ABSTRACT
A reliable method of cell tracing is essential in evaluating potential therapeutic procedures based on stem cell transplantation. Here we present data collected using neural stem cells isolated from a transgenic mouse line Thy1-YFP. When transplanted into a stroke affected brain these cells give rise to neurons that express a fluorescent signal which can be used for their detection and tracing. Observed processes were compared with those taking place during normal embryonic neurogenesis as well as during in vitro differentiation. Since the same neurogenic patterns were observed, we confirm that neural stem cell transplantation fits well into the paradigm of neuronal birth and differentiation.
Keywords: cell tracing, neural stem cells, stroke, Th1 YFP-16
The lack of effective therapeutic approaches in treatment of brain diseases represents an enormous burden on society and has invoked an increased interest in translational research. Since stem cells provide a viable option for the repair of damaged brain tissue, there has been a rapid development of procedures based on stem cell transplantation.1,2 In the process of standardization of these procedures the ongoing challenge is the lack of precise control of stem cell behavior after transplantation.3 Improved knowledge on post-transplantation events is thus recognized as a major prerequisite necessary to develop a successful and functional stem cell therapy for patients.4 Upon contact with a host tissue, transplanted immature progenitor cells undergo morphological and functional maturation. Although traditionally the visual tracing of cells has been accomplished using exogenous dyes, it has been shown that the majority of commercially available exogenous dyes were unsuitable for tracing stem cell migration, survival and differentiation due to rapid signal deterioration.5,6 Methods based on transgenic constructs which allow for much longer signal presence were thus found to be a good alternative since they also provided valuable insight into expression of specific stem cell markers.7 The first successful tracing of a whole neuron, including its cell body, axon, dendrites and their synapses using a florescent construct was achieved by Feng et al. by designing a mouse strain that expressed a fluorescent reporter gene controlled by the Thy1 promoter.8 Thy1 (THYmocyte differentiation antigen 1) is a member of the immunoglobulin family normally expressed on the surface of neurons as well as several non-neuronal cell types including thymocytes, fibroblasts, endothelial cells and smooth muscle cells.9,10 We as well as others have observed that during the period of 7 days, 21.88 ± 0.53% of progenitor cells originating from the B6.Cg-Tg(Thy1-YFP)16Jrs/J (Thy1 YFP-16) neural stem cells, express yellow fluorescent protein.8,11-13
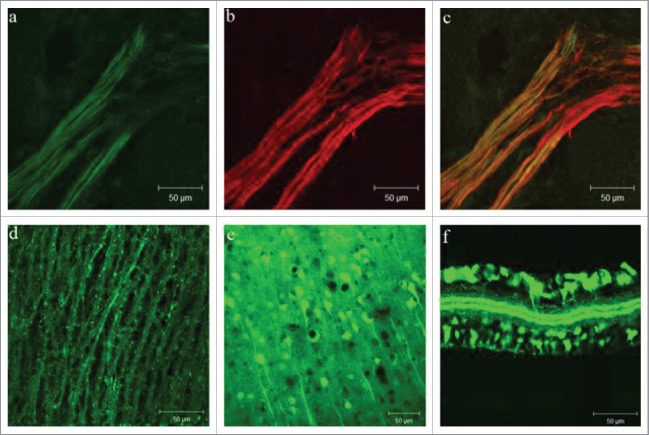
Here we validate the application of Thy1 YFP-16 transgenic mouse strain in stem cell tracing. We analyzed the Thy1 YFP expression on 3 different levels: during embryonic development, differentiation of neurons in vitro and after transplantation to brain tissue affected by stroke. Based on a detailed characterization of cells originating from the Thy1 mouse strain, we were able to perform precise tracking of cell fates after transplantation for a prolonged period of time. We confirmed that the expression of fluorescent signal in Thy1 neurons mirrors that of the normal embryonic development with the first expression observed at E12.5 in the central and peripheral nervous system. During early embryonic development (E12.5) and during cell differentiation in vivo, Thy1-YFP positive cells expressed YFP in all parts of the cell including the nucleus, perikaryon and processes and co-localized with specific neuron markers such as β3-tubulin (see Fig. 1A-C for neuronal processes). Moreover, we observed strong expression of YFP in the spines of early neurons at E15.5 (see spines in Fig. 1D). This signal was present until birth as well as in adult mice. The most important finding was that the first sign of expression was visible in the neural progenitors. During embryonic development expression was increased in both CNS (spinal cord, prosencephalon and rhombencephalon) (see expression in brain cortex in Fig. 1E) and in PNS (cranial and spinal nerves). Strong expression was detectable in facial, maxillary and mandibular nerves and consequently in the roots of developing teeth. A very strong expression in CNS was observed in the spinal cord where neurons were completely positive in all parts of the cell including the spines. Moreover, expression in PNS was detectable simultaneously in both brachial and lumbosacral plexus, while the expression in retina was detectable at E17.5 with a steady increase in postnatal days. In adult mice ganglion cells of retina were observed to be Thy1-YFP positive (see expression in retina in Fig. 1F). Based on the data collected during embryonic development,13 we analyzed the expression patterns observed during in vitro differentiation of Thy1-YFP cells. Expression of the Thy1-YFP positive cells was monitored during 14 d of differentiation. As hypothesized, it indeed followed the pattern observed during embryonic development. Expression of Thy1-YFP was observed in progenitor as well as differentiated neurons and was specific for the neuronal cell lineage. Interestingly, during cell differentiation the number of Thy1-YFP positive cells was constant and was around 22%, on both immunofluorescence and RT-PCR levels. All Thy1-YFP positive cells co-localized with neuronal markers (MAP2, ß3-tubulin, NeuN and Doublecortin) but they never co-localized with GFAP. When compared with the primary culture of neurons, we observed the same percentage of Thy1-YFP positive cells. Primary neuronal cultures were isolated at E17.5 and included more glial progenitors in their original population when compared with the neural stem cells isolated at the earlier time points (until E14.5). Therefore, it follows that the number of astrocytes in primary neural cultures was increased. At the same time, all neurons in the primary culture, albeit mature, expressed Doublecortin which co-localized with Thy1-YFP, MAP2, ß3-tubulin and NeuN. Despite the fact that NeuN is a typical marker of neuronal nuclei, under our conditions NeuN indicated granular positivity in neuronal processes, too. To analyze the events following transplantation of neural stem cells in the brain tissue affected by stroke, cells were labeled with PKH26 dye and transplanted near the hippocampus of the wild type mouse brain affected by stroke and in its sham operated control.13 Brains were isolated at 2, 4, 8, 14 weeks, and 6 months after transplantation. At earlier time points (2 and 4 weeks) cells were PKH26 positive and they migrated toward the injury. Majority of the cells differentiated at the site of injection, marked by high expression of Gap43 and Casp314 and they were Thy1-YFP positive. Eight and 14 weeks after transplantation PKH26 dye lost its efficiency, similar as shown by Li et al.,5 but Thy1-YFP positive cells were completely recognizable: they differentiated and incorporated into the host hippocampus. Neurons expressed YFP in all their parts, including the spines and were comparable to those differentiated in the cell culture or during embryonic development. Six months after transplantation cells almost completely filled in the defect present in the stroke affected tissue. Although some cells died, approximately 70% of cells survived for analyzed period of 6 months. Processes of Thy1-YFP positive cells were pronounced and thick, they penetrated the scar and established connections between healthy and stroke affected tissue (Fig 2C, D). Finally, we have been able to confirm that transplanted Thy1-YFP stem cells follow the same pattern observed during embryo and in vitro development. Cells which differentiated into neurons revealed co-localization with Map2 (Fig 3).
Figure 1.
Thy1-YFP expression during embryonic development and in the postnatal life: (A-C) Spinal nerve in E12.5, Thy1-YFP (green) and ß3-tubulin (red); (D) lateral section of the spinal cord in E15.5; (E) brain cortex and (F) retina in adult mice.
Figure 2.
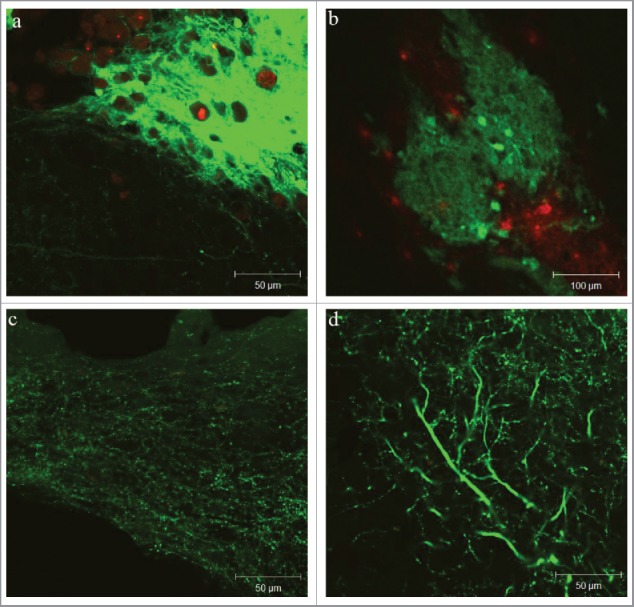
Thy1-YFP expression after transplantation into the mouse brain: spindle shaped graft in stroke affected brain (A – higher and B – lower magnification); scar and brain tissue permeated with Thy1-YFP processes (C and D).
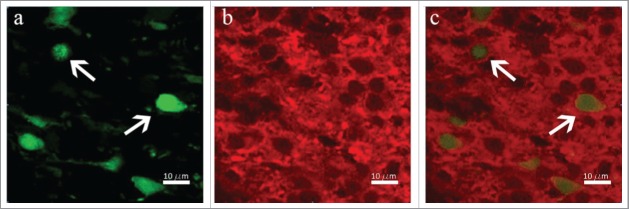
Figure 3.
Transplanted Thy1 cells follow the same pattern like during embryonic or in vitro differentiation – co-localization of Thy1 and Map2 is visible. Green – transplanted Thy1-YFP (A and C), Red – Map2 cells in the host nervous tissue (B and C).
Combination of in vitro and in vivo experiments using theThy1 YFP-16 strain yielded several important results. In vitro results suggested that neural stem cells follow differentiation pathway identical to that observed during embryonic development. This was shown in our previous work,13 and confirmed in this study suggesting that Thy1 YFP cells could potentially be useful in future preclinical and clinical studies based on the stem cell transplantation. Moreover, we clearly showed that nervous tissue originating either from various sources15 or isolated in various stages exhibited different features. The primary neuronal culture when compared with the neural stem cells differentiation, exhibited a higher number of astrocytes. The reason for this is most likely a higher number of glial progenitors in the later stages. Since they require a longer time for differentiation this can result in the increased number of astrocytes. This has been recently reported as physiologically important since astroglia can potentially be converted toward the neuronal lineage upon injury of the nervous system.16 Here we can confirm that all neurons in primary cultures expressed Doublecortin which co-localized with Thy1-YFP, MAP2, ß3-tubulin and NeuN. A high potential to obtain a uniform cell culture population is an important prerequisite for any future studies based on in vitro produced cell populations. It was therefore important to analyze the behavior of in vitro generated cells after transplantation to the brain tissue injured by stroke. It was very interesting to observe how the niche formed by an ischemic event attracted neural stem cells and how the axons growing out of the transplanted cells penetrated the tissue affected by ischemia. This suggested that in vitro cells could be used in future brain tissue repair studies. In addition, our observation on the high potential of the transgenic strain Thy1 YFP-16 has been recognized by other groups. Some of the additional applications include visualization of neuromuscular junctions,17 experimental tumors, inflammation and wound healing.18 Taken altogether, our results do confirm a high potential of Thy1-YFP strain for cell tracing upon transplantation. Moreover, we propose usage of Thy1-YFP mouse as a robust model for estimation of brain regeneration, following various therapeutic approaches.
Materials and methods
Animals and stem cells
Mice strains that were used in this research include B6.Cg-Tg(Thy1-YFP)16Jrs/J(Thy1 YFP-16) and C57Bl/6NCrl (wild type control) (The JacksonLaboratory, Bar Harbor, ME, USA). The animals were bred and kept at the animal facility at the Croatian Institute for Brain Research. All experiments on animals received approval of the Internal Review Board of the Ethical Committee of the School of Medicine University of Zagreb (04–77/2010–238), Faculty of Veterinary Medicine (251/61–01/139–13–4) and they were performed in accordance with the EU Directive 2010/63/EU on the protection of animals used for scientific purposes.
Neural stem cells were isolated from telencephalic wall of E14.5 while the cells for primary neuronal culture were isolated from telencephalic wall of E17.5 Thy1 YFP-16 fetuses. Neural stem cells were differentiated 7 d while the primary neuronal cultures were differentiated 14 d. A whole procedure of isolation and manipulation of neural stem cells and neurons has been described previously.13,19
Mouse stroke model by middle cerebral artery occlusion (MCAO) and cell transplantation
Ischemic brain injury (stroke) was induced by transient left middle cerebral artery occlusion (MCAO) in wild type, age 3 months and weight 25–30 g as described previously.14,20 Intraluminal filament (Doccol Company) was inserted through common carotid artery (CCA) into internal carotid artery (ICA) to the origin of middle cerebral artery (MCA) and left for 90 minutes followed by removal of the filament and reperfusion. An i.p. injection of analgetic buprenorphine (0.03 mg/kg) was administered.
Cells for intracerebral transplantation were labeled with PKH26 fluorescent dye (PKH26GL-1KT, Sigma) following manufacturer's instructions. For stereotactic transplantation we used 2 groups of mice: healthy and stroke-affected. Since we used 10 mice for each of 5 time points, in total we used 50 mice. Injections were performed using KOPF stereotactic apparatus (900LS) and Hamilton syringe needle 1μL at stereotactic coordinates near hippocampus: AP −1.3, ML+2.0 and DV −1.5.
Tissue isolation and immunofluorescence
Mice were anesthetized using Avertin (0.5 g/kg) and then perfused transcardially with PBS and subsequently 4% paraformaldehyde in PBS (pH 7.4). Brains were isolated and further fixed by immersion in the same fixative at 4°C overnight. For embryo isolation pregnant Thy1 YFP-16 females were used, killed at different stages of gestation (from 9th to 17th day). Embryos were isolated by cutting uterine wall and foetal membranes, fixed in 4% PFA (pH 7.4). Tissue was washed in PBS and transferred to 10% sucrose followed by 30% sucrose in PBS at 4°C. Followed cryopreservation 20 μm thick sections were cut using cryostat and mounted on Superfrost Plus (Menzel Glaser) coated slides.
Cells and tissues were immunolabelled using specific primary antibodies and secondary antibodies. The method has been described previously.13 Primary and secondary antibodies as well as assays used for RT-PCR are listed in Tables 1, 2 and 3 (Supplemented). Fluorescent analysis was made with confocal microscope Zeiss LSM510 Meta and Olympus Provis AX70 microscope.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Funding
This manuscript has been supported by projects of the Croatian National Foundation (IP-2016–06–9451) and YoungBrain (EU-ESF 3.2.01–0180) awarded to Dinko Mitrečić, and FP7 Glowbrain project REGPOT-2012-CT2012–316120 awarded to Srećko Gajović.
References
- [1].Mitrecic D. Current advances in intravascular administration of stem cells for neurological diseases: a new dose of rejuvenation injected. Rejuvenation Res 2011; 14(5):553-5; PMID:21951133; http://dx.doi.org/ 10.1089/rej.2011.1209 [DOI] [PubMed] [Google Scholar]
- [2].Lunn JS, Sakowski SA, Hur J, Feldman EL. Stem cell technology for neurodegenerative diseases. Ann Neurol 2011; 70(3):353-61; http://dx.doi.org/ 10.1002/ana.22487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mitrecic D, Nicaise C, Gajovic S, Pochet R. Distribution, differentiation, and survival of intravenously administered neural stem cells in a rat model of amyotrophic lateral sclerosis. Cell Transplant 2010; 19(5):537-48; PMID:20350352; http://dx.doi.org/ 10.3727/096368910X498269 [DOI] [PubMed] [Google Scholar]
- [4].Barker RA, Parmar M, Kirkeby A, Bjorklund A, Thompson L, Brundin P. Are stem cell-based therapies for parkinson's disease ready for the clinic in 2016? J Parkinsons Dis 2016; 6(1):57-63; PMID:27003785; http://dx.doi.org/ 10.3233/JPD-160798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li P, Zhang R, Sun H, Chen L, Liu F, Yao C, Du M, Jiang X. PKH26 can transfer to host cells in vitro and vivo. Stem Cells Dev 2013; 22(2):340-4; PMID:22913652; http://dx.doi.org/ 10.1089/scd.2012.0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Silva AK, Wilhelm C, Kolosnjaj-Tabi J, Luciani N, Gazeau F. Cellular transfer of magnetic nanoparticles via cell microvesicles: impact on cell tracking by magnetic resonance imaging. Pharm Res 2012; 29(5):1392-403; PMID:22271049; http://dx.doi.org/ 10.1007/s11095-012-0680-1 [DOI] [PubMed] [Google Scholar]
- [7].Kosi N, Alic I, Kolacevic M, Vrsaljko N, Jovanov Milosevic N, Sobol M, Philimonenko A, Hozak P, Gajovic S, Pochet R, et al.. Nop2 is expressed during proliferation of neural stem cells and in adult mouse and human brain. Brain Res 2015; 1597:65-76; PMID:25481415; http://dx.doi.org/ 10.1016/j.brainres.2014.11.040 [DOI] [PubMed] [Google Scholar]
- [8].Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 2000; 28(1):41-51; PMID:11086982; http://dx.doi.org/ 10.1016/S0896-6273(00)00084-2 [DOI] [PubMed] [Google Scholar]
- [9].Morris R. Thy-1 in developing nervous tissue. Dev Neurosci 1985; 7(3):133-60; PMID:2866949; http://dx.doi.org/ 10.1159/000112283 [DOI] [PubMed] [Google Scholar]
- [10].Gordon JW, Chesa PG, Nishimura H, Rettig WJ, Maccari JE, Endo T, Seravalli E, Seki T, Silver J. Regulation of Thy-1 gene expression in transgenic mice. Cell 1987; 50(3):445-52; PMID:2886226; http://dx.doi.org/ 10.1016/0092-8674(87)90498-3 [DOI] [PubMed] [Google Scholar]
- [11].Keller-Peck CR, Walsh MK, Gan WB, Feng G, Sanes JR, Lichtman JW. Asynchronous synapse elimination in neonatal motor units: studies using GFP transgenic mice. Neuron 2001; 31(3):381-94; PMID:11516396; http://dx.doi.org/ 10.1016/S0896-6273(01)00383-X [DOI] [PubMed] [Google Scholar]
- [12].Bannerman PG, Hahn A, Ramirez S, Morley M, Bonnemann C, Yu S, Zhang GX, Rostami A, Pleasure D. Motor neuron pathology in experimental autoimmune encephalomyelitis: studies in THY1-YFP transgenic mice. Brain 2005; 128(Pt 8):1877-86; PMID:15901645; http://dx.doi.org/ 10.1093/brain/awh550 [DOI] [PubMed] [Google Scholar]
- [13].Alic I, Kosi N, Kapuralin K, Gorup D, Gajovic S, Pochet R, Mitrecic D. Neural stem cells from mouse strain Thy1 YFP-16 are a valuable tool to monitor and evaluate neuronal differentiation and morphology. Neurosci Lett 2016; 634:32-41. [DOI] [PubMed] [Google Scholar]
- [14].Gorup D, Bohacek I, Milicevic T, Pochet R, Mitrecic D, Kriz J, Gajovic S. Increased expression and colocalization of GAP43 and CASP3 after brain ischemic lesion in mouse. Neurosci Lett 2015; 597:176-82; PMID:25929184; http://dx.doi.org/ 10.1016/j.neulet.2015.04.042 [DOI] [PubMed] [Google Scholar]
- [15].Mitrečić D, Kostović-Knežević L, Gajović S. Morphological features of tail bud development in truncate mouse mutants. Cells Tiss Org 2004; 178(1):23-32; PMID:15550757; http://dx.doi.org/ 10.1159/000081090 [DOI] [PubMed] [Google Scholar]
- [16].Noristani HN, Sabourin JC, Boukhaddaoui H, Chan-Seng E, Gerber YN, Perrin FE. Spinal cord injury induces astroglial conversion towards neuronal lineage. Mol Neurodegener 2016; 11(1):68; PMID:15550757; http://dx.doi.org/ 10.1186/s13024-016-0133-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Blizzard CA, Lee KM, Dickson TC. Inducing chronic excitotoxicity in the mouse spinal cord to investigate lower motor neuron degeneration. Front Neurosci 2016; 10:76; PMID:26973454; http://dx.doi.org/ 10.3389/fnins.2016.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Josvay K, Winter Z, Katona RL, Pecze L, Marton A, Buhala A, Szakonyi G, Olah Z, Vizler C. Besides neuro-imaging, the Thy1-YFP mouse could serve for visualizing experimental tumours, inflammation and wound-healing. Sci Rep 2014; 4:6776; PMID:25345415; http://dx.doi.org/ 10.1038/srep06776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kapuralin K, Curlin M, Mitrecic D, Kosi N, Schwarzer C, Glavan G, Gajovic S. STAM2, a member of the endosome-associated complex ESCRT-0 is highly expressed in neurons. Mol Cell Neurosci 2015; 67:104-15; PMID:26101075; http://dx.doi.org/ 10.1016/j.mcn.2015.06.009 [DOI] [PubMed] [Google Scholar]
- [20].Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 1996; 27(9):1616-22; PMID:8784138; http://dx.doi.org/ 10.1161/01.STR.27.9.1616 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.