Abstract
Arrhythmias are the common complications following cardiac surgery and contribute to hemodynamic instability, cognitive impairment, thromboembolic events, and congestive heart failure. Prevention of atrial fibrillation following cardiac surgery reduces morbidity and among the many available preventive approaches dexmedetomidine shows many positive effects on cardiovascular stability. Even though many studies indicated the beneficial effects of dexmedetomidine, the power of the analysis and conclusion of these studies is rather weak due to relatively smaller number of patients in these studies. In the present meta-analysis, we included a large number of patients, both children and adults, undergoing cardiac surgery, to address the efficacy of dexmedetomidine. Several databases were searched to identify clinical studies comparing the efficacy of dexmedetomidine in myocardial protection in patients undergoing cardiac surgery. Cardiac function related parameters including heart rate, blood pressure, tachycardia, arrhthmias, and bradycardia were measured. In accordance with the selection criteria, a total of 18 studies published between 2003 and 2016, with a total of 19,225 patients were included in the present meta-analysis. Dosage of dexmedetomidine was in the range of 0.5–1 µg/kg body weight loading followed by continuous infusion at a rate of 0.2–0.7 µg/kg/h. Dexmedetomidine treatment was found to lower heart rate, systolic blood pressure, incidence of tachycardia and arrhythmias in both adult and pediatric patients, but elevated the risk of bradycardia. In conclusion, results of this meta-analysis indicate that dexmedetomidine is an efficacious cardioprotective drug in adults and children undergoing cardiac surgery.
Keywords: dexmedetomidine, cardiac surgery
Introduction
Atrial arrhythmias are the most common complications following cardiac surgery and contribute to hemodynamic instability, cognitive impairment, thromboembolic events, and congestive heart failure and prevention of atrial fibrillation following cardiac surgery reduces morbidity and mortality (1,2). During surgery and anesthesia, many patients undergo hemodynamic depression and this is more often seen in patients with hypertension or myocardial insufficiency (3). Many of the available clinical approaches for addressing this are not completely safe and dexmedetomidine has been shown to have many positive effects on cardiovascular stability in patients undergoing cardiac surgery (4,5). Dexmedetomidine, a highly selective and potent α2-adrenoreceptor agonist, with analgesic as well as anaesthetic effects also has other beneficial effects including reduction in the release of catecholamine (6), incidence of postoperative delirium (7,8), and also need for anesthetics (9,10). Because of these multiple beneficial effects dexmedetomidine is widely used in various surgeries and intensive care units. Many studies have shown that dexmedetomidine lowers the myocardial complications following cardiovascular surgery in adults (11,12). It has been reported that postoperative myocardial injury and arrhythmic events were decreased in patients administered dexmedetomidine (13).
Besides its use in adult patients, dexmedetomidine has also been found to be effective in children undergoing surgery for congestive heart failure in reducing post-operative cardiac complications (14). It has been observed that dexmedetomidine usage in children led to more stable intraoperative hemodynamics, reduced mechanical ventilation times and analgesia requirements along with lower incidence of agitation and delirium, similar to the effects in adults. However, perioperative bradycardia and hypotension are common in patients receiving dexmedetomidine and care must be taken to control the extent of bradycardia and hypotension. Considering that hypotension and bradycardia are often seen in patients undergoing cardiac surgery, the possibility that dexmedetomidine may further aggravate their incidence exists (3). Even though many studies with a smaller number of patients and also some meta-analysis studies indicated the beneficial effects of dexmedetomidine, the power of the analysis and conclusion of the studies is rather weak due to relatively smaller number of patients and included studies.
Materials and methods
Objectives
In the present meta-analysis, we included studies from 2003 to 2016 and a large number of patients, both children and adults, undergoing cardiac surgery, to address the efficacy of dexmedetomidine to control adverse effects of surgery and the results are strongly suggest that dexmedetomidine has many beneficial effects both in children as well as adults, following cardiac surgery.
Methods
Criteria for considering studies for this review
In the present meta-analysis, both randomized and non-randomized studies that examined and compared the efficacy of dexmedetomidine in patients of all ages, undergoing different types of cardiac surgery are included. All the studies included a control group of patients, receiving either placebo or other analgesic/anaesthetic, for comparison with dexmedetomidine group. Studies that addressed the effectiveness of dexmedetomidine during non-cardiac surgeries were not included. In order to increase the strength of the analysis, we included studies on children as well as adults as the type of surgeries and the outcomes measured were the same and thus relate to the effectiveness of dexmedetomidine.
Search methods
Publications describing the relevant information were searched in PubMed, Google Scholar, Scopus and Web of Science databases. Search MeSH terms included dexmedetomidine, cardiac surgery, atrial fibrillation, arrhythmias, myocardial infarction, cardiac protection, tachycardia, bradycardia, hypotension and systolic blood pressure. Papers published in English language in the last 20 years were searched. All the authors of these meta-analysis citations initially screened the articles for relevance at the title and abstract level and full reports and supplemental information files were retrieved as per the relevance of the selected study.
Data collection and analysis and quality assessment
The data extracted from the included studies are as follows: Institutional details where the study was conducted and the authors, publication details, the total number of patients studied and the number of patients in dexmedetomidine treated and control groups, age, and gender of the studied patients. Also, the type of cardiac surgery and the dosage of dexmedetomidine employed were collected. Besides, results on heart rate and systolic blood pressure before and after dexmedetomidine treatment, and tachycardia, bradycardia and atrial fibrillation events in control and dexmedetomidine treated groups were collected. Co-authors of this meta-analysis study independently screened all the data items and the full texts of all selected studies. The collected information was then combined and reviewed collectively and final data collected were decided by discussion and consensus.
Statistical analysis
The statistical analyses were performed by Review Manager (RevMan) version 5.3 (The Cochrane Collaboration, London, UK). The comparative effect of dexmedetomidine administration versus control (placebo or other anaesthetic/analgesic) was analyzed by Mantel-Haenszel statistics in the random-effect model. For heart rate and systolic blood pressure effects, mean difference was calculated and for tachycardia, bradycardia and atrial fibrillation analyses, odds ratios (OR) were derived at 95% confidence intervals (CI). P<0.05 was considered to indicate a statistically significant difference.
Results
A total of 1,005 studies were identified in database search, that contained the search terms, in title, abstract or main text. All the authors of this meta-analysis participated in deciding on the studies to be included the present meta-analysis. In accordance with the selection criteria, a total of 18 studies (13,15–31) published between 2003 and 2015 were selected. A total of 19,225 patients were included in these studies and formed part of the present meta-analysis. Flow chart of study selection (Fig. 1) gives the details of selected studies based on inclusion and exclusion criteria. The total number of patients treated with dexmedetomidine was 2,045 and the number of control patients was 17,734.
Figure 1.
Study selection flow chart.
The demographic characteristics of patients included in this study are given in Table I. In most of the included studies, there was a higher proportion of males and the age of patients ranged from less than a year to 71 years, as the age was not an exclusion criteria. All the patients were suffering from cardiac abnormalities and underwent surgery for ventricle or septal repair, or aortic defects, cardiopulmonary bypass, coronary artery bypass grafting or off-pump coronary artery bypass grafting surgery. Dosage of dexmedetomidine was in the range of 0.5–1 µg/kg body weight loading followed by continuous infusion at a rate of 0.2–0.7 µg/kg/h. The body weights of patients ranged from 3 to 89 kg (Table I).
Table I.
Demographic characteristics of patients in the included studies.
| Study Author (Refs.) | Year | Patients on Dex (% males) | Age | BW (kg) | Dex dose/administration | Cardiac diagnosis |
|---|---|---|---|---|---|---|
| Herr et al (15) | 2003 | 148 | 1.0 µg/kg loading; 0.2–0.2 µg/kg/h infusion | Cardiac surgeries | ||
| Corbett et al (16) | 2005 | 43 | 63 years | 89 | 1 µg/kg loading; 0.4 µg/kg continuous infusion | Coronary artery bypass grafting |
| Chrysostomou et al (17) | 2008 | 14 (79) | 2 months | 4 | 1.1±0.5 µg/kg loading; 0.9 µg/kg continuous infusion | Arrhythmias |
| Shehabi et al (20) | 2009 | 152 | 60 years | 0.1–0.1 µg/kg/h | Cardiac surgeries | |
| Chrysostomou et al (18) | 2010 | 51 (61) | 0.5 year | 3.4 | 1 µg/kg loading; 0.9 µg/kg continuous infusion | Ventricle and septal defects repair |
| Anger et al (21) | 2010 | 28 | 0.6±0.1 µg/kg/h | Cardiac surgeries | ||
| Hosokawa et al (22) | 2010 | 56 | 1 year | 0.4–0.4 µg/kg/h | Cardiac surgeries | |
| Chrysostomou et al (19) | 2011 | 32 (66) | 4.8 months | 5.3 | 1 µg/kg loading; 0.5 µg/kg continuous infusion | Ventricle, aortic, septal defects repair |
| Ji et al (23) | 2013 | 568 (72) | 63 years | 0.24–0.24 µg/kg/h | Cardiopulmonary bypass | |
| Ren et al (13) | 2013 | 81 (31) | 60 years | 0.2–0.2 µg/kg/h | Coronary artery bypass grafting | |
| Tosun et al (24) | 2013 | 18 (72) | 60.4 years | 77.5 | 0.5 µg/kg loading; 0.5 µg/kg/min continuous infusion | Coronary artery bypass grafting |
| Gu et al (25) | 2014 | 14 (86) | 5.2 years | 22.5 | 1 µg/kg loading; 0.01 µg/kg/min continuous infusion | Laparoscopic surgery |
| Rajput et al (26) | 2014 | 110 (86) | 2.8 years | 10 | 0.5 µg/kg loading; 0.5 µg/kg/min | Tetralogy of Fallot continuous infusion |
| Turan et al (27) | 2014 | 765 (70) | 58 years | Cardiac surgeries | ||
| Narisawa et al (28) | 2015 | 16 (75) | 71.3 years | 0.3±0.2 µg/kg/h during night time | Cardiac surgeries | |
| Jiang et al (29) | 2015 | 77 | 17.7 years | 0.25–0.25 µg/kg/h | Congenital heart disease | |
| Cheng et al (30) | 2015 | 29 | 6.6 months | 5.5 | 0.5–0.5 µg/kg/h | Congenital heart disease |
| Chi et al (31) | 2016 | 34 (65) | 56 years | 69 | 1 µg/kg loading; 0.6 µg/kg continuous infusion | Off-pump coronary artery bypass grafting surgery |
Comparative analysis of cardiac parameters before administration of dexmedetomidine, preoperatively and following treatment with dexmedetomidine post-operatively revealed that heart rate in most studies decreased significantly in dexmedetomidine receiving patients, both young and old (Table II and Fig. 2). Mean difference analysis showed that dexmedetomidine treatment favors lower heart rate (P<0.00001). Similarly, another cardiac parameter, systolic blood pressure, showed a decline in many studies and mean difference analysis that dexmedetomidine has a strong tendency for lowering systolic blood pressure in both adult and pediatric patients (Table II; Fig. 3, P<0.0006). These analyses confirmed the earlier meta-analysis results in a small group of studies with much lower number of pediatric patients (14).
Table II.
Effect of dexmedetomidine treatment on heart rate and systolic blood pressure of patients undergoing cardiac surgery.
| Heart rate | Systolic blood pressure | ||||
|---|---|---|---|---|---|
| Study Author (Refs.) | Year | Before Dex | Post-Dex | Before Dex | Post-Dex |
| Chrysostomou et al (17) | 2008 | 145±12 | 124±8 | 80.5±17.3 | 73.4±10.1 |
| Chrysostomou et al (18) | 2010 | 140±22 | 115±23 | – | – |
| Chrysostomou et al (19) | 2011 | 144±5 | 130±4 | 87±5 | 84±4 |
| Ren et al (13) | 2013 | 76±8 | 64±15 | 135±11 | 105±9 |
| Tosun et al (24) | 2013 | 84±16 | 76±12 | 155±20 | 106±24 |
| Gu et al (25) | 2014 | 115±33 | 86±15 | 83.5±23 | 85.2±21 |
| Rajput et al (26) | 2014 | 132±18 | 122±14 | 82±13 | 73±12 |
| Narisawa et al (28) | 2015 | 62.4±11.1 | 69.9±11.3 | – | – |
| Cheng et al (30) | 2015 | 145±22 | 110±13 | 85±15 | 75±15 |
| Chi et al (31) | 2016 | 85±10 | 72±5 | 101±5 | 84±6 |
Figure 2.
Effect of dexmedetomidine treatment on heart rate in patients with cardiac surgery. Forest plot of mean difference analyzed by inverse variance (IV) analysis in random-effect model at 95% confidence intervals (CI).
Figure 3.
Effect of dexmedetomidine treatment on systolic blood pressure in patients with cardiac surgery. Forest plot of mean difference analyzed by inverse variance (IV) analysis in random-effect model at 95% confidence intervals (CI).
Incidence of tachycardia and arrhythmias is common during surgery due to the procedures involved such as tracheal intubation, which elevates sympathetic and sympathoadrenal activity. The increased sympathoadrenal activity not only leads to tachycardia and arrhythmias, but also to increased myocardial oxygen consumption and ischemia (32). In the present meta-analysis, we found that the number of tachycardia events (Table III; Fig. 4, P<0.00001) were significantly lower in patients receiving dexmedetomidine, as compared to control patients (OR, 7.2; 95% CI limits 3.12, 16.64). The number needed to treat (NNT) is 431, indicating a significant benefit by dexmedetomidine treatment. However, the number of atrial fibrillation events (Table III; Fig. 5, P=0.23) in dexmedetomidine treated group is not significantly different from control patients, even though there was a tendency towards a lower incidence (OR, 2.51; 95% CI limits 0.56, 11.22). This is presumably because only three studies were included in this meta-analysis as other studies did not report the incidence of atrial fibrillation events in their patients and because of this, the power of this analysis for atrial fibrillation events was less.
Table III.
Effect of dexmedetomidine treatment on post-operative cardiac parameters in patients undergoing cardiac surgery.
| Control patients | Dex-treated patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Study Author (Refs.) | No. of patients | Tachycardia events | Bradycardia events | Atrial fibrillation events | No. of patients | Tachycardia events | Bradycardia events | Atrial fibrillation events |
| Herr et al (15) | 147 | 7 | 2 | – | 148 | 0 | 2 | – |
| Corbett et al (16) | 46 | 1 | – | – | 43 | 0 | – | – |
| Shehabi et al (20) | 147 | 2 | 9 | – | 152 | 2 | 25 | – |
| Chrysostomou et al (18) | 25 | 0 | 0 | – | 51 | 0 | 1 | – |
| Anger et al (21) | 28 | – | 4 | – | 28 | – | 5 | – |
| Hosokawa et al (22) | 85 | – | 7 | – | 56 | – | 12 | – |
| Chrysostomou et al (19) | 20 | 10 | 2 | – | 32 | 2 | 2 | – |
| Ren et al (13) | 81 | 12 | – | 5 | 81 | 2 | – | 1 |
| Rajput et al (26) | 110 | 22 | – | 110 | 2 | – | ||
| Turan et al (27) | 17,011 | – | – | 2,756 | 765 | – | – | 124 |
| Jiang et al (29) | 5 | – | 0 | – | 9 | – | 6 | – |
| Narisawa et al (28) | 29 | – | – | 10 | 16 | – | – | 1 |
Figure 4.
Prevention of tachycardia by dexmedetomidine in patients undergoing cardiac surgery compared to control patients. Forest plot of odds ratio, analyzed by Mantel-Haenszel statistics in the random-effect model.
Figure 5.
Prevention of atrial fibrillation by dexmedetomidine in patients undergoing cardiac surgery compared to control patients. Forest plot of odds ratio, analyzed by Mantel-Haenszel statistics in the random-effect model.
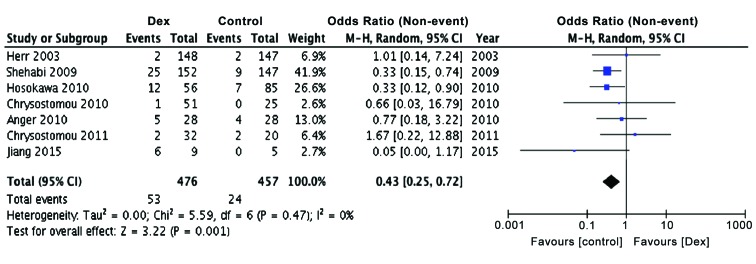
Since it has been reported earlier that perioperative bradycardia and hypotension are common in patients receiving dexmedetomidine (3) and considering that hypotension and bradycardia are often seen in patients undergoing cardiac surgery, we examined the effect of dexmedetomidine treatment on the incidence of bradycardia, both in children as well as adults. The present meta-analysis also showed a significant preponderance of patients with bradycardia in dexmedetomidine group, as compared to controls (Table III; Fig. 6, P<0.001), confirming earlier observations, but adding more power to the conclusions reached (OR, 0.43; 95% CI limits 0.25, 0.72). The Relative Risk of developing bradycardia was found to be 26.3 (95% CI limits, 16.3, 42.4; P<0.0001) and NNT was 29.3, indicating strong possibility for the development for bradycardia in dexmedetomidine treated patients.
Figure 6.
Increased incidence of bradycardia in patients undergoing cardiac surgery treated with dexmedetomidine compared to control patients. Forest plot of odds ratio, analyzed by Mantel-Haenszel statistics in the random-effect model.
Discussion
There have been few meta-analysis studies addressing the effects of dexmedetomidine specifically in cardiac surgery patients, even though there are many clinical studies indicating the beneficial effects of this drug in cardiac protection following surgery. Also, there were few studies indicating a lack of cardiac protection effects by dexmedetomidine in adult patients undergoing coronary artery bypass grafting with cardiopulmonary bypass (24). This raised questions regarding the wider usefulness of dexmedetomidine, as a cardioprotective drug during cardiac surgery. We addressed this issue with respect to few important parameters, known to be influenced by dexmedetomidine, such as heart rate, systolic blood pressure, tachycardia, arrhthmias and bradycardia. In a meta-analysis of patients undergoing non-cardiac surgeries, it was noted that dexmedetomidine improved cardiac outcomes including myocardial ischemia and non-fatal myocardial infarction while increasing peri-operative hypotension and bradycardia (33). In another meta-analysis of studies including randomized clinical trials of non-cardiac surgery patients, it was observed that dexmedetomidine significantly lowered the length of ICU stay, shortened the duration of mechanical ventilation, even though this drug increased the incidence of bradycardia (34). In patients with cardiac surgery, a meta-analysis showed that dexmedetomidine greatly reduces the length of mechanical ventilation and also lowers risk of delirium and ventricular tachycardia, following surgery (7). However, heart rate and systolic blood pressure were not analyzed in this study. A more recent meta-analysis on pediatric clinical studies with patients undergoing surgery for congenital heart disease, concluded that dexmedetomidine likely improves cardiac function in children; but the included studies were few and are mostly observational studies and thus the conclusions of this analysis were based on less number of patients and studies.
The present meta-analysis included much larger number of studies and patients were undergoing only cardiac related surgeries, where cardiac protection is the major issue. In this meta-analysis, we noted that the long-suspected beneficial effects of dexmedetomidine are true both for adults as well as children, undergoing cardiac surgery. While dexmedetomidine improves heart rate, systolic blood pressure and lowers tachycardia and arrhythmias, this drug also increased the incidence of bradycardia, indicating that care must be taken while administering dexmedetomidine, particularly to patients who are in shock and those with a transduction block. However, earlier studies suggested that the increased bradycardia did not increase the length of hospital stay (14).
Overall, this meta-analysis suggests that the use of dexmedetomidine in cardiac surgery patients has many beneficial cardioprotective effects, irrespective of the age group of the patients. The major weakness of this meta-analysis is the inclusion of both randomized and non-randomized clinical studies, but this also increased the power of analysis by increasing the number of included studies and patients to reach valid conclusions with regard to the beneficiary effects of dexmedetomidine. Another possible weakness is to combine pediatric and adult patients in the same analysis and we did this particularly in increase the number of included and analyzed studies and to enhance the predictive power of this analysis. This also indicates that there is a need for larger number of randomized clinical trial studies in either adult or pediatric patient population, with more number of enrolled patients. It is interesting to note that only in 2008, the US Food and Drug Administration (FDA) approved the use of dexmedetomidine for sedation in adults and this drug represents only 4% of the currently used drugs outside the operating room (35). Even though dexmedetomidine has not yet been approved by FDA for use in pediatric patients, clinicians have found it to be an important drug for cardioprotection and as an adjunctive anesthetic agent in children undergoing surgery for repair of congenital heart defects. Despite the setbacks of this meta-analysis, described above, the results indicate that dexmedetomidine is an efficacious cardioprotective drug in adults and children.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant nos. 81373498 and 81060277).
References
- 1.Rostagno C, La Meir M, Gelsomino S, Ghilli L, Rossi A, Carone E, Braconi L, Rosso G, Puggelli F, Mattesini A, et al. Atrial fibrillation after cardiac surgery: incidence, risk factors, and economic burden. J Cardiothorac Vasc Anesth. 2010;24:952–958. doi: 10.1053/j.jvca.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Ad N, Barnett SD, Haan CK, O'Brien SM, Milford-Beland S, Speir AM. Does preoperative atrial fibrillation increase the risk for mortality and morbidity after coronary artery bypass grafting? J Thorac Cardiovasc Surg. 2009;137:901–906. doi: 10.1016/j.jtcvs.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Zhao X, Wang Y. Dexmedetomidine: a review of applications for cardiac surgery during perioperative period. J Anesth. 2015;29:102–111. doi: 10.1007/s00540-014-1857-z. [DOI] [PubMed] [Google Scholar]
- 4.Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H, Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Ann Card Anaesth. 2012;15:39–43. doi: 10.4103/0971-9784.91480. [DOI] [PubMed] [Google Scholar]
- 5.Kunisawa T, Nagata O, Nagashima M, Mitamura S, Ueno M, Suzuki A, Takahata O, Iwasaki H. Dexmedetomidine suppresses the decrease in blood pressure during anesthetic induction and blunts the cardiovascular response to tracheal intubation. J Clin Anesth. 2009;21:194–199. doi: 10.1016/j.jclinane.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian J Anaesth. 2011;55:352–357. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin YY, He B, Chen J, Wang ZN. Can dexmedetomidine be a safe and efficacious sedative agent in post-cardiac surgery patients? a meta-analysis. Crit Care. 2012;16:R169. doi: 10.1186/cc11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasin L, Febres D, Testa V, Frati E, Borghi G, Landoni G, Zangrillo A. Dexmedetomidine vs midazolam as preanesthetic medication in children: a meta-analysis of randomized controlled trials. Paediatr Anaesth. 2015;25:468–476. doi: 10.1111/pan.12587. [DOI] [PubMed] [Google Scholar]
- 9.Mariappan R, Ashokkumar H, Kuppuswamy B. Comparing the effects of oral clonidine premedication with intraoperative dexmedetomidine infusion on anesthetic requirement and recovery from anesthesia in patients undergoing major spine surgery. J Neurosurg Anesthesiol. 2014;26:192–197. doi: 10.1097/ANA.0b013e3182a2166f. [DOI] [PubMed] [Google Scholar]
- 10.Martin E, Ramsay G, Mantz J, Sum-Ping ST. The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. J Intensive Care Med. 2003;18:29–41. doi: 10.1177/0885066602239122. [DOI] [PubMed] [Google Scholar]
- 11.Ji F, Li Z, Young N, Moore P, Liu H. Perioperative dexmedetomidine improves mortality in patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2014;28:267–273. doi: 10.1053/j.jvca.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wijeysundera DN, Naik JS, Beattie WS. Alpha-2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta-analysis. Am J Med. 2003;114:742–752. doi: 10.1016/S0002-9343(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 13.Ren J, Zhang H, Huang L, Liu Y, Liu F, Dong Z. Protective effect of dexmedetomidine in coronary artery bypass grafting surgery. Exp Ther Med. 2013;6:497–502. doi: 10.3892/etm.2013.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan W, Wang Y, Lin L, Zhou G, Hua X, Mo L. Outcomes of dexmedetomidine treatment in pediatric patients undergoing congenital heart disease surgery: a meta-analysis. Paediatr Anaesth. 2016;26:239–248. doi: 10.1111/pan.12820. [DOI] [PubMed] [Google Scholar]
- 15.Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17:576–584. doi: 10.1016/S1053-0770(03)00200-3. [DOI] [PubMed] [Google Scholar]
- 16.Corbett SM, Rebuck JA, Greene CM, Callas PW, Neale BW, Healey MA, Leavitt BJ. Dexmedetomidine does not improve patient satisfaction when compared with propofol during mechanical ventilation. Crit Care Med. 2005;33:940–945. doi: 10.1097/01.CCM.0000162565.18193.E5. [DOI] [PubMed] [Google Scholar]
- 17.Chrysostomou C, Beerman L, Shiderly D, Berry D, Morell VO, Munoz R. Dexmedetomidine: a novel drug for the treatment of atrial and junctional tachyarrhythmias during the perioperative period for congenital cardiac surgery: a preliminary study. Anesth Analg. 2008;107:1514–1522. doi: 10.1213/ane.0b013e318186499c. [DOI] [PubMed] [Google Scholar]
- 18.Chrysostomou C, Komarlu R, Lichtenstein S, Shiderly D, Arora G, Orr R, Wearden PD, Morell VO, Munoz R, Jooste EH. Electrocardiographic effects of dexmedetomidine in patients with congenital heart disease. Intensive Care Med. 2010;36:836–842. doi: 10.1007/s00134-010-1782-z. [DOI] [PubMed] [Google Scholar]
- 19.Chrysostomou C, Sanchez-de-Toledo J, Wearden P, Jooste EH, Lichtenstein SE, Callahan PM, Suresh T, O'Malley E, Shiderly D, Haney J, et al. Perioperative use of dexmedetomidine is associated with decreased incidence of ventricular and supraventricular tachyarrhythmias after congenital cardiac operations. Ann Thorac Surg. 2011;92:964–972. doi: 10.1016/j.athoracsur.2011.04.099. discussion 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shehabi Y, Grant P, Wolfenden H, Hammond N, Bass F, Campbell M, Chen J. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study) Anesthesiology. 2009;111:1075–1084. doi: 10.1097/ALN.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- 21.Anger KE, Szumita PM, Baroletti SA, Labreche MJ, Fanikos J. Evaluation of dexmedetomidine versus propofol-based sedation therapy in mechanically ventilated cardiac surgery patients at a tertiary academic medical center. Crit Pathw Cardiol. 2010;9:221–226. doi: 10.1097/HPC.0b013e3181f4ec4a. [DOI] [PubMed] [Google Scholar]
- 22.Hosokawa K, Shime N, Kato Y, Taniguchi A, Maeda Y, Miyazaki T, Hashimoto S. Dexmedetomidine sedation in children after cardiac surgery. Pediatr Crit Care Med. 2010;11:39–43. doi: 10.1097/PCC.0b013e3181b062d7. [DOI] [PubMed] [Google Scholar]
- 23.Ji F, Li Z, Nguyen H, Young N, Shi P, Fleming N, Liu H. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;127:1576–1584. doi: 10.1161/CIRCULATIONAHA.112.000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tosun Z, Baktir M, Kahraman HC, Baskol G, Guler G, Boyaci A. Does dexmedetomidine provide cardioprotection in coronary artery bypass grafting with cardiopulmonary bypass? A pilot study. J Cardiothorac Vasc Anesth. 2013;27:710–715. doi: 10.1053/j.jvca.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Gu H, Liu J, Wu C. Impact of dexmedetomidine versus propofol on cardiac function of children undergoing laparoscopic surgery. Int J Clin Exp Med. 2014;7:5882–5885. [PMC free article] [PubMed] [Google Scholar]
- 26.Rajput RS, Das S, Makhija N, Airan B. Efficacy of dexmedetomidine for the control of junctional ectopic tachycardia after repair of tetralogy of Fallot. Ann Pediatr Cardiol. 2014;7:167–172. doi: 10.4103/0974-2069.140826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turan A, Bashour CA, You J, Kirkova Y, Kurz A, Sessler DI, Saager L. Dexmedetomidine sedation after cardiac surgery decreases atrial arrhythmias. J Clin Anesth. 2014;26:634–642. doi: 10.1016/j.jclinane.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Narisawa A, Nakane M, Kano T, Momose N, Onodera Y, Akimoto R, Kobayashi T, Iwabuchi M, Okada M, Miura Y, et al. Dexmedetomidine sedation during the nighttime reduced the incidence of postoperative atrial fibrillation in cardiovascular surgery patients after tracheal extubation. J Intensive Care. 2015;3:26. doi: 10.1186/s40560-015-0092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang L, Ding S, Yan H, Li Y, Zhang L, Chen X, Yin X, Liu S, Tang X, Zhang J. A retrospective comparison of dexmedetomidine versus midazolam for pediatric patients with congenital heart disease requiring postoperative sedation. Pediatr Cardiol. 2015;36:993–999. doi: 10.1007/s00246-015-1110-z. [DOI] [PubMed] [Google Scholar]
- 30.Cheng X, Zuo Y, Zhao Q, Gu E, Huang Y. Comparison of the effects of dexmedetomidine and propofol on hemodynamics and oxygen balance in children with complex congenital heart disease undergoing cardiac surgery. Congenit Heart Dis. 2015;10:E123–E130. doi: 10.1111/chd.12228. [DOI] [PubMed] [Google Scholar]
- 31.Chi X, Liao M, Chen X, Zhao Y, Yang L, Luo A, Yang H. Dexmedetomidine attenuates myocardial injury in off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2016;30:44–50. doi: 10.1053/j.jvca.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Mikawa K, Nishina K, Maekawa N, Obara H. Comparison of nicardipine, diltiazem and verapamil for controlling the cardiovascular responses to tracheal intubation. Br J Anaesth. 1996;76:221–226. doi: 10.1093/bja/76.2.221. [DOI] [PubMed] [Google Scholar]
- 33.Biccard BM, Goga S, de Beurs J. Dexmedetomidine and cardiac protection for non-cardiac surgery: a meta-analysis of randomised controlled trials. Anaesthesia. 2008;63:4–14. doi: 10.1111/j.1365-2044.2007.05306.x. [DOI] [PubMed] [Google Scholar]
- 34.Constantin JM, Momon A, Mantz J, Payen JF, De Jonghe B, Perbet S, Cayot S, Chanques G, Perreira B. Efficacy and safety of sedation with dexmedetomidine in critical care patients: a meta-analysis of randomized controlled trials. Anaesth Crit Care Pain Med. 2016;35:7–15. doi: 10.1016/j.accpm.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Tobias JD. Dexmedetomidine and ketamine: an effective alternative for procedural sedation? Pediatr Crit Care Med. 2012;13:423–427. doi: 10.1097/PCC.0b013e318238b81c. [DOI] [PubMed] [Google Scholar]