Abstract
Motor activity in humans and other animals possesses fractal temporal fluctuations that co-exists with circadian or daily activity rhythms. The perturbations in fractal activity patterns are often accompanied by altered circadian/daily rhythms. The goal of this study is to test whether fractal regulation in motor activity provides physiological information independent from 24-h/circadian rhythmicity. To achieve the goal, we studied locomotor activity recordings of rats with the lesion of the suprachiasmatic nucleus (SCN) that are known to have diminished circadian/daily activity rhythms and perturbed fractal regulation. By restricting feeding time (i.e., food was only availability in the dark period of the 12h: 12h light-dark cycles), we found that mean activity levels in these animals displayed significant 24-h rhythms. In contrast, the restricted feeding had no influences on the perturbed fractal regulation in these SCN-lesioned animals, i.e., activity fluctuations in these animals remained random over a wide range of time scales from 2–20h. Our results indicate that 24-h rhythm of food availability can restore/improve circadian/daily rhythms in the SCN-lesioned animals but not necessarily improve the disrupted fractal activity regulation in these animals. This study provides clear and direct evidence that fractal activity patterns offer complementary information about motor activity regulation at multiple time scales that is beyond 24-h rhythm control.
Keywords: Motor activity, Fractal activity regulation, Food restriction, Circadian rhythm, Inter-daily stability (IS)
INTRODUCTION
Motor activity possesses fractal patterns as characterized by similar structures and statistical properties in temporal activity fluctuations at different time scales from seconds up to 24 hours. The same fractal patterns present in many species such as drosophila [1] mice [2,3], rats [4] and humans [5] and the patterns are independent of mean activity levels and persist even without external driving forces [5]. These results indicate the existence of a universal control mechanism—fractal regulation (FR) in motor activity. It is accepted that FR reflects high complexity in the system control that is a consequence of system integrity and adaptability, indicating healthy physiology [6]. Strong fractal activity patterns are observed in healthy young animals and humans but the fractal patterns are altered or disturbed with aging and in diseases such as dementia [3,7,8].
Although the neural circuitry of FR and the pathological pathways for the alterations of FR with aging and diseases are still yet to be elucidated, a series of studies have indicated the important role of the endogenous circadian system in FR. In mammals, the circadian system is a complex network of coupled central neural nodes with the central clock or the circadian pacemaker in the suprachiasmatic nucleus (SCN). The circadian system generates and orchestrates the circadian rhythms of ~24 hours in a wide range of physiological functions including sleep, even in the absence of 24-h external stimuli (light-dark cycles). In rats, lesioning the SCN caused the breakdown of FR in motor activity with totally random behavior at time scales >4h [4]. Consistently, our human postmortem study showed a strong association between the perturbation of FR and the reduction of SCN neurotransmitters (e.g., vasopressin and neurotensin) in patients with dementia [9] These results strongly indicated that the SCN acts as a major neural control node that is responsible for not only the circadian rhythms but also the generation of FR at multiple time scales. In addition, using animal models, we also found that forcing the animals to be active during their normal inactive periods (e.g., sleep deprivation or restricted feeding during the light phase of the light-dark cycles) caused perturbed FR with activity patterns similar to SCN-lesioned animals and humans with dementia [10]. These results further suggest that the normal circadian control is essential for maintaining FR in motor activity control.
In most of previous studies of FR in motor activity, the observed alterations in FR are always associated with reduced 24-h rhythms, thus raising two important questions: 1) whether FR is simply another measure for 24-h or circadian rhythmicity or it provides complementary information about motor activity control; and 2) whether degraded fractal regulation due to the dysfunction of the circadian system can be rescued partially or fully by restoring 24-h activity rhythm with food restriction. To address these questions, we studied rats after the lesion of the SCN that is known to cause degraded fractal regulation and diminished circadian rhythms [4,11,12]. We examined fractal activity regulation in these animals under the 12:12h light-dark cycles in two different protocols: (1) ad libitum food access with food available any time; and (2) restricted feeding with food access only during the 12-h dark phase. We hypothesized that the 24-h rhythm of food availability (restricted feeding) improves the 24-h rhythm in motor activity but cannot restore fractal activity patterns.
MATERIALS AND METHODS
Ethics statement
All animal experiments were performed according to the guide for care and use of animal experimentation in Universidad Nacional Autónoma de México and the international guidelines for animal handling. The study was approved by the ethical committee at the Instituto de Investigaciones Biomédicas.
Animals and protocols
To test our hypothesis, we studied 5 Wistar rats that underwent a surgery in which the SCN was removed (see below). After the recovery from the surgery, animals were placed in individual cages under 12h: 12h light-dark cycles. In the first 10 days, animals were not disturbed with ad libitum food access. Then the same animals underwent a 24-day scheduled food restriction protocol in which food was only available during the dark phase (Zeitgeber time 12–24 hours).
Lesions
For the SCN lesion, animals were anesthetized with 200 μL Ketamine (10 mg/100 g) and 165 μL Xilazine (1 mg/100 g) and mounted in a stereotaxic frame. Lesions were aimed to the SCN with either kainic acid (a bilateral injection of 1% kainic acid, 100 nL Sigma) or with electrolytic current (a constant current of 0.2 μmAmp of 6V was used for 1 min bilaterally).
Data acquisition
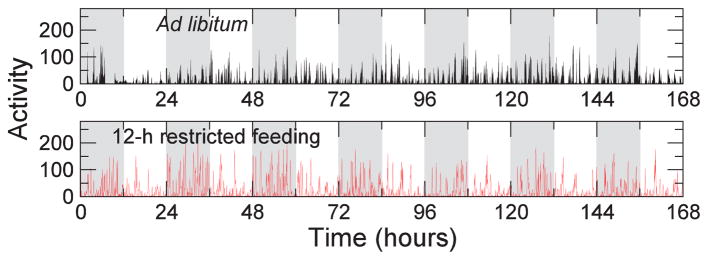
Locomotor activity data were collected using motion sensors located at the bottom of cages that were designed to continuously monitor the animal motion via the vibration of the cages. Data were collected at 1-minute intervals and were re-sampled with the epoch length of 4 minutes (Figure 1). To minimize transitional artifacts and training effects, only data during the last 7 days in each food protocol were used for data analysis.
Figure 1.
Two 7-day locomotor activity recordings of a rat with the lesioned SCN. (A) The animal had ad libitum food access. (B) The animal had restricted feeding during the 12-h dark phase of the light-dark cycles.
Rhythmicity assessment
To assess the strength of 24-h rhythm, we compared the mean activity levels during the dark phase and the light phase. To determine further the stability of the 24-h rhythm, we performed the inter-daily stability (IS) of activity data [13]. Data were first resampled to hourly bins before performing the IS analysis.
Assessment of fractal activity patterns
To quantify the fractal patterns in activity fluctuations, detrended fluctuation analysis (DFA) was used to examine temporal correlations in activity fluctuations at time scales from 20 min to 20 hours [5]. DFA calculates the fluctuation amplitude F (n) at each time scale n. To better remove linear trends, the 2nd polynomial function was used in the analysis to fit the data in each window of the size n in order to obtain the fluctuations along the trend (14). A fractal temporal structure in activity fluctuations is indicated by a power-law form: F (n)~nα which is a straight line on the log-log plane of F(n) versus time scale n. The scaling exponent α quantifies the temporal correlation properties: i) α=0.5 indicates no correlation in the fluctuations (white noise); and ii) α>0.5 suggests there are positive correlations (i.e., large activity values are more likely to be followed by large activity values and vice versa). Most healthy physiological systems are characterized by α of ~1[6,15], which demonstrates a well balance between uncorrelated randomness and excessive regularity—highly complex. Pathological conditions are commonly accompanied with perturbed balance, or complexity, under which the scaling exponent α can become either too small (towards random) or too large (towards regular). Previous studies of rats with the lesion of SCN have identified different variations of scaling exponents α in two regions of time scales with a crossover at ~4h [4]. Thus, we obtained α in the two regions, separately, i.e., α1 at 0.2–2h and α2 at 4–20h. Note that we skipped the transition region at 2–4h.
Statistical Analysis
Results are presented as mean ± standard deviation (SD). To assess the effects of time-restricted feeding and time scale region on fractal activity patterns, we used a mixed model in which α was included as the outcome variable, food condition (ad libitum vs. restricted feeding), time scale region (0.2–2h vs. 4–20h), and their interaction as fixed effects, and subject as a random factor. To assess the effects of time-restricted feeding and light/dark on mean activity levels, we used a similar mixed models in which food condition (ad libitum vs. restricted feeding), light condition (light vs. dark), and their interaction as the fixed effects, and subject as a random factor. To examine the effect of food restriction on stability, we used a mixed model in which IS was included as the outcome variable, food condition (ad libitum vs. restricted feeding) as the fixed effect, and subject as a random factor. All the statistical analyses were performed using JMP 12 pro (SAS Institute Inc, North Carolina).
RESULTS
Rhythmicity
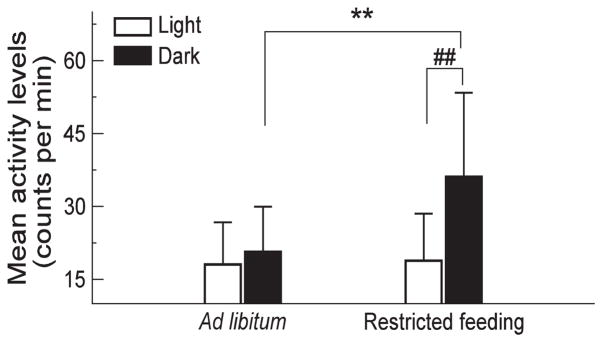
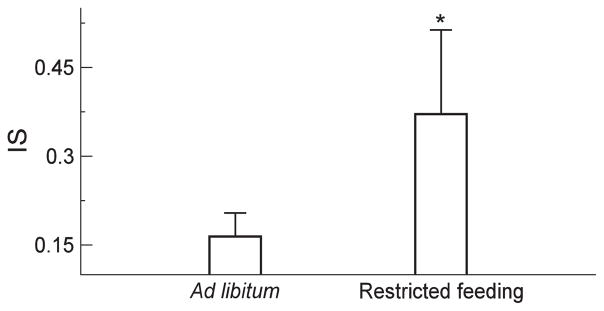
Under the ad libitum condition, these SCN-lesioned animals showed no significant 24-h rhythms as expected, i.e., mean activity levels were 21 ± 9 during the dark phase and during the light phase 19 ± 9 (p>0.5) (Figures 1 and 2). With the restricted feeding, significant 24-h rhythms appeared in these animals with higher activity levels during the dark (feeding) phase (36 ± 17) as compared to the levels during the light phase (19 ± 9) (p=0.0019). Consistently, the mixed model showed that there was a significant interaction between the effects of the light/dark rhythm and the food availability condition (p=0.03). The difference in the 24-h light-dark rhythm between the two food access conditions was mainly due to the increased activity levels during the dark phase with restricted feeding (p=0.004). The restricted feeding did not affect the mean activity levels during the light phase (p>0.8). In addition to the increased 24-h activity rhythm, restricted feeding caused a significant increase in IS (Figure 3), i.e., 0.16 ± 0.04 under the ad libitum condition, and 0.37 ± 0.14 under the restricted feeding condition (p=0.04).
Figure 2.
Group averages of mean activity levels during the light phase and during the dark phase. Restricted feeding caused a significant increase in the mean activity levels during the dark phase (indicated by **: p<0.01) but not during the light phase. Such effects of restricted feeding led to a significant light-dark rhythm (indicated by ##: p<0.01).
Figure 3.
Effect of restricted feeding on inter-daily stability (IS) of 24-h activity rhythm. IS was significantly higher when food was only available during the 12-h dark phase (indicated by *: p<0.05).
Fractal patterns
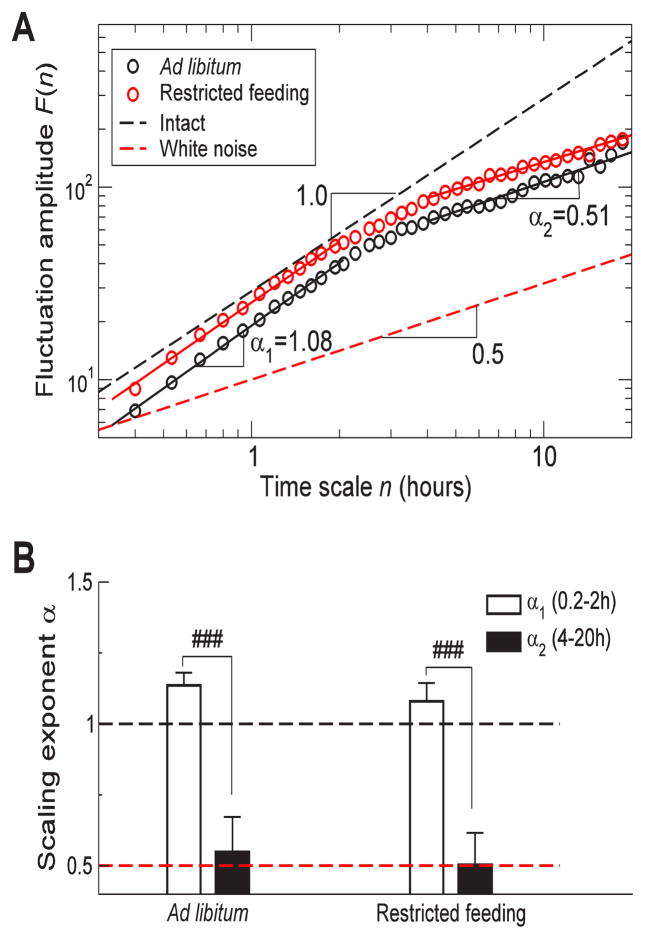
Under the adlibitum condition, activity fluctuations showed strong correlations at <2h, as characterized by the scaling exponent that was much greater than 0.5 (α1=1.14 ± 0.04), and random behavior at >4h, as characterized by the scaling exponent that was close to and not significantly different from 0.5 (α2=0.55 ± 0.12; p>0.5). The significant difference between the correlations in the two scale regions (α1>α2; p<0.0001) indicates a breakdown of fractal patterns and, thus, a perturbation in fractal regulation. The restricted feeding had no significant influences on the perturbed fractal patterns (Figure 4). Both α1 (α1=1.08 ± 0.06) and α2 (α2=0.50 ± 0.11) under the restricted feeding condition were similar to the values under the ad libitum condition (both p values >0.4).
Figure 4.
Different temporal activity correlations at different time scales in SCN-lesioned rats. (A) Fluctuation functions of the activity recordings of an SCN-lesioned rat under ad libitum and restricted feeding conditions. Data are shown on log-log plots. Results were obtained from the signals shown in Figure 1 using the detrended fluctuation analysis. (B) Group averages of scaling exponents at time scales of 0.2–2h and at 4–20h. ### indicates the significant difference between the two scale regions (p<0.0001). The dashed black line indicates the average behavior of intact rats with a scaling exponent of 1.0. The dashed red line indicates the scaling exponent for white noise with random fluctuations.
DISCUSSION
In this study, we confirmed that rats with the SCN lesioned had perturbed activity regulation at multiple time scales. Under the ad libitum condition, these animals had abolished 24-h rhythms and a breakdown of fractal patterns, leading to random fluctuations at time scales >4h. We further showed that restricted feeding during the dark phase caused a significant light-dark activity rhythm with an increased inter-daily stability in the same animals. These results indicate that the timing of feeding can provide an important time cues and that a 24-h rhythm of food availability improve daily rhythms in SCN-lesioned animals. Despite the restored 24-h activity rhythm, we found that the perturbed fractal activity patterns in these animals remained unchanged under the restricted feeding condition. These results indicate that fractal activity patterns provide complementary information of activity regulation at multiple time scales that is different from 24-h activity rhythms.
Unlikely rhythm measures such as circadian and ultradian rhythms that are focused on oscillations/fluctuations at discrete time scales; fractal regulation is focused on the interrelationship between activity oscillations/fluctuations at different time scales, i.e. a delicate orchestration of the amplitudes of these multi-scale fluctuations. Thus, fractal patterns mathematically represent a temporal organization that is different from that of rhythmicity. This theoretic concept has been supported by our previous animal study of mice and rats, in which we found that the neural activity fluctuations of the SCN in vitro in both species show a circadian rhythm but without the presence of fractal patterns [16]. However, in vitro studies may not fully represent the complex network regulation of physiological outputs, even for the circadian rhythm. For instance, the non-fractal patterns of the neural activity of the SCN in vitro are dramatically different from the perturbed fractal patterns of motor activity as observed in the SCN-lesioned animals or in humans with dementia; and the circadian profile of the neural activity is even different between the SCN in vivo and in vitro. The current study provides more direct evidence that restoring circadian/daily rhythms in the SCN-lesion animals may not necessarily improve disrupted fractal activity regulation.
It is worth noting that the restricted feeding used in this study did not fully restore the 24-h rhythm as we observed in the intact rats. In a previous study of the same type of rats without the SCN lesion, we showed that the mean activity level during the dark phase was 3.5 times of the level during the light phase [17]. In this study, the ratio was only 1.9 when the animals were in the restricted feeding protocol (Figure 2). Thus, it is yet to be determined whether a full restoration of 24-h rhythms can improve fractal activity regulation in the SCN-lesioned rats. In addition, the restricted feeding protocol we used in this study is different from the other RF protocols that are intended to introduce food anticipatory behaviors (i.e., increased activity a few hours before the feeding period). For instance, food was typically provided in a period less than 5 hours each day (e.g., 2 hours during the light phase) [17]. It is necessary to examine how different durations and different timings of food availability affect 24-h rhythmicity and fractal activity regulation. Nevertheless, our findings clearly indicate that fractal activity patterns are not simply associated with 24-h rhythmicity measures.
With the established physiological importance of fractal regulation, there are many follow-up questions regarding fractal regulation in motor activity control. One of them is how to understand the co-existence of the alterations in circadian/daily rhythms and fractal activity patterns with aging and under pathological conditions in humans and animals. In order to address this question, it is necessary to understand the mechanisms of fractal activity patterns. Based on the mathematical models of physical systems, fractal regulation requires an integrated network of multiple control nodes that function at different time scales [15]. Supporting this network theory, we have recently found that fractal activity patterns are also perturbed in the animals after lesioning the dorso medial hypothalamic nucleus—a major neural node involved in the food anticipatory activity—despite that the amplitude of the circadian/daily rhythm remained intact [17]. In addition, forcing intact animals to be active during the light phase can cause disturbances in fractal activity patterns that are similar to those in SCN-lesioned animals [10]. All these results suggest that the normal function of the whole circadian network (i.e., the individual neural nodes and their coupling) is important for fractal regulation. To reveal the neural circuitry for fractal regulation, animal models are required to further identify other neural nodes in the circadian system and their interactions that are important for maintaining fractal activity patterns.
Another important question is how to prevent or restore the degraded fractal activity regulation. There is not much information about the possible interventions for improving fractal regulation. Our recent animal study showed that exercise might be a beneficial approach to slow down the effect of aging on fractal activity regulation [3]. In that study, we found that the degradation of fractal activity patterns in the older mice was strongly associated with the physical activity levels and the association was stronger than the effect of age. In the same study, we also found that depriving the opportunity of exercise caused perturbed fractal activity regulation in both aged and young mice, suggesting possible causal relationship between exercise and fractal activity regulation. It is warranted for future studies to test whether or not the beneficial effect of exercise on fractal activity regulation is through its influence on the circadian system.
To conclude, fractal regulation is linked to the circadian control system such that its degradation is associated with the alterations of 24-h rhythmicity in certain cases when the circadian control is perturbed. However, the fractal activity measure provides additional information of motor activity control that does not simply reflect the 24-h activity rhythmicity.
Acknowledgments
This research was supported by National Institutes of Health grants R00HL102241, R01AG048108, National Science Council in Taiwan (ROC) grant NSC 100-2911-I-008-001, and grants from Mexico DGAPA IN-209711 and CONACYT 79797. PL was supported by the International Postdoctoral Exchange Fellowship 20150042 from the China Postdoctoral Council.
ABBREVIATIONS
- FR
Fractal Regulation
- IS
Inter-Daily Stability
- SCN
Suprachiasmatic Nucleus
References
- 1.Cole BJ. Fractal time in animal behaviour: the movement activity of Drosophila. Animal Behaviour. 1995;50:1317–1324. [Google Scholar]
- 2.Lima GZ, Lobao-Soares B, do Nascimento GC, Franca AS, Muratori L, Ribeiro S, et al. Mouse activity across time scales: fractal scenarios. PLoS ONE. 2014;9:e105092. doi: 10.1371/journal.pone.0105092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu C, Coomans CP, Hu K, Scheer FA, Stanley HE, Meijer JH. Lack of exercise leads to significant and reversible loss of scale invariance in both aged and young mice. Proc Natl Acad Sci U S A. 2015;112:2320–2324. doi: 10.1073/pnas.1424706112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu K, Scheer FA, Ivanov P, Buijs RM, Shea SA. The suprachiasmatic nucleus functions beyond circadian rhythm generation. Neuroscience. 2007;149:508–517. doi: 10.1016/j.neuroscience.2007.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu K, Ivanov P, Chen Z, Hilton MF, Stanley HE, Shea SA, et al. Non-random fluctuations and multi-scale dynamics regulation of human activity. Physica A. 2004;337:307–318. doi: 10.1016/j.physa.2004.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberger AL, Amaral LA, Hausdorff JM, Ivanov P, Peng CK, Stanley HE. Fractal dynamics in physiology: Alterations with disease and aging. Proc Natl Acad Sci U S A. 2002;99:2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu K, Riemersma-van der Lek RF, Patxot M, Li P, Shea SA, Scheer FA, et al. Progression of dementia assessed by temporal correlations of physical activity: Results from a 3.5-year, longitudinal randomized controlled trial. Sci Rep. 2016;6:27742. doi: 10.1038/srep27742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu K, Van Someren EJ, Shea SA, Scheer FA. Reduction of scale invariance of activity fluctuations with aging and Alzheimer’s disease: Involvement of the circadian pacemaker. Proc Natl Acad Sci U S A. 2009;106:2490–2494. doi: 10.1073/pnas.0806087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu K, Harper DG, Shea SA, Stopa EG, Scheer FA. Noninvasive fractal biomarker of clock neurotransmitter disturbance in humans with dementia. Sci Rep. 2013;3:2229. doi: 10.1038/srep02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh WH, Escobar C, Yugay T, Lo MT, Pittman-Polletta B, Salgado-Delgado R, et al. Simulated shift work in rats perturbs multiscale regulation of locomotor activity. J R Soc Interface. 2014;11:20140318. doi: 10.1098/rsif.2014.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 13.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27:563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 14.Hu K, Ivanov PC, Chen Z, Carpena P, Stanley HE. Effect of trends on detrended fluctuation analysis. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;64:011114. doi: 10.1103/PhysRevE.64.011114. [DOI] [PubMed] [Google Scholar]
- 15.Pittman-Polletta BR, Scheer FA, Butler MP, Shea SA, Hu K. The role of the circadian system in fractal neurophysiological control. Biol Rev Camb Philos Soc. 2013;88:873–894. doi: 10.1111/brv.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu K, Meijer JH, Shea SA, vanderLeest HT, Pittman-Polletta B, Houben T, et al. Fractal patterns of neural activity exist within the suprachiasmatic nucleus and require extrinsic network interactions. PLoS ONE. 2012;7:e48927. doi: 10.1371/journal.pone.0048927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo MT, Chiang WY, Hsieh WH, Escobar C, Buijs RM, Hu K, et al. Interactive effects of dorsomedial hypothalamic nucleus and time-restricted feeding on fractal motor activity regulation. Front Physiol. 2016;7:174. doi: 10.3389/fphys.2016.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]