Abstract
Metabolic acidosis has been proved to be a risk factor for the progression of chronic kidney disease, but its relation to acute kidney injury (AKI) has not been investigated. In general, a diagnosis of metabolic acidosis is based on arterial blood gas (ABG) analysis, but the diagnostic role of carbon dioxide combining power (CO2CP) in the venous blood may also be valuable to non-respiratory patients. This retrospective study included all adult non-respiratory patients admitted consecutively to our hospital between October 01, 2014 and September 30, 2015. A total of 71,089 non-respiratory patients were included, and only 4,873 patients were evaluated by ABG analysis at admission. In patients with ABG, acidosis, metabolic acidosis, decreased HCO3− and hypocapnia at admission was associated with the development of AKI, while acidosis and hypocapnia were independent predictors of hospital mortality. Among non-respiratory patients, decreased CO2CP at admission was an independent risk factor for AKI and hospital mortality. ROC curves indicated that CO2CP was a reasonable biomarker to exclude metabolic acidosis, dual and triple acid-base disturbances. The effect sizes of decreased CO2CP on AKI and hospital mortality varied according to age and different underlying diseases. Metabolic acidosis is an independent risk factor for the development of AKI and hospital mortality. In non-respiratory patient, decreased CO2CP is also an independent contributor to AKI and mortality and can be used as an indicator of metabolic acidosis.
Keywords: acid-base, metabolic acidosis, carbon dioxide combining power, acute kidney injury, mortality
Introduction
Acidosis is the most common acid-base disturbance, with metabolic acidosis potentially indicating a more severe course and worse outcome. The actual incidence and prevalence of metabolic acidosis have not been established in critically ill and general patients. Often this disorder is a marker for underlying pathology, and the most commonly encountered causes of metabolic acidosis are renal insufficiency, sepsis, and diabetic ketoacidosis. Although recent studies showed that metabolic acidosis is associated with high mortality and increased the length of stay in the hospital and ICU (1,2), it remains uncertain whether or not there is a true cause relationship between metabolic acidosis and adverse clinical outcomes.
The kidney is a principally responsible organ for retention and excretion of electrolytes and maintaining acid-base homeostasis in healthy individuals (3). Both acute kidney injury (AKI) and chronic kidney disease (CKD) can cause metabolic acidosis. But on the other hand, accumulating evidence identifies metabolic acidosis not only as a consequence of but as a contributor to, the progression of kidney dysfunction in patients with CKD (4). The mechanisms may be that metabolic acidosis can reduce renal blood flow in healthy human volunteers (5) and increase inflammatory mediator release (6). A recent experimental study proved that metabolic acidosis exacerbates ischemia/reperfusion-induced AKI (7). However, Limited data exist about the harmful effect of metabolic acidosis on the development of AKI.
In general, a diagnosis of metabolic acidosis is based on arterial blood gas (ABG) analysis after an arterial puncture which may lead to local hematoma and other complications. Carbon dioxide combining power (CO2CP) in the venous blood which is a measure of the alkali reserve, can help in the diagnosis of the metabolic types of acidosis and alkalosis. Even though CO2CP does not give any idea of the ratio between carbonic acid and bicarbonate, it enables timely diagnosis and proper corrective therapy in metabolic acidosis if respiratory types of acid-base disturbances do not exist.
In this study, we hypothesized that metabolic acidosis might be associated with the development of AKI and hospital mortality, and decreased CO2CP can be used to an indicator of metabolic acidosis in non-respiratory patients. The study was aimed at identifying the variables in acid-base status at admission which were associated with the occurrence of renal dysfunction and hospital mortality. The secondary aims were to determine the relationship between CO2CP and other variables in the electrolyte and acid-base status and to screen high-risk patients of AKI and hospital mortality when decreased CO2CP occurs.
Materials and methods
Study population and data collection
This retrospective study included all adult patients admitted consecutively to Zhongshan Hospital, Fudan University in Shanghai, China, between October 01, 2014 and September 30, 2015. This study was approved by the institutional review board of the ethics committee, Zhongshan Hospital, Fudan University, Shanghai China. The requirement for informed consent was waived for this observational survey. The patient records and information were anonymized and de-identified before analysis.
Exclusion criteria were the following: Age of fewer than 18 years, serum creatinine (SCr) at admission >115 µmol/l, CO2CP at admission >29 mmol/l, history of CKD and respiratory diseases, hospital admissions for respiratory diseases. The respiratory diseases included inflammatory lung diseases, restrictive lung diseases, respiratory tract infections, lung tumors, pleural cavity diseases and pulmonary vascular diseases.
All the data were collected from an electronic medical record database. The data included demographics, categories of underlying diseases, mean blood pressure (MBP) and laboratory values at admission. However, we focused on the data of electrolyte and acid-base status in all included patients. The incidence of AKI, hospital mortality, hospital stay, and costs was also recorded.
Definitions and calculation
AKI was defined using Kidney Disease: Improving Global Outcomes criteria (8) as an increase in serum creatinine (SCr) ≥0.3 mg/dl (≥26.5 µmol/l) within 48 h, or an increase in SCr to ≥1.5 times baseline known or presumed to have occurred within the prior seven days. The patients who developed AKI during the following hospitalization were divided into AKI group, while those who did not be split into the non-AKI group.
All samples were analyzed in the central laboratory of the Institution. Anion gap (AG) was calculated by the standard formula (9): AG=[Na+]−[Cl−]−[HCO3−], with an elevated AG defined as greater than or equal to 16 mmol/l. Strong ion difference (SID) is the sum of strong cations minus the sum of strong anions. Strong ion gap (SIG) is the difference between the apparent SID (SIDa) and the effective SID (SIDe). SIG represents the sum of any unmeasured strong positive and negative ions with an elevated SIG defined as greater than 2 mmol/l. SIDa=[Na+]+[K+]+[Ca2+]+[Mg2+]-[Cl−]. SIDe=[HCO3−]+(0.123xpH-0.631)x[Alb]+(pH-0.469)x[Pi]. SIG=SIDa-SIDe (10). Calculated osmolality=2x[Na+] +[glucose]+[urea], with the normal range from 280 to 310 mOsm/l. Hypernatremia, hyponatremia, hyperkalemia, hypokalemia, hyperchloremia, hypochloremia, hypercalcemia, hypocalcemia, hyperphosphatemia, hypophosphatemia, hypermagnesemia, hypomagnesemia, hyperuricemia and hypouricemia were defined according to the reference ranges provided by the central laboratory. Metabolic acidosis includes simple and complex metabolic acidosis, and the latter may exist in dual or triple acid-base disturbances. Simple, dual and triple acid-base disturbances were diagnosed according to the flow diagrams described by Milford Fulop (11). Decreased CO2CP was defined when it was lower than 23 mmol/l (normal range: 23–29 mmol/l).
Statistical methods
The data were analyzed using SPSS version 24.0 software (SPSS, Chicago, IL, USA). Continuous variables are presented as mean ± SD if they were statically normally distributed and categorical variables as numbers and percentages. Moreover, they were compared using the Student t-test for continuous variables and the χ2 test for categorical variables. Medians and interquartile ranges are presented when variables are not normally distributed, and they were compared using Mann-Whitney U test. Multiple regression binary logistic regression with the Wald forward stepwise method was performed to evaluate the independent risk factors for the occurrence of AKI and hospital mortality, and the results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). We also used Cox proportional hazard model with the Wald forward stepwise method to analyze the independent predictors of AKI and hospital mortality after propensity scores matching, and the results are presented as hazard ratios (HRs) and 95% CIs. The significant acceptance and removal levels for a covariate were set at 0.05 and 0.1, respectively. Multiple linear regression analysis with the stepwise method was used to determine the relationship of CO2CP and other variables. The ability of CO2CP to predict AKI and hospital mortality, and to diagnose acid-base disturbances was assessed by plotting the receiver operating characteristic (ROC) curves and further reported as areas under the curve (AUC) with 95% CIs. An AUC-ROC value of >0.7 was taken to indicate reasonable biomarker performance (12). ROC curve optimal cut-off values were defined as the point that maximized the Youden index, defined as (sensitivity + specificity)-1 (13). A P-value <0.05 was considered to indicate statistical significance.
Propensity score matching
To reduce the impact of potential confounding factors in an observational study, we also performed propensity score matching for each AKI using a multivariable logistic regression analysis model based on the following covariates: Age, sex, SCr and BUN at admission. Propensity scores were then employed using the nearest neighbor matching algorithm. Matching was performed with a computer application implemented in SPSS 24.0 and R software version 3.2.2 to select for the most similar propensity scores and to create 1:2 matched pairs (matching the non-AKI to AKI). To identify independent risk factors of AKI and hospital mortality in patients with ABG, we conducted three Cox proportional hazard models. We included all potential factors associated with AKI and hospital mortality in model 1, the level of HCO3− was not included in model 2, and PaCO2 was not considered in model 3.
Results
Patient characteristics
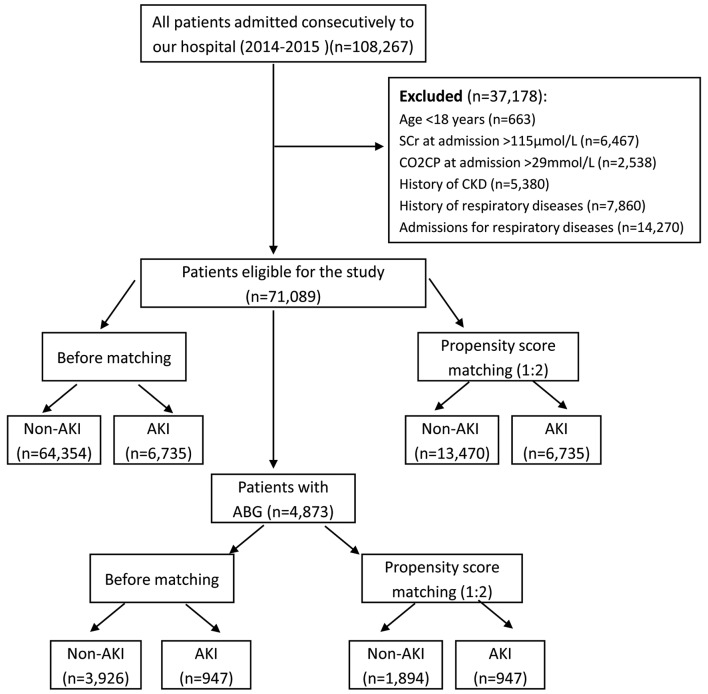
After screening, a total of 71,089 patients met the inclusion criteria, and there were 4,873 patients with ABG analysis. The flow-chart of this study was showed in Fig. 1. In all included patients, the incidence of AKI was 9.5%, and the hospital mortality was 0.7%, while the rates in patients with ABG were 19.4 and 1.6%, respectively. The top four underlying diseases with a high incidence of AKI were cardiothoracic surgery (40.7%), cancer (15.8%), general surgery (10.3%) and neurological diseases (10.1%), while the incidence of AKI in cardiovascular patients was 4.3%. Characteristics of all subjects before and after propensity score matching were listed in Table I, while the details of patients with ABG were showed in Table II. By the propensity score, patients in AKI group were successfully matched to patients in the non-AKI group with the ratio of 1:2 in all patients and patients with ABG. After propensity score matching, no statistically significant baseline characteristics in age, sex, SCr and BUN at admission between AKI and non-AKI groups were found. After propensity matching of all patients, the hospital mortality in AKI group was eight times higher than that in the non-AKI group (4.0 vs. 0.5%, respectively, P<0.001). The hospital stay was longer (P<0.001), and the cost was higher (P<0.001) in AKI group than these in the non-AKI group.
Figure 1.
Flowchart of the study. All patients and patients with ABG were analyzed respectively, and propensity score matching was used to obtain more reliable conclusions. SCr, serum creatinine; CO2CP, carbon dioxide combining power; AKI, acute kidney injury; ABG, arterial blood gas analysis; CKD, chronic kidney disease.
Table I.
Baseline characteristics of all included patients before and after propensity score matching.
| Before matching | Propensity score matching (1:2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Non-AKI (n=64,354) | AKI (n=6,735) | P-value | Non-AKI (n=13,470) | AKI (n=6,735) | P-value | ||
| Age, yr | 57.0±14.3 | 59.3±14.6 | <0.001a | 59.1±14.5 | 59.3±14.6 | 0.275a | ||
| Male sex, n (%) | 38,131(59.3) | 4,274 (63.5) | <0.001 | 8467 (62.9) | 4274 (63.5) | 0.404 | ||
| Renal function at admission | ||||||||
| SCr, µmol/l | 70.0 (59.0–82.0) | 76.0 (63.0–91.0) | <0.001 | 76.0 (63.0–89.0) | 76.0 (63.0–91.0) | 0.086 | ||
| BUN, mmol/l | 4.8 (3.9–5.9) | 5.2 (4.1–6.1) | <0.001 | 5.3 (4.2–6.2) | 5.2 (4.1–6.1) | 0.096 | ||
| Underlying diseases, n (%) | ||||||||
| Cardiovascular | 16,543 (25.7) | 741 (11.0) | <0.001 | 3,886 (28.8) | 741 (11.0) | <0.001 | ||
| General surgery | 9,323 (14.5) | 1,073 (15.9) | 0.001 | 1,771 (13.1) | 1,073 (15.9) | <0.001 | ||
| Digestive | 7,895 (12.3) | 326 (4.8) | <0.001 | 1,565 (11.6) | 326 (4.8) | <0.001 | ||
| Cancer | 7,297 (11.3) | 1,371 (20.4) | <0.001 | 1,410 (10.5) | 1,371 (20.4) | <0.001 | ||
| Orthopedic surgery | 3,533 (5.5) | 147 (2.2) | <0.001 | 700 (5.2) | 147 (2.2) | <0.001 | ||
| Cardiothoracic surgery | 2,549 (4.0) | 1,752 (26.0) | <0.001 | 589 (4.4) | 1,752 (26.0) | <0.001 | ||
| Endocrine | 2,297 (3.6) | 58 (0.9) | <0.001 | 457 (3.4) | 58 (0.9) | <0.001 | ||
| Hematological | 2,199 (3.4) | 352 (5.2) | <0.001 | 407 (3.0) | 352 (5.2) | <0.001 | ||
| Renal | 1,941 (3.0) | 114 (1.7) | <0.001 | 476 (3.5) | 114 (1.7) | <0.001 | ||
| Neurological | 1,835 (2.9) | 207 (3.1) | 0.299 | 382 (2.8) | 207 (3.1) | 0.344 | ||
| Gynecological | 1,779 (2.8) | 104 (1.5) | <0.001 | 312 (2.3) | 104 (1.5) | <0.001 | ||
| Others | 7,163 (11.1) | 490 (7.3) | <0.001 | 1,515 (11.2) | 490 (7.3) | <0.001 | ||
| Clinical data at admission | ||||||||
| CO2CP, mmol/l | 24.0 (23.0–26.0) | 24.0 (22.0–26.0) | <0.001 | 24.0 (23.0–26.0) | 24.0 (22.0–26.0) | <0.001 | ||
| MBP, mmHg | 93.3 (86.7–98.7) | 92.7 (83.3–97.3) | <0.001 | 93.3 (86.7–98.7) | 92.7 (83.3–97.3) | <0.001 | ||
| WBC, 109/l | 5.9 (4.7–7.7) | 6.1 (4.8–7.8) | <0.001 | 5.9 (4.8–7.8) | 6.1 (4.8–7.8) | <0.001 | ||
| Hemoglobin, g/l | 131.0 (119.0–143.0) | 126.0 (111.0–139.0) | <0.001 | 131.0 (118.0–143.0) | 126.0 (111.0–139.0) | <0.001 | ||
| AST, U/l | 20.0 (16.0–28.0) | 23.0 (17.0–36.0) | <0.001 | 20.0 (16.0–28.0) | 23.0 (17.0–36.0) | <0.001 | ||
| ALT, U/l | 19.0 (13.0–30.0) | 20.0 (13.0–36.0) | <0.001 | 19.0 (13.0–30.0) | 20.0 (13.0–36.0) | <0.001 | ||
| TBIL, µmol/l | 9.5 (7.0–13.0) | 10.5 (7.4–15.4) | <0.001 | 9.5 (7.0–13.0) | 10.5 (7.4–15.4) | <0.001 | ||
| Albumin, g/l | 40.0 (37.0–43.0) | 38.0 (35.0–41.0) | <0.001 | 40.0 (37.0–43.0) | 38.0 (35.0–41.0) | <0.001 | ||
| SUA, mmol/l | 297.0 (240.0–357.0) | 305.0 (239.0–377.0) | <0.001 | 311.0 (251.0–376.0) | 305.0 (239.0–377.0) | <0.001 | ||
| Glucose, mmol/l | 5.2 (4.7–6.7) | 5.3 (4.7–6.7) | 0.126 | 5.3 (4.8–6.8) | 5.3 (4.7–6.7) | 0.315 | ||
| Na, mmol/l | 141.0 (139.0–143.0) | 141.0 (138.0–143.0) | <0.001 | 141.0 (139.0–143.0) | 141.0 (138.0–143.0) | <0.001 | ||
| K, mmol/l | 4.0 (3.8–4.8) | 4.0 (3.8–4.8) | 0.780 | 4.0 (3.9–4.9) | 4.0 (3.8–4.8) | 0.001 | ||
| Cl, mmol/l | 103.0 (101.0–105.0) | 103.0 (100.0–105.0) | <0.001 | 103.0 (101.0–105.0) | 103.0 (100.0–105.0) | <0.001 | ||
| Mg, mmol/l | 0.91 (0.86–0.86) | 0.90 (0.84–0.84) | <0.001 | 0.91 (0.86–0.86) | 0.90 (0.84–0.84) | <0.001 | ||
| Ca, mmol/l | 2.31 (2.23–2.23) | 2.28 (2.16–2.16) | <0.001 | 2.31 (2.23–2.23) | 2.28 (2.16–2.16) | <0.001 | ||
| P, mmol/l | 1.12 (0.99–1.99) | 1.12 (0.95–1.95) | <0.001 | 1.11 (0.98–1.98) | 1.12 (0.95–1.95) | 0.255 | ||
| Osmolality, mOsm/l | 293.3 (289.6–296.6) | 292.9 (288.5–297.5) | <0.001 | 293.9 (290.1–297.1) | 292.9 (288.5–297.5) | <0.001 | ||
| Death, n (%) | 256 (0.4) | 268 (4.0) | <0.001 | 68 (0.5) | 268 (4.0) | <0.001 | ||
| Hospital stay, days | 5.0 (2.5–8.5) | 12.5 (8.0–19.0) | <0.001 | 5.0 (3.0–8.0) | 12.5 (8.0–19.0) | <0.001 | ||
| Hospital cost, RMB | 14,803.5 | 52,479.7 | <0.001 | 14,961.9 | 52,479.7 | <0.001 | ||
| (8,020.7-35,359.8) | (22,202.9-108,512.1) | (7,993.8-37,631.1) | (22,202.9-108,512.1) | |||||
Unpaired Student's t-test. AKI, acute kidney injury; SCr, serum creatinine; BUN, blood urea nitrogen; CO2CP, carbon dioxide combining power; MBP, mean blood pressure; WBC, white blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; SUA, serum uric acid; RMB, Ren Min Bi.
Table II.
Baseline characteristics of included patients with arterial blood gas analysis before and after propensity score matching.
| Before matching | Propensity score matching (1:2) | |||||
|---|---|---|---|---|---|---|
| Variable | Non-AKI (n=3,926) | AKI (n=947) | P-value | Non-AKI (n=1,894) | AKI (n=947) | P-value |
| Age, yr | 58.9±12.6 | 59.3±13.3 | 0.461a | 59.5±12.7 | 59.3±13.3 | 0.583a |
| Male sex, n (%) | 2,366 (60.3) | 637 (67.3) | <0.001 | 1242 (65.6) | 637 (67.3) | 0.370 |
| Renal function at admission | ||||||
| SCr, µmol/l | 62.0 (55.0–72.0) | 64.0 (56.0–74.0) | 0.060 | 63.0 (56.0–72.0) | 64.0 (56.0–74.0) | 0.162 |
| BUN, mmol/l | 4.6 (3.8–5.8) | 4.8 (3.8–5.8) | 0.001 | 4.9 (3.9–5.9) | 4.8 (3.8–5.8) | 0.501 |
| Clinical data at admission | ||||||
| CO2CP, mmol/l | 24.1±2.5 | 23.9±2.6 | 0.002a | 24.1±2.5 | 23.9±2.6 | 0.028a |
| MBP, mmHg | 93.3 (86.0–96.0) | 93.0 (84.3–96.3) | 0.457 | 92.7 (85.3–96.3) | 93.0 (84.3–96.3) | 0.795 |
| WBC, 109/l | 5.9 (4.7–7.7) | 6.0 (4.8–7.8) | 0.093 | 5.9 (4.7–7.7) | 6.0 (4.8–7.8) | 0.128 |
| Hemoglobin, g/l | 130.0 (118.0–141.0) | 129.0 (115.0–141.0) | 0.071 | 130.0 (118.0–142.0) | 129.0 (115.0–141.0) | 0.045 |
| AST, U/l | 19.0 (15.0–27.0) | 21.0 (16.0–33.0) | <0.001 | 19.0 (15.0–27.0) | 21.0 (16.0–33.0) | <0.001 |
| ALT, U/l | 18.0 (12.0–29.0) | 20.0 (13.0–34.0) | <0.001 | 18.0 (12.0–29.0) | 20.0 (13.0–34.0) | <0.001 |
| TBIL, µmol/l | 9.5 (7.1–13.1) | 10.7 (7.6–15.6) | <0.001 | 9.5 (7.1–13.1) | 10.7 (7.6–15.6) | <0.001 |
| Albumin, g/l | 39.0 (37.0–42.0) | 39.0 (35.0–41.0) | <0.001 | 39.0 (36.0–42.0) | 39.0 (35.0–41.0) | <0.001 |
| SUA, mmol/l | 278.0 (226.8–332.8) | 276.0 (212.0–341.0) | 0.398 | 280.0 (229.0–334.0) | 276.0 (212.0–341.0) | 0.188 |
| Glucose, mmol/l | 5.8±2.1 | 5.9±2.2 | 0.422a | 5.9±2.3 | 5.9±2.2 | 0.970a |
| Na, mmol/l | 141.0 (140.0–143.0) | 141.0 (139.0–143.0) | 0.003 | 141.0 (140.0–143.0) | 141.0 (139.0–143.0) | 0.029 |
| K, mmol/l | 4.0 (3.8–4.8) | 4.0 (3.7–4.7) | 0.255 | 4.0 (3.8–4.8) | 4.0 (3.7–4.7) | 0.126 |
| Cl, mmol/l | 103.0 (101.0–105.0) | 103.0 (100.0–105.0) | 0.002 | 103.0 (101.0–105.0) | 103.0 (100.0–105.0) | 0.008 |
| Mg, mmol/l | 0.92 (0.86–0.86) | 0.90 (0.84–0.84) | <0.001 | 0.92 (0.86–0.86) | 0.90 (0.84–0.84) | <0.001 |
| Ca, mmol/l | 2.29 (2.20–2.20) | 2.27 (2.15–2.15) | <0.001 | 2.28 (2.19–2.19) | 2.27 (2.15–2.15) | 0.003 |
| P, mmol/l | 1.12 (0.99–1.99) | 1.10 (0.94–1.94) | 0.003 | 1.11 (0.99–1.99) | 1.10 (0.94–1.94) | 0.128 |
| Osmolality, mOsm/l | 293.3 (289.6–296.6) | 292.9 (288.5–296.5) | 0.026 | 293.3 (289.0–296.0) | 292.9 (288.5–296.5) | 0.008 |
| PH | 7.42 (7.40–7.40) | 7.42 (7.39–7.39) | 0.870 | 7.42 (7.40–7.40) | 7.42 (7.39–7.39) | 0.783 |
| PaCO2, mmHg | 40.0 (36.0–43.0) | 39.0 (35.4–42.4) | <0.001 | 40.0 (26.0–43.0) | 39.0 (35.4–42.4) | 0.001 |
| HCO3-, mmol/l | 25.4 (23.4–27.4) | 24.4 (21.7–26.7) | <0.001 | 23.3 (25.4–27.4) | 24.4 (21.7–26.7) | <0.001 |
| BE, mmol/l | 1.2 (−0.7–2.7) | 0.6 (−1.9–2.9) | <0.001 | 1.2 (−0.8–2.8) | 0.6 (−1.9–2.9) | <0.001 |
| AG, mmol/l | 14.0 (13.0–16.0) | 14.0 (13.0–16.0) | 0.172 | 14.2±2.6 | 14.4±2.9 | 0.091a |
| SIDa, mmol/l | 48.4 (46.7–50.7) | 48.2 (46.3–50.3) | 0.046 | 48.4 (46.6–49.6) | 48.2 (46.3–50.3) | 0.222 |
| SIDe, mmol/l | 44.2 (41.5–46.5) | 42.8 (39.0–45.0) | <0.001 | 44.0 (41.4–46.4) | 42.8 (39.0–45.0) | <0.001 |
| SIG, mmol/l | 4.3 (2.1–6.1) | 5.6 (3.0–8.0) | <0.001 | 4.4 (2.2–6.2) | 5.6 (3.0–8.0) | <0.001 |
| Death, n (%) | 28 (0.7) | 48 (5.1) | <0.001 | 17 (0.9) | 48 (5.1) | <0.001 |
| Hospital stay, days | 10.0 (7.0–13.0) | 15.5 (11.5–23.5) | <0.001 | 10.0 (7.0–23.0) | 15.5 (11.5–23.5) | <0.001 |
| Hospital cost, RMB | 39,559.3 (19,529.7-58,308.5) | 73,051.7 (45,782.8-121,322.4) | <0.001 | 39,481.1 (19,587.8-58,471.2) | 73,051.7 (45,782.8-121,322.4) | <0.001 |
Unpaired Student's t-test. AKI, acute kidney injury; SCr, serum creatinine; BUN, blood urea nitrogen; CO2CP, carbon dioxide combining power; MBP, mean blood pressure; WBC, white blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; SUA, serum uric acid; BE, base excess; AG, anion gap; SIDa, apparent strong ion difference; SIDe, effective strong ion difference; SIG, strong ion gap; RMB, Ren Min Bi.
Clinical data at admission after propensity matching
In all included patients, the patients in AKI group had lower mean blood pressure, hemoglobin, albumin and a higher number of white blood cell and indexes of liver function (P<0.001). There was significant differences in the levels of Na (P<0.001), K (P=0.001), Cl (P<0.001), Mg (P<0.001), Ca (P<0.001), osmolality (P<0.001) and serum uric acid (P<0.001) between AKI and non-AKI group. Although the median of CO2CP in these two groups was same, the lower interquartile was lower in AKI group than that in the non-AKI group, and there was a significant difference (P<0.001) (Table I).
In 4,873 patients with ABG, those in AKI group had still lower hemoglobin and albumin, higher indexes of liver function than these in the non-AKI group. There were significant differences in the levels of electrolytes, including Na (P=0.029), Cl (P=0.008), Mg (P<0.001), and Ca (P=0.003). The osmolality in AKI group was lower than that in the non-AKI group (P=0.0008). Even though there was no significant difference in pH and AG between these two groups, the levels of PaCO2 (P=0.001), HCO3− (P<0.001), BE (P<0.001), SIDe (P<0.001) and SIG (P<0.001) were significantly different. The CO2CP in AKI group was lower than that in the non-AKI group (P=0.028).
Independent risk factors for incidence of AKI
For patients with ABG, multivariate logistic regression analysis, and three Cox proportional hazards models were used to determine the independent risk factors of incidence of AKI. For the final multiple logistic analysis, 13 variables were kept in the model for AKI, including male sex, hyponatremia, pH>7.45, HCO3−<22 mmol/l, HCO3−>27 mmol/l, BE<-3 mmol/l, BE>3 mmol/l and so on. To cut off the interaction of acid-base indexes, we conducted three Cox proportional hazards models. In model 1, all potential variables were included for final analysis, and ten variables were identified including pH<7.35 (HR 1.810, 95% CI: 1.298–2.524, P<0.001), HCO3−<22 mmol/l (HR 2.051, 95% CI: 1.498–2.809, P<0.001), hypomagnesemia, hypermagnesemia and so forth. In model 2, the variable of HCO3− was not included. Further, two variables were kept: metabolic acidosis (HR 1.160, 95% CI: 1.001–1.344, P=0.049) and PaCO2<35 mmHg (HR 1.253, 95% CI: 1.053–1.492, P=0.011). In model 3, the variable of PaCO2 was not included; the final analysis was similar to model 1. The detailed results were listed in Table III.
Table III.
Independent risk factors for acute kidney injury including patients with arterial blood gas analysis.
| Multiple logistic analysis | Cox proportional hazards model 1 | Cox proportional hazards model 2 | Cox proportional hazards model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | OR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value |
| Male sex | 1.427 (1.214–1.214) | <0.001 | ||||||
| Hyponatremia | 0.510 (0.271–0.271) | 0.037 | ||||||
| Hypernatremia | 0.653 (0.332–1.332) | 0.215 | ||||||
| Hypomagnesemia | 0.601 (0.467–0.467) | <0.001 | 3.399 (1.578–7.578) | 0.002 | 2.191 (1.299–3.299) | 0.003 | 2.123 (1.257–3.257) | 0.005 |
| Hypermagnesemia | 2.040 (1.065–3.065) | 0.032 | 1.965 (1.509–2.509) | <0.001 | 1.631 (1.303–2.303) | <0.001 | 1.593 (1.272–1.272) | <0.001 |
| Hypophosphatemia | 0.728 (0.576–0.576) | 0.008 | ||||||
| Hyperphosphatemia | 0.911 (0.679–1.679) | 0.532 | ||||||
| Hypouricemia | 0.596 (0.445–0.445) | 0.001 | 1.270 (1.023–1.023) | 0.031 | 1.165 (0.986–1.986) | 0.073 | 1.150 (0.973–1.973) | 0.101 |
| Hyperuricemia | 0.730 (0.521–1.521) | 0.068 | 1.768 (1.272–2.272) | 0.001 | 1.397 (1.098–1.098) | 0.006 | 1.399 (1.101–1.101) | 0.006 |
| Metabolic acidosis | 1.160 (1.001–1.001) | 0.049 | ||||||
| pH<7.35 | 1.022 (0.816–1.816) | 0.849 | 1.810 (1.298–2.298) | <0.001 | 1.386 (1.107–1.107) | 0.004 | 1.357 (1.072–1.072) | 0.011 |
| pH>7.45 | 1.853 (1.306–2.306) | 0.001 | 1.029 (0.805–1.805) | 0.820 | 1.010 (0.832–1.832) | 0.922 | 1.034 (0.856–1.856) | 0.727 |
| HCO3- <22 mmol/l | 1.553 (1.179–2.179) | 0.002 | 2.051 (1.498–2.498) | <0.001 | 1.497 (1.194–1.194) | <0.001 | ||
| HCO3- >27 mmol/l | 3.072 (2.142–4.142) | <0.001 | 0.640 (0.476–0.476) | 0.003 | 0.745 (0.587–0.587) | 0.015 | ||
| PaCO2 <35 mmHg | 1.253 (1.053–1.053) | 0.011 | ||||||
| PaCO2> 45 mmHg | 1.097 (0.884–1.884) | 0.399 | ||||||
| BE <-3 mmol/l | 0.631 (0.478–0.478) | 0.001 | 0.754 (0.518–1.518) | 0.141 | 0.866 (0.661–1.661) | 0.296 | ||
| BE >3 mmol/l | 0.513 (0.339–0.339) | 0.002 | 1.639 (1.213–2.213) | 0.001 | 1.369 (1.084–1.084) | 0.008 | ||
| AST, per 40 U/l increase | 1.140 (1.075–1.075) | <0.001 | 1.148 (1.073–1.073) | <0.001 | 1.060 (1.018–1.018) | 0.005 | 1.068 (1.026–1.026) | 0.001 |
| Albumin, per 5 g/l decrease | 1.211 (1.110–1.110) | <0.001 | 1.184 (1.077–1.077) | 0.001 | 1.106 (1.029–1.029) | 0.006 | 1.103 (1.028–1.028) | 0.006 |
BE, base excess; AST, aspartate aminotransferase; OR, odds ratio; HR, hazard ratio; CI, confidence interval.
In all included patients, multivariate logistic regression analysis and Cox proportional hazards model were used to determine the independent risk factors of incidence of AKI during the following hospitalization. For the final multiple logistic analysis, 22 variables were kept in the model for AKI, including age, male sex, CO2CP <23 mmol/l (OR 1.220, 95% CI: 1.150–1.295, P<0.001), mean blood pressure <70 mmHg, hyponatremia, hypernatremia, and so on. After propensity matching, 14 variables were selected in Cox proportional hazards model for the development of AKI, including CO2CP <23 mmol/l (HR 1.099, 95% CI: 1.041–1.161, P=0.001), mean blood pressure <70 mmHg, hypernatremia, and so on. The detailed results were listed in Table IV.
Table IV.
Independent risk factors for acute kidney injury including all patients.
| Multiple logistic analysis | Cox proportional hazards model | |||
|---|---|---|---|---|
| Variable | OR (95%CI) | P-value | HR (95%CI) | P-value |
| Age, per 10-year increase | 1.077 (1.057–1.057) | <0.001 | ||
| Male sex | 1.141 (1.079–1.079) | <0.001 | ||
| CO2CP<23 mmol/l | 1.220 (1.150–1.150) | <0.001 | 1.099 (1.041–1.041) | 0.001 |
| MBP<70 mmHg | 2.232 (1.771–2.771) | <0.001 | 1.360 (1.130–1.130) | 0.001 |
| Hyponatremia | 1.159 (1.052–1.052) | 0.003 | 1.085 (0.999–1.999) | 0.054 |
| Hypernatremia | 1.590 (1.224–2.224) | 0.001 | 1.522 (1.244–1.244) | <0.001 |
| Hypokalemia | 1.109 (1.006–1.006) | 0.037 | ||
| Hyperkalemia | 1.843 (1.197–2.197) | 0.006 | ||
| Hypochloremia | 1.375 (1.241–1.241) | <0.001 | 1.197 (1.096–1.096) | <0.001 |
| Hyperchloremia | 0.905 (0.689–1.689) | 0.473 | 0.862 (0.692–1.692) | 0.185 |
| Hypocalcemia | 1.658 (1.523–1.523) | <0.001 | 1.319 (1.224–1.224) | <0.001 |
| Hypercalcemia | 1.007 (0.803–1.803) | 0.949 | 0.992 (0.803–1.803) | 0.944 |
| Hypomagnesemia | 2.373 (1.862–3.862) | <0.001 | 1.319 (1.090–1.090) | 0.005 |
| Hypermagnesemia | 1.340 (1.193–1.193) | <0.001 | 1.085 (0.977–1.977) | 0.126 |
| Hypophosphatemia | 1.181 (1.085–1.085) | <0.001 | 1.113 (1.032–1.032) | 0.005 |
| Hyperphosphatemia | 1.604 (1.472–1.472) | <0.001 | 1.347 (1.246–1.246) | <0.001 |
| Hypouricemia | 0.863 (0.797–0.797) | <0.001 | 1.021 (0.952–1.952) | 0.568 |
| Hyperuricemia | 1.765 (1.632–1.632) | <0.001 | 1.160 (1.081–1.081) | <0.001 |
| Hypoosmolality | 0.935 (0.807–1.807) | 0.368 | ||
| Hyperosmolality | 3.255 (2.334–4.334) | <0.001 | ||
| Hemoglobin, per 10 g/l decrease | 1.073 (1.052–1.052) | <0.001 | 1.021 (1.004–1.004) | 0.018 |
| WBC<4×109/l | 0.996 (0.922–1.922) | 0.924 | 1.094 (1.019–1.019) | 0.014 |
| WBC>12×109/l | 1.727 (1.546–1.546) | <0.001 | 1.242 (1.130–1.130) | <0.001 |
| AST, per 40 U/l increase | 1.088 (1.071–1.071) | <0.001 | 1.033 (1.022–1.022) | <0.001 |
| TBIL, per 20 µmol/l increase | 1.023 (1.006–1.006) | 0.007 | ||
| Albumin, per 5 g/l decrease | 1.220 (1.179–1.179) | <0.001 | 1.120 (1.087–1.087) | <0.001 |
CO2CP, carbon dioxide combining power; MBP, mean blood pressure; WBC, white blood cell; AST, aspartate aminotransferase; TBIL, total bilirubin; OR, odds ratio; HR, hazard ratio; CI, confidence interval.
Independent risk factors for hospital mortality
For patients with ABG, multivariate logistic regression analysis, and three Cox proportional hazards models were used to determine the independent risk factors for hospital mortality. For the final multiple logistic analysis, four variables were kept in the model for hospital mortality, including age, pH>7.45, AST, and albumin. Three Cox proportional hazards models were also conducted as above; six variables were kept in more than one model, including age, pH<7.35, PaCO2 <35 mmHg, hypo-osmolality, AST, and decreased albumin. The detailed results were listed in Table V.
Table V.
Independent risk factors for hospital mortality including patients with arterial blood gas analysis.
| Multiple logistic analysis | Cox proportional hazards model 1 | Cox proportional hazards model 2 | Cox proportional hazards model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age, per 10-year increase | 1.500 (1.233–1.233) | <0.001 | 1.360 (1.103–1.103) | 0.004 | 1.333 (1.092–1.092) | 0.005 | 1.346 (1.103–1.103) | 0.003 |
| pH<7.35 | 0.674 (0.370–1.370) | 0.198 | 5.064 (2.438–10.438) | <0.001 | 3.915 (1.966–7.966) | <0.001 | 4.533 (2.328–8.328) | <0.001 |
| pH>7.45 | 2.827 (1.316–6.316) | 0.008 | 1.793 (0.928–3.928) | 0.082 | 1.879 (1.005–3.005) | 0.048 | 2.295 (1.258–4.258) | 0.007 |
| PaCO2<35 mmHg | 0.551 (0.246–1.246) | 0.148 | 2.636 (1.406–4.406) | 0.003 | 2.491 (1.360–4.360) | 0.003 | ||
| PaCO2>45 mmHg | 1.307 (0.556–3.556) | 0.539 | 2.103 (0.892–4.892) | 0.089 | 1.951 (0.866–4.866) | 0.107 | ||
| Hypoosmolality | 1.192 (1.032–1.032) | 0.072 | 2.233 (1.109–4.109) | 0.024 | 1.870 (0.988–3.988) | 0.054 | 2.052 (1.091–3.091) | 0.026 |
| Hyperosmolality | 0.480 (0.075–3.075) | 0.438 | 4.032 (0.669–24.669) | 0.128 | 3.610 (0.818–15.818) | 0.090 | 3.921 (0.876–17.876) | 0.074 |
| AST, per 40 U/l increase | 1.462 (1.245–1.245) | <0.001 | 1.220 (1.095–1.095) | <0.001 | 1.173 (1.065–1.065) | 0.001 | 1.217 (1.106–1.106) | <0.001 |
| Albumin, per 5 g/l decrease | 1.688 (1.348–2.348) | <0.001 | 1.647 (1.296–2.296) | <0.001 | 1.507 (1.217–1.217) | <0.001 | 1.425 (1.120–1.120) | 0.004 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; TBIL, total bilirubin; OR, odds ratio; HR, hazard ratio; CI, confidence interval.
In all included patients, multivariate logistic regression analysis and Cox proportional hazards model were used to identify the independent risk factors for hospital mortality. For the final multiple logistic analysis, 14 variables were kept in the model for hospital mortality, including age, male sex, CO2CP <23 mmol/l (OR 1.344, 95% CI: 1.107–1.632, P=0.003), hyponatremia, hypochloremia, and so on. After propensity matching, 11 variables were selected in Cox proportional hazards model for hospital mortality. CO2CP<23 mmol/l was also an independent risk factor for hospital mortality (HR 1.294, 95% CI: 1.026–1.632, P=0.030). The detailed results were listed in Table VI.
Table VI.
Independent risk factors for hospital mortality including all patients.
| Multiple logistic analysis | Cox proportional hazards model | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | P-value | HR (95% CI) | P-value |
| Age, per 10-year increase | 1.611 (1.504–1.504) | <0.001 | ||
| Male sex | 1.364 (1.124–1.124) | 0.002 | ||
| CO2CP<23 mmol/l | 1.344 (1.107–1.107) | 0.003 | 1.294 (1.026–1.026) | 0.030 |
| Hyponatremia | 1.563 (1.190–2.190) | 0.001 | 1.592 (1.147–2.147) | 0.005 |
| Hypernatremia | 1.916 (0.935–3.935) | 0.076 | 1.647 (0.749–3.749) | 0.214 |
| Hypokalemia | 1.453 (1.081–1.081) | 0.013 | ||
| Hyperkalemia | 1.487 (0.685–3.685) | 0.315 | ||
| Hypochloremia | 1.968 (1.484–2.484) | <0.001 | 1.877 (1.349–2.349) | <0.001 |
| Hyperchloremia | 0.728 (0.378–1.378) | 0.342 | 0.676 (0.323–1.323) | 0.299 |
| Hypomagnesemia | 0.295 (0.090–0.090) | 0.044 | 0.350 (0.111–1.111) | 0.072 |
| Hypermagnesemia | 1.565 (1.104–2.104) | 0.012 | 1.594 (1.112–2.112) | 0.011 |
| Hypouricemia | 1.283 (1.025–1.025) | 0.030 | 1.278 (0.980–1.980) | 0.070 |
| Hyperuricemia | 1.742 (1.324–2.324) | <0.001 | 1.520 (1.127–2.127) | 0.006 |
| Hypoosmolality | 0.936 (0.684–1.684) | 0.679 | 1.086 (0.761–1.761) | 0.650 |
| Hyperosmolality | 5.029 (2.549–9.549) | <0.001 | 3.588 (1.767–7.767) | <0.001 |
| Hemoglobin, per 10 g/l decrease | 1.204 (1.145–1.145) | <0.001 | 1.128 (1.067–1.067) | <0.001 |
| WBC<4×109/l | 1.064 (0.817–1.817) | 0.645 | 0.889 (0.635–1.635) | 0.546 |
| WBC>12×109/l | 2.917 (2.274–3.274) | <0.001 | 2.096 (1.580–2.580) | <0.001 |
| AST, per 40 U/l increase | 1.126 (1.094–1.094) | <0.001 | 1.068 (1.039–1.039) | <0.001 |
| TBIL, per 20 µmol/l increase | 1.032 (1.001–1.001) | 0.043 | ||
| Albumin, per 5 g/l decrease | 1.492 (1.355–1.355) | <0.001 | 1.294 (1.026–1.026) | <0.001 |
CO2CP, carbon dioxide combining power; WBC, white blood cell; AST, aspartate aminotransferase; TBIL, total bilirubin; OR, odds ratio; HR, hazard ratio; CI, confidence interval.
ROC curves of CO2CP
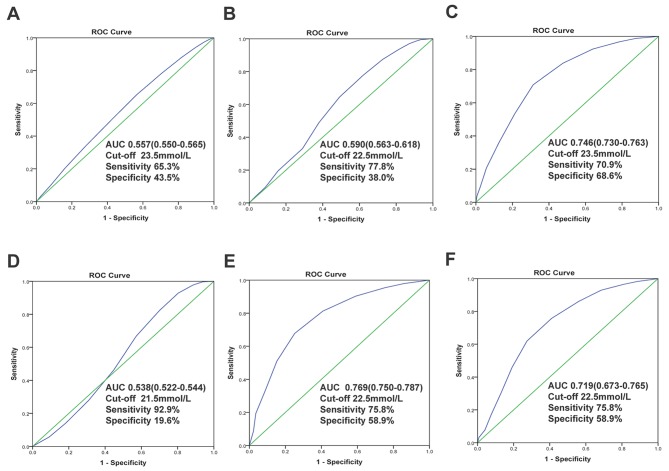
In all included patients, the area under the ROC of CO2CP was 0.557 (95% CI: 0.550–0.565, P<0.001) for predicting of not developing AKI and 0.590 (95% CI: 0.563–0.618, P<0.001) for predicting of no hospital death. And the optimal cut-off values of CO2CP were 23.5 (sensitivity 65.3%, specificity 43.5%) and 22.5 mmol/l (sensitivity 77.8%, specificity 38.0%), respectively (Fig. 2A and B). In patients with ABG, the area under the ROC of CO2CP was 0.746 (95% CI: 0.730–0.763, P<0.001) for diagnosis of no metabolic acidosis and the optimal cut-off values of CO2CP were 23.5 (sensitivity 70.9%, specificity 68.6%), indicating reasonable biomarker performance (Fig. 2C). We also conduct ROC curves of CO2CP for excluding the diagnosis of any acid-base disturbances (Fig. 2D), simple, dual and triple acid-base disturbances. There were reasonable biomarker performances to exclude diagnosis of dual (AUC 0.769, 95%: CI 0.750–0.787, P<0.001, Fig. 2E) and triple (AUC 0.719, 95%: CI 0.673–0.765, P<0.001, Fig. 2F) acid-base disturbances with 22.5 mmol/l as the optimal cut-off value.
Figure 2.
Receiver operator characteristic curves of carbon dioxide combining power. For predicting of not developing of (A) acute kidney injury and (B) no hospital death; For excluding the diagnosis of (C) metabolic acidosis, (D) any acid-base disturbances, (E) dual and (F) triple acid-base disturbances. The AUC, cut-off values of CO2CP, sensitivity and specificity for diagnosis were provided. ROC, receiver operator characteristic; AUC, areas under the curve.
Subgroup analysis of CO2CP as a contributor of AKI and hospital mortality
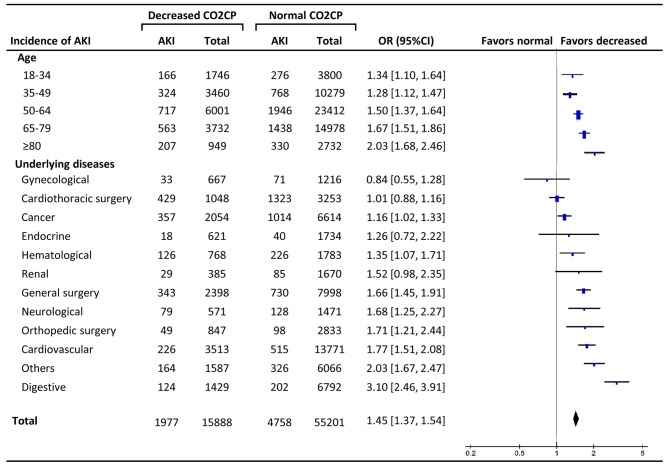
The total incidence of AKI in decreased CO2CP group was significantly higher than normal CO2CP group (12.4% and 8.6, respectively, OR 1.45, 95% CI: 1.37–1.54, P<0.001). Subgroup analysis showed that the ORs increased along with older age with significant differences at every age stage (18–34 years, P=0.004; other stages, P<0.001). The effect sizes of decreased CO2CP on AKI varied according to different diseases, and there were significant differences in digestive (OR 3.10, P<0.001), cardiovascular diseases (OR 1.77, P<0.001), orthopedic surgery (OR 1.71, P=0.002), neurological diseases (OR 1.68, P=0.001), general surgery (OR 1.66, P<0.001), hematological diseases (OR 1.35, P=0.012), cancer (OR 1.16, P=0.026) and others (OR 2.03, P<0.001) (Fig. 3).
Figure 3.
Subgroup analysis of decreased carbon dioxide combining power (<23 mmol/l) as a contributor to acute kidney injury. The effect of different age subgroups and underlying diseases were analyzed. AKI, acute kidney injury; CO2CP, carbon dioxide combining power; OR, odds ratio; CI, confidence interval.
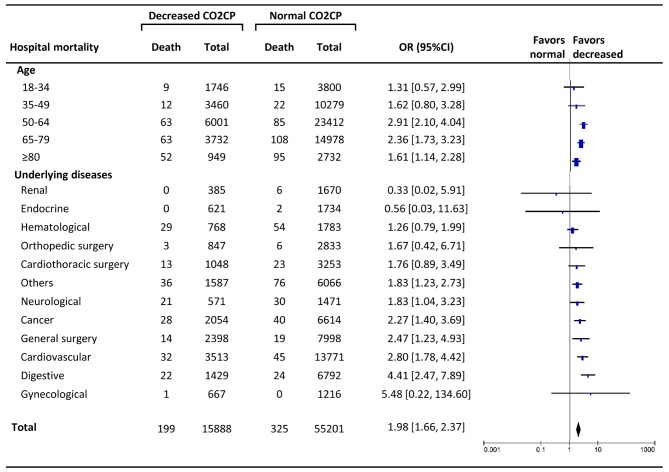
The total hospital mortality in decreased CO2CP group was 1.3%, significantly higher than normal CO2CP group (0.6%) (OR 1.98, 95% CI: 1.66–2.37, P<0.001). The increasing trend of ORs along with older age was not present, and there were significant differences only at the age stages of 50–64 years (OR 2.91, P<0.001), 65–79 years (OR 2.36, P<0.001), and >80 years (OR 1.61, P=0.007). The effect sizes of decreased CO2CP on hospital mortality were significantly different in digestive (OR 4.41, P<0.001), cardiovascular diseases (OR 2.80, P<0.001), general surgery (OR 2.47, P=0.008), cancer (2.27 1.61, P=0.001), neurological diseases (OR 1.83, P=0.033), and others (OR 1.83, P=0.003) (Fig. 4).
Figure 4.
Subgroup analysis of decreased carbon dioxide combining power (<23 mmol/l) as a contributor to hospital mortality. The effect of different age subgroups and underlying diseases were analyzed. AKI, acute kidney injury; CO2CP, carbon dioxide combining power; OR, odds ratio; CI, confidence interval.
Discussion
The present study focuses on the variables in the electrolyte and acid-base balance among non-respiratory patients associated with the occurrence of AKI and hospital mortality using a propensity score matching method. Primary analysis in patients with ABG demonstrated that several variables which could result in decreased CO2CP, such as acidosis (pH<7.35), metabolic acidosis, decreased HCO3− and hypocapnia were associated with the development of AKI, while acidosis and hypocapnia were independent predictors of hospital mortality. Further analysis found decreased CO2CP was an independent risk factor for AKI and hospital mortality. AUC-ROC indicated that CO2CP was a reasonable biomarker to exclude metabolic acidosis, dual and triple acid-base disturbances with 22.5 or 23.5 mmol/l as optimal cut-off values. Furthermore, the effect sizes of decreased CO2CP on AKI and hospital mortality varied according to age and different underlying diseases.
Whether acid-base disturbances are contributors to acute kidney injury or rather are simple epiphenomena has rarely been discussed in previous studies. The role of serum bicarbonate level as a risk factor for renal outcomes (end-stage renal disease or 50% reduction in evaluated glomerular filtration rate) has been evaluated in patients with CKD. Aſter adjustment for covariates, the risk of developing a renal endpoint is 3% lower per 1 mM increase in serum bicarbonate level (HR 0.97, 95% CI: 0.94–0.99) (14). However, it is not known whether metabolic acidosis affects the development of AKI in a clinical setting. Our research found that acidosis, especially metabolic acidosis, hypocapnia, and decreased CO2CP were contributors to the incidence of AKI. Besides, the previous study (1) showed that patients with acidosis are 4 to 5 times as likely to die as patients who do not, and the mortality among patients with metabolic acidosis is highest for patients with lactic acidosis (high AG) and SIG acidosis. We found that acidosis, hypocapnia, and decreased CO2CP were predictors of hospital mortality, and significantly higher SIG was observed in AKI group than the non-AKI group, indicating that SIG should also be carefully monitored, especially in critically ill patients. Although the roles of metabolic acidosis in the development of AKI and death were confirmed, the benefits and administration timing of sodium bicarbonate for prevention and treatment of AKI are still not clear now (15).
In this study, we restricted our analysis to patients without respiratory diseases and patients with increased CO2CP at admission were also excluded, for several reasons. As discussed above, CO2CP does not reflect the actual state of the acid-base balance when respiratory acid-base balances exist. Two major acid-base disturbances, respiratory acidosis, and metabolic alkalosis, both of which can result in increased CO2CP, are not uncommon in respiratory patients. If respiratory diseases, such as chronic obstructive pulmonary diseases, are excluded, the impact of respiratory acid-base balances to CO2CP can be minimized. On the other hand, reduced CO2CP suggests metabolic acidosis (1) or respiratory alkalosis (16), both of which are indicators of poor outcomes. Although ABG analysis has become an essential test to diagnose acid-base disturbances, only a small percentage of patients received this test for its invasive procedure and complications. In this respect, CO2CP is easier for patients to obtain from venous blood than ABG analysis from arterial blood. Furthermore, if integrated with clinical and electrolytes for full consideration, more exact information about acid-base disturbances can be obtained from CO2CP.
Most often, decreased CO2CP signifies the presence of metabolic acidosis, but it also could reflect a decline in the bicarbonate concentration as compensation for respiratory alkalosis. Respiratory alkalosis is a disturbance in acid and base balance due to alveolar hyperventilation. Respiratory alkalosis is the most common acid-base disturbance observed in patients who are critically ill. When respiratory alkalosis is present, the cause may be a minor non-life-threatening disorder. However, more serious disease processes may exist, especially in critically ill patients. In our study, hypocapnia at admission in non-respiratory patients was associated with poor outcomes, including the development of AKI and hospital mortality. Furthermore, in non-respiratory patients, AUC-ROC stated that CO2CP was a reasonable biomarker to exclude metabolic acidosis, dual and triple acid-base disturbances with 22.5 or 23.5 mmol/l as optimal cut-off values.
The relationship of electrolyte disturbances and poor outcomes is complex. They may often be a para-phenomenon, as an indicator of the severity of the underlying diseases. However, according to our multiple logistic regression and propensity-matched analysis, some disorders were independent contributors to hospital mortality, such as hyponatremia, hypochloremia, hyperkalemia, hypermagnesemia, and hyperosmolality. We also found that several types of electrolyte disturbances were associated with AKI (hyponatremia, hypernatremia, hypochloremia, hypocalcemia, hypomagnesemia, hypophosphatemia and hyperphosphatemia).
However, some limitations must be considered. Firstly, although we attempted to control for confounders by using multiple logistic regression analysis and Cox proportional hazards model after propensity score matching, this retrospective observational design does not establish causal relationships of acid-base disturbances with the development of AKI and hospital mortality. Secondly, only non-respiratory patients were considered in this study, which may lead to potential bias and we could not give a reasonable explanation for different effect sizes of decreased CO2CP on various underlying diseases in our study. Thirdly, although we tried to collect the data about the intervention of acid-base disturbances, for example, the administration of sodium bicarbonate and fluid management, it was too hard to obtain these data for a retrospective study. Further well-designed and prospective research is needed.
Metabolic acidosis is an independent risk factor for the development of AKI and hospital mortality. In non-respiratory patients without ABG, decreased CO2CP is also an independent contributor to AKI and mortality, and can be used as an indicator of metabolic acidosis. Further ABG test is needed, and close attention should be paid to high-risk patients with decreased CO2CP.
Acknowledgements
This study was supported by the Shanghai Key Discipline Construction Project on the Fourth Round of Three-year Action Plan for Public Health Systems: Subject of Dialysis and Body Fluids (15GWZK0502), Grant of Chinese Ministry of Health 2013 and 14DZ2260200 from Shanghai Science and Technology Committee Foundation (Shanghai Key Laboratory of Kidney Diseases and Blood Purification).
References
- 1.Gunnerson KJ, Saul M, He S, Kellum JA. Lactate versus non-lactate metabolic acidosis: A retrospective outcome evaluation of critically ill patients. Crit Care. 2006;10:R22. doi: 10.1186/cc3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva J, únior GB, Edf F Daher, Mota RM, Menezes FA. Risk factors for death among critically ill patients with acute renal failure. Sao Paulo Med J. 2006;124:257–263. doi: 10.1590/S1516-31802006000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bockenkamp B, Vyas H. Understanding and managing acute fluid and electrolyte disturbances. Current Paediatrics. 2003;13:520–528. doi: 10.1016/j.cupe.2003.08.008. [DOI] [Google Scholar]
- 4.Chen W, Abramowitz MK. Metabolic acidosis and the progression of chronic kidney disease. BMC Nephrol. 2014;15:55. doi: 10.1186/1471-2369-15-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 6.Peppicelli S, Bianchini F, Contena C, Tombaccini D, Calorini L. Acidic pH via NF-κB favours VEGF-C expression in human melanoma cells. Clin Exp Metastasis. 2013;30:957–967. doi: 10.1007/s10585-013-9595-4. [DOI] [PubMed] [Google Scholar]
- 7.Magalhães PA, de Brito TS, Freire RS, da Silva MT, dos Santos AA, Vale ML, de Menezes DB, Martins AM, Libório AB. Metabolic acidosis aggravates experimental acute kidney injury. Life Sci. 2016;146:58–65. doi: 10.1016/j.lfs.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 9.Engle JE. Clinical physiology of acid-base and electrolyte disorders. JAMA. 1990;263:2375–2376. doi: 10.1001/jama.1990.03440170097050. [DOI] [Google Scholar]
- 10.Fencl V, Jabor A, Kazda A, Figge J. Diagnosis of metabolic acid-base disturbances in critically ill patients. Am J Respir Crit Care Med. 2000;162:2246–2251. doi: 10.1164/ajrccm.162.6.9904099. [DOI] [PubMed] [Google Scholar]
- 11.Fulop M. Flow diagrams for the diagnosis of acid-base disorders. J Emerg Med. 1998;16:97–109. doi: 10.1016/S0736-4679(97)00247-3. [DOI] [PubMed] [Google Scholar]
- 12.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 13.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Dobre M, Yang W, Chen J, Drawz P, Hamm LL, Horwitz E, Hostetter T, Jaar B, Lora CM, Nessel L, et al. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: A report from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. 2013;62:670–678. doi: 10.1053/j.ajkd.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewitt J, Uniacke M, Hansi NK, Venkat-Raman G, McCarthy K. Sodium bicarbonate supplements for treating acute kidney injury. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD009204.pub2. CD009204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, Tsurumi M, Matsushita M, Okazaki H, Yamamoto Y, Yokoyama S, et al. Clinical significance of acid-base balance in an emergency setting in patients with acute heart failure. J Cardiol. 2012;60:288–294. doi: 10.1016/j.jjcc.2012.06.004. [DOI] [PubMed] [Google Scholar]