Abstract
The present study aimed to investigate the neuroprotective effects of phenylethanol glycosides (PhGs) on H2O2- and β-amyloid peptide (Aβ)1–42-induced injury of PC12 cells as an in vitro model of Alzheimer's disease (AD). The optimal induction conditions were established through screening of various incubation times and concentrations. PC12 cells were treated with 0.5 µM Aβ1–42 and H2O2 in the presence of PhGs for 24 h and the cell viability was then evaluated by an MTT assay; lactate dehydrogenase (LDH) release and malondialdehyde (MDA) content were also measured. The optimal conditions for establishing the AD model were the treatment of PC12 cells with 0.5 µM Aβ1–42 for 48 h, or with 25 µM H2O2 dissolved in DMEM with PBS. PhGs at concentrations of 5, 25 and 50 µg/ml increased the viability and decreased LDH and MDA release by PC12 cells injured with Aβ1–42 or H2O2. In conclusion, the model of Aβ1–42- and H2O2-induced PC12 cell injury was successfully established. PhGs were shown to have a significant neuroprotective effect against Aβ1–42- or H2O2-induced cell injury.
Keywords: phenylethanol glycosides, PC12 cells, H2O2, β-amyloid peptide1–42, neuroprotection
Introduction
Alzheimer's disease (AD), a neurodegenerative disorder with the clinical characteristics of progressive memory loss and cognitive function impairment (1), is the most common cause of dementia worldwide (2). The financial costs are immense due to the prevalence of AD (3). Hence, it is urgent to develop appropriate means for the management and prevention of AD.
The pathogenesis of AD is closely associated with the accumulation of neurofibrillary tangles and senile plaques (SPs) in affected brain regions (4,5). β-amyloid peptide (Aβ), the major component of SPs, has been reported to have a causative role in the progression of AD as has a toxic effect on neuronal cells (6). Aβ fragments, including Aβ1–40, Aβ25–35 and Aβ1–42, have been generated through the split of amyloid precursor protein (7). The neurotoxicity of Aβ1–42 was found to be significantly higher than that of Aβ25–35 and Aβ1–40, and Aβ1–42 is able to induce an AD model (1,8–10). Oxidative stress may be involved in the pathogenesis of AD and is the major mechanism underlying Aβ-induced neurotoxicity (11–13). Several studies suggested that Aβ1–42 caused intracellular accumulation of reactive oxygen species (ROS), leading to lipid and protein oxidation, DNA damage and activation of cell cycle checkpoint signaling (1,14,15). Excessive amounts of H2O2 may lead to oxidative damage and induce apoptosis of PC12 cells (16). Therefore, targeting of oxidative stress may be a promising approach for the development of therapeutic strategies for inhibiting Aβ-induced neurotoxicity in AD.
Herba (H.) Cistanche, a Chinese herbal medicine commonly used in mainland China for nourishing the kidneys and replenishing essence and blood, has been used to treat memory loss and senile constipation (17). Phenylethanoid glycoside (PhG), one of the major constituents in H. Cistanche, improves the impairment of neuronal apoptosis caused by Aβ25–35 via its anti-oxidant effects (18,19). A previous study identified five major components from total PhGs, namely acteoside, 2′-acetylacteoside, echinacoside, cistanosides and isoacteoside (20). Among these components, acteoside and echinacoside have been reported to have neuroprotective effects on Aβ25–35- or H2O2-induced neurotoxicity (21–23). For instance, Wu et al (24) suggested that acteoside and echinacoside ameliorated cognitive dysfunction caused by Aβ1–42. The present study aimed to investigate the protective effects of PhGs in an in vitro rat cell model of AD.
Materials and methods
Preparation of PhGs
Total PhGs were extracted from H. Cistanche as previously described (25). The air-dried stem of H. Cistanche was powdered and extracted by percolation with 80% EtOH. The percolate was evaporated under reduced pressure, followed by re-suspension in an appropriate amount of H2O2 (100 µmol/l). The mixture was isolated on an SP-825 macroporous resin column (Mitsubishi Chemical, Tokyo, Japan) and eluted with 0, 30, 50, 70 and 90% EtOH in water. To obtain the PhG-rich fraction, the 30–50% EtOH eluents were concentrated and dried under reduced pressure. Ultraviolet (UV) spectrophotometry was performed to determine the total PhGs. The content of echinacoside and acteoside was determined by high-pressure liquid chromatography according to a previous protocol (26). A Hypersil ODS-2 column (4.6×250 mm, 5 µm; Dalian Elite Analytical Instruments, Co., Ltd., Dalian, China) was used and maintained at room temperature. The mobile phases were methyl cyanides and water containing 0.4% phosphoric acid (v/v; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The flow rate was 0.75 ml/min and the wavelength was set to 333 nm.
Cell culture and drug treatment
The PC12 rat pheochromocytoma cell line was provided by Dr He Chunhui, the Medical School, Xinjiang Medical University (Urumqi, China). Cells were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (Sangon Biotech Co. Ltd., Shanghai, China), 100 U/ml penicillin and 100 U/ml streptomycin in an incubator at 37°C containing 5% CO2 and 95% air. Upon reaching 80% confluence, the cells were treated with 0.25% trypsin and passaged.
In order to eliminate the interference of the drug itself on the growth of PC12 cells, a toxicity experiment was performed. In brief, PC12 cells (3×104 cells/ml) were seeded in 96-well plates at 100 µl/well and incubated at 37°C overnight. After discarding the supernatant, 200 µl complete DMEM was added in the blank group, while cells in the intervention groups were treated by PhGs at various doses (5, 25, 50, 75, 100, 125, 150, 175 and 200 µg/ml). Following incubation of the cells at 37°C for 48 h, 20 µl MTT solution (Sigma-Aldrich; Merck KGaA) was added to each well. After incubation for 4 h, the supernatant was discarded and 150 µl dimethylsulfoxide was added per well, followed by agitation for 10 min. The optical density (OD) values at 490 nm were detected using an ELISA plate reader.
Aβ1–42-induced PC12 cell injury
Aβ1–42 peptide purchased from Bioss Biotech (Beijing, China) was dissolved in water (100 µg/ml). Subsequently, the mixture was incubated at 37°C for 4 days and stored at 4°C prior to use.
PC12 cells were seeded in 96-well plates (3x104 cells in 100 µl per well). After culture for 24 h for adherence, 50 µl Aβ1–42 at various final concentrations (0, 0.25, 0.5, 1, 1.5 or 2 µM) dissolved in serum-free DMEM was added, followed by incubation for 24, 48, 72 or 96 h. Cell viability was evaluated by an MTT assay. The optimal Aβ1–42 concentration was 0.5 determined to be µM.
PC12 cells (3x104 cells per well) were treated with various doses of PhGs (0, 0.5, 5, 25 or 50 µg/ml) in the presence of 0.5 µM Aβ1–42 for 24 h. Cell viability was evaluated by an MTT assay.
H2O2-induced PC12 cell injury
PC12 cells were plated seeded in 96-well plates (3x104 cells in 100 µl per well). After culture for 24 h for adherence, 100 µl H2O2 at various final concentrations (0, 25, 50, 100, 200, 300, 400 and 500 µM) dissolved in DMEM with or without PBS (0.01 mol/l) was added, followed by incubation for 24 h. Cell viability was evaluated by an MTT assay. The optimal H2O2 concentration and solvent was determined to establish the in vitro model of AD.
PC12 cells (3x104 cells per well) were treated with various doses of PhGs (0, 0.5, 5, 25 and 50 µM). After culture for 24 h for adherence, PC12 cells were treated with 100 µl H2O2 dissolved in DMEM with PBS in the presence of PhGs for 24 h. The cell viability was evaluated by an MTT assay.
Lactate dehydrogenase (LDH) release assay
Cell injury was assessed through measuring the LDH activity in the supernatant of PC12 cells using an LDH kit according to the manufacturer's protocol (cat. no. 20150604; Nanjing Jiancheng Bioengineering Institute, Nanjing, China). In brief, double-distilled H2O, 0.2 µmol/ml pyruvic acid, matrix buffer and coenzyme I buffer were added in sequence at 48 h after drug treatment. After incubation at 37°C for 15 min, 2,4-dinitro-phenylhydrazine was added. Subsequently, 250 µl of a 0.4 M NaOH solution was added to each well. The supernatant was collected after incubation for 30 min at room temperature. The absorbance at 450 nm was then measured with a microplate reader.
Measurement of malondialdehyde (MDA)
MDA was measured in the supernatant of PC12 cells using commercial kit (cat. no. 20150604; Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's protocol In brief, dehydrated alcohol and other reagents were added in order, followed by incubation in a water bath at 95°C for 40 min. The mixture was centrifuged at 1,006 × g and 25°C for 10 min after cooling. The supernatant was then used to determine the MDA content. Absorbance was subsequently measured with a microplate reader at 532 nm.
Assessment of protective effects of echinacoside and acteoside against AD in vitro
PC12 cells were seeded at a density of 3x104 cells/well in 96-well plates (100 µl/well). Cells were incubated with drugs including echinacoside (cat. no. 111670-200503; National Institutes for Food and Drug Control, Beijing, China) and acteoside (cat. no. 111530-200505; National Institutes for Food and Drug Control) at various concentrations (0.5, 25 and 50 µg/ml). Subsequently, the cells were treated with Aβ1–42 or H2O2 for 24 h and the cell viability was measured by an MTT assay.
Statistical analysis
Values are expressed as the mean ± standard deviation. Student's t-test was used for inter-group comparisons. Statistical analyses were performed using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Quantification of PhGs from H. Cistanches
The UV spectra of the PhGs extracted as well as standard solutions of echinacoside and acteoside were recorded, and the results showed that the UV spectra were consistent (Fig. 1). UV spectrophotometry showed that the PhG content was 87.6%. The HPLC results showed that the contents of echinacoside and acteoside were 37.7 and 17.8%, respectively (Fig. 2).
Figure 1.
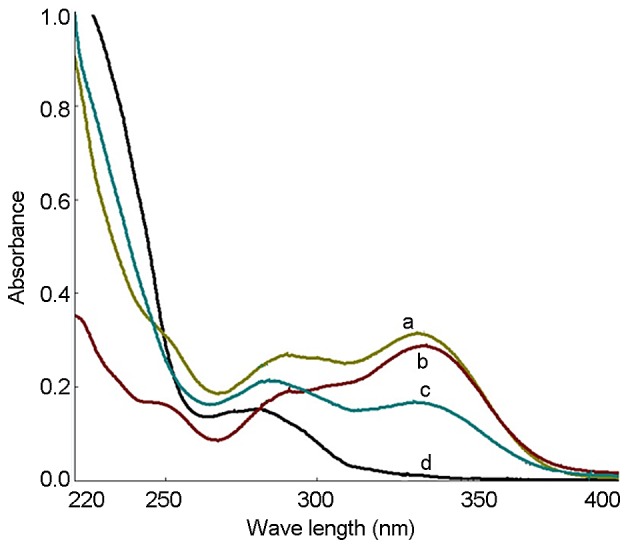
Ultraviolet spectrum of (a) echinacoside, (b) acteoside (c) phenylethanoid glycosides extracted in the present study, and (d) blank control.
Figure 2.
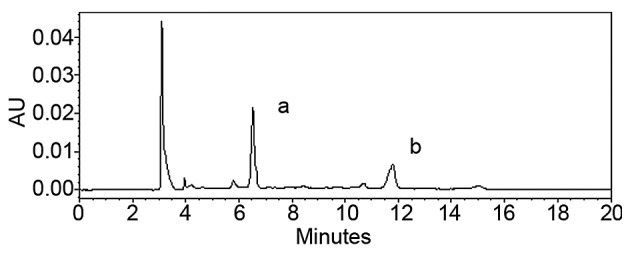
High-performance liquid chromatography spectrum showing (a) echinacoside and (b) acteoside.
Determination of the ideal PhG concentration
Compared with the blank group, PhG at 75, 100, 125, 150, 175 and 200 µg/ml had a significant inhibitory effect on PC12 cells (P<0.05), while PhG at 5, 25 and 50 µg/ml showed low toxicity on PC12 cells, and the cell viability was >80% (Fig. 3). Thus, PhGs at the concentration of 5, 25 and 50 µg/ml was used for treating PC12 cells in subsequent experiments due to not affecting the cell viability.
Figure 3.
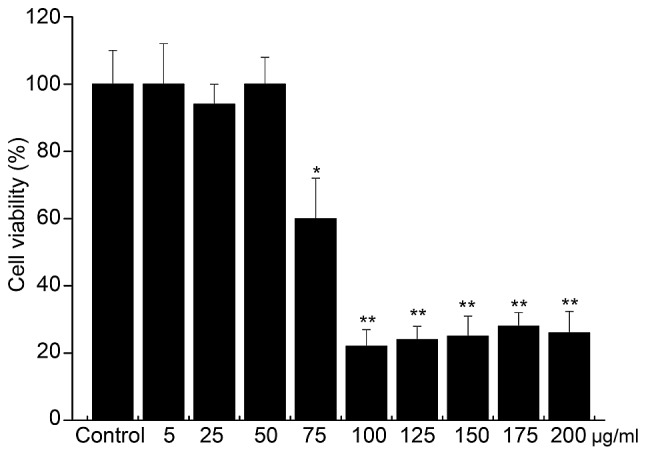
Dose screening of phenylethanoid glycosides to determine an optimal concentration to not affect cell viability. *P<0.05, vs. control; **P<0.05, vs. control.
Aβ1–42-induced PC12 cell injury
Compared with that in the control group, the cell viability in the 0.5 µM Aβ1–42 injury group was 63% (P<0.05). The cell viability was decreased by Aβ1–42 in a concentration-dependent manner, and the viability was <50% in the 1, 1.5 and 2 µM Aβ1–42 injury groups (Fig. 4). Thus, treatment with 0.5 µM Aβ1–42 for 48 h was determined to be the optimal condition for establishing the in vitro AD model.
Figure 4.
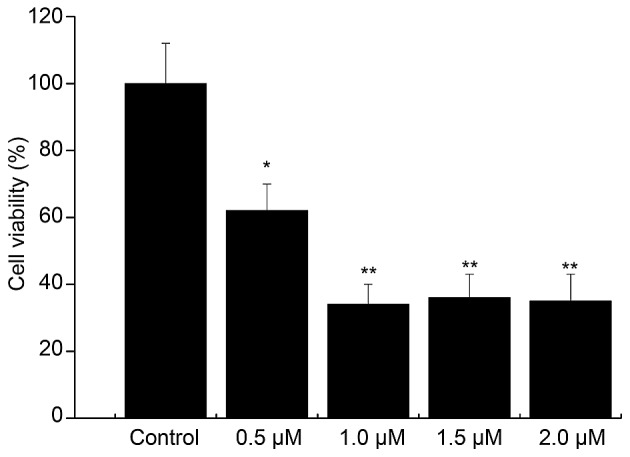
Screening of â-amyloid peptide1–42 damage conditions. *P<0.05, vs. control; **P<0.05, vs. control.
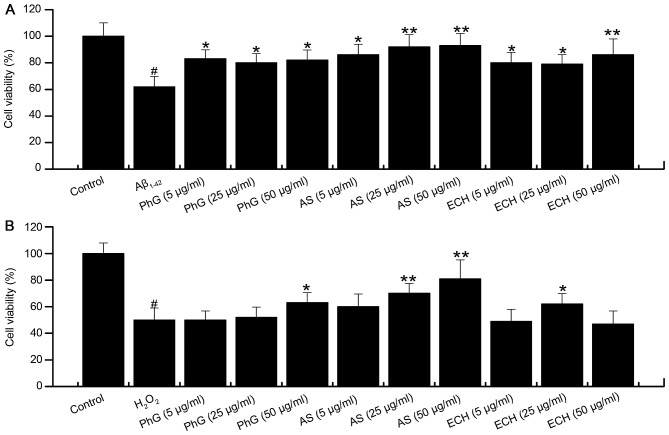
The activity of PC12 cells treated with 0.5 µM Aβ1–42 in the presence of safe doses of PhGs (5, 25 and 50 µg/ml) for 24 h was also determined. Compared with the model group (P<0.01), PhGs showed a significant neuroprotective effect on PC12 cells. The cell viability was rescued by PhGs in a dose-dependent manner (Fig. 5).
Figure 5.
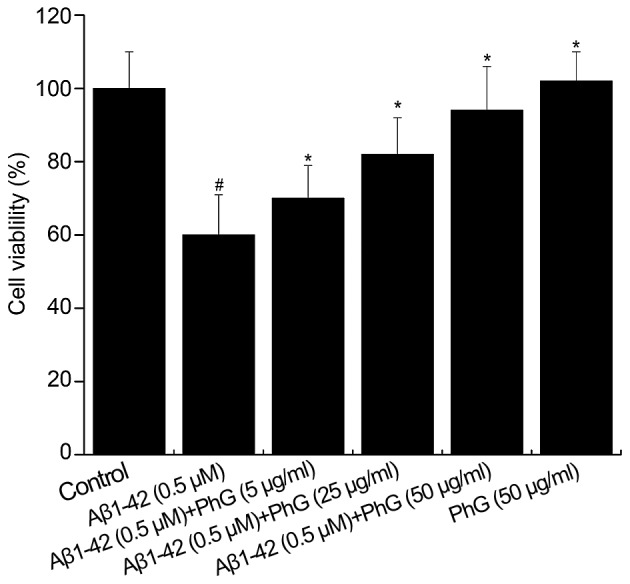
Cell viability of PC12 cells treated with Aβ1–42 and PhGs. #P<0.05, vs. control group; *P<0.05, vs. model group. Aβ, â-amyloid peptide; PhGs, phenylethanoid glycosides.
H2O2-induced PC12 cell injury
The viability of PC12 cells treated with 25 µM H2O2 dissolved in DMEM with PBS was 56.43%. The viability of PC12 cells treated with 200 µM H2O2 dissolved in DMEM without PBS was 71.64% (Table I). Thus, PC12 cells treated with 25 µM H2O2 dissolved in DMEM with PBS was the selected as the optimal condition for establishing the AD model.
Table I.
Cell viability (%) after H2O2 treatment.
| Solvent | ||
|---|---|---|
| H2O2 concentration (µM) | DMEM+PBS | DMEM |
| 0 | 100 | 100 |
| 25 | 56.43a | 107.61 |
| 50 | 54.81a | 108.13 |
| 100 | 52.79a | 105.01 |
| 200 | 46.30a | 71.64a |
| 300 | 53.90a | 60.06a |
| 400 | 44.28a | 61.00a |
| 500 | 43.68 | 60.17a |
P<0.05 vs. control group. PhGs, phenylethanoid glycosides; DMEM, Dulbecco's modified Eagle's medium; PBS, phosphate-buffered saline.
Compared with the control group, the cell viability in the model group was 48.8% (P<0.05). Compared with the model group, PhGs had a significant neuroprotective effect on PC12 cells. The cell viability was dose-dependently increased by PhGs, and the viability of PC12 cells treated with PhGs at concentrations of 5, 25 and 50 µg/ml was 54, 57 and 64%, respectively (Table II).
Table II.
Cell viability after drug interference.
| Group | Cell viability (%) |
|---|---|
| Control | 100 |
| Model | 48.83a |
| PhG 5 µg/ml | 53.94b |
| PhG 25 µg/ml | 57.39b |
| PhG 50 µg/ml | 64.00c |
P<0.05, vs. control group
P<0.05, vs. model group
P<0.05, vs. model group. PhGs, phenylethanoid glycosides.
PhGs inhibit injury-induced LDH release by PC12 cells
Compared with the control group, the LDH content of the supernatant of injured PC12 cells was increased, which was inhibited by PhGs in a concentration-dependent manner. This result indicated that PhGs have a significant neuroprotective effect on PC12 cells (Fig. 6).
Figure 6.
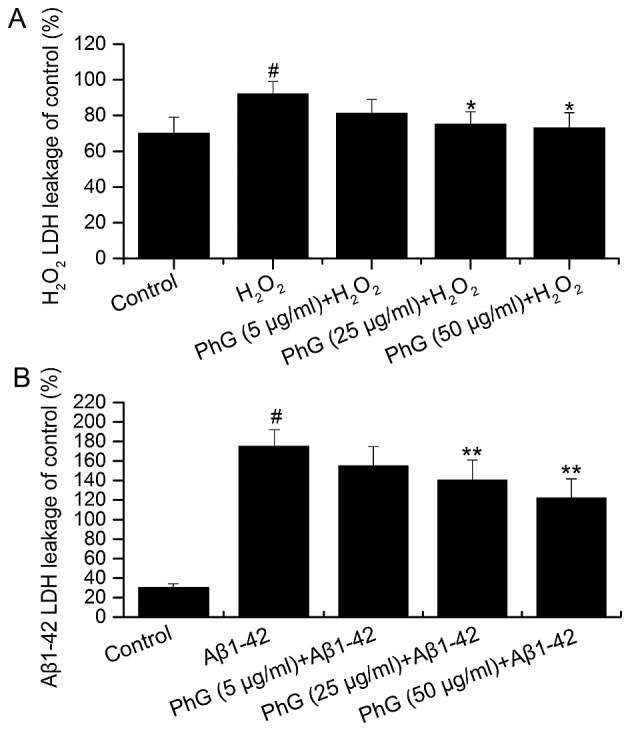
LDH release after (A) H2O2 interference and (B) Aβ1–42 interference in an in vitro model of Alzheimer's disease. #P<0.05, vs. control group; *P<0.05, vs. model group; **P<0.05, vs. model group. LDH, lactate dehydrogenase; Aβ, â-amyloid peptide; PhGs, phenylethanoid glycosides.
PhGs inhibit injury-induced MDA production by PC12 cells
Compared with the control group, the MDA content in the supernatant of injured PC12 cells was increased, which was inhibited by PhGs in a concentration-dependent manner. This result indicated that PhGs have a significant neuroprotective effect on PC12 cells (Fig. 7).
Figure 7.
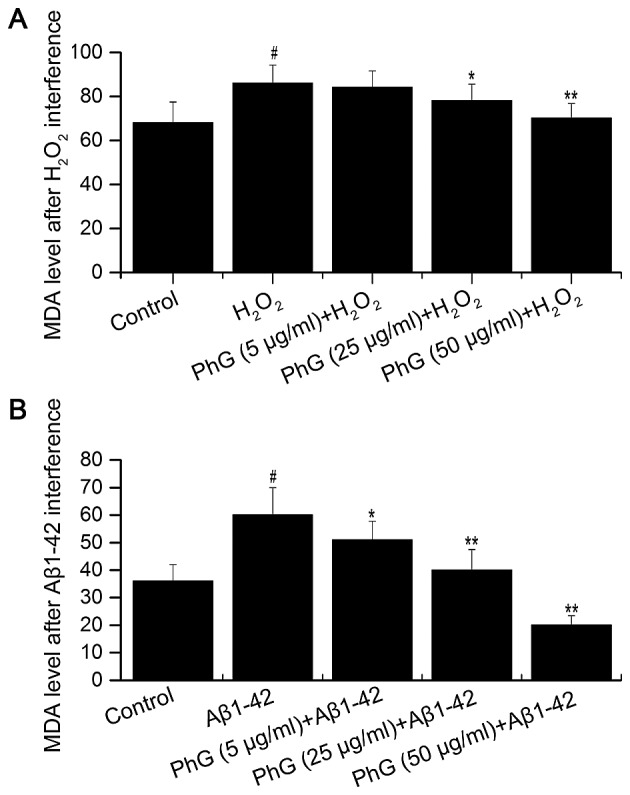
Determination of MDA after (A) H2O2 interference and (B) Aβ1–42 interference in an in vitro model of Alzheimer's disease. #P<0.05, vs. control group; *P<0.05, vs. model group; **P<0.05, vs. model group. MDA, malondialdehyde; Aβ, â-amyloid peptide; PhGs, phenylethanoid glycosides.
PhG and its components echinacoside and acteoside rescue the viability of injured PC12 cells
Compared with the model group, treatment with acteoside significantly increased the viability of Aβ1–42-injured PC12 cells in a dose dependent manner. PhGs and echinacoside also significantly increased the viability of Aβ1–42-injured PC12 cells at all concentrations tested (Fig. 8A).
Figure 8.
Protective effects of PhGs, ECH and AS on the cells treated with (A) Aβ1–42 and (B) H2O2. #P<0.05, vs. control group; *P<0.05, vs. model group; **P<0.05, vs. model group. Aβ, â-amyloid peptide; PhGs, phenylethanoid glycosides; ECH, echinacoside; AS, acteoside.
Compared with the model group, acteoside significantly increased the viability of PC12 cells treated with H2O2. PhGs also increased the viability of PC12 cells treated with H2O2, while the effect was not significant at concentrations of 5 and 25 µg/ml (Fig. 8B). In addition, echinacoside increased the cell viability at 25 µg/ml.
In conclusion, acteoside, PhGs and echinacoside exerted significant neuroprotective effects on PC12 cells subjected to injury with Aβ1–42 or H2O2.
Discussion
Oxidative stress is the major mechanism underlying Aβ-mediated neurotoxicity in AD (11–13). Therefore, targeting oxidative stress may represent an approach for the treatment of AD. In the present study, an in vitro model of AD comprising Aβ1–42- and H2O2-induced PC12 cell injury was successfully established. Results of the MTT, LDH and MDA assays showed that PhGs increased the cell viability, and decreased LDH and MDA release by PC12 cells subjected to injury. It can be concluded that PhGs have significant neuroprotective effects on PC12 cells.
In order to reduce the effects of PhGs themselves on PC12 cell growth and prevent abnormal proliferation, the safe dose of PhGs was determined in a screening assay. The results showed that PhGs at 75, 100, 125, 150, 175 and 200 µg/ml had a significant inhibitory effect on PC12 cells (P<0.05, P<0.01), while cell viability remained >80% at concentrations of 5, 25 and 50 µg/ml. Thus, PhGs at the concentration of 5, 25 and 50 µg/ml were safe for PC12 cells.
The injury by Aβ1–42 was affected by certain factors, including the solvent, incubation time and product quality. In the present study, Aβ1–42 peptide was dissolved in water (100 µg/ml) and incubated at 37°C for 4 days in a CO2 incubator prior to use. PC12 cells were treated with Aβ1–42 at concentrations of 0.5, 1, 1.5 and 2 µM. The results showed that the cell viability was decreased with the increase of Aβ1-42, and the viability was <50% in the 1, 1.5 and 2 µM Aβ1–42 injury groups. Thus, treatment of PC12 cells with 0.5 µM Aβ1–42 for 48 h was determined to be the optimal condition for establishing the AD model. Aβ25–35 has been commonly used to establish AD models due to low cost and simple operation (27–29). The neurotoxicity of Aβ1–42 is significantly higher than that of Aβ25–35, and Aβ1–42 is therefore the optimal Aβ fragment for establishing an AD model (1,8–10).
H2O2 is an oxidizer and excessive H2O2 may cause oxidative damage and induce cell apoptosis (30). In the present study, PC12 cells were treated with 25–500 µM H2O2 dissolved in DMEM with or without PBS. The results showed that H2O2 dissolved in DMEM without PBS caused abnormal proliferation of PC12 cells. Thus, treatment of PC12 cells with 25 µM H2O2 dissolved in DMEM with PBS was the optimal condition for establishing the AD model. Aβ1–42-induced injury was greater than H2O2-induced injury due to poor stability of H2O2 and solvent effects.
When the cell is damaged, LDH leakage into the culture medium is significantly increased. ROS is known to cause the production of MDA. The content of MDA and LDH therefore reflect the amount of oxidative damage. In the present study, damage-induced LDH and MDA activity was decreased with increasing doses of PhGs. These results indicated that PhGs have a significant neuroprotective effect on PC12 cells. The MTT assay showed that PhGs exhibited a dose-dependent neuroprotective effect on PC12 cells.
In conclusion, an in vitro model of AD comprising Aβ1–42- and H2O2-induced PC12 cell injury was successfully established. Treatment with PhGs increased the cell viability, and decreased LDH and MDA release by PC12 cells treated with Aβ1–42 or H2O2. PhGs had a significant neuroprotective effect on Aβ1–42- or H2O2-induced cell injury.
References
- 1.Qu M, Zhou Z, Xu S, Chen C, Yu Z, Wang D. Mortalin overexpression attenuates beta-amyloid-induced neurotoxicity in SH-SY5Y cells. Brain Res. 2011;1368:336–345. doi: 10.1016/j.brainres.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 2.Uzun S, Kozumplik O, Folnegović-Smalc V. Alzheimer's dementia: current data review. Collegium antropologicum. 2011;35:1333–1337. [PubMed] [Google Scholar]
- 3.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimer's & dementia. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 4.Castellani RJ, Rolston RK, Smith MA. Alzheimer disease. Disease-a-month. 2010;56:484–546. doi: 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gouras GK, Tsai J, Naslund J, et al. Intraneuronal Aβ42 accumulation in human brain. The American journal of pathology. 2000;156:15–20. doi: 10.1016/S0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen C, Chen Y, Liu H, et al. Hydrogen peroxide promotes Aβ production through JNK-dependent activation of γ-secretase. Journal of Biological Chemistry. 2008;283:17721–17730. doi: 10.1074/jbc.M800013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shih P-H, Wu C-H, Yeh C-T, Yen G-C. Protective effects of anthocyanins against amyloid β-peptide-induced damage in neuro-2A cells. Journal of agricultural and food chemistry. 2011;59:1683–1689. doi: 10.1021/jf103822h. [DOI] [PubMed] [Google Scholar]
- 9.Figueiredo CP, Bicca MA, Latini A, Prediger R, Medeiros R, Calixto JB. Folic acid plus α-tocopherol mitigates amyloid-β-induced neurotoxicity through modulation of mitochondrial complexes activity. Journal of Alzheimer's disease: JAD. 2010;24:61–75. doi: 10.3233/JAD-2010-101320. [DOI] [PubMed] [Google Scholar]
- 10.Dumont M, Lin MT, Beal MF. Mitochondria and antioxidant targeted therapeutic strategies for Alzheimer's disease. Journal of Alzheimer's disease: JAD. 2010;20:S633. doi: 10.3233/JAD-2010-100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnen JA, Breitner JC, Lovell MA, Markesbery WR, Quinn JF, Montine TJ. Free radical-mediated damage to brain in Alzheimer's disease and its transgenic mouse models. Free Radical Biology and Medicine. 2008;45:219–230. doi: 10.1016/j.freeradbiomed.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 13.Trushina E, McMurray C. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145:1233–1248. doi: 10.1016/j.neuroscience.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 14.Huang S-H, Lin C-M, Chiang B-H. Protective effects of Angelica sinensis extract on amyloid β-peptide-induced neurotoxicity. Phytomedicine. 2008;15:710–721. doi: 10.1016/j.phymed.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 15.Burhans WC, Heintz NH. The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free Radical Biology and Medicine. 2009;47:1282–1293. doi: 10.1016/j.freeradbiomed.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Xue HY, Gao GZ, Lin QY, Jin LJ, Xu YP. Protective effects of aucubin on H2O2-induced apoptosis in PC12 cells. Phytotherapy Research. 2012;26:369–374. doi: 10.1002/ptr.3562. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Gou C, Yang H, Qiu J, Gu T, Wen T. Echinacoside ameliorates D-galactosamine plus lipopolysaccharide-induced acute liver injury in mice via inhibition of apoptosis and inflammation. Scandinavian journal of gastroenterology. 2014;49:993–1000. doi: 10.3109/00365521.2014.913190. [DOI] [PubMed] [Google Scholar]
- 18.Liu, F-X, Wang X-w, Luo L, Xin H, Na B, Wang X-F. The effects of glycosides of cistanche on learning and memory in beta-amyloid peptide induced Alzheimers disease in mice and its possible mechanism. Chinese Pharmacological Bulletin. 2006;22:595. [Google Scholar]
- 19.Bao B, Tang X, Tian H, Tong Y, Wu W, Hong Y. Antioxidant activity of extracts from desert living Cistanche tubulosa (Schrenk) R. Wright Shanghai J Tradit Chin Med. 2010;44:68–71. [Google Scholar]
- 20.Jiang Y, Li S, Wang Y, Chen X, Tu P. Differentiation of Herba Cistanches by fingerprint with high-performance liquid chromatography-diode array detection-mass spectrometry. Journal of Chromatography A. 2009;1216:2156–2162. doi: 10.1016/j.chroma.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Xu Y, Yan J, et al. Acteoside protects human neuroblastoma SH-SY5Y cells against β-amyloid-induced cell injury. Brain research. 2009;1283:139–147. doi: 10.1016/j.brainres.2009.05.101. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Q, Gao J, Li W, Cai D. Neurotrophic and neurorescue effects of Echinacoside in the subacute MPTP mouse model of Parkinson's disease. Brain research. 2010;1346:224–236. doi: 10.1016/j.brainres.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Kuang R, Sun Y, Yuan W, Lei L, Zheng X, Food Z. Protective effects of echinacoside, one of the phenylethanoid glycosides, on H2 O2-induced cytotoxicity in PC12 cells. neurodegenerative diseases. 2009;8:9. doi: 10.1055/s-0029-1185806. [DOI] [PubMed] [Google Scholar]
- 24.Wu C-R, Lin H-C, Su M-H. Reversal by aqueous extracts of Cistanche tubulosa from behavioral deficits in Alzheimer's disease-like rat model: relevance for amyloid deposition and central neurotransmitter function. BMC complementary and alternative medicine. 2014;14:202. doi: 10.1186/1472-6882-14-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai RL, Yang MH, Shi Y, Chen J, Li YC, Qi Y. Antifatigue activity of phenylethanoid-rich extract from Cistanche deserticola. Phytotherapy research. 2010;24:313–315. doi: 10.1002/ptr.2927. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Zong C, Liu F, et al. Evaluation of the Intestinal Transport of a Phenylethanoid Glycoside-Rich Extract from Cistanche deserticola across the Caco-2 Cell Monolayer Model. PloS one. 2015;10:e0116490. doi: 10.1371/journal.pone.0116490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoon J-H, Youn K, Ho C-T, Karwe MV, Jeong W-S, Jun M. p-Coumaric Acid and Ursolic Acid from Corni fructus Attenuated β-Amyloid 25–35-induced Toxicity through Regulation of the NF-κB Signaling Pathway in PC12 cells. Journal of agricultural and food chemistry. 2014;62:4911–4916. doi: 10.1021/jf501314g. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Sun X, Gong T, He Q, Zhang Z. Antioxidant and Antiapoptotic Effects of 1, 1′-(Biphenyl-4,4′-diyl)-bis (3- (dimethylamino)-propan-1-one) on protecting PC12 cells from Aβ-induced injury. Molecular pharmaceutics. 2013;11:428–435. doi: 10.1021/mp400395g. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Su Y, Run X, et al. Pretreatment of PC12 cells with 17β-estradiol prevents Aβ-induced down-regulation of CREB phosphorylation and prolongs inhibition of GSK-3β. Journal of Molecular Neuroscience. 2013;50:394–401. doi: 10.1007/s12031-012-9938-7. [DOI] [PubMed] [Google Scholar]
- 30.Jiang B, Liu J, Bao Y, An L. Catalpol inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Toxicon. 2004;43:53–59. doi: 10.1016/j.toxicon.2003.10.017. [DOI] [PubMed] [Google Scholar]