Abstract
Receptors for glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) are present in vascular endothelial cells. Previous studies investigating euglycemic status have demonstrated that GIP is directly involved in the physiology of blood vessels by controlling the blood flow rate of portal veins and that GLP-1 has a protective effect on blood vessels by acting on endothelial cells. However, to the best of our knowledge, the effects of GIP and GLP-1 on endothelial cells in patients with hyperglycemia remain unknown. Therefore, the present study investigated whether the effect of the incretin hormones GLP-1 and GIP differed with regards to the reversal of endothelial cell dysfunction caused by hyperglycemia. The production of nitric oxide (NO) was measured using the Griess reagent system kit and the expression of cyclic adenosine monophosphate (cAMP) in the cell was measured at a wavelength of 405 nm with the ELISA reader using the cyclic AMP EIA kit. Exposure of human umbilical vein endothelial cells (HUVEC) to a high glucose concentration decreased NO and endothelial nitric oxide synthase (eNOS) levels but increased inducible NOS (iNOS) levels. However, when HUVECs were pretreated with GLP-1, a reduction of iNOS expression was observed and the expression of eNOS and NO were increased, as opposed to pretreatment with GIP. The results differed according to the response of cAMP, the second messenger of incretin hormones: The GIP pretreatment group did not exhibit an increase in cAMP levels while the GLP-1 pretreatment group did. The results of the present study provide evidence that GLP-1, but not GIP, has a protective effect on endothelial function associated with cardiovascular disease, as it is associated with increased eNOS expression and the levels of NO. This effect may be due to an increase in the cAMP concentration during hyperglycemic events.
Keywords: cyclic adenosine monophosphate response, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, nitric oxide
Introduction
Hyperglycemic conditions evoked by diabetes mellitus disrupt the role of endothelial cells in the protection of blood vessels and is therefore associated with cardiovascular disease. Diabetic macrovascular complications, such as myocardial infarction and stroke, are a major cause of morbidity and mortality (1). Once atherosclerosis has developed, it is irreversible and the progression of endothelial damage continues. Thus, even if blood sugar levels are well controlled thereafter, the natural course of macrovascular complications cannot be altered (2). In early stage diabetes mellitus, nitric oxide (NO), an endothelium-derived relaxing factor, is important in relaxing and protecting the blood vessels. NO is produced as a result of an enzymatic reaction of the L-arginine amino acid, facilitated by nitric oxide synthase (NOS). Endothelial cells possess two forms of NOS: Constitutive or endothelial NOS (cNOS or eNOS; type III) and inducible NOS (iNOS; type II) (3). Under normal conditions, NO has anti-inflammatory and anti-proliferative effects, which protect blood vessels by inhibiting smooth muscle hyperplasia and scavenging superoxide anion. NO also inhibits leukocyte adhesion to vascular endothelium, exerting an anti-thrombotic effect (3). However, abnormal conditions, such as inflammation and hyperglycemia, lead to a decrease in the synthesis of NO. This may lead to the development of vascular diseases including inflammation, thrombosis, vascular hypertrophy, stenosis and vasoconstriction (4).
Diabetic vascular complications are caused by endothelial cell dysfunction. Initially, the damage caused by lesions is reversible (4), thus early treatment to correct endothelial dysfunction is important to prevent diabetic macrovascular complications, and reduce the morbidity and mortality caused by cardiovascular diseases (5).
Incretin hormones, such as glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), have become the subjects of attention. GIP and GLP-1 are intestinal hormones that control blood sugar by stimulating insulin release from the pancreas (6). Incretin hormones have receptors in a number of organs and have been assessed with regards to control of blood sugar levels (7). GIP and GLP-1 receptors are present in vascular endothelial cells. It has been demonstrated that GIP is directly involved in the physiology of blood vessels; controlling the blood flow rate of the hepatic portal veins and increasing nutrient absorption (8). GLP-1 has a protective effect on blood vessels by acting on the endothelial cells (9). However, to the best of our knowledge, the current data do not sufficiently clarify the effects that GIP and GLP-1 have on endothelial cells in patients with hyperglycemia.
Therefore, the present study aimed to investigate whether the incretin hormones GLP-1 and GIP improve endothelial cell dysfunction caused by hyperglycemia.
Materials and methods
Cell culture and treatments
Hamster-derived insulin-secreting HIT-T15 cells (Calbiochem; EMD Millipore, Billerica, MA, USA) were maintained in RPMI 1,640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 11.1 mM glucose supplemented with 10% heat-inactivated fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), penicillin (100 U/ml), and streptomycin (100 µg/ml), in a humidified atmosphere containing 5% CO2 at 37°C. These cells were used as a comparison when determining whether GIP and GLP-1 were expressed in HUVECs.
Passage 6–10 cells of human umbilical vein endothelial cells (HUVECs) supplied by Lonza (Walkersville, MD, USA) were cultured with medium from the EGM-2™ Bullet kit™ (Lonza). The kit contained 10% fetal bovine serum, vascular endothelial growth factor, human fibroblastic growth factor B, hydrocortisone, R3-insulin-like growth factor-I, ascorbic acid, GA-1,000, human epidermal growth factor and heparin. The conditions for culture were maintained at 37°C and 95% humidity in 5% CO2. When the confluence reached 80–90%, subcultures were prepared following washing with phosphate-buffered saline (PBS) and processing with trypsin-EDTA. The collected cells were underwent centrifugation at room temperature, 500 × g for 10 min in order to produce pellets. The pellets were gently resuspended in EGM-2™ Bullet kit™ medium once the supernatant was discarded. This medium was replaced every 2 days until confluence was reached (3–5 days). Following treatment with GIP (Sigma-Aldrich, Merck kGaA, Darmstadt, Germany), GLP-1 (Bachem Americas, Inc., Torrance, CA, USA), or Exendin 9–39, a specific GLP-1 receptor antagonist (Bachem Americas) in 5.5 or 30 mM glucose, analysis was completed. Exendin 9–39 GLP-1 receptor antagonist (Saxon Biochemicals, Hannover, Germany) was used to determine if the change in iNOS and eNOS was due to the GLP-1 receptor agonist. HUVECs were pretreated for 1 h with 1 nM GIP or 3 nM GLP-1, and 50 µM DPPIV inhibitor (Sigma-Aldrich; Merck kGaA, Darmstadt, Germany). HUVECs were then cultured in medium containing 5.5 or 30 mM glucose for 48 h.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR was used to determine the presence of GIP or GLP-1 receptors in HUVECs. Total RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's protocol. The reverse transcription reaction was completed at 42°C for 60 min using ImProm-II™ reverse transcription system (20 µl; Promega Corporation, Madison, WI, USA), containing AMV reverse transcriptase, MgCl2 25 mM, reverse transcription 10X buffer, dNTP mixture 10 mM, recombinant RNasin ribonuclease inhibitor and oligo (dT)15 primer, before heating them to 70°C for 5 min followed immediately by cooling on ice so that reverse transcription enzymes were inactivated and cDNA remained in a linear strand state. cDNA (1 µg) was amplified in final volume 20 µl with iTaq™ DNA polymerase (Bio-Rad Laboratories Inc., Hercules, CA, USA) using the following primer sets: GIP receptor: Forward, 5′-AACGAAGTCAAGGCCATTTG-3′ and reverse, 5′-GTCCTCAGCTTGGACAGGAG-3′; GLP-1 receptor: Forward 5′-GTTCCCCTGCTGTTTGTTGT-3′ and reverse, 5′-TGGCCTTCAGTTTGGATACC-3′; GAPDH forward, 5′-AAGGGTCATCATCTCTGCCC-3′ and reverse, 5′-GTGATGGCATGGACTGTGGT-3′. PCR reaction procedure began with heating at 95°C for 5 min prior to cycle starts. Cycles consisted of denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec and elongation at 72°C for 30 sec, repeated 30 times, followed by the extension of generated strands at 72°C for 5 min. Following the PCR reaction process, the extended DNA was subjected to electrophoresis at 100V for 20 min using 1.2% agarose gel.
Reverse transcription-quantitative PCR (RT-qPCR)
Extraction of total RNA and cDNA synthesis were performed using the aforementioned method in the previous section. RT-qPCR primers were synthesized, ~100 bps based on the base sequence of GenBank (https://www.ncbi.nlm.nih.gov/genbank). Sequences of the synthesized primers were as follows: iNOS, forward, 5′-ACAAGCCTACCCCTCCAGAT-3′ and reverse, 5′-TCCCGTCAGTTGGTAGGTTC-3′; eNOS, forward, 5′-CCCTTCAGTGGCTGGTACAT-3′ and reverse 5′-TATCCAGGTCCATGCAGACA-3′; GAPDH, forward 5′-AAGGGTCATCATCTCTGCCC-3′ and reverse 5′-GTGATGGCATGGACTGTGGT-3′. qPCR was performed with a 20 µl reaction mixture containing 1 µg cDNA, 10 pmol forward primer, 10 pmol reverse primer and 10 µl Fast SYBR Green Master Mix (Invitrogen; Thermo Fisher Scientific, Inc.) using the iQ™5 Optical system (Bio-Rad Laboratories, Inc.). The reaction procedure was completed as follows: Heating at 95°C for 5 min prior to denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and elongation at 72°C for 30 sec for 40 cycles. This was followed by the extension of generated strands at 72°C for 5 min. Melting curve analysis was performed at a temperature between 65–95°C and a Cq value for each was used to normalize the data to GAPDH mRNA value following compensation (10).
Western blot analysis
Cells were harvested and then pelleted by centrifugation at 13,000 rpm for 10–20 sec. Cells were resuspended in 400 µl PRO-PREP™ solution (Intron Biotechnology, Inc., Seongnam, Korea), and mix well. Cell lysis was induced by incubation for 10–20 min on freezer at −20°C. Centrifugation was then performed at 2,000 × g at 4°C for 5 min, and supernatant was transferred to a fresh 1.5 ml tube. The extracted protein was quantified using a Bradford protein assay kit (Bio-Rad Laboratories, Inc.) and equal amounts of protein (20 µg/lane) were separated by 20% SDS-PAGE and transferred to a nitrocellulose membrane (Invitrogen; Thermo Fisher Scientific, Inc.). The membrane was incubated in 5% non-fat dry milk-PBS Tween-20 (PBST; 0.01% Tween 20 in PBS) for 1 h at room temperature to block the non-specific bonding between antibody and protein. Primary antibodies; anti-iNOS (sc-49055; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-eNOS (9572; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-phospho-eNOS (Ser1177) (9517; Cell Signaling Technology, Inc.), anti-GIP receptor (ab30679; Abcam, Cambridge, UK), anti-GLP-1 receptor (ab36598; Abcam) and anti-β-actin (internal control; a5441; Sigma-Aldrich; Merck kGaA) were incubated with the protein at room temperature for 2 h (anti-β-actin was diluted to 1:5,000 and other antibodies to 1:500 with blocking buffer). Following washing with PBST twice for 10 min, the secondary antibodies, anti-mouse immunoglobulin (Ig) G (sc-51993) and anti-rabbit IgG (sc-358919; Santa Cruz Biotechnology, Inc.) conjugated with horseradish peroxidase (HRP) were diluted to 1:500 with blocking buffer and incubated at room temperature for 1 h. The protein obtained following the reaction was examined for specific bands following exposure to light on X-ray film using the Chemiluminescent Reagent kit (ab79907; Abcam).
MTT assay
An MTT assay (Vybrant® MTT Cell Proliferation Assay kit, Invitrogen; Thermo Fisher Scientific, Inc.) was completed.
Assessment of NO production
The activity of NO was determined by measuring the nitrite (NO2-) concentration in the culture media using the Griess reagent system (Promega Corporation). Each medium supernatant (100 ml) was mixed with 50 ml 1% sulfanilamide (in 5% phosphoric acid, Sigma-Aldrich; Merck kGaA) and 50 ml 0.1% N-(1-Naphthyl) ethylenediamine dihydrochloride (Santa Cruz Biotechnology, Inc.) and incubated in the dark at room temperature for 10 min. Absorbance was measured using a SpectraMax L Microplate Reader (Molecular Devices, LLC, Sunnyvale, CA, USA) at 540 nm. NO2 concentration was determined based on the nitrogen standard curve.
Measurement of cyclic adenosine monophosphate (cAMP)
Cultured cells were washed with cold PBS twice and lysed with 0.1 M hydrochloric acid at room temperature for 20 min. Cells were then collected using a scraper and subjected to centrifugation at 1,000 × g for 10 min at room temperature. The supernatant was transferred to new tubes and used for experimentation in a 96-well plate. cAMP levels in the cells were measured at a wavelength of 405 nm using an ELISA microplate reader (Molecular Devices LLC.) using a cyclic AMP ELISA kit (581001; Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturers protocol.
Statistical analysis
Each experiment was repeated four times and data were indicated as mean ± standard deviation. All statistical analysis was performed using the Statistical Package for the Social software (SPSS; Korean version 20.0; IBM SPSS, Armonk, NY, USA). Statistical differences were analyzed using one-way analysis of variance followed by Tukey's test. P<0.05 was determined to indicate a statistically significant difference.
Results
Expression of GIP and GLP-1 receptors in HUVEC
Prior to investigating the action of GIP or GLP-1 in endothelial cells, RT-PCR and western blot analysis were used to assess whether GIP and GLP-1 receptors were expressed in HUVEC. The expression of GIP and GLP-1 receptors in HUVECs is lower than in islet cells (Fig. 1A). Furthermore, in HUVEC cells, the GLP-1 receptor is more highly expressed than the GIP receptor (Fig. 1B). Islet cells were used as positive control. An MTT assay was completed to eliminate cell death from high glucose. During the low and high glucose exposure for up to 48 h, the HUVECs survived and the maintenance of cell morphology was confirmed by MTT and light microscopy (data not shown).
Figure 1.
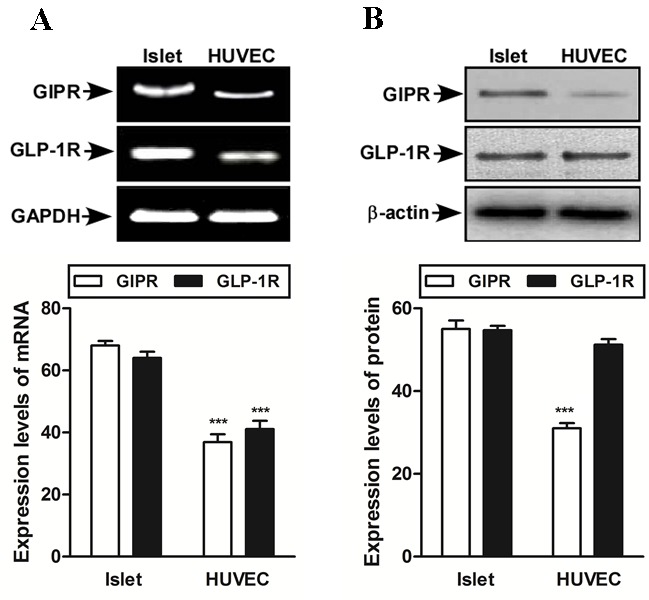
Expression of GIPR and GLP-1R mRNA in HUVECs using reverse transcription-polymerase chain reaction and western blot analysis. (A) mRNA expression of GIP and GLP-1 receptors present in HUVEC and (B) protein expression level of GIP and GLP-1 receptors present in HUVEC is lower than in islet cells. GAPDH and β-actin were used as controls. Data are presented as mean ± standard deviation. ***P<0.001 vs. control cells, GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; HUVEC, human umbilical vein endothelial cells; GIPR, GIP receptor; GLP-1R, GLP-1 receptor.
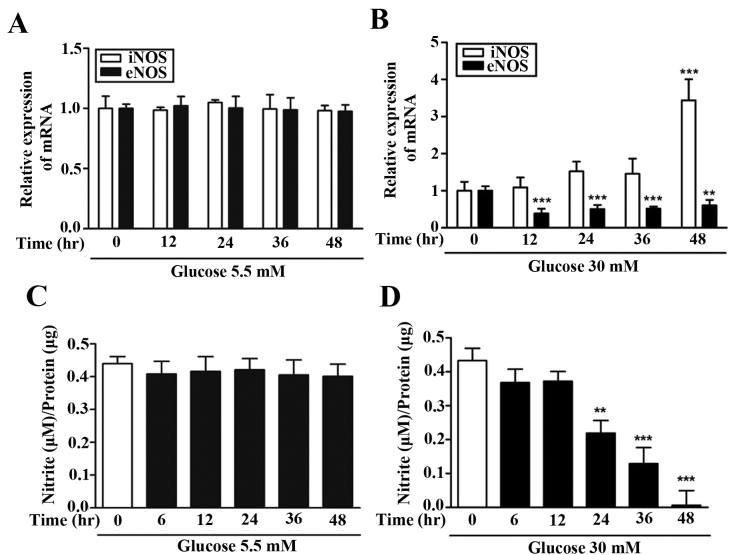
Effect of high glucose on iNOS and eNOS mRNA expression and NO production
To compare NOS expression against high glucose levels, expression of iNOS mRNA and eNOS mRNA were measured by RT-qPCR. The expression of iNOS and eNOS mRNA was not affected in normal conditions with 5.5 mM glucose (Fig. 2A). However, the expression of eNOS mRNA significantly decreased by >50% 12 h following treatment with 30 mM glucose, indicating a fast reaction (P<0.001; Fig. 2B). Following treatment with 30 mM glucose, the expression of iNOS mRNA did not increase during the initial 0–36 h but significantly increased compared with the baseline value following 48 h (P<0.01; Fig. 2B). The production of nitric oxide, which is associated with vascular endothelial cell function, was not affected in normal conditions with 5.5 mM glucose (Fig. 2C) but decreased significantly from 24 h onwards under the high glucose (30 mM) condition (P<0.01; Fig. 2D).
Figure 2.
Time-dependent analysis of iNOS, eNOS and NO in low and high-glucose conditions. Expression of iNOS and eNOS in (A) 5.5 and (B) 30 nM glucose determined by reverse transcriptase-quantitative polymerase chain reaction. Activity of nitric oxide with (C) 5.5 and (D) 30 nM glucose examined by Griess reagent system kit. Data are presented as the means ± standard deviation of six independent experiments. **P<0.01, ***P<0.001 vs. control cells. iNOS, inducible nitric oxide synthase; eNOS, endothelial nitric oxide synthase; NO, nitric oxide.
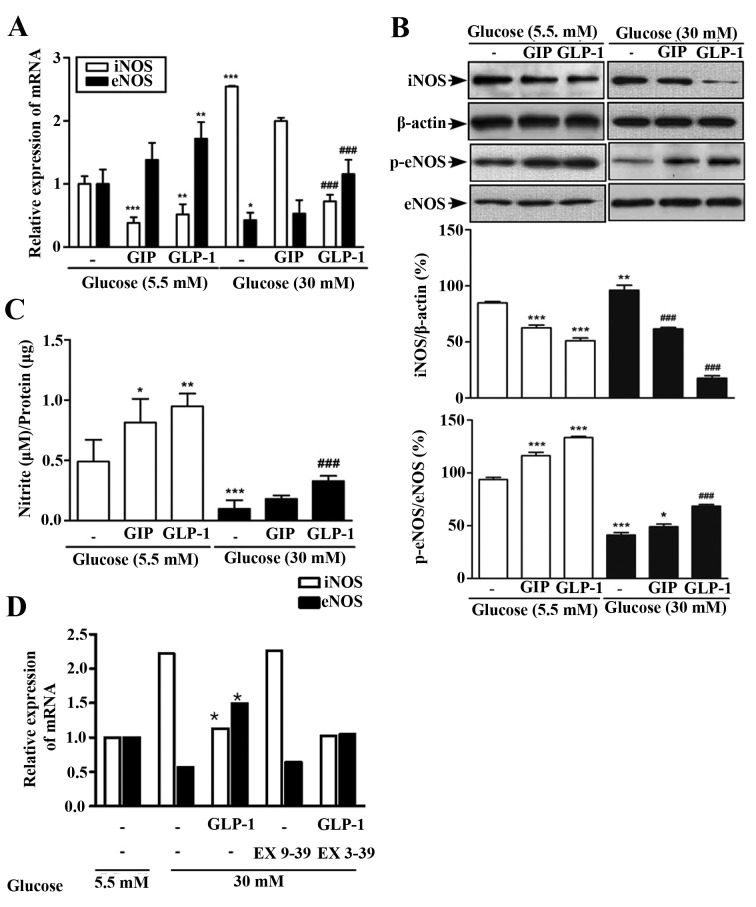
Effects of GIP or GLP-1 on the expression of iNOS and eNOS and the production of NO in high glucose concentrations
Similar to the results described in Fig. 2, a high glucose concentration (30 mM) had different effects on eNOS and iNOS in HUVEC and led to a decrease in NO production. In order to determine whether incretin hormones have a protective effect on the reaction of endothelial cells to hyperglycemia, the experiment was performed following treatment with GIP or GLP-1. HUVECs pre-treated with GLP-1 exhibited decreased expression of iNOS mRNA (P<0.01) and an increase in eNOS mRNA expression under hyperglycemic conditions (both P<0.001; Fig. 3A). However, HUVECs pre-treated with GIP did not exhibit any change in iNOS and eNOS mRNA expressions (Fig. 3A). Similar results were obtained regarding the expression of iNOS and eNOS protein (Fig. 3B). The production of NO also differed between these two treatment groups, demonstrating a significant increase of NO expression in the GLP-1 treatment group compared with control group (P<0.001; Fig. 3C). However, there was no significant difference between the NO expression in cells treated with GIP and control cells in the hyperglycemic condition. To evaluate whether the GLP-1 acts through GLP-1 receptor or not, Exendin (9–39) was used. This determined that the effect of GLP-1 on iNOS and eNOS in HUVEC was reduced significantly compared with treatment with GLP-1 alone. (Fig. 3D).
Figure 3.
Effect of GIP or GLP-1 on iNOS, eNOS and NO in normal glucose (5.5 mM) or high-glucose (30 mM). (A) Expression of iNOS and eNOS mRNA was determined by reverse transcription-quantitative polymerase chain reaction. (B) Expression of iNOS, p-eNOS and eNOS proteins was determined by western blot analysis. β-actin was used as a loading control. (C) Activity of NO was examined using a Griess reagent system kit. (D) Following co-treatments with Exendin (9–39), a GLP-1 receptor antagonist, and GLP-1, the effect of GLP-1 on iNOS and eNOS expression in HUVEC was observed to decrease in high-glucose cells (30 mM), compared with treatment with GLP-1 alone. *P<0.05, **P<0.01, ***P<0.001 vs. control cells of normal glucose (5.5 mM). ###P<0.001 vs. control cells of high-glucose (30 mM). Data are presented as the mean ± standard deviation of four independent experiments. GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; iNOS, inducible nitric oxide synthase; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; HUVEC, human umbilical vein endothelial cells; p-eNOS, phosphorylated eNOS; DPPIV, dipeptidyl peptidase-4.
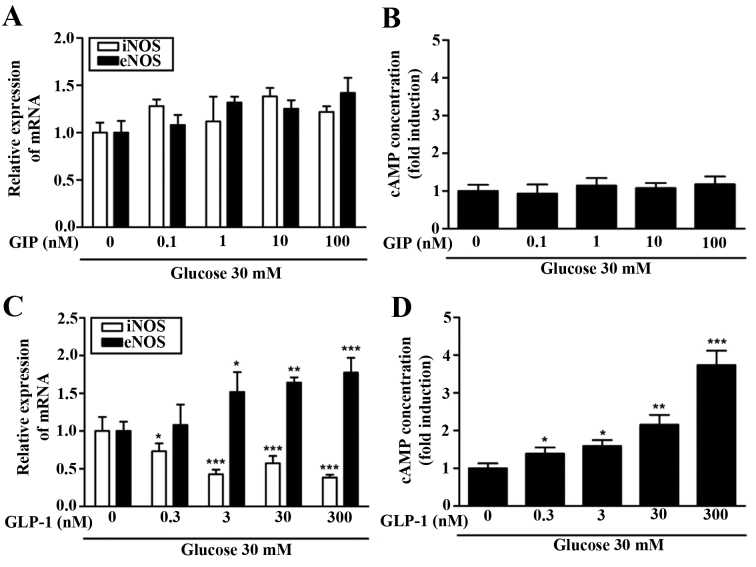
Effect of GIP or GLP-1 on cAMP concentration in HUVEC
Due to the differing effects of GIP and GLP-1 observed regarding the expression of NO, eNOS and iNOS, the change in the concentration of cAMP was assessed. Increasing GIP concentration in the hyperglycemic condition did not alter the expression of iNOS and eNOS mRNA (Fig. 4A). An increased GIP concentration did not result in any change in cAMP concentration (Fig. 4B). By contrast, it was observed that the expression of iNOS and eNOS mRNA differed significantly depending on the concentration of GLP-1. At higher concentrations of GLP-1 (>0.3 nM), iNOS mRNA expression decreased significantly (P<0.05) and at concentrations of GLP-1 >3 nM, eNOS mRNA increased significantly (P<0.05; Fig. 4C). Furthermore, cAMP concentration increased in a GLP-1-dependent manner (Fig. 4D). cAMP concentration increased ~x3 in hyperglycemic condition (30 mM glucose) compared with a normal glucose condition (5.5 mM). The increase in cAMP concentrations following pre-treatment with GLP-1, but not with GIP treatment, indicates that there is an association between the two.
Figure 4.
iNOS and eNOS expression and change in cAMP levels according to GIP or GLP-1 dose in high-glucose (30 mM). HUVEC was pretreated with GIP or GLP-1 in the indicated doses and 50 µM DPPIV inhibitor for 1 h. HUVEC was maintained in medium containing 30 mM glucose for 48 h. Expression of iNOS mRNA and eNOS mRNA was determined following treatment with (A) GIP or (B) GLP-1 by reverse transcription-quantitative polymerase chain reaction. (C) cAMP concentration was examined following treatment with GIP or (D) GLP-1 using a cAMP EIA kit. *P<0.05, **P<0.01, ***P<0.001 vs. control cells of high-glucose (30 mM). Data are presented as the mean ± standard deviation of four independent experiments. iNOS, inducible nitric oxide synthase; eNOS, endothelial nitric oxide synthase; cAMP, cyclin adenoside monophosphate; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; HUVEC, human umbilical vein endothelial cells; DPPIV, dipeptidyl peptidase-4.
Discussion
The current study demonstrates that GLP-1 induced cAMP activity serves a pivotal role in GLP-1 mediated vascular protection by increasing NO levels. However, GIP has no effect in GIP associated with increasing cAMP activity in high glucose condition. Thus, the results of the present study indicate that cAMP signaling is required in the protective effect of GLP-1 in endothelial cells.
It has been demonstrated that vascular endothelial cells express GIP and GLP-1 receptors and this has been interpreted to mean that incretin hormones may be useful in the treatment of vascular diseases (11,12). Therefore, the current study aimed to examine how the incretin hormones GIP and GLP-1 act to protect endothelial cells under high glucose conditions and to identify their mechanism of action using a HUVEC cell line.
GLP-1 receptors are widely expressed in pancreatic islets, brain, heart, kidney, endothelial cells and the gastrointestinal tract (13). Some of the functions of GLP-1 in certain organs have been reported but its precise functions have not yet been established (14). Since one of the functions of GLP-1 is to inhibit glucagon and stimulate glucose-induced insulin secretion, it is used as a fasting or postprandial hypoglycemic agent. A protective effect of GLP-1 on vascular endothelial cells has been identified, but its mechanism in endothelial cells remains unknown (13). A previous clinical study demonstrated that GLP-1 may lead to the improvement of aorta pulse wave velocity (PWV) in obese diabetic patients during short-term treatment (15). Aorta PWV is the velocity at which the arterial pulse propagates through the circulatory system and is used clinically as a measure of arterial stiffness (16). It was demonstrated that aorta PWV is directly associated with cardiovascular disease and the early endothelial cell dysfunction marker in patients with diabetes mellitus (17). In the current study, it was suggested that GLP-1 protects against hyperglycemia and vascular endothelial cell dysfunction in hyperglycemic conditions by increasing eNOS expression and thus increasing levels of NO in endothelial cells. This protects the endothelial cells from being damaged by the effects of hyperglycemia. The increase of NO expression in endothelial cells is associated with an increase in eNOS activation by GLP-1, which is dependent on the GLP-1 receptor (18). The results of the present study also demonstrated a GLP-1 dose-dependent increase of eNOS; this effect was significant at low (5.5 mM) and high (30 nM) glucose concentrations. By contrast, the effect of GIP was significant at a low glucose concentration (5.5 mM; P<0.05) but did not cause a significant change in the amount of iNOS and eNOS at higher glucose levels (Fig. 3).
Since the two incretin hormones demonstrated different endothelial cell reactions to a high glucose concentration, the expression of cAMP was measured. The difference in cAMP expression between the two hormones at a high concentration of glucose indicates that GLP-1 is superior to GIP in protecting endothelial cells. Treatment with GLP-1 also resulted in a dose-dependent increase of cAMP occurring. Although the effect of GIP on endothelial vasoconstriction or dilatation may be explained in connection with the types of vascular endothelial cells or the expression of receptors, it exhibits the same result that occurs when blood sugar is controlled normally. As such reactions may be inhibited at high glucose levels due to unresponsive cAMP, GLP-1 is effective even in hyperglycemic conditions.
GIP and GLP-1 stimulate insulin secretion through Ca2+ and cAMP pathways in beta cells (19). In endothelial cells, GIP increases the level of calcium in a dose-dependent manner, however the magnitude of this response differs depending on the endothelial cell type (20). cAMP also increases insulin secretion in beta cells but it inhibits the mitogenic effect and bovine fibroblast growth in endothelial cells (21). By contrast, with GIP, cAMP levels did not increase in endothelial cell lines therefore; the inhibition of cell proliferation caused by cAMP does not occur (22). The results of the present study demonstrated no increase of cAMP upon increasing the GIP concentration at high glucose levels, but GLP-1 increased the cAMP concentration proportional to its concentration. A previous study indicated that there was a significant decrease in proliferation and an increase in the apoptosis of smooth muscle cells in vitro following treatment with exendin-4, this effect appears to be mediated through cAMP signaling (23). Ge et al (24) demonstrated the protective effect of GLP-1 using microvascular endothelial cells whereas the present study used HUVEC as a macrovascular cell line. GLP-1 also protects cardiac microvessels against apoptosis and oxidative stress (24). The protective effects of GLP-1 are dependent on the downstream inhibition of Rho in a cAMP/Protein kinase A-dependent manner (25).
Vascular endothelial cells serve an important function in maintaining vascular homeostasis by controlling vasomotor, blood coagulation and decomposition, and the proliferation and migration of inflammatory cells or vascular smooth muscle (26). In patients with diabetes however, hyperglycemia causes endothelial dysfunction and it is difficult to maintain vascular homeostasis. An early complication of diabetes is lesions of dysfunctional endothelial cells, which are initially reversible, however early detection and treatment is required (27). NO is an important factor for endothelial cell dysfunction in the form of lesions that appear in the early stage of vascular disease in patients with diabetes (28). Controlling blood sugar and NO levels in diabetes patients may help prevent and treat vascular complications by normalizing the endothelial function at the early stage of vascular disease (29).
In conclusion, to the best of our knowledge, the present study is the first to identify a dose-dependent association between GIP or GLP-1 and hyperglycemia in HUVEC endothelial cells. This is associated with the generation of eNOS, iNOS and NO at different cAMP concentrations.
Acknowledgements
The present study was supported by a grant (L.D.M., 2011) from the Korean Diabetes Association.
References
- 1.Rask-Madsen C, King GL. Mechanisms of disease: Endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- 2.ACCORD Study Group. Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr., Probstfield JL, Cushman WC, Ginsberg HN, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Förstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H. Nitric oxide synthase isozymes: Characterization, purification, molecular cloning and functions. Hypertension. 1994;23:1121–1131. doi: 10.1161/01.HYP.23.6.1121. [DOI] [PubMed] [Google Scholar]
- 4.Wright E, Jr., Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int J Clin Pract. 2006;60:308–314. doi: 10.1111/j.1368-5031.2006.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schäfer A, Bauersachs J. Endothelial dysfunction, impaired endogenous platelet inhibition and platelet activation in diabetes and atherosclerosis. Curr Vasc Pharmacol. 2008;6:52–60. doi: 10.2174/157016108783331295. [DOI] [PubMed] [Google Scholar]
- 6.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 7.Vilsbøll T, Krarup T, Madsbad S, Holst JJ. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept. 2003;114:115–121. doi: 10.1016/S0167-0115(03)00111-3. [DOI] [PubMed] [Google Scholar]
- 8.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60:470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kogire M, Inoue K, Sumi S, Doi R, Yun M, Kaji H, Tobe T. Effects of gastric inhibitory polypeptide and glucagon on portal venous and hepatic arterial flow in conscious dogs. Dig Dis Sci. 1992;37:1666–1670. doi: 10.1007/BF01299856. [DOI] [PubMed] [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Ojima A, Matsui T, Maeda S, Takeuchi M, Yamagishi S. Glucose-dependent insulinotropic polypeptide (GIp) inhibits signaling pathways of advanced glycation end products (AGEs) in endothelial cells via its antioxidative properties. Horm Metab Res. 2012;44:501–505. doi: 10.1055/s-0032-1312595. [DOI] [PubMed] [Google Scholar]
- 12.Forst T, Weber MM, Pfützner A. Cardiovascular benefits of GLP-1-based therapies in patients with diabetes mellitus type 2: Effects on endothelial and vascular dysfunction beyond glycemic control. Exp Diabetes Res. 2012;2012:635472. doi: 10.1155/2012/635472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 14.Hansotia T, Maida A, Flock G, Yamada Y, Tsukiyama K, Seino Y, Drucker DJ. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight and energy expenditure. J Clin Invest. 2007;117:143–152. doi: 10.1172/JCI25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong JY, Park KY, Kim BJ, Hwang WM, Kim DH, Lim DM. Effects of short-term exenatide treatment on regional fat distribution, glycated hemoglobin levels and aortic pulse wave velocity of obese type 2 diabetes mellitus patients. Endocrinol Metab (Seoul) 2016;31:80–85. doi: 10.3803/EnM.2016.31.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S–10S. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Anderson TJ. Arterial stiffness or endothelial dysfunction as a surrogate marker of vascular risk. Can J Cardiol. 2006;22(Suppl B):72B–80B. doi: 10.1016/S0828-282X(06)70990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding L, Zhang J. Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol Sin. 2012;33:75–81. doi: 10.1038/aps.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51(Suppl 3):S434–S442. doi: 10.2337/diabetes.51.2007.S434. [DOI] [PubMed] [Google Scholar]
- 20.Zhong Q, Bollag RJ, Dransfield DT, Gasalla-Herraiz J, Ding KH, Min L, Isales CM. Glucose-dependent insulinotropic peptide signaling pathways in endothelial cells. Peptides. 2000;21:1427–1432. doi: 10.1016/S0196-9781(00)00287-4. [DOI] [PubMed] [Google Scholar]
- 21.D'Angelo G, Lee H, Weiner RI. CAMP-dependent protein kinase inhibits the mitogenic action of vascular endothelial growth factor and fibroblast growth factor in capillary endothelial cells by blocking raf activation. J Cell Biochem. 1997;67:353–366. doi: 10.1002/(SICI)1097-4644(19971201)67:3<353::AID-JCB7>3.3.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Ding KH, Zhong Q, Isales CM. Glucose-dependent insulinotropic peptide stimulates thymidine incorporation in endothelial cells: Role of endothelin-1. Am J Physiol Endocrinol Metab. 2003;285:E390–E396. doi: 10.1152/ajpendo.00509.2002. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson L, Saxelin R, Röhl S, Roy J, Caidahl K, Nyström T, Hedin U, Razuvaev A. Glucagon like peptide-1 receptor activation does not affect re-endothelization but reduces intimal hyperplasia via direct effects on smooth muscle cells in a nondiabetic model of arterial injury. J Vasc Res. 2015;52:41–52. doi: 10.1159/000381097. [DOI] [PubMed] [Google Scholar]
- 24.Ge GH, Dou HJ, Yang SS, Ma JW, Cheng WB, Qiao ZY, Hou YM, Fang WY. Glucagon-like peptide-1 protects against cardiac microvascular endothelial cells injured by high glucose. Asian Pac J Trop Med. 2015;8:73–78. doi: 10.1016/S1995-7645(14)60191-7. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Luo P, Wang Y, Li W, Wang C, Sun D, Zhang R, Su T, Ma X, Zeng C, et al. Glucagon-like peptide-1 protects against cardiac microvascular injury in diabetes via a cAMP/PKA/Rho-dependent mechanism. Diabetes. 2013;62:1697–1708. doi: 10.2337/db12-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lüscher TF, Barton M. Biology of the endothelium. Clin Cardiol. 1997;20(11 Suppl 2):II3–II10. [PubMed] [Google Scholar]
- 27.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 28.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: Testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 29.Belz GG, Mohr-Kahaly S. Cacoa and dark chocolate in cardiovascular prevention? Dtsch Med Wochenschr. 2011;136:2657–2663. doi: 10.1055/s-0031-1292826. (In German) [DOI] [PubMed] [Google Scholar]