Abstract
The present study aimed to investigate the expression of complement component 1, q subcomponent-binding protein (C1QBP) in colon cancer cells, and identify proteins that interact with C1QBP. Total proteins were extracted from both the tumor and normal tissues of 22 patients with colon cancer and analyzed using liquid chromatography-mass spectrometry (LC-MS) to identify proteins that were differentially-expressed in tumor tissues. C1QBP overexpression was induced in 293T cells using a pFLAG-CMV2 expression vector. Overexpressed FLAG-tagged C1QBP protein was then immunoprecipitated using anti-FLAG antibodies and C1QBP-interacting proteins were screened using LC-MS analysis of the immunoprecipitates. The C1QBP-interacting proteins were confirmed using reverse-immunoprecipitation and the differential expression of C1QBP in tissues and cell lines was confirmed using western blot analysis. LC-MS analysis revealed that C1QBP exhibited a typical tumor expression pattern. Two immune-reactive signals (33 and 14 kDa) were detected in normal and tumor tissues from 19 patients. Furthermore, 14 kDa C1QBP protein was upregulated in the tumors of 15 patients. In total, 39 proteins were identified as candidate C1QBP-interacting proteins, and an interaction between C1QBP and apolipoprotein A-I was confirmed. The present study indicates that C1QBP is involved in colon cancer carcinogenesis, and that the mechanisms underlying the established anti-tumor properties of apolipoprotein A-I may include interacting with and inhibiting the activity of C1QBP.
Keywords: complement component 1, q subcomponent-binding protein, colon cancer, apolipoprotein A-I, proteomics
Introduction
Complement component 1, q subcomponent binding protein (C1QBP) is a multifunctional protein. Its sequence is identical to that of p32, a protein that co-purifies with splicing factor SF2, and that of hyaluronan-binding protein 1, a member of the hyaladherin family (1). Its terminal α-helices are important in protein-protein interactions and regulate its multifunctional nature. C1QBP is commonly localized in the mitochondrial matrix, although it exhibits differential localization among cell lines under various physiological conditions (1). Moreover, it serves important roles in various biological processes, including inflammation, infection, cell signaling and chemotaxis (2–4).
However, it remains unclear whether C1QBP exerts anti- or pro-tumorigenic effects during cancer pathogenesis (5). Upregulated expression of C1QBP has been reported in various different types of cancer, including breast, prostate, thyroid, colon, pancreatic, gastric, esophageal and lung cancer (6–9). This elevated expression has been linked to the metastasis of ovarian cancer (10), poor prognosis or metastasis of breast cancer (7,9) and early relapse following surgery in prostate cancer (11). By contrast, other studies have reported that C1QBP is required for the induction of mitochondria-dependent apoptotic cell death (12,13). The constitutive expression of C1QBP in normal fibroblast cells perturbs its growth characteristics and induces apoptosis (14). In addition, it has been demonstrated that C1QBP is upregulated following the induction of apoptosis in HeLa cells in response to a chemotherapeutic agent (cisplatin), suggesting that C1QBP may be involved in apoptosis (15,16).
Over one million people worldwide develop colorectal cancer annually, and the disease has a mortality rate of ~33% (17). Carcinogenesis and biology of colorectal cancer have been recognized as multistep processes involving various molecular alterations (18). Knowledge of the specific molecular markers or pathways that are responsible for disease progression and poor prognosis may be beneficial in the development of more effective treatment.
The present proteomic study revealed that C1QBP was cleaved in the tumor tissues of patients with colon cancer, and that apolipoprotein A-I (ApoA-I) interacts with C1QBP. Furthermore, the possible role of C1QBP in colon cancer via its interaction with ApoA-I is discussed.
Materials and methods
Tissues from colorectal cancer patients
Fresh tissues (normal colon mucosa and primary colon tumor) were obtained from 22 patients with colon cancer (8 female and 14 male, 61.5±10.5 years old) who were enrolled in the current study between January 2006 and May 2007 at the National Cancer Center, Republic of Korea. Following dissection of necrotic exudates and stromal components, the overall cellularity of the normal epithelium and tumors was >75%. The present study was approved and performed in accordance with the guidelines of the Institutional Review Board of the National Cancer Center (Goyang, Republic of Korea), and informed consent was obtained from all patients.
Human cancer cell lines
The human colon cancer cell lines (SNU-81, SNU-407, SNU-C4, NCI-H498, NCI-H508, CaCo2, DLD-1, HCT-116, LoVo and SW620), a cervical cancer cell line (HeLa) and an embryonic kidney cell line (293T) were all obtained from the Korean Culture Line Bank (Seoul, Korea).
Mass analysis
Mass analysis was performed as described previously (19). The bands of SDS-PAGE corresponding to potential proteins of interest were excised, destained using 50% acetonitrile in 0.1 M ammonium bicarbonate (Thermo Fisher Scientific, Inc., Waltham, MA USA) and dried using a SpeedVac evaporator (Savant SVC-100H, Thermo Fisher Scientific, Inc.). Dried gel pieces were swollen with 30 µl of 25 mM sodium bicarbonate (pH 8.8) (Thermo Fisher Scientific, Inc.) containing 50 ng trypsin (Promega Corporation, Madison, WI, USA) at 37°C overnight. Samples were subsequently desalted using Zip-Tips C18 (EMD Millipore, Billerica, MA, USA) and dissolved in 10 µl of 2% acetonitrile (Thermo Fisher Scientific, Inc.) in 0.1% formic acid (Thermo Fisher Scientific, Inc.). Analyses were performed using a linear trap quadrupole (LTQ) XL linear ion trap mass spectrometer (MS; Thermo Fisher Scientific, Inc.) in the Proteomics Core of the National Cancer Center (Goyang, Republic of Korea). The mass spectrometer was set for nanospray ionization (NSI) in the positive mode. Moreover, a syringe pump was used to introduce the calibration solution for the automatic tuning and calibration of the LTQ in an NSI-positive ion mode. The infusion of trypsin-digested samples into the ionization source of the mass spectrometer was performed following liquid chromatographic separation. The spray voltage was set at +1.1 kV, the temperature of the capillary apparatus was maintained at 200°C, the capillary voltage was set at +20 V and the tube lens voltage was +100 V. Moreover, the auxiliary gas was set to zero. Full scan experiments were performed to linear trap in the m/z range of 150–2,000. Systematic MS/MS experiments were performed by changing the relative collision energy and monitoring the intensities of the fragment ions. All MS/MS samples were analyzed using Sequest (version v.27, rev.11; Thermo Fisher Scientific) to search the uniprot_sprot (http://www.uniprot.org) and IPI human databases (European Bioinformatics Institute, Hinxton, UK; ftp://ftp.ebi.ac.uk/pub/databases/IPI/last_release/current/) assuming digestion with trypsin. Sequest was searched with a fragment ion mass tolerance of 1.00 Da and a parent ion tolerance of 1.2 Da. Methionine oxidation was specified as a variable modification.
Western blot analysis
Western blotting was performed as described previously (20). Briefly, cell homogenates containing equivalent quantities (20 µg) of protein were centrifuged at 4,000 × g (4°C) for 5 min and the supernatant fractions were separated using 4–10% gradient SDS-PAGE. Following electrophoresis, the proteins were transferred to polyvinylidene fluoride membranes (EMD Millipore), blocked for 2 h at 4°C in 1% Tween 20-Tris buffered saline buffer containing 1.5% non-fat dry milk (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and 1 mM MgCl2. The blocked membranes were then incubated for 2 h at room temperature with primary antibodies against C1QBP (dilution 1:1,000, cat no. ab131284, Abcam, Cambridge, MA, USA), ApoA-I (dilution 1:1,000, cat no. ab52945, Abcam) or β-actin (dilution 1:2,000, cat no. 04-1116, Merck Millipore, Darmstadt, Germany). They were then washed three times in blocking solution (1% Tween 20-TBS buffer containing 1.5% non-fat dry milk and 1 mM MgCl2) for 15 min each and incubated with diluted horseradish-peroxidase conjugated secondary antibody (dilution 1:2,000, cat no. 4010-05, Southern Biotech Associates, Inc., Birmingham, AL, USA) for 1 h at room temperature. Membranes were then washed with blocking solution (3×15 min), incubated with WEST-ZOL® plus chemiluminescence reagent (iNtRON Biotechnology, Inc., Gyeonggi, Korea) for 1 min and exposed to film (Kodak Blue XB-1, Kodak, Rochester, NY, USA).
Overexpression of C1QBP in 293T cells and immunoprecipitation
In order to generate a pFLAG-CMV2-C1QBP plasmid containing the full-length coding sequence of human C1QBP, polymerase chain reaction (PCR) was performed from a human brain cDNA library (Clontech Laboratories, Inc., Rochester, NY, USA) with the following oligomers: Sense, 5′-GGAATTCTATGCTGCCTCTGCTGCGCTGC-3′ and antisense, 5′-TAACCCGGGCTACTGGCTCTTGACAAAACTCTTGAGG-3′. The amplified PCR product was digested using EcoRI-XmaI (Clontech Laboratories), then inserted into pFLAG-CMV2 (Merck Millipore). The 293T cells were transfected with control or FLAG-C1QBP plasmids. At 48 h after transfection, cells were harvested and aliquots of the total protein from each sample were analyzed using western blotting, as indicated.
For immunoprecipitation-coupled MS analyses, cells were lysed in immunoprecipitation buffer containing 150 mM NaCl, 25 mM HEPES-KOH (pH 7.5), 10% (v/v) glycerol, 1 mM MgCl2, 2 mM sodium orthovanadate, 2 mM glycerophosphate, 1 mM PMSF, 1 mM DTT, 2 mM EDTA, 0.5% Triton X-100 and 1X protease inhibitor cocktail (Roche Applied Science, Madison, WI, USA). Following a brief sonication and incubation on ice, the lysates were centrifuged at 15,000 × g for 5 min to remove the insoluble materials. The lysates were then incubated with anti-FLAG M2 affinity agarose beads (Sigma-Aldrich; Merck Millipore) for 2 h at 4°C, and the collected beads were washed four times with washing buffer (0.05% Triton X-100 immunoprecipitation buffer without a protease inhibitor cocktail), and boiled in SDS sample buffer for protein immunoprecipitation-coupled MS analysis.
Results
Differential expression of C1QBP in tissues from colon cancer patients
Whole proteins were extracted from both the tumor and normal tissues of three patients and were separated by SDS-PAGE (Fig. 1). Sections of gel containing proteins were then cut according to their molecular weight and subjected to proteomic analysis. Proteins of 19–35-kDa were analyzed using the linear ion trap MS system, which identified C1QBP to be present only in tumor tissues (Fig. 1 and Table I).
Figure 1.
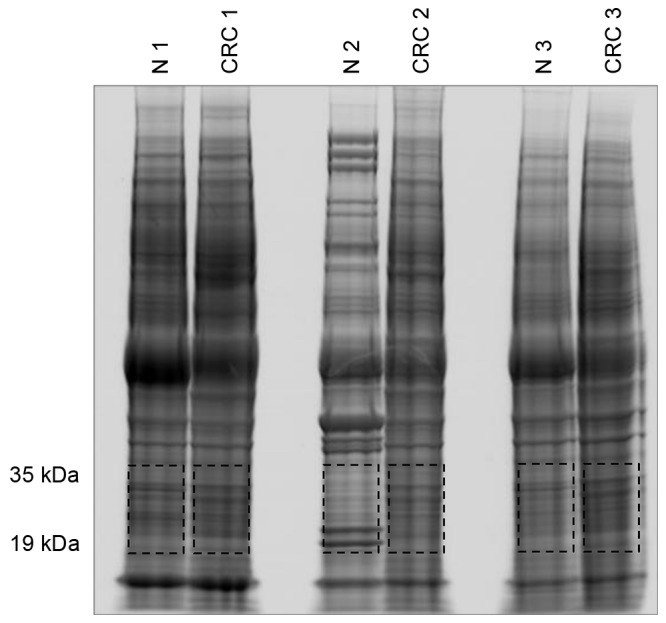
Image of an SDS-PAGE gel containing whole proteins extracted from paired tumor (CRC 1–3) and normal (N 1–3) tissues of three colon cancer patients. Red boxes indicate the gel slices used for mass analysis to identify proteins of 19–35 kDa.
Table I.
Proteins identified in the range of molecular weight between 19 to 35 kDa indicated in Fig. 1. Proteins were extracted from the paired tissues (tumor, CRC; normal, N) obtained from three colon cancer patients. ‘O’ indicates successful identification of protein.
| Total identification no. | CRC | Normal | ||||||
|---|---|---|---|---|---|---|---|---|
| Protein accession number and name | CRC | Normal | CRC1 | CRC2 | CRC3 | N1 | N2 | N3 |
| IPI:IPI00014230.1 C1QBP Complement component 1 Q subcomponent-binding protein, mitochondrial | 3 | 0 | O | O | O | |||
| IPI:IPI00010204.1 SRSF3 Serine/arginine-rich splicing factor 3 | 3 | 1 | O | O | O | O | ||
| IPI:IPI00021266.1 SNORD4A;RPL23A 60S ribosomal protein L23a | 3 | 1 | O | O | O | O | ||
| IPI:IPI00215914.5 ARF1 ADP-ribosylation factor 1 | 3 | 1 | O | O | O | O | ||
| IPI:IPI00219622.3 PSMA2 Proteasome subunit alpha type-2 | 3 | 1 | O | O | O | O | ||
| IPI:IPI00306332.4 RPL24 60S ribosomal protein L24 | 3 | 1 | O | O | O | O | ||
| IPI:IPI00003881.5 HNRNPF Heterogeneous nuclear ribonucleoprotein F | 2 | 0 | O | O | ||||
| IPI:IPI00007321.2 LYPLA1 cDNA FLJ60607, highly similar to Acyl-protein thioesterase 1 | 2 | 0 | O | O | ||||
| IPI:IPI00010105.1 EIF6 Eukaryotic translation initiation factor 6 | 2 | 0 | O | O | ||||
| IPI:IPI00016568.1 AK4 Adenylate kinase isoenzyme 4, mitochondrial | 2 | 0 | O | O | ||||
| IPI:IPI00031691.1 RPL9 60S ribosomal protein L9 | 2 | 0 | O | O | ||||
| IPI:IPI00179964.5 PTBP1 Isoform 1 of Polypyrimidine tract-binding protein 1 | 2 | 0 | O | O | ||||
| IPI:IPI00219097.4 HMGB2 High mobility group protein B2 | 2 | 0 | O | O | ||||
CRC, colorectal cancer.
Western blot analysis was then used to confirm the expression of C1QBP in normal and tumor tissues from patients with colon cancer (Fig. 2A). Two immunoreactive signals at 33 and 14 kDa were detected using the anti-C1QBP antibody (Fig. 2A). In contrast to the proteomic analysis, 33 kDa C1QBP exhibited no typical expression pattern in tumor tissues (Fig. 2A). However, among the 19 pairs of tissues analyzed, 15 showed upregulation of 14 kDa C1QBP in tumor compared with normal tissues (Fig. 2A). In human colon cancer cell lines, the expression of 33 kDa C1QBP was variable, whereas the short form was not detected (Fig. 2B).
Figure 2.
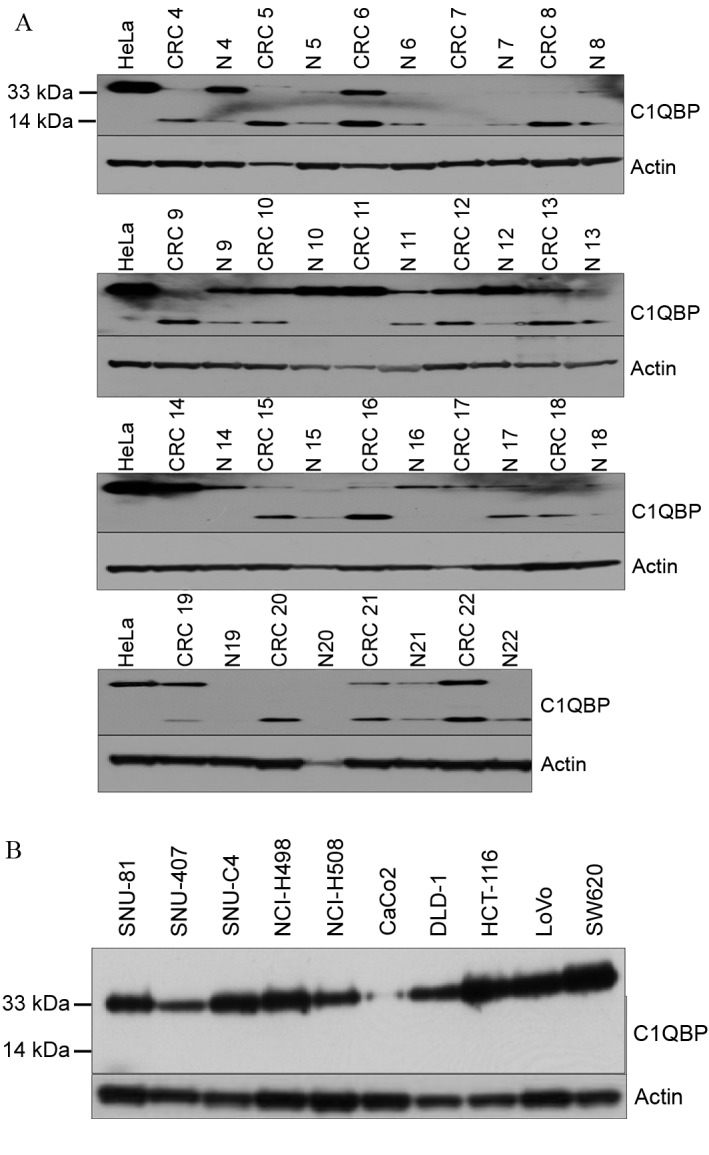
Expression of C1QBP in tissues from colon cancer patients and colon cancer cell lines. (A) Immunoreactive signals were detected at 33 and 14 kDa in tissues from colon cancer patients. There was no typical expression pattern of C1QBP in tumor tissues, but increased expression of 14 kDa C1QBP was found in 15 tumors. (B) Only 33 kDa C1QBP was detected in human colon cancer cell lines. C1QBP, complement component 1, q subcomponent-binding protein.
Candidate C1QBP-interacting proteins
In order to further evaluate the role of C1QBP in colon cancer at the molecular level, C1QBP-interacting proteins were screened for in immunoprecipitates from FLAG-tagged C1QBP-overexpressing 293T cells. Fig. 3 presents the results of immunoprecipitation using FLAG antibodies in whole cell lysates from C1QBP-overexpressing 293T cells (pFLAG_CMV2_C1QBP). FLAG-tagged C1QBP was immunoprecipitated successfully using anti-FLAG antibodies, and was detected as a band at 33 kDa on the SDS-PAGE gel (Fig. 3). As a control, immunoprecipitation was performed using 293T cells overexpressing FLAG alone (pFLAG_CMV2_MOCK_293T). The appropriate bands were excised from the gels as described in Fig. 3 and the proteins therein were subjected to in-gel tryptic digestion and MS. The proteins that co-precipitated with C1QBP are listed in Table II after exclusion of proteins also identified in the control lane. In total 39 proteins, including fibrinogen α-, β- and γ-chains, complement C3, complement C4-A, ApoA-I and ApoA-II, were identified as possible C1QBP-interacting proteins (Table II).
Figure 3.
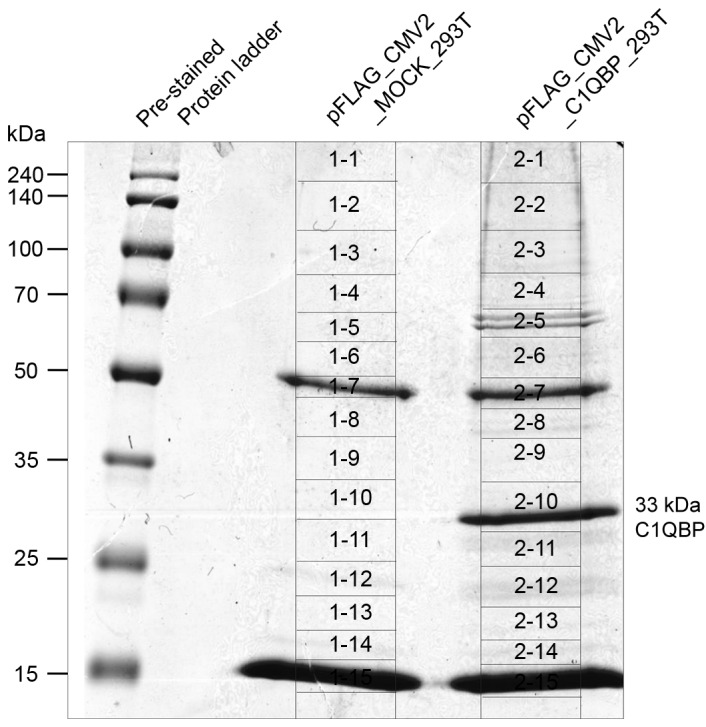
SDS-PAGE gel after C1QBP immunoprecipitation. Immunoprecipitation of whole cell lysates from 293T cells overexpressing C1QBP (pFLAG_CMV2_C1QBP) was performed using FLAG antibodies. The FLAG-tagged C1QBP protein was immunoprecipitated efficiently using anti-FLAG antibodies and stained in a ‘2-10’ numbered slice of an SDS-PAGE gel. 293T cells overexpressing only FLAG (pFLAG_CMV2_MOCK_293T) were used in control immunoprecipitation. The indicated slices were excised from SDS-PAGE gels and subjected to mass analyses to identify the proteins that co-precipitated with C1QBP. C1QBP, complement component 1, q subcomponent-binding protein.
Table II.
Candidate proteins interacting with complement component 1, q subcomponent-binding protein.
| MS/MS sample | Protein name | Accession number | Molecular weight, Da | Identification probability (%) | Total spectra, % | Sequence coverage, % |
|---|---|---|---|---|---|---|
| 2–01 | General transcription factor 3C polypeptide 1 | TF3C1_HUMAN | 238,860.60 | 100.00 | 0.06 | 1.19 |
| 2–01 | Insulin receptor substrate 4 | IRS4_HUMAN | 133,751.30 | 99.80 | 0.06 | 2.07 |
| 2–02 | Heterogeneous nuclear ribonucleoprotein U | HNRPU_HUMAN | 90,567.20 | 100.00 | 0.23 | 9.09 |
| 2–02 | General transcription factor 3C polypeptide 2 | TF3C2_HUMAN | 100,652.80 | 99.80 | 0.07 | 1.98 |
| 2–02 | ATP-dependent RNA helicase A | DHX9_HUMAN | 140,902.50 | 100.00 | 0.07 | 2.44 |
| 2–02 | Ubiquitin-associated protein 2-like | UBP2L_HUMAN | 116,780.70 | 100.00 | 0.13 | 4.34 |
| 2–02 | Thyroid hormone receptor-associated protein 3 | TR150_HUMAN | 108,650.90 | 99.80 | 0.07 | 1.99 |
| 2–03 | General transcription factor 3C polypeptide 3 | TF3C3_HUMAN | 101,258.20 | 100.00 | 0.27 | 6.77 |
| 2–03 | Nucleolar RNA helicase 2 | DDX21_HUMAN | 87,328.00 | 99.80 | 0.11 | 2.55 |
| 2–04 | Alpha-1-antitrypsin | A1AT_HUMAN | 46,719.90 | 100.00 | 0.11 | 8.37 |
| 2–04 | Apolipoprotein A-I | APOA1_HUMAN | 30,760.50 | 100.00 | 0.11 | 12.40 |
| 2–04 | Protein LAS1 homolog | LAS1L_HUMAN | 83,047.50 | 100.00 | 0.14 | 6.40 |
| 2–06 | Lamin-B receptor | LBR_HUMAN | 70,688.30 | 99.80 | 0.10 | 3.09 |
| 2–08 | RNA methyltransferase-like protein 1 | RMTL1_HUMAN | 47,002.50 | 100.00 | 0.24 | 7.62 |
| 2–08 | Keratin, type I cytoskeletal 14 | K1C14_HUMAN | 51,544.50 | 100.00 | 0.12 | 9.11 |
| 2–09 | Interleukin enhancer-binding factor 2 | ILF2_HUMAN | 43,044.70 | 100.00 | 0.17 | 11.50 |
| 2–10 | Heterogeneous nuclear ribonucleoproteins C1/C2 | HNRPC_HUMAN | 33,652.50 | 100.00 | 0.37 | 22.20 |
| 2–10 | Glutaminyl-peptide cyclotransferase-like protein | QPCTL_HUMAN | 42,907.60 | 100.00 | 0.12 | 8.12 |
| 2–11 | rRNA 2′-O-methyltransferase fibrillarin | FBRL_HUMAN | 33,766.10 | 99.90 | 0.09 | 7.48 |
| 2–11 | Polymerase delta-interacting protein 2 | PDIP2_HUMAN | 42,015.00 | 100.00 | 0.20 | 20.10 |
| 2–12 | THO complex subunit 4 | THOC4_HUMAN | 26,870.70 | 100.00 | 0.26 | 21.00 |
| 2–13 | Alpha-1-antichymotrypsin | AACT_HUMAN | 47,634.90 | 99.80 | 0.04 | 4.73 |
| 2–13 | Alpha-2-macroglobulin | A2MG_HUMAN | 163,271.90 | 100.00 | 0.56 | 18.80 |
| 2–13 | Plasma protease C1 inhibitor | IC1_HUMAN | 55,137.50 | 99.70 | 0.04 | 5.60 |
| 2–13 | Serotransferrin | TRFE_HUMAN | 77,046.20 | 100.00 | 0.16 | 13.50 |
| 2–13 | Hemopexin | HEMO_HUMAN | 51,658.50 | 100.00 | 0.05 | 8.23 |
| 2–13 | Haptoglobin | HPT_HUMAN | 45,186.90 | 100.00 | 0.16 | 13.10 |
| 2–13 | Fibrinogen alpha chain | FIBA_HUMAN | 94,955.40 | 100.00 | 0.18 | 14.40 |
| 2–13 | Fibrinogen beta chain | FIBB_HUMAN | 55,910.60 | 100.00 | 0.15 | 21.60 |
| 2–13 | Fibrinogen gamma chain | FIBG_HUMAN | 51,495.30 | 100.00 | 0.11 | 14.10 |
| 2–13 | Ig gamma-1 chain C region | IGHG1_HUMAN | 36,087.00 | 100.00 | 0.33 | 20.00 |
| 2–13 | Ig kappa chain C region | IGKC_HUMAN | 11,590.50 | 99.80 | 0.18 | 30.20 |
| 2–13 | Ig lambda-2 chain C regions | LAC2_HUMAN, | 11,275.20 | 99.80 | 0.13 | 32.10 |
| 2–13 | Ig mu chain C region | IGHM_HUMAN | 49,287.70 | 100.00 | 0.15 | 22.80 |
| 2–13 | Complement C3 | CO3_HUMAN | 187,131.10 | 100.00 | 0.15 | 7.76 |
| 2–13 | Complement C4-A | CO4A_HUMAN | 192,754.80 | 100.00 | 0.05 | 2.52 |
| 2–13 | Apolipoprotein A-II | APOA2_HUMAN | 11,202.40 | 99.80 | 0.07 | 21.00 |
| 2–14 | 60S ribosomal protein L13 | RL13_HUMAN | 24,244.20 | 99.80 | 0.08 | 11.40 |
Interaction between C1QBP and ApoA-I
Reverse immunoprecipitation was subsequently used to confirm the interaction between C1QBP and candidate proteins. Among the proteins listed in Table II, ApoA-I was selected for confirmation of its interaction with C1QBP. Human SW620 colon cancer cells were used because they express high levels of C1QBP (Fig. 2B, and mouse IgG or antibodies against C1QBP and ApoA-I were added to SW620 whole-cell lysates. Immunoprecipitation was then performed, followed by western blotting. Immunoreactive signals produced by monoclonal antibodies against ApoA-I were detected in immunoprecipitates prepared using both anti-ApoA-I and -C1QBP antibodies (Fig. 4). In addition, SW620 whole cell lysates were immunoreactive for ApoA-I (Fig. 4).
Figure 4.
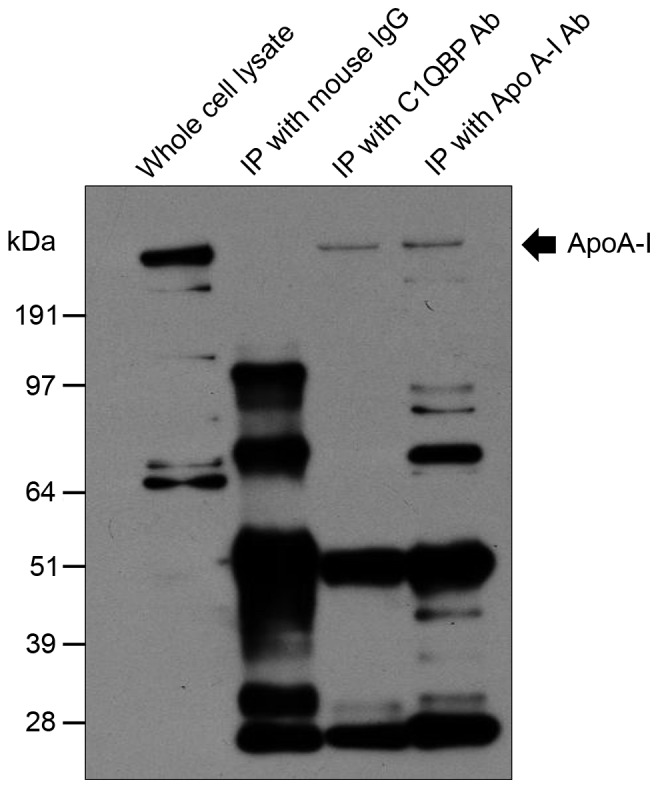
Reverse immunoprecipitation of C1QBP. Among the proteins listed in Table II, ApoA-I was selected for confirmation of interaction with C1QBP. Mouse IgG or antibodies against either C1QBP or ApoA-I were added to whole cell lysates of SW620 cells expressing high levels of C1QBP. Following immunoprecipitation, immunoreactive signals produced by monoclonal antibodies against ApoA-I were detected in immunoprecipitates prepared using ApoA-I antibody (indicated by arrow). C1QBP, complement component 1, q subcomponent-binding protein; ApoA-I, apolipoprotein A-I.
Discussion
The present study revealed that the cleaved form of C1QBP (14 kDa) is upregulated in colon cancer compared with normal cells, which is consistent with previous reports of the pro-tumorigenic properties of C1QBP (5,6,8). Rubinstein et al (8) compared the C1QBP protein levels among several adenocarcinomas. Immunohistochemical staining of histological tissue sections revealed pronounced differences in the expression in colon adenocarcinoma (as well as thyroid, pancreatic, gastric, esophageal and lung cancer) vs. non-malignant tissues. Dembitzer et al (6) revealed strong C1QBP expression in epithelial breast, prostate, liver, lung, colon and skin tumors. However, increased C1QBP staining was also detected in inflammatory and proliferative lesions of the same cell types, as well as in normal and continuously dividing cells. Moreover, McGee et al (5) reported that C1QBP expression was upregulated in breast, colon and lung cancer compared with normal control tissues. Altogether, these data indicate that C1QBP is important in colon cancer tumorigenicity.
A novel observation of the present study is that C1QBP interacts with ApoA-I. Among the many candidate-binding proteins identified, ApoA-I was selected for confirmation because it has a relatively well-established role in colon cancer. The lipid metabolism is closely associated with cancer (21,22). In particular, it has been speculated that lipids and lipoproteins are associated with neoplastic processes, including inflammation, oxidative stress and insulin resistance (23). Moreover, ApoA-I is a major component of high-density lipoprotein in plasma, which exerts protective anti-inflammatory, anti-oxidant and anti-microbial functions, and it is important in innate immunity (21). ApoA-I is synthesized primarily in the liver (80%) and small intestine (10%) (24), and it is known to be important in reverse cholesterol transport and for promoting cholesterol efflux from tissues by acting as a cofactor for lecithin cholesterol acyltransferase (24). In a study of a cohort of >520,000 participants from 10 European countries, high serum concentrations of HDL and ApoA-I were associated with a decreased risk of colon cancer (23). In addition, HDL mimetics inhibited tumor development in both induced and spontaneous mouse models of colon cancer, possibly by inhibiting angiogenesis (21). Zhang et al (25) analyzed the lipid levels of 206 patients with colorectal cancer, 70 patients with benign colorectal disease, and 300 healthy participants, and revealed that serum ApoA-I and ApoB levels were significantly lower in colorectal cancer patients (25). In addition to colon cancer, ApoA-I inhibited tumor development in a mouse model of ovarian cancer (26). Significantly decreased serum levels of ApoA-I were found in patients with cholangiocarcinoma (27), and increased levels in serum were associated with a favorable prognosis in patients with metastatic nasopharyngeal carcinoma (28). These anti-tumor properties of ApoA-I may be associated with its binding to and subsequent inhibition of C1QBP. As such, HDL has received attention as a promising therapeutic strategy for colon cancer. A novel finding in the present study, that C1QBP binds to ApoA-I, may assist the future development of such therapeutic strategies.
Nevertheless, the physiological role of the interaction between ApoA1 and C1QBP requires further investigation. Furthermore, one contradictory study indicated that the expression of ApoA-I was associated with colon adenocarcinoma progression, and that ApoA-I is a potential marker of tumor aggression (29). However, the novel observations in the present study may facilitate identification of the molecular mechanisms underlying the roles of ApoA-I and C1QBP in colon cancer.
Acknowledgements
The present study was supported by the Soonchunhyang University Research Fund.
References
- 1.Chowdhury AR, Ghosh I, Datta K. Excessive reactive oxygen species induces apoptosis in fibroblasts: Role of mitochondrially accumulated hyaluronic acid binding protein 1 (HABP1/p32/gC1qR) Exp Cell Res. 2008;314:651–667. doi: 10.1016/j.yexcr.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 2.Majumdar M, Meenakshi J, Goswami SK, Datta K. Hyaluronan binding protein 1 (HABP1)/C1QBP/p32 is an endogenous substrate for MAP kinase and is translocated to the nucleus upon mitogenic stimulation. Biochem Biophys Res Commun. 2002;291:829–837. doi: 10.1006/bbrc.2002.6491. [DOI] [PubMed] [Google Scholar]
- 3.Ghebrehiwet B, Peerschke EI. cC1q-R (calreticulin) and gC1q-R/p33: Ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol Immunol. 2004;41:173–183. doi: 10.1016/j.molimm.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Tahtouh M, Garçon-Bocquet A, Croq F, Vizioli J, Sautière PE, van Camp C, Salzet M, Nagnan-le Meillour P, Pestel J, Lefebvre C. Interaction of HmC1q with leech microglial cells: Involvement of C1qBP-related molecule in the induction of cell chemotaxis. J Neuroinflammation. 2012;9:37. doi: 10.1186/1742-2094-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGee AM, Douglas DL, Liang Y, Hyder SM, Baines CP. The mitochondrial protein C1qbp promotes cell proliferation, migration and resistance to cell death. Cell Cycle. 2011;10:4119–4127. doi: 10.4161/cc.10.23.18287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dembitzer FR, Kinoshita Y, Burstein D, Phelps RG, Beasley MB, Garcia R, Harpaz N, Jaffer S, Thung SN, Unger PD, et al. gC1qR expression in normal and pathologic human tissues: Differential expression in tissues of epithelial and mesenchymal origin. J Histochem Cytochem. 2012;60:467–474. doi: 10.1369/0022155412440882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Zhang F, Guo L, Wang Y, Zhang P, Wang R, Zhang N, Chen R. Interactome analysis reveals that C1QBP (complement component 1, q subcomponent binding protein) is associated with cancer cell chemotaxis and metastasis. Mol Cell Proteomics. 2013;12:3199–3209. doi: 10.1074/mcp.M113.029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubinstein DB, Stortchevoi A, Boosalis M, Ashfaq R, Ghebrehiwet B, Peerschke EI, Calvo F, Guillaume T. Receptor for the globular heads of C1q (gC1q-R, p33, hyaluronan-binding protein) is preferentially expressed by adenocarcinoma cells. Int J Cancer. 2004;110:741–750. doi: 10.1002/ijc.20105. [DOI] [PubMed] [Google Scholar]
- 9.Chen YB, Jiang CT, Zhang GQ, Wang JS, Pang D. Increased expression of hyaluronic acid binding protein 1 is correlated with poor prognosis in patients with breast cancer. J Surg Oncol. 2009;100:382–386. doi: 10.1002/jso.21329. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Liu Q, Xin T, Xing L, Dong G, Jiang Q, Lv Y, Song X, Teng C, Huang D, et al. Elevated expression of hyaluronic acid binding protein 1 (HABP1)/P32/C1QBP is a novel indicator for lymph node and peritoneal metastasis of epithelial ovarian cancer patients. Tumour Biol. 2013;34:3981–3987. doi: 10.1007/s13277-013-0986-6. [DOI] [PubMed] [Google Scholar]
- 11.Amamoto R, Yagi M, Song Y, Oda Y, Tsuneyoshi M, Naito S, Yokomizo A, Kuroiwa K, Tokunaga S, Kato S, et al. Mitochondrial p32/C1QBP is highly expressed in prostate cancer and is associated with shorter prostate-specific antigen relapse time after radical prostatectomy. Cancer Sci. 2011;102:639–647. doi: 10.1111/j.1349-7006.2010.01828.x. [DOI] [PubMed] [Google Scholar]
- 12.Itahana K, Zhang Y. Mitochondrial p32 is a critical mediator of ARF-induced apoptosis. Cancer Cell. 2008;13:542–553. doi: 10.1016/j.ccr.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunayama J, Ando Y, Itoh N, Tomiyama A, Sakurada K, Sugiyama A, Kang D, Tashiro F, Gotoh Y, Kuchino Y, Kitanaka C. Physical and functional interaction between BH3-only protein Hrk and mitochondrial pore-forming protein p32. Cell Death Differ. 2004;11:771–781. doi: 10.1038/sj.cdd.4401418. [DOI] [PubMed] [Google Scholar]
- 14.Meenakshi J, Goswami SK Anupama, Datta K. Constitutive expression of hyaluronan binding protein 1 (HABP1/p32/gC1qR) in normal fibroblast cells perturbs its growth characteristics and induces apoptosis. Biochem Biophys Res Commun. 2003;300:686–693. doi: 10.1016/S0006-291X(02)02788-2. [DOI] [PubMed] [Google Scholar]
- 15.Kamal A, Datta K. Upregulation of hyaluronan binding protein 1 (HABP1/p32/gC1qR) is associated with Cisplatin induced apoptosis. Apoptosis. 2006;11:861–874. doi: 10.1007/s10495-006-5396-4. [DOI] [PubMed] [Google Scholar]
- 16.Hosseinimehr SJ, Nobakht R, Ghasemi A, Pourfallah TA. Radioprotective effect of mefenamic acid against radiation-induced genotoxicity in human lymphocytes. Radiat Oncol J. 2015;33:256–260. doi: 10.3857/roj.2015.33.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han S, Bui NT, Ho MT, Kim YM, Cho M, Shin DB. Dexamethasone inhibits TGF-β1-induced cell migration by regulating the ERK and AKT pathways in human colon cancer cells via CYR61. Cancer Res Treat. 2016;48:1141–1153. doi: 10.4143/crt.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim ST, Park KH, Kim JS, Shin SW, Kim YH. Impact of KRAS mutation status on outcomes in metastatic colon cancer patients without anti-epidermal growth factor receptor therapy. Cancer Res Treat. 2013;45:55–62. doi: 10.4143/crt.2013.45.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JH, Kim KH, Park JW, Chang HJ, Kim BC, Kim SY, Kim KG, Lee ES, Kim DY, Oh JH, et al. Low-mass-ion discriminant equation: A new concept for colorectal cancer screening. Int J Cancer. 2014;134:1844–1853. doi: 10.1002/ijc.28517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim KH, Yeo SG, Kim WK, Kim DY, Yeo HY, Hong JP, Chang HJ, Park JW, Kim SY, Kim BC, Yoo BC. Up-regulated expression of l-caldesmon associated with malignancy of colorectal cancer. BMC Cancer. 2012;12:601. doi: 10.1186/1471-2407-12-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su F, Grijalva V, Navab K, Ganapathy E, Meriwether D, Imaizumi S, Navab M, Fogelman AM, Reddy ST, Farias-Eisner R. HDL mimetics inhibit tumor development in both induced and spontaneous mouse models of colon cancer. Mol Cancer Ther. 2012;11:1311–1319. doi: 10.1158/1535-7163.MCT-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho O, Hwang HS, Lee BS, Oh YT, Kim CH, Chun M. Met inactivation by S-allylcysteine suppresses the migration and invasion of nasopharyngeal cancer cells induced by hepatocyte growth factor. Radiat Oncol J. 2015;33:328–336. doi: 10.3857/roj.2015.33.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, Jenab M, Pischon T, Jansen EH, Frohlich J, Ayyobi A, Overvad K, Toft-Petersen AP, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European prospective investigation into cancer and nutrition. Gut. 2011;60:1094–1102. doi: 10.1136/gut.2010.225011. [DOI] [PubMed] [Google Scholar]
- 24.Halley P, Kadakkuzha BM, Faghihi MA, Magistri M, Zeier Z, Khorkova O, Coito C, Hsiao J, Lawrence M, Wahlestedt C. Regulation of the apolipoprotein gene cluster by a long noncoding RNA. Cell Rep. 2014;6:222–230. doi: 10.1016/j.celrep.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Zhao XW, Liu DB, Han CZ, Du LL, Jing JX, Wang Y. Lipid levels in serum and cancerous tissues of colorectal cancer patients. World J Gastroenterol. 2014;20:8646–8652. doi: 10.3748/wjg.v20.i26.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su F, Kozak KR, Imaizumi S, Gao F, Amneus MW, Grijalva V, Ng C, Wagner A, Hough G, Farias-Eisner G, et al. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc Natl Acad Sci USA. 2010;107:19997–20002. doi: 10.1073/pnas.1009010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Dai S, Zhang Z, Liu L, Wang J, Xiao X, He D, Liu B. Characterization of apolipoprotein A-I as a potential biomarker for cholangiocarcinoma. Eur J Cancer Care (Engl) 2009;18:625–635. doi: 10.1111/j.1365-2354.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 28.Jiang R, Yang ZH, Luo DH, Guo L, Sun R, Chen QY, Huang PY, Qiu F, Zou X, Cao KJ, et al. Elevated apolipoprotein A-I levels are associated with favorable prognosis in metastatic nasopharyngeal carcinoma. Med Oncol. 2014;31:80. doi: 10.1007/s12032-014-0080-y. [DOI] [PubMed] [Google Scholar]
- 29.Tachibana M, Ohkura Y, Kobayashi Y, Sakamoto H, Tanaka Y, Watanabe J, Amikura K, Nishimura Y, Akagi K. Expression of apolipoprotein A1 in colonic adenocarcinoma. Anticancer Res. 2003;23:4161–4167. [PubMed] [Google Scholar]


