Abstract
Introduction
The nicotine-metabolite ratio (NMR) predicts treatment response and is related to treatment side effect severity. Sleep disturbance may be one important side effect, but understanding sleep disturbance effects on smoking cessation is complicated by the fact that nicotine withdrawal also produces sleep disturbance.
Aims
To evaluate the effects of withdrawal and treatment side effects on sleep disturbance.
Methods
This is a secondary analysis of data from a clinical trial (Lerman et al., 2015) of 1,136 smokers randomised to placebo (n = 363), transdermal nicotine (TN; n = 381), or varenicline (n = 392) and stratified based on NMR (559 slow metabolisers; 577 normal metabolisers). Sleep disturbance was assessed at baseline and at 1-week following the target quit date (TQD). We also examined whether sleep disturbance predicted 7-day point-prevalence abstinence at end-of-treatment (EOT).
Results
The varenicline and TN groups exhibited greater increases in sleep disturbance (vs. placebo; treatment × time interaction; p = 0.005), particularly among those who quit smoking at 1-week post-TQD. There was a main effect of NMR (p = 0.04), but no interactions with treatment. TN and varenicline attenuated withdrawal symptoms unrelated to sleep (vs. placebo). Greater baseline sleep disturbance predicted relapse at EOT (p = 0.004).
Conclusions
Existing treatments may not mitigate withdrawal-related sleep disturbance and adjunctive treatments that target sleep disturbance may improve abstinence rates.
Introduction
Despite the fact that cigarette smoking continues to be the greatest preventable cause of morbidity and mortality, there are currently only three FDA-approved medications for nicotine dependence: nicotine replacement therapy (NRT; TN, nasal spray, gum, lozenge), bupropion, and varenicline. TN and bupropion produce comparable EOT quit rates of ∼30% (Hughes, Stead, & Lancaster, 2007), but lower than the more recently approved nicotinic receptor partial agonist, varenicline, which produces EOT quit rates of roughly 50% (Gonzales et al., 2006; Jorenby et al., 2006). Because of the considerable variability in treatment response and side effects across smokers (Lerman et al., 2004; Swan, Jack, Javitz, McAfee, & McClure, 2008; Swan & Lessov-Schlaggar, 2009), recent work has focussed on identifying strategies to optimise treatments. Lerman and colleagues (2015) demonstrated that a genetically informed biomarker of nicotine metabolism, the ratio of 3′-hydroxycotinine to cotinine and referred to as the NMR, may be a valuable approach to improving nicotine dependence treatment. Specifically, smokers who metabolise nicotine more quickly (normal metabolisers) were more likely to quit smoking with varenicline than TN. Among slow metabolisers, these two treatments were equally efficacious. However, slow metabolisers reported more severe side effects on varenicline than normal metabolisers, which may undermine the potential benefits of this medication in this sub-group of smokers.
Evidence suggests that sleep disturbance may be one important side effect to consider when providing treatment recommendations to smokers. First, sleep disturbance reported prior to a cessation attempt predicts smoking relapse in clinical trials and laboratory models of relapse (Augustson et al., 2008; Boutou et al., 2008; Peters, Fucito, Novosad, Toll, & O’Malley, 2011). Although it is not clear whether these sleep problems predated the onset of smoking behaviour or occurred as a consequence of smoking, these findings indicate that sleep disturbance may be important to address prior to a quit attempt. Second, existing nicotine dependence treatments may not mitigate sleep disturbance during a quit attempt. Smokers using NRT (Gourlay, Forbes, Marriner, Pethica, & McNeil, 1995; Hurt et al., 1994; Jaehne, Loessl, Barkai, Riemann, & Hornyak, 2009; Schnoll et al., 2010) or varenicline (Foulds et al., 2013) commonly report problems falling and staying asleep. Using TN at night may also result in abnormal dreams or nightmares (Fredrickson et al., 1995; Jorenby et al., 1996). However, assessment of sleep disturbance in clinical trials is complicated by the fact that sleep disturbance, including difficulty falling asleep and increased number of awakenings, is also a symptom of nicotine withdrawal (Hughes et al., 2007; Soldatos, Kales, Scharf, Bixler, & Kales, 1980; Wetter, Fiore, Baker, & Young, 1995). Thus, among those who quit smoking, the use of a placebo arm could help ascertain whether sleep disturbance is due to withdrawal from nicotine or a side effect of treatment. In contrast, among those who relapse (or never quit), increased sleep disturbance is more likely a side effect of treatment. These observations suggest that it may be important to identify circumstances under which sleep disturbance occurs (e.g., withdrawal vs. treatment) and identify individuals more likely to experience sleep disturbance (e.g., slow vs. normal metabolisers) if we are to consider the use of adjunctive treatments that target sleep disturbance to augment the therapeutic benefits of treatments for smoking cessation.
Thus, in this secondary analysis of data from a large biomarker-stratified placebo-controlled trial (Lerman et al., 2015), we evaluated the effects of tobacco withdrawal and treatment side effects among 1,136 adult smokers who were randomised to receive placebo, TN, or varenicline and stratified based on their NMR status (normal vs. slow metabolisers). We focussed on the 1-week post-TQD time point because withdrawal symptoms typically peak in the first week (Hughes, Keely, &Naud, 2004; Shiffman et al., 2006) and this is a vulnerable time during which most smokers relapse (Ashare, Wileyto, Perkins, & Schnoll, 2013; Garvey, Bliss, Hitchcock, Heinold, & Rosner, 1992). We predicted that sleep disturbance would increase following a quit attempt and that this increase would be larger for active treatment (TN, varenicline) vs. placebo, consistent with a lack of treatment-mitigation of this side effect. We also predicted that NMR group would moderate treatment effects on sleep disturbance (i.e., slow metabolisers on varenicline would report more sleep disturbance than normal metabolisers). If increases in sleep disturbance are due to withdrawal, we predicted that sleep disturbance would be attenuated among those who relapsed (or never quit smoking). Last, we evaluated whether sleep disturbance predicted relapse to smoking at EOT in order to further assess the importance of this issue in clinical smoking cessation trials.
Materials and Methods
Participants
This is a secondary analysis; study participants and procedures are described in detail elsewhere (Lerman et al., 2015). Briefly, treatment-seeking smokers were screened for eligibility from November 2010 to September 2013 at four academic medical centres. Smokers ages 18–65 who reported smoking at least 10 cigarettes per day for the past 6 months were included. Exclusion criteria included use of non-cigarette tobacco products, e-cigarettes, or current smoking treatment; history of substance abuse treatment, current use of cocaine or methamphetamine, or >25 alcoholic drinks per week; medical contraindications (e.g., pregnancy, uncontrolled hypertension); history of DSM-IV Axis 1 psychiatric disorder or suicide risk score > 1 on the MINI International Neuropsychiatric Interview (Sheehan et al., 1997), or current major depression; current use of antipsychotics, stimulants, or medications altering CYP2A6 activity (e.g., MAO inhibitors, tricyclic antidepressants). All participants provided written informed consent.
Procedures
Participants at all sites received identical assessments of demographics, smoking rate, and nicotine dependence (FTND) (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) and provided a blood sample for the NMR assay. Participants were randomly assigned to one of three treatment groups: placebo (placebo pill plus placebo patch); nicotine patch (placebo pill plus active patch); or varenicline (active pill plus placebo patch) in a 1:1:1 ratio. Randomisation was stratified by study site and baseline NMR status and a cut-off of 0.31 was used to differentiate between slow metabolisers (NMR < 0.31) and normal metabolisers (NMR ≥ 0.31). All participants completed a pre-quit group counselling session 1 week before the TQD (week 0). As recommended, varenicline (or matching placebo) titration began 1 week before the TQD and nicotine patch (or matching placebo) was initiated on the morning of the TQD. Varenicline was delivered for 12 weeks as in previous trials (Gonzales et al., 2006): days 1–3 (0.5 mg once daily); days 4–7 (0.5 mg twice daily); and days 8–84 (1.0 mg twice daily). Varenicline typically reaches steady state in 4 instructions on the use of medication at weeks 0 [TQD], 1, 4, and 8. Selfreported smoking status was assessed using a standard timeline follow-back procedure (Brown et al., 1998) and biochemically verified. The institutional review boards at all sites approved the protocol.
Measures
Sleep scale
A self-report checklist (SEC) measured the severity of common side-effects (none [0] to severe [3]) (Schnoll et al., 2009). Both measures were collected at pre-quit (1 week prior to the TQD), weeks 0 (TQD), 1, and 4. The time frame for all assessments was ‘past week’. The current analyses are restricted to the pre-quit and week 1 assessments to enable assessment of all participants at baseline (before initiation of any treatment) and after both active treatment groups were on medication for at least one week. Thus, the week 1 assessment occurred 2 weeks after the pre-quit visit. Three items from the SEC (sleep problems, insomnia, abnormal dreams) were used to assess self-reported sleep disturbance. The three items were averaged to compute a summary score for each visit. Internal consistency of the sleep scale, assessed via Cronbach’s alpha, was 0.63 and 0.66 at the pre-quit and 1-week post-TQD visits, respectively. These values indicate acceptable scale reliability at both time points.
Withdrawal symptoms (non-sleep related)
Self-reported withdrawal symptoms were measured with the Minnesota Nicotine Withdrawal Scale (MNWS; none [0] to severe [4]) (Hughes et al., 1984). To determine whether changes in sleep were independent of changes in overall withdrawal symptoms, the MNWS (minus two items ‘insomnia/sleep problems’ and ‘dreaming/nightmares’) was used as a secondary outcome variable. To compare the magnitude of the effects on overall withdrawal to sleep disturbance, z-scores were created for the modified MNWS using similar procedures to those described for the sleep scale.
Quit status at week 1
Because we were interested in isolating the effects of withdrawal from the effects of treatment on sleep disturbance, we determined quit status at week 1. Participants were coded as abstinent if they reported no smoking (not even a puff) since the TQD and if they provided biochemical verification (i.e., CO < 8 ppm) (Hughes et al., 2003).
Smoking cessation outcomes
Based on guidelines for smoking cessation trials, the primary endpoint was 7-day point prevalence abstinence at EOT (week 11) (Hughes et al., 2003). Abstinence was defined as no self-reported smoking (not even a puff) for at least 7 days immediately before the follow-up time-point and biochemically verified (i.e., CO < 8 ppm) (Hughes et al., 2003). Participants lost to follow-up or who failed to provide a sample for biochemical verification were coded as non-abstinent (Hughes et al., 2003).
Data Analysis
Modelling of self-reported sleep disturbance was conducted using random effects maximum likelihood regression (Stata xtreg; Stata Corp., College Station, TX). Models included terms for the main effects of NMR group (slow vs. normal), treatment arm (placebo, TN, and varenicline), quit status at week 1, and visit (pre-quit vs. week 1). Covariates included sex, age, and race, since these variables were associated with sleep disturbance (see below). First, the NMR group × time, treatment × time, and the three-way NMR group × treatment × time interactions were examined. Second, we conducted separate regression models similar to those described above for those who were abstinent at week 1 and those who relapsed. Next, identical parallel models were conducted using the modified MNWS as the outcome variable. Last, we used logistic regression models to examine whether sleep disturbance predicted abstinence status at EOT. First, pre-quit sleep disturbance was entered as a predictor. In a subsequent model, we included the change in sleep disturbance from pre-quit to week 1 as a predictor. Models also included terms for NMR group, treatment arm, quit status at week 1, sex, age, and race.
Results
Participant Characteristics
Demographic and smoking history by NMR group and treatment arm are depicted in (Table 1). Of the 1,246 participants in the full sample, 1,136 had complete data at the pre-quit and week 1 visits. Overall, 46% (n = 514) of the sample was female and 55% (n = 630) was Caucasian. On average, participants were 45.6 years old (SD = 11), smoked 18 cigarettes per day (SD = 7), were moderately nicotine dependent (FTND M = 5.2, SD = 2), and 45% (n = 509) met criteria for abstinence at week 1. There were no baseline differences across treatment arm. Fewer people in the placebo group (31%) were abstinent compared to the TN (48%) and varenicline (54%) groups at week 1 (p < 0.001). Similar to prior analyses (Chenoweth et al., 2014), normal metabolisers were older (p = 0.01), more likely to be white (p < 0.001), more likely to be female (p = 0.001), and smoked more cigarettes per day (p = 0.0002), compared to slow metabolisers. Last, age was positively associated with sleep disturbance (p = 0.01), African-Americans reported less sleep disturbance (p = 0.04), and women reported more sleep disturbance than men at pre-quit (p = 0.02). These variables were included as covariates.
Table 1.
Demographic and smoking history by NMR group and treatment arm (n = 1,136)
| Placebo
|
Nicotine Patch
|
Varenicline
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SM (n = 182) |
NM (n = 181) |
All (n = 363) |
SM (n = 184) |
NM (n = 197) |
All (n = 381) |
SM (n = 193) |
NM (n = 199) |
All (n = 392) |
|
| Sex (n, % female) | 69 (38%) | 93 (51%) | 162 (45%) | 83 (42%) | 90 (49%) | 173 (45%) | 81 (41%) | 98 (51%) | 179 (46%) |
| Race (n, % White) | 81 (46%) | 118 (67%) | 199 (57%) | 98 (51%) | 118 (66%) | 216 (58%) | 88 (45%) | 127 (67%) | 215 (56%) |
| Age (years) | 47.5 (10.9) | 44.4 (11.3) | 46.0 (11.2) | 45.9 (10.7) | 45.4 (10.8) | 45.7 (10.7) | 45.8 (11.3) | 44.4 (12.1) | 45.1 (11.7) |
| Cigarettes per day | 19.3 (8.6) | 17.5 (7.3) | 18.4 (8.0) | 18.6 (7.1) | 17.5 (7.1) | 18.0 (7.1) | 18.4 (6.3) | 16.7 (5.5) | 17.5 (6.0) |
| FTND score | 5.4 (2.0) | 5.3 (1.9) | 5.3 (2.0) | 5.2 (1.9) | 5.1 (2.0) | 5.2 (2.0) | 5.1 (2.0) | 5.1 (2.0) | 5.1 (2.0) |
| Week 1 quit status (n, % abstinent) | 57 (31%) | 57 (31%) | 114 (31%) | 103 (52%) | 79 (43%) | 182 (48%) | 114 (57%) | 99 (51%) | 213 (54%) |
Note. Data are n (%) or mean (SD). SM = slow metaboliser; NM = normal metaboliser; NMR = nicotine metabolite ratio; FTND = Fagerström Test for Nicotine Dependence.
NMR and Treatment Effects on Sleep
There were no baseline differences in sleep disturbance between normal and slow metabolisers or between treatment arms (ps > 0.6). There was a significant increase in sleep disturbance from pre-quit to 1-week post-TQD (p < 0.001) and slow metabolisers reported significantly more sleep disturbance (p = 0.04). In addition, the TN group (p = 0.04), reported more sleep disturbance (vs. placebo), but the varenicline group did not (p = 0.14). The three-way NMR group by treatment by visit interaction was not significant (p = 0.55) and was dropped from subsequent models. As shown in (Figure 1), there was a significant treatment × visit interaction (β = 0.4, p = 0.005), indicating that, although all groups exhibited an increase in sleep disturbance, the increase in the varenicline and TN groups (mean difference = 0.47 and 0.40, respectively; ps < 0.001) was more than double the increase in the placebo group (mean difference = 0.18; p < 0.001). These changes did not differ by NMR group, sex, or age.
Figure 1.
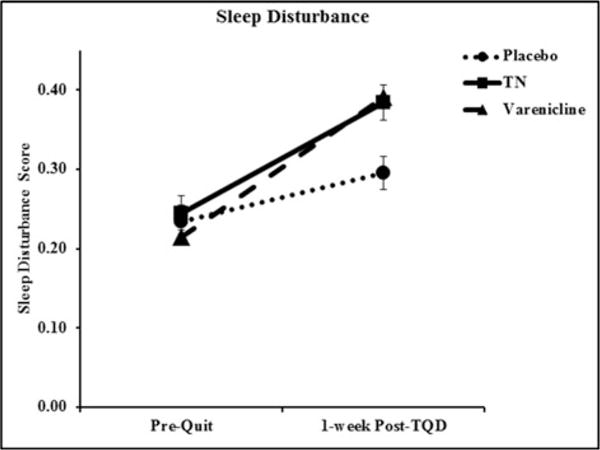
Sleep disturbance score at baseline (Pre-Quit) and 1-week post-TQD by treatment arm among all subjects. Data presented are reaw values. Error bars are standard error
The Role of Treatment vs. Withdrawal on Sleep
Because we were interested in understanding the role of withdrawal vs. treatment side effects on sleep disturbance, we evaluated whether quit status at 1-week post-TQD moderated the effect of treatment over time. Although the three-way treatment arm × quit status × visit interaction was not significant (p = 0.2), we separated individuals who were abstinent at 1-week post-TQD from those who either never quit or relapsed1. Among individuals who were abstinent, only active treatment groups exhibited an increase in sleep disturbance (TN and varenicline groups, βs> 0.54, ps < 0.001), whereas the placebo group did not (β = 0.18, p = 0.15; treatment × visit interaction, p = 0.02) (Figure 2). In contrast, among those who either never quit smoking or relapsed, there was no treatment × visit interaction (p = 0.25). Rather, all groups exhibited an increase in sleep disturbance between baseline and 1-week post-TQD (βs = 0.18–0.38, ps < 0.05).
Figure 2.
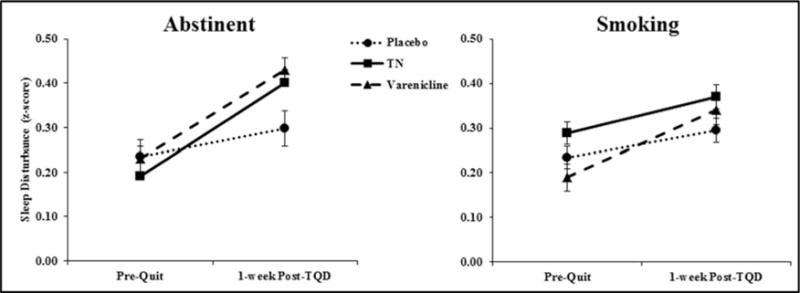
Sleep disturbance score at pre-quit and 1-week Post-TQD by treatment arm and abstinence status at 1-week Post-TQD (smoking n = 627 vs. abstinent n = 509). Among those who were abstinent, all groups exhibited an increase in sleep disturbance, but the effect was significantly large in the TN and varenicline groups, compared to the placebo group (treatment arm × visit interaction, p = 0.03). In contrast, among those who either never quit smoking or relapsed, there was no treatment × visit interaction (p = 0.25). The three-way NMR × treatment arm × abstinence status interaction was not significant. Mean +/−standard error
Unique Effects of Treatment on Sleep Disturbance
As expected, there was a significant increase in withdrawal from pre-quit to 1-week post-TQD (β = 0.32, p < 0.001), but there were no main effects of treatment arm or NMR group (ps > 0.1). Likewise, the three-way NMR group by treatment by visit interaction was not significant (p = 0.6) and was dropped from subsequent models. Similar to changes in sleep disturbance, there was a significant treatment × visit interaction (p = 0.004). However, the largest increase in withdrawal was observed in the placebo group (mean difference = 0.47, p < 0.001; (Figure 3)) compared to the varenicline and TN groups (mean difference = 0.21 and 0.28, respectively; ps < 0.001). These changes did not differ by NMR group, sex, or age.
Figure 3.
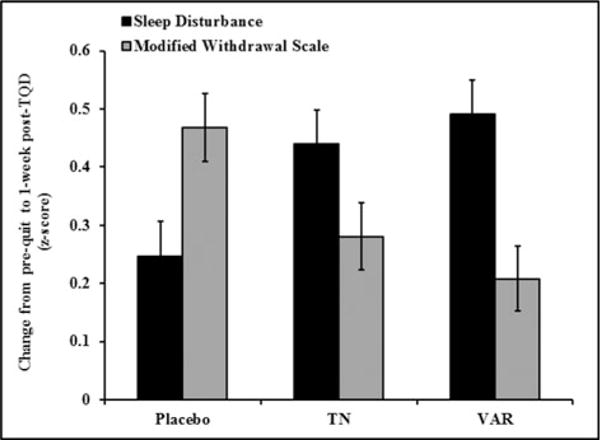
Change in sleep disturbance and modified MNWS from baseline (Pre-Quit) to 1-week post-TQD by treatment arm among all subjects. Error bars are standard error
Role of Sleep Disturbance in Relapse
In the logistic regression model predicting abstinence at EOT, pre-quit sleep disturbance was significantly negatively related to abstinence. Individuals who reported more sleep disturbance at baseline were less likely to be abstinent, controlling for treatment and NMR group (OR = 0.79 95% CI 0.67–0.93, p = 0.004). There were no interactions with treatment or NMR group (ps > 0.2). The change in sleep disturbance from pre-quit to 1-week post-TQD was not related to relapse (p = 0.39).
Discussion
In this secondary analysis of data from a large biomarker-stratified placebo-controlled randomised trial (Lerman et al., 2015), we sought to understand the independent and interactive effects of treatment and abstinence on self-reported sleep disturbance. Our data suggest that individuals who received active smoking cessation treatment experienced greater increases in sleep disturbance than those who received placebo. Moreover, increased sleep disturbance in both active treatment groups was most pronounced among those who successfully quit smoking for the first week following the TQD. These data indicate that existing treatments do not attenuate sleep disturbance during tobacco withdrawal. Thus, sleep disturbance may be a useful therapeutic target to improve cessation rates.
The fact that treatment altered the effect of quitting smoking on sleep disturbance might suggest that the observed increase in self-reported sleep problems is a side effect of treatment. Indeed, individuals treated with TN or varenicline reported a greater increase in sleep disturbance 1-week following the TQD compared to those receiving placebo. Although this finding is consistent with several previous studies (Foulds et al., 2013; Gourlay et al., 1995; Hurt et al., 1994; Jaehne et al., 2009; Schnoll et al., 2010), it is important to consider that at this time point, approximately half the sample reported no smoking at all in the last 7 days. For instance, among those who were able to quit smoking for the first week, we found that those treated with TN or varenicline reported significantly more sleep disturbance than the placebo group. In contrast, among those who relapsed during the first week, treatment had no effect on the magnitude of change in sleep disturbance. Although the quit status at 1-week post-TQD did not significantly moderate the interaction between treatment and visit, this finding suggests that the observed changes in sleep disturbance may have additively increased as a function of both withdrawal and as a side effect of treatment. Specifically, those who quit smoking and use either TN or varenicline to aid their quit attempt may experience greater increases in sleep disturbance than those who continue smoking or make an unaided quit attempt.
Although difficulty falling asleep and increased number of nighttime awakenings are commonly reported withdrawal symptoms, other affective, physical, and cognitive symptoms are also important (Hughes et al., 2007; Soldatos et al., 1980; Wetter et al., 1995). Moreover, suppression of withdrawal symptoms may be one mechanism by which smoking cessation treatments exert their efficacy (Ferguson, Shiffman, & Gwaltney, 2006; Lerman et al., 2002). Therefore, we sought to evaluate whether the pattern of increased sleep disturbance was unique or whether it was simply a part of the broader withdrawal syndrome. Our findings indicated that, contrary to our findings for sleep disturbance, TN and varenicline attenuated withdrawal symptoms unrelated to sleep disturbance, relative to placebo. Thus, to the extent that sleep disturbance may play an important role in relapse, addressing these potential side effects with adjunctive treatments may be an effective strategy for improving abstinence rates.
We also examined the association between sleep disturbance and the ability to quit smoking and found that greater sleep disturbance at baseline was associated with a higher likelihood of relapse at EOT. This effect remained significant after controlling for NMR group and treatment arm and is consistent with prior studies (Augustson et al., 2008; Boutou et al., 2008; Peters et al., 2011). Perhaps, increased sleep disturbance during a quit attempt exacerbates other symptoms of withdrawal, such as negative mood and difficulty concentrating, and may indirectly contribute to increased risk of relapse (Colrain, Trinder, & Swan, 2004; Hughes et al., 2007). Thus, it may be important to address sleep disturbance prior to or during a quit attempt.
Finally, we were somewhat surprised that although slow metabolisers reported more sleep disturbance overall, there were no interactions of NMR group with treatment on sleep disturbance. However, previous studies have shown that normal and slow metabolisers do not differ in withdrawal symptoms (Lerman et al., 2006; Patterson et al., 2008; Schnoll et al., 2009). The reported heightened side effects among slow metabolisers treated with varenicline may be a function of a broad constellation of side effects, rather than just sleep disturbance.
One limitation of this post-hoc analysis is that this study was not specifically designed to examine sleep disturbance. For instance, frequent nighttime awakenings may be more specific to nicotine withdrawal, whereas difficulty falling asleep may be due to medications (Colrain et al., 2004; Hughes et al., 2007). A more sensitive measure, such as the Pittsburgh Sleep Quality Index (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), which includes subscales of different sleep problems (e.g., sleep latency, sleep efficiency, daytime dysfunction) would have allowed us to distinguish specific patterns of sleep disturbance. We did include baseline sleep in our analyses but a more comprehensive measure of sleep could better control for pre-existing sleep conditions. Moreover, objective measures (e.g., actigraphy) would have allowed us to assess changes in sleep patterns independent of participants’ expectancies for sleep disturbance during withdrawal or as a side effect of treatment. Another possibility is that the most pronounced withdrawal-related sleep disturbance occurred in the first 24–72 hours following the TQD but our assessment did not occur until 7 days following the TQD. Future studies should include more thorough assessments including self-reported daily sleep diaries and objective measures to capture changes in sleep (Buysse, Ancoli-Israel, Edinger, Lichstein, & Morin, 2006; Colrain et al., 2004).
Conclusions
The current findings have two important implications for smoking cessation treatment. First, active treatment with either TN or varenicline does not mitigate withdrawal-related sleep disturbance and these effects are similar for slow and normal metabolisers. As the field approaches personalised treatment, behavioural or pharmacological treatments that improve sleep may be useful adjunctive smoking cessation treatments regardless of NMR status. For example, cognitive behavioural treatment for insomnia may increase time to relapse among smokers with insomnia (Fucito et al., 2014). In addition, we are currently evaluating whether an FDA-approved insomnia medication attenuates withdrawal-related sleep disturbance and improves short-term abstinence rates. Second, pre-quit sleep disturbance predicted relapse at EOT. Perhaps, inoculating against potential sleep disturbance before a quit attempt would be a useful strategy to improve abstinence rates among these individuals. Thus, sleep disturbance may be an important, yet overlooked, therapeutic target for smoking cessation and warrants further investigation.
Acknowledgments
Financial Support
This research was supported by a grant from the National Institute on Drug Abuse, the National Cancer Institute, the National Institute of General Medical Sciences, and the National Human Genome Research Institute (PGRN U01 DA020830) and byK23 DA035295.
Footnotes
The group of smokers who relapsed in the first week included those who never quit and those who were able to quit for a day or more, which may have increased the variability within this group. We ran a separate set of analyses comparing those who were abstinent at 1-week post-TQD and those who never quit (i.e., were not able to maintain abstinence for 24 h; n = 355). With the exception of an NMR group by visit interaction (p = 0.04), all results remained unchanged. The interaction suggested that slow metabolisers reported a significant increase in sleep disturbance from pre-quit to 1-weekpost-TQD (p = 0.01), whereas the normal metabolisers did not (p = 0.58).
Conflict of Interest
CL received study medication and placebo, and support for medication packaging, from Pfizer; she has also consulted to Gilead, and has been a paid expert witness in litigation against tobacco companies. PC served on the scientific advisory board of Pfizer Pharmaceuticals, did educational talks sponsored by Pfizer on smoking cessation from 2006 to 2008, and has received grant support from Pfizer. RAS received medication and placebo free of charge from Pfizer for a different project, and has consulted to Pfizer and GlaxoSmithKline. TPG has had both investigator-initiated and industry-sponsored grants from Pfizer in the past 12 months, and serves on a data monitoring committee for Novartis. RFT has acted as a consultant to pharmaceutical companies, primarily on smoking cessation. The remaining authors declare no competing interests.
Ethical Standards
The Institutional Review Boards at all sites approved this protocol. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Declaration of Helsinki.
References
- Ashare RL, Wileyto EP, Perkins KA, Schnoll RA. The first 7 days of a quit attempt predicts relapse: Validation of a measure for screening medications for nicotine dependence. J Addict Med. 2013;7(4):249–254. doi: 10.1097/ADM.0b013e31829363e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustson EM, Wanke KL, Rogers S, Bergen AW, Chatterjee N, Synder K, et al. Predictors of sustained smoking cessation: A prospective analysis of chronic smokers from the alpha-tocopherol Beta-carotene cancer prevention study. American Journal of Public Health. 2008;98(3):549–555. doi: 10.2105/AJPH.2005.084137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutou AK, Tsiata EA, Pataka A, Kontou PK, Pitsiou GG, Argyropoulou P. Smoking cessation in clinical practice: Predictors of six-month continuous abstinence in a sample of Greek smokers. Prim Care Respir J. 2008;17(1):32–38. doi: 10.3132/pcrj.2008.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12(2):101–112. [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chenoweth MJ, Novalen M, Hawk LW, Schnoll RA, George TP, Cinciripini PM, et al. Known and novel sources of variability in the Nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiology Biomarkers & Prevention. 2014;23(9):1773–1782. doi: 10.1158/1055-9965.EPI-14-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Trinder J, Swan GE. The impact of smoking cessation on objective and subjective markers of sleep: Review, synthesis, and recommendations. Nicotine & Tobacco Research. 2004;6(6):913–925. doi: 10.1080/14622200412331324938. [DOI] [PubMed] [Google Scholar]
- Faessel HM, Obach RS, Rollema H, Ravva P, Williams KE, Burstein AH. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clin Pharmacokinet. 2010;49(12):799–816. doi: 10.2165/11537850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. J Consult Clin Psychol. 2006;74(6):1153–1161. doi: 10.1037/0022-006X.74.6.1153. [DOI] [PubMed] [Google Scholar]
- Foulds J, Russ C, Yu CR, Zou KH, Galaznik A, Franzon M, et al. Effect of varenicline on individual nicotine withdrawal symptoms: A combined analysis of eight randomized, placebo-controlled trials. Nicotine Tob Res. 2013;15(11):1849–1857. doi: 10.1093/ntr/ntt066. [DOI] [PubMed] [Google Scholar]
- Fredrickson PA, Hurt RD, Lee GM, Wingender L, Croghan IT, Lauger G, et al. High dose transdermal nicotine therapy for heavy smokers: Safety, tolerability and measurement of nicotine and cotinine levels. Psychopharmacology (Berl) 1995;122(3):215–222. doi: 10.1007/BF02246542. [DOI] [PubMed] [Google Scholar]
- Fucito LM, Redeker NS, Ball SA, Toll BA, Ikomi JT, Carroll KM. Integrating a behavioural sleep intervention into smoking cessation treatment for smokers with insomnia: A randomised pilot study. J Smok Cessat. 2014;9(1):31–38. doi: 10.1017/jsc.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among selfquitters: A report from the Normative aging study. Addict Behav. 1992;17(4):367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers. BMJ. 1995;311(7001):363–366. doi: 10.1136/bmj.311.7001.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine Tob Res. 2007;9(3):315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;1(1):CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami D, Pickens R, Krahn D, Malin S, Luknic A. Effect of nicotine on the tobacco withdrawal syndrome. Psychopharmacology (Berl) 1984;83(1):82–87. doi: 10.1007/BF00427428. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Dale LC, Fredrickson PA, Caldwell CC, Lee GA, Offord KP, et al. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow-up. One-year outcome and percentage of nicotine replacement. JAMA. 1994;271(8):595–600. [PubMed] [Google Scholar]
- Jaehne A, Loessl B, Barkai Z, Riemann D, Hornyak M. Effects of nicotine on sleep during consumption, withdrawal and replacement therapy. Sleep Med Rev. 2009;13(5):363–377. doi: 10.1016/j.smrv.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hatsukami DK, Smith SS, Fiore MC, Allen S, Jensen J, et al. Characterization of tobacco withdrawal symptoms: Transdermal nicotine reduces hunger and weight gain. Psychopharmacology (Berl) 1996;128(2):130–138. doi: 10.1007/s002130050118. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Lerman C, Kaufmann V, Rukstalis M, Patterson F, Perkins K, Audrain-McGovern J, et al. Individualizing nicotine replacement therapy for the treatment of tobacco dependence: A randomized trial. Ann Intern Med. 2004;140(6):426–433. doi: 10.7326/0003-4819-140-6-200403160-00009. [DOI] [PubMed] [Google Scholar]
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend. 2002;67(2):219–223. doi: 10.1016/s0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- Lerman C, Schnoll RA, Hawk LW, Cinciripini P, George TP, Wileyto EP, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: A randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3(2):131–138. doi: 10.1016/S2213-2600(14)70294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clinical Pharmacology and Therapeutics. 2006;79(6):600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward personalized therapy for smoking cessation: A randomized placebo-controlled trial of bupropion. Clinical Pharmacology and Therapeutics. 2008;84(3):320–325. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- Peters EN, Fucito LM, Novosad C, Toll BA, O’Malley SS. Effect of night smoking, sleep disturbance, and their co-occurrence on smoking outcomes. Psychol Addict Behav. 2011;25(2):312–319. doi: 10.1037/a0023128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, et al. Effectiveness of extended-duration transdermal nicotine therapy: A randomized trial. Ann Intern Med. 2010;152(3):144–151. doi: 10.7326/0003-4819-152-3-201002020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: A validation study. Pharmacology, Biochemistry and Behavior. 2009;92(1):6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Janavs J, Weiller E, Keskiner A, et al. The validity of the Mini International Neuropsychiatric Interview(MINI) according to the SCID-P and its reliability. European Psychiatry. 1997;12(5):232–241. [Google Scholar]
- Shiffman S, Patten C, Gwaltney C, Paty J, Gnys M, Kassel J, et al. Natural history of nicotine withdrawal. Addiction. 2006;101(12):1822–1832. doi: 10.1111/j.1360-0443.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- Soldatos CR, Kales JD, Scharf MB, Bixler EO, Kales A. Cigarette smoking associated with sleep difficulty. Science. 1980;207(4430):551–553. doi: 10.1126/science.7352268. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Javitz HS, McAfee T, McClure JB. Predictors of 12-month outcome in smokers who received bupropion sustained-release for smoking cessation. CNS Drugs. 2008;22(3):239–256. doi: 10.2165/00023210-200822030-00004. [DOI] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN. Tobacco addiction and pharmacogenetics of nicotine metabolism. Journal of neurogenetics. 2009;23(3):262–271. doi: 10.1080/01677060802572903. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Fiore MC, Baker TB, Young TB. Tobacco withdrawal and nicotine replacement influence objective measures of sleep. Journal of Consulting and Clinical Psychology. 1995;63(4):658–667. doi: 10.1037//0022-006x.63.4.658. [DOI] [PubMed] [Google Scholar]


