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder, G. L., Allen, P. B., Fienberg, A. A., Valle, C. G., Huganir, R. L., Nairn, A. C. & Greengard, P. (2000) J. Neurosci. 20, 4480-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukamoto, T., Iyo, M., Tani, K., Sekine, Y., Hashimoto, K., Ohashi, Y., Suzuki, K., Iwata, Y. & Mori, N. (2001) Psychopharmacology 158, 107-113. [DOI] [PubMed] [Google Scholar]
- 10.Halpain, S., Girault, J. A. & Greengard, P. (1990) Nature 343, 369-372. [DOI] [PubMed] [Google Scholar]
- 11.Manji, H. K., Gottesman, I. I. & Gould, T. D. (2003) Sci. STKE 207, pe49. [DOI] [PubMed] [Google Scholar]
- 12.Kandel, E. R. (2001) Science 294, 1030-1038. [DOI] [PubMed] [Google Scholar]
- 13.Mazzucchelli, C., Vantaggiato, C., Ciamei, A., Fasano, S., Pakhotin, P., Krezel, W., Welzl, H., Wolfer, D. P., Pages, G., Valverde, O., et al. (2002) Neuron 34, 807-820. [DOI] [PubMed] [Google Scholar]
- 14.Einat, H., Yuan, P., Gould, T. D., Li, J., Du, J., Zhang, L., Manji, H. K. & Chen, G. (2003) J. Neurosci. 23, 7311-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhalla, U. S., Ram, P. T. & Iyengar, R. (2002) Science 297, 1018-1023. [DOI] [PubMed] [Google Scholar]
- 16.Pozzi, L., Hakansson, K., Usiello, A., Borgkvist, A., Lindskog, M., Greengard, P. & Fisone, G. (2003) J. Neurochem. 86, 451-459. [DOI] [PubMed] [Google Scholar]
- 17.Valjent, E., Pages, C., Herve, D., Girault, J. A. & Caboche, J. (2004) Eur. J. Neurosci. 19, 1826-1836. [DOI] [PubMed] [Google Scholar]